Abstract
Background
Though vitamin C supplementation has shown no observed effects on stroke prevention in several clinical trials, uncertainty remains as to whether long‐term, low‐dose intake influences the development of stroke among general populations. Furthermore, the association between circulating vitamin C and the risk of stroke is also unclear. For further clarification of these issues, we conducted a meta‐analysis of prospective studies.
Methods and Results
PubMed and EMBASE databases were searched, and the bibliographies of the retrieved articles were also reviewed to identify eligible studies. Summary relative risk (RRs) with corresponding 95% confidence intervals (CIs) were computed with a random‐effects model. The summary RR for the high‐versus‐low categories was 0.81 (95% CI: 0.74 to 0.90) for dietary vitamin C intake (11 studies), and 0.62 (95% CI: 0.49 to 0.79) for circulating vitamin C (6 studies). The summary RR for each 100 mg/day increment in dietary vitamin C was 0.83 (95% CI: 0.75 to 0.93) (10 studies), and for each 20 μmol/L increment in circulating vitamin C was 0.81 (95% CI: 0.75 to 0.88) (5 studies). Few studies reported results for vitamin C supplements (RR for high‐versus‐low intake=0.83, 95% CI: 0.62 to 1.10, 3 studies).
Conclusions
This meta‐analysis suggests significant inverse relationships between dietary vitamin C intake, circulating vitamin C, and risk of stroke.
Keywords: antioxidants, diet, meta‐analysis, prevention, stroke
Introduction
Stroke remains the second leading cause of death globally and the most common cause of disability in adults in most regions. In the United States alone, it is estimated that there are 795 000 people who experience a new or recurrent stroke each year,1 and the direct and indirect cost of stroke for 2010 is $73.7 billion.1
Multiple lines of evidence have demonstrated that a high consumption of fruits and vegetables, which are a major dietary source of antioxidants, is associated with a reduction in the risk of stroke,2 leading to great interest in the role of antioxidants in the etiology of stroke. Vitamin C is an effective antioxidant shown to have blood pressure‐lowering effects.3–4 While several phase‐III randomized controlled trials (RCTs) have suggested that vitamin C supplementation has no effect on stroke prevention,5 the trials have tended to enroll high‐risk populations, have short duration, and use high doses. Therefore, they cannot answer the question of whether long‐term and low‐dose exposure influences the development of this disease among general populations.
A number of epidemiologic studies to date have provided us with encouraging, but still inconclusive findings that vitamin C intake may reduce risk of stroke. In particular, some have focused on how diet may be associated with a decreased risk of stroke.6–17 Furthermore, circulating vitamin C, a good indicator of a diet rich in fruits and vegetables,18 has also been indicated to be inversely associated with stroke risk,9,19–22 but the reported results so far have not totally been consistent. To better understand these subjects, we took up this meta‐analysis of prospective studies in order to characterize the association between vitamin C intake, circulating vitamin C, and risk of stroke.
Methods
Literature Search
We performed a literature search through April 2013 on PubMed (http://www.ncbi.nlm.nih.gov/pubmed) and EMBASE (http://www.embase.com/home) using the search terms “vitamin C or ascorbate or antioxidant combined with stroke or cerebrovascular disorders or cerebrovascular disease or intracranial hemorrhage or brain hemorrhage and cohort or prospective or follow‐up or nested case‐control” with no language restrictions imposed. We also comprehensively reviewed the reference lists of the retrieved publications to identify any additional relevant studies. We also contacted authors of the primary articles for additional information.
Study Selection
Studies were included if they met the following criteria: (1) the study had a prospective design; (2) the exposure of interest was vitamin C intake or circulating vitamin C; (3) the outcome of interest was stroke; and (4) the relative risk (RR) estimates (or odds ratios [OR] in nested case‐control studies) with corresponding 95% confidence interval (CI) were provided. When multiple published publications from the same study were available, we included the one with the largest events in the meta‐analysis. Studies on multivitamin use only were excluded.
Data Extraction
The following data were extracted from each included eligible study using a standardized data‐collection form: the first author's last name, publication year, study location, length of follow‐up, sex and age of participants, number of cases and participants, stroke characteristics (fatal or nonfatal), sources of vitamin C, levels of exposure, the maximally adjusted RR or OR of stroke and corresponding 95% CI for each category of exposure, and variables controlled for in the analysis. Literature search and data extraction were conducted independently by 2 authors (Drs. Chen and Liu) with any disagreements resolved by consensus.
Statistical Analysis
We used a DerSimonian and Laird random‐effects model,23 which considers both within‐ and between‐study variation to calculate the summary‐risk estimate. The ORs in the nested case‐control study were considered approximations of RRs. In case of studies that did not report overall risk estimates, but separately presented results for stroke subtypes10 or for men and women,12 we combined the results using a fixed‐effects model and then included the pooled RR estimates in the primary analysis. For one study19 on circulating vitamin C, which presented results by plasma carotene levels, we also combined the results. For better comparability between studies, we pooled RR estimates by sources of vitamin C intake (foods or supplements). We also performed a sensitivity analysis using the results of total vitamin C in 2 studies6,15 combined with the results of dietary intake in others to test the stability of the summary risk estimates.
We also conducted several subanalyses according to geographic area, length of follow‐up, number of cases, characteristics of stroke (fatal or nonfatal), sex, and subtypes of stroke to examine the potential effect modification of these factors when there were sufficient number of studies.
We also conducted a dose‐response analysis using the method proposed by Greenland and Longnecker24 and Orsini et al.25 This method requires the number of cases/person‐years (or controls in nested case‐control studies) as well as the risk estimates with their variance estimates for at least 3 quantitative exposure categories. In cases where the studies did not report these data, we contacted relevant authors to request the data. If we did not receive a response, we estimated them according to the reported information. When the risk estimates were reported as a continuous variable,21 the reported results were used in the analysis. We also examined a potential nonlinear relationship by modeling vitamin C intake or circulating vitamin C using restricted cubic splines with 3 knots at 10%, 50%, and 90% of the distribution.26 A P value for nonlinearity was calculated by testing the null hypothesis that the coefficient of the second spline was equal to zero. Dose‐response analysis would be performed when the number of eligible studies was ≥3.
A heterogeneity test was performed by use of Q and I2 statistics.27 For the Q statistic, a P value of <0.1 was considered statistically significant heterogeneity. Potential publication bias was investigated by use of Egger's regression asymmetry test.28 This test was conducted when the number of studies was ≥5. All statistical analyses were done using STATA software, version 11.0 (STATA corp.). All P values were 2‐sided and the level of significance was <0.05, unless explicitly stated.
Results
Literature Search
A flow chart showing the details of study selection is shown in Figure 1. Briefly, 14 articles on vitamin C intake and 7 articles on circulating vitamin C in relation to risk of stroke were identified. One article29 on vitamin C intake was excluded because it was a duplicate report of another paper15 with a larger event; 1 publication30 concerning circulating vitamin C was excluded for the same reason. We further excluded 1 article31 on multivitamin use. At last, 12 prospective studies6–17 on vitamin C intake and 6 prospective studies9,19–22 on circulating vitamin C were included in the meta‐analysis.
Figure 1.
Flow chart of study selection.
Study Characteristics
Table 1 shows the characteristics of the 12 prospective studies on vitamin C intake. These studies contained a total of 217 454 participants and 3762 stroke events, and were published between 1995 and 2011. Half of the 12 studies were conducted in Europe, 3 were carried out in the United States, and the remaining 3 were from Asia. The outcome was fatal stroke only in 4 studies, and was both fatal and nonfatal strokes in 8 studies. Five studies included men only, 5 studies included both men and women, and 2 studies included women only. The study duration ranged between 6.1 and 30 years. The average dietary vitamin C intake in the high categories ranged between 45 and 375.8 mg/day. Two studies also reported results for total vitamin C intake and the average intake in the high categories was 1167 mg/day in the US study by Ascherio et al6 and 678.7 mg/day in the US study by Yochum et al.15
Table 1.
Characteristics of the Included Prospective Studies on Vitamin C Intake and Stroke Risk
| Study | Year | Location | Duration, Years | Participants | End‐Points | Sources | Vitamin C Intake, mg/day | RR (95% CI), High vs Low | Adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | |||||||||
| Gale et al9 | 1995 | UK | 20 | 730 M/F | 124 TS (fatal) | Foods | 53.4 (T3) | 19.4 (T1) | 0.5 (0.3 to 0.8) | Age, sex, DBP, and serum cholesterol. |
| Keli et al11 | 1996 | Netherlands | 15 | 552 M | 42 TS (Fatal+nonfatal) | Foods | 131.2 (T3) | 59.3 (T1) | 1.21 (0.55 to 2.66) | Age, SBP, serum cholesterol, smoking, intakes of fish, alcohol, and energy. |
| Ross et al13 | 1997 | China | 8 | 1470 M | 245 TS (fatal) | Foods | 45.6 (T3)* | 15.2 (T1)* | 1.1 (0.7 to 1.6) | BMI, education, marital status, smoking, alcohol, and hypertension. |
| Daviglus et al7 | 1997 | USA | 30 | 1843 M | 222 TS (Fatal+nonfatal) | Foods | 258 (Q4) | 48 (Q1) | 0.71 (0.47 to 1.05) | Age, SBP, smoking, BMI, serum cholesterol, intakes of total energy alcohol, and diabetes. |
| Ascherio et al6 | 1999 | USA | 8 | 43738 M | 328 TS, 210 IS, 70 HS (Fatal+nonfatal) | Total | 1167 (Q5) | 95 (Q1) | TS: 0.95 (0.66 to 1.35) IS: 1.03 (0.66 to 1.59) HS: 0.82 (0.36 to 1.89) |
Age, calendar time, smoking, intakes of total energy and alcohol, hypertension, parental history of MI, profession, BMI and physical activity. |
| Supplements | 850 (Q5) | 0 (Q1) | TS: 0.85 (0.59 to 1.24) IS: 0.93 (0.60 to 1.45) HS: 0.67 (0.28 to 1.60) |
|||||||
| Hirvonen et al10 | 2000 | Finland | 6.1 | 26539 M | 736 IS, 95 ICH, 83 SAH (Fatal+nonfatal) | Foods | 141 (Q4) | 52 (Q1) | IS: 0.89 (0.72 to 1.09) ICH: 0.39 (0.21 to 0.74) SAH: 1.16 (0.62 to 2.18) |
Age, supplementation group, SBP, DBP, serum total cholesterol and HDL cholesterol, BMI, height, smoking, history of diabetes or CHD, alcohol intake, and education. |
| Yochum et al15 | 2000 | USA | 11 | 34492 F | 215 TS (fatal) | Total | 678.7 (Q5) | 82.4 (Q1) | 1.23 (0.76 to 1.90) | Age, BMI, waist‐to‐hip ratio, hypertension, diabetes, estrogen replacement therapy, education, marital status, smoking, physical activity, intakes of total energy, cholesterol, alcohol, saturated fat, fish, vitamin E, carotenoids, dietary fiber, and whole grains. |
| Foods | 247.9 (Q5) | 67.2 (Q1) | 0.99 (0.58 to 1.72) | |||||||
| Supplements | 1120 (Q5) | 0 (Q1) | 0.90 (0.36 to 2.19) | |||||||
| Voko et al14 | 2003 | Netherlands | 6.4 | 5159 M/F | 227 (Fatal+nonfatal) | Foods | T3 | T1 | 0.66 (0.46 to 0.93) | Age, sex, total energy intake, smoking, hypertension, diabetes mellitus, history of CHD, transient ischemic attacks, and, in case of vitamin E, polyunsaturated fatty acid intake. |
| Supplements | Yes | No | 0.77 (0.47 to 1.26) | |||||||
| Marniemi et al16 | 2005 | Finland | 10 | 755 M/F | 70 TS (Fatal+nonfatal) | Foods | 113.8 (T3)* | 57.8 (T1)* | 0.99 (0.56 to 1.76) | Age, sex, smoking, functional capacity, and weight‐ adjusted energy intake |
| Weng et al17 | 2008 | Taiwan | 10.6 | 1772 M/F | 132 IS (Fatal+nonfatal) | Foods | 375.8 (Q4+Q3) | 180.7 (Q1) | 0.73 (0.47 to 1.12) | Age, sex, area, smoking, BMI, central obesity, physical activity, diabetes, hypertension, use of antihypertensive drugs, self‐reported heart disease, hypercholesterolemia, hypertriglyceridemia, fibrinogen, apolipoprotein B, plasminogen, alcohol |
| Del Rio et al8 | 2011 | Italy | 7.9 | 41620 M/F | 194 TS, 112 IS, 48 HS (Fatal+nonfatal) | Foods | 201 (T3) | 83 (T1) | TS: 0.89 (0.6 to 1.32) IS: 0.53 (0.31 to 0.89) HS: 1.83 (0.81 to 4.13) |
Age, center, sex, hypertension, smoking, education, energy intake, alcohol, waist circumference, obesity, and physical activity. |
| Kubota et al12 | 2011 | Japan | 16.5 | 23119 M/F | 1227 TS (fatal) | Foods | Men: 145 (Q5) Women: 150 (Q5) |
Men: 52 (Q1) Women: 65 (Q1) |
Men: 0.84 (0.62 to 1.13) Women: 0.70 (0.54 to 0.92) |
Age, hypertension, diabetes, smoking, BMI, mental stress, walking, sports, education, intakes of total energy, alcohol, cholesterol, saturated fatty acids, n‐3 fatty acids, and sodium. |
BMI indicates body mass index; CHD, coronary heart disease; CI, confidence interval; DBP, diastolic blood pressure; F, females; HDL, high‐density lipoprotein; HS, hemorrhagic stroke; ICH, intracerebral hemorrhagic; IS, ischemic stroke; M, males; MI, myocardial infarction; Q, quartile/quintile; RR, relative risk; SAH, subarachnoid hemorrhagic; SBP, systobic blood pressure; T, tertile; TS, total stroke.
The midpoint vitamin intake in the lowest and highest tertiles was estimated as the mean intake (30.4 mg/day)±half of the mean intake among noncases.
The midpoint vitamin intake in the lowest and highest tertiles was estimated as the mean intake (85.8 mg/day)±half of the SD (56 mg/day) among noncases.
Table 2 presents the characteristics of the 6 prospective studies on circulating vitamin C. These studies involved 989 stroke cases and 29 648 participants. They were published between 1993 and 2008 and followed up for 9.5 to 20 years. Five of the 6 studies were from Europe, and the remaining 1 was carried out in Japan. The exposure of interest was serum vitamin C in 2 studies, and plasma vitamin C in the remaining studies. Four studies included both men and women, and 2 studies consisted entirely of men. All provided multivariable‐adjusted risk estimates (Tables 1 and 2).
Table 2.
Characteristics of the Included Prospective Studies on Blood (Plasma or Serum) Vitamin C Levels and Stroke Risk
| Study | Year | Location | Duration, years | Participants | End‐points | Sources | Circulating Vitamin C, μmol/L | RR (95% CI) | Adjustment | |
|---|---|---|---|---|---|---|---|---|---|---|
| High | Low | |||||||||
| Gey et al19 | 1993 | Switzerland | 12 | 2974 M | 31 (fatal) | Plasma | “Normal” | “Low” | Normal carotene: 0.78 (0.24 to 2.5); Low carotene: 0.24 (0.10 to 0.60) |
Age, smoking, BP, cholesterol and carotene. |
| Gale et al9 | 1995 | UK | 20 | 730 M/F | 124 TS (fatal) | Plasma | 35.77 (T3) | 3.96 (T1) | 0.7 (0.4 to 1.1) | Age, sex, DBP, and serum cholesterol. |
| Yokoyama et al22 | 2000 | Japan | 20 | 880 M/F | 196 TS, 109 IS, 54 HS (nonfatal) | Serum | 69.5 (Q4) | 35 (Q1) | TS: 0.71 (0.45 to 1.14) IS: 0.63 (0.34 to 1.18) HS: 0.59 (0.24 to 1.46) |
Age, sex, BP, serum cholesterol, BMI, presence of a trial fibrillation, use of antihypertensive medication, personal history of IHD, physical activity, smoking, and alcohol drinking. |
| Kurl et al20 | 2002 | Finland | 10.4 | 2419 M | 120 TS (Fatal+nonfatal) | Plasma | 73.36 (Q4) | 18.8 (Q1) | 0.48 (0.26 to 0.85) | Age, examination months, BMI, smoking, alcohol, SBP, serum total cholesterol, diabetes, and myocardial ischemia during exercise. |
| Marniemi et al16 | 2005 | Finland | 10 | 755 M/F | 70 TS (Fatal+nonfatal) | Serum | 6.5 (T3)* | 3.6 (T1)* | 1.07 (0.59 to 1.93) | Age, sex, smoking, functional capacity. |
| Myint et al21 | 2008 | UK | 9.5 | 20649 M/F | 448 TS (Fatal+nonfatal) | Plasma | 71.5 (Q4) | 35 (Q1) | 0.57 (0.42 to 0.76) | Age, sex, smoking, BMI, SBP, cholesterol, physical activity, MI, diabetes, social class, vitamin supplement use, and intakes of alcohol, fruit and vegetable. |
BMI indicates body mass index; BP, blood pressure; CI, confidence interval; DBP, diastolic blood pressure; F, females; HS, hemorrhagic stroke; IHD, ischemic heart disease; IS, ischemic stroke; M, males; MI, myocardial infarction; Q, quartile; RR, relative risk; SBP, systobic blood pressure; T, tertile; TS, total stroke.
The midpoint circulating vitamin C in the lowest and highest tertiles was estimated as the mean value (5.05 μmol/L)±half of the SD (2.9 μmol/L) among noncases.
Dietary Vitamin C
Eleven studies7–17 examined the association of vitamin C intake from foods with risk of stroke. The summary RR for the high compared with low dietary vitamin C intake was 0.81 (95% CI: 0.74 to 0.90), with no evidence of heterogeneity (Pheterogeneity=0.71, I2=0.0%) (Figure 2A). There was little evidence of publication bias with Egger's test (PEgger's=0.39).
Figure 2.
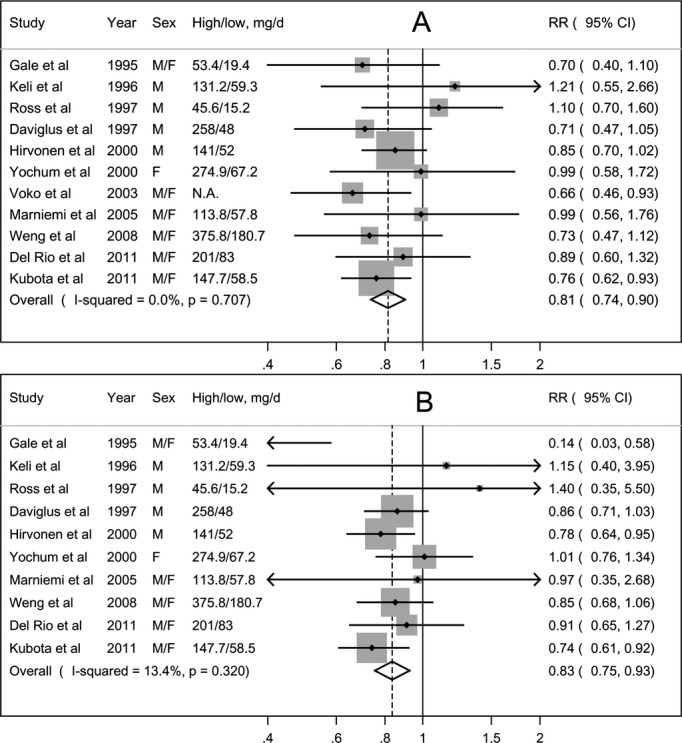
Meta‐analysis of dietary vitamin C intake and risk of stroke. A, high vs low analysis; (B) dose‐response analysis. F indicates women; M, men; N.A., not available; RR, relative risk.
One study14 was not eligible for the dose‐response analysis. The combined results of the remaining 10 studies suggested a summary RR of 0.83 (95% CI: 0.75 to 0.93) for an increment in dietary vitamin C intake of 100 mg/day, with little heterogeneity (Pheterogeneity=0.32, I2=13.4%) (Figure 2B). There was no evidence of a nonlinear association (Pnonlinearity=0.17).
Supplemental Vitamin C
Three studies6,14–15 covering 770 stroke cases reported results for supplemental vitamin C intake. The summary RR for the high‐versus‐low intake was 0.83 (95% CI: 0.62 to 1.10), with no heterogeneity (Pheterogeneity=0.94, I2=0.0%). Only 2 studies6,15 provided adequate data for the dose‐response analysis.
Circulating Vitamin C
A pooled analysis of 6 studies showed that participants with a higher level of circulating vitamin C had a 38% lower risk of stroke (RR=0.62, 95% CI: 0.49 to 0.79) compared with those with a lower level, with low heterogeneity (Pheterogeneity=0.23, I2=27.6%) (Figure 3A). Further omitting one small study16 with a very narrow range of exposure, the summary RR was 0.58 (95% CI: 0.48 to 0.71). There was no suggestion of publication bias (PEgger's=0.93).
Figure 3.
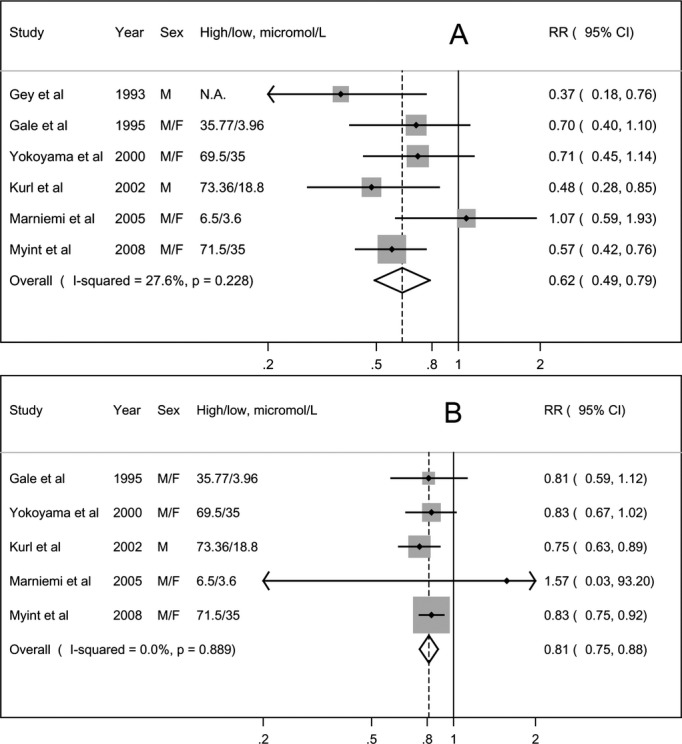
Meta‐analysis of circulating vitamin C and risk of stroke. A, high vs low analysis; (B) dose‐response analysis. F indicates women; M, men; N.A., not available; RR, relative risk.
Five studies9,16,20–22 were included in the dose‐response analysis. Combined results suggested that a 20 μmol/L increment in circulating vitamin C was associated with a 19% decreased risk of stroke (RR=0.81, 95% CI: 0.75 to 0.88), with no evidence of heterogeneity (Pheterogeneity=0.89, I2=0.0%) (Figure 3B). We observed no evidence of a nonlinear association (Pnonlinearity=0.13).
Subgroup and Sensitivity Analyses (for Vitamin C Intake)
The observed inverse association between high dietary vitamin C intake and risk of stroke was not significantly modified by geographic area, length of follow‐up, number of cases, characteristics of stroke (fatal or nonfatal), sex, and subtypes of stroke (Table 3). In addition, omitting any single study did not remarkably alter the summary risk estimates (RRs ranged between 0.80 and 0.83). Too few studies reported results for women and for hemorrhagic stroke.
Table 3.
Subgroup Analyses of Dietary Vitamin C Intake and Stroke, High vs Low Intake
| N | RR (95% CI) | Heterogeneity | P interaction | ||
|---|---|---|---|---|---|
| P Value | I2 (%) | ||||
| All studies | 11 | 0.81 (0.74 to 0.90) | 0.71 | 0.0 | |
| Geographic Area | |||||
| Europe | 6 | 0.83 (0.72 to 0.95) | 0.63 | 0.0 | 0.78 |
| Other areas | 5 | 0.80 (0.69 to 0.93) | 0.46 | 0.0 | |
| Years of Follow‐Up | |||||
| ≥10 years | 7 | 0.78 (0.68 to 0.91) | 0.79 | 0.0 | 0.49 |
| <10 years | 4 | 0.85 (0.72 to 1.00) | 0.32 | 14.9 | |
| Number of Cases | |||||
| >200 | 6 | 0.81 (0.72 to 0.91) | 0.42 | 0.0 | 0.77 |
| <200 | 5 | 0.84 (0.67 to 1.05) | 0.71 | 0.0 | |
| Outcome | |||||
| Fatal | 5 | 0.81 (0.69 to 0.95) | 0.50 | 0.0 | 0.99 |
| Fatal and nonfatal | 7 | 0.81 (0.71 to 0.93) | 0.69 | 0.0 | |
| Sex | |||||
| Men | 5 | 0.86 (0.75 to 0.99) | 0.56 | 0.0 | 0.29 |
| Women | 2 | 0.77 (0.57 to 1.03) | 0.26 | 20.6 | |
| Men and women | 5 | 0.76 (0.63 to 0.93) | 0.70 | 0.0 | |
| Stroke Subtypes | |||||
| Ischemic stroke | 4 | 0.77 (0.64 to 0.92) | 0.30 | 18.5 | 0.55 |
| Hemorrhagic stroke | 2 | 1.07 (0.38 to 3.00) | 0.03 | 79.3 | |
CI indicates confidence interval; RR, relative risk.
We conducted a separate analysis using the results of total vitamin C in 2 studies6,15 and those of dietary vitamin C in other studies. Thus, this separate analysis was based on 12 prospective studies.6–17 The summary RR for the high‐versus‐low vitamin C intake was 0.83 (95% CI, 0.76 to 0.92). In the dose‐response analysis, an increase in vitamin C intake of 100 mg/day was marginally associated with a reduced risk of stroke (RR=0.91, 95% CI: 0.83 to 1.00), with considerable heterogeneity (Pheterogeneity=0.001, I2=66.9%). There was a somewhat U‐shaped association between vitamin C intake and risk of stroke (Pnonlinearity=0.0001) (Figure 4), with the greatest risk reduction observed at an intake of ≈200 mg/day, and remained protective until an intake of ≈550 mg/day. However, data points become especially sparse above intakes of 200 mg/day, and so the results for higher intakes should be treated with caution.
Figure 4.
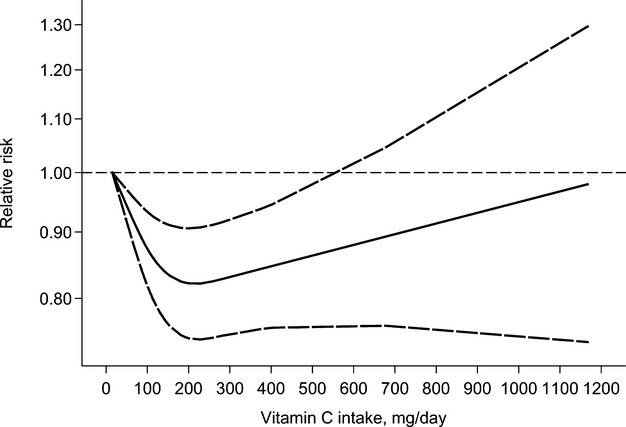
Relative risk (solid line) with 95% confidence interval (long dashed lines) for the association between vitamin C intake (total or dietary intake) and risk of stroke in a restricted cubic spline random‐effects meta‐analysis. The lowest intake of 15.2 mg/day was used to estimate all relative risks.
Discussion
The findings from this meta‐analysis of prospective studies show that both dietary vitamin C intake and circulating vitamin C are significantly inversely associated with the risk of stroke in a dose‐response manner. Supplemental vitamin C intake was not significantly related to a reduced risk of stroke, but the analysis was based on a limited number of studies (3 studies) and stroke cases (770 cases).
Several putative mechanisms whereby vitamin C protects against stroke have been proposed. Vitamin C is a strong antioxidant, and has been shown to reduce the oxidation of low‐density lipoproteins, to inhibit the proliferation of smooth muscle, to protect membrane from peroxidation, and ultimately to slow the progression of atherosclerosis.32–35 There is also growing evidence that systemic inflammation is involved in stroke etiology and pathology,36 and plasma or dietary vitamin C has been suggested to have antiinflammatory properties.37 Vitamin C intake in plasma has also been demonstrated to be inversely associated with blood pressure.4 Hence, vitamin C may reduce stroke risk through its pressure‐lowering effects.
A large body of human clinical trials have been conducted to assess the effect of vitamin C or other traditional antioxidant‐related vitamins or minerals on prevention of a multitude of chronic diseases, such as type 2 diabetes,38 stroke and other cardiovascular diseases,5 and certain cancers.39 However, the trials generally failed to produce convincing evidence to justify the effectiveness of these nutrients.
There are only 4 RCTs40–43 that evaluate the role of vitamin C alone, or in combination with other antioxidants, for primary or secondary prevention of stroke, and all but one42 of them have reported negative findings. Even then, it is noteworthy that there are several differences between the observational studies and the RCTs which may, to some extent, explain the disparate findings between them. Individuals who took vitamin C supplementation in the RCTs were usually high‐risk rather than general populations. Besides, dietary intake represents long‐term habitual exposure, whereas supplementation is generally characterized by shorter duration and higher dose. Thus, if the effect of vitamin C is restricted to the early stage of the disease, the trials enrolling high‐risk participants or with insufficient follow‐up duration may not be able to detect any significant associations.
Furthermore, circulating vitamin C, a more accurate indicator of body vitamin C status, has been shown to be saturable, with a linear increase only observed at vitamin C intakes of <100 mg/day; above 100 mg/day, there is little change in blood concentration despite large changes in dose.44 In this respect, for subjects who already have high or saturating vitamin C in their blood (obtained from diet), supplementation may not be expected to provide additional benefits, and those with high concentrations may also be categorized as a placebo group (this is particularly likely to have occurred in the RCTs that tended to recruit health‐conscious individuals), which could have limited the statistical power of the trials. Further support for this threshold hypothesis comes from the data of the Linxian General Population Nutrition Intervention Trial.42 In this trial, a nutritionally deprived population (probably with low concentrations of circulating vitamin C) taking 120 mg/day of vitamin C supplementation (combined with molybdenum of 30 μg/day) had a significantly lower risk of death from stroke (RR=0.92, 95% CI: 0.86 to 0.99). While a recent meta‐analysis documented that vitamin C supplementation with a median dose of 500 mg/day may have been effective on reducing blood pressure, the duration of the primary RCTs was very short (median duration was 8 weeks).3
On the other hand, it is vitally important to notice that observational studies lack the experimental random allocation of the intervention necessary to test exposure—outcome hypotheses optimally, and so the inverse associations observed in this meta‐analysis may be confounded by unmeasured social and behavioral factors. Those subjects with higher dietary vitamin C intake or higher circulating vitamin C levels would also generally engage in other healthy behaviors; by contrast, those with lower intake or low circulating levels tended to be more likely to have unfavorable lifestyle habits. Although most primary studies had adjusted for multiple variables including smoking, alcohol drinking, dietary cholesterol/circulating cholesterol levels, body mass index/obesity, history of diabetes, and the potential intermediate of hypertension/blood pressure, nearly all studies did not control for many other potential key dietary confounders that may influence the development of stroke, such as dietary fiber, whole grains, nuts, salt, and red and processed meat, etc. Therefore, the observed effect of vitamin C on stroke reduction may simply be a proxy for specific foods (eg, fruits and vegetables) that causally lower stroke, or a proxy for specific foods that themselves are markers for other factors (other dietary or lifestyle habits) that causally lower stroke, but not due to vitamin C per se.
Furthermore, it is clear that dietary or circulating vitamin C marks fruit/vegetable intake.18,45 In light of the neutral effects of vitamin C on stroke prevention documented in RCTs,5 even if vitamin C is one of the causal components in fruits and vegetables, it is also plausible that its consumption as part of a matrix of other nutrients in foods may be essential for its benefits.
This meta‐analysis has several strengths. All studies included are of a prospective design, which eliminates the possibility of recall and selection biases. In addition, most of the studies included had a long‐duration of follow‐up. As the single prospective studies were mainly of limited power to prove statistical significance, this meta‐analysis involving a large number of stroke cases enhances the statistical power to assess the long‐term effects of dietary vitamin C intake and circulating vitamin C on stroke development.
However, apart from the previously described limitations, there are a number of other limitations that merit discussion. First, most included studies assessed exposure of interests only at baseline, and recorded diet intake with a self‐administered food frequency questionnaire, which may have led to some misclassification of exposure and, therefore, resulted in an underestimation of the risk estimates. Second, the characteristics of subjects in the primary studies were not always comparable, and the reference categories also varied widely. Third, some of the stratified analyses such as sex‐specific and stroke subtypes analyses for dietary vitamin C intake were based on a limited number of studies. Fourth, dietary intake in the high category was in general <250 mg/day in the primary studies, which could limit the generalizability of our findings. Finally, publication bias could be of concern as this meta‐analysis was based on published literature. In this meta‐analysis, however, little evidence of such bias was found.
Our findings may be of several implications. To date, the recommended dietary allowance for vitamin C has been largely inconsistent among countries, generally ranging from 40 to 110 mg/day.46 Though current clinical evidence does not recommend vitamin C supplementation to prevent stroke, from a public health point of view, for populations with low intake and for those who are at high risk, increasing consumption of vitamin C‐rich foods (eg, fruits and vegetables) and adhering to other healthy dietary habits and lifestyles can lead to substantial reductions in the burden of stroke and other cardiovascular diseases. Furthermore, given that the established risk factors for stroke appear to be responsible for only half of the incident cases,47 our findings indicate that circulating concentrations of vitamin C may serve as a good predictor of stroke risk and diet status.
Our findings also raised several intriguing questions: Is there a threshold for vitamin C intake to prevent stroke? Is the beneficial effect of vitamin C against stroke (if any) limited to populations with nutritional deficiencies (or with low vitamin C concentrations in the blood)? Future prospective epidemiologic studies conducted among high‐intake populations with better adjustment for potential confounders, as well as well‐designed RCTs using high‐circulating vitamin C concentrations as an exclusion criteria may help to further elucidate these issues.
In summary, findings from this meta‐analysis suggest significant inverse dose‐response relationships between dietary vitamin C intake, circulating vitamin C, and risk of stroke. In view of evidence from these RCTs, it is clearly premature to recommend supplementation of vitamin C (or other antioxidants) to prevent stroke, and the prevention of this disease should largely lie in the modification of lifestyle habits, as well as effective therapies lowering risk factors for stroke.
Sources of Funding
This study is supported in part by Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).
Disclosures
None.
Acknowledgments
We thank the authors who kindly provided us with original unpublished data.
References
- 1.Lloyd‐Jones D, Adams RJ, Brown TM, Carnethon M, Dai S, De Simone G, Ferguson TB, Ford E, Furie K, Gillespie C, Go A, Greenlund K, Haase N, Hailpern S, Ho PM, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott MM, Meigs J, Mozaffarian D, Mussolino M, Nichol G, Roger VL, Rosamond W, Sacco R, Sorlie P, Thom T, Wasserthiel‐Smoller S, Wong ND, Wylie‐Rosett J. Heart disease and stroke statistics—2010 update: a report from the American Heart Association. Circulation. 2010; 121:e46-e215 [DOI] [PubMed] [Google Scholar]
- 2.He FJ, Nowson CA, MacGregor GA. Fruit and vegetable consumption and stroke: meta‐analysis of cohort studies. Lancet. 2006; 367:320-326 [DOI] [PubMed] [Google Scholar]
- 3.Juraschek SP, Guallar E, Appel LJ, Miller ER., III Effects of vitamin C supplementation on blood pressure: a meta‐analysis of randomized controlled trials. Am J Clin Nutr. 2012; 95:1079-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Block G, Jensen CD, Norkus EP, Hudes M, Crawford PB. Vitamin C in plasma is inversely related to blood pressure and change in blood pressure during the previous year in young Black and White women. Nutr J. 2008; 7:35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Myung SK, Ju W, Cho B, Oh SW, Park SM, Koo BK, Park BJ. Efficacy of vitamin and antioxidant supplements in prevention of cardiovascular disease: systematic review and meta‐analysis of randomised controlled trials. BMJ. 2013; 346:f10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascherio A, Rimm EB, Hernan MA, Giovannucci E, Kawachi I, Stampfer MJ, Willett WC. Relation of consumption of vitamin E, vitamin C, and carotenoids to risk for stroke among men in the United States. Ann Intern Med. 1999; 130:963-970 [DOI] [PubMed] [Google Scholar]
- 7.Daviglus ML, Orencia AJ, Dyer AR, Liu K, Morris DK, Persky V, Chavez N, Goldberg J, Drum M, Shekelle RB, Stamler J. Dietary vitamin C, beta‐carotene and 30‐year risk of stroke: results from the Western Electric Study. Neuroepidemiology. 1997; 16:69-77 [DOI] [PubMed] [Google Scholar]
- 8.Del Rio D, Agnoli C, Pellegrini N, Krogh V, Brighenti F, Mazzeo T, Masala G, Bendinelli B, Berrino F, Sieri S, Tumino R, Rollo PC, Gallo V, Sacerdote C, Mattiello A, Chiodini P, Panico S. Total antioxidant capacity of the diet is associated with lower risk of ischemic stroke in a large Italian cohort. J Nutr. 2011; 141:118-123 [DOI] [PubMed] [Google Scholar]
- 9.Gale CR, Martyn CN, Winter PD, Cooper C. Vitamin C and risk of death from stroke and coronary heart disease in cohort of elderly people. BMJ. 1995; 310:1563-1566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hirvonen T, Virtamo J, Korhonen P, Albanes D, Pietinen P. Intake of flavonoids, carotenoids, vitamins C and E, and risk of stroke in male smokers. Stroke. 2000; 31:2301-2306 [DOI] [PubMed] [Google Scholar]
- 11.Keli SO, Hertog MG, Feskens EJ, Kromhout D. Dietary flavonoids, antioxidant vitamins, and incidence of stroke: the Zutphen study. Arch Intern Med. 1996; 156:637-642 [PubMed] [Google Scholar]
- 12.Kubota Y, Iso H, Date C, Kikuchi S, Watanabe Y, Wada Y, Inaba Y, Tamakoshi A. Dietary intakes of antioxidant vitamins and mortality from cardiovascular disease: the Japan Collaborative Cohort Study (JACC) study. Stroke. 2011; 42:1665-1672 [DOI] [PubMed] [Google Scholar]
- 13.Ross RK, Yuan JM, Henderson BE, Park J, Gao YT, Yu MC. Prospective evaluation of dietary and other predictors of fatal stroke in Shanghai, China. Circulation. 1997; 96:50-55 [DOI] [PubMed] [Google Scholar]
- 14.Voko Z, Hollander M, Hofman A, Koudstaal PJ, Breteler MM. Dietary antioxidants and the risk of ischemic stroke: the Rotterdam Study. Neurology. 2003; 61:1273-1275 [DOI] [PubMed] [Google Scholar]
- 15.Yochum LA, Folsom AR, Kushi LH. Intake of antioxidant vitamins and risk of death from stroke in postmenopausal women. Am J Clin Nutr. 2000; 72:476-483 [DOI] [PubMed] [Google Scholar]
- 16.Marniemi J, Alanen E, Impivaara O, Seppänen R, Hakala P, Rajala T, Rönnemaa T. Dietary and serum vitamins and minerals as predictors of myocardial infarction and stroke in elderly subjects. Nutr Metab Cardiovasc Dis. 2005; 15:188-197 [DOI] [PubMed] [Google Scholar]
- 17.Weng L‐C, Yeh W‐T, Bai C‐H, Chen H‐J, Chuang S‐Y, Chang H‐Y, Lin B‐F, Chen K‐J, Pan W‐H. Is ischemic stroke risk related to folate status or other nutrients correlated with folate intake? Stroke. 2008; 39:3152-3158 [DOI] [PubMed] [Google Scholar]
- 18.Block G, Norkus E, Hudes M, Mandel S, Helzlsouer K. Which plasma antioxidants are most related to fruit and vegetable consumption? Am J Epidemiol. 2001; 154:1113-1118 [DOI] [PubMed] [Google Scholar]
- 19.Gey KF, Stahelin HB, Eichholzer M. Poor plasma status of carotene and vitamin C is associated with higher mortality from ischemic heart disease and stroke: Basel Prospective Study. Clin Investig. 1993; 71:3-6 [DOI] [PubMed] [Google Scholar]
- 20.Kurl S, Tuomainen TP, Laukkanen JA, Nyyssonen K, Lakka T, Sivenius J, Salonen JT. Plasma vitamin C modifies the association between hypertension and risk of stroke. Stroke. 2002; 33:1568-1573 [DOI] [PubMed] [Google Scholar]
- 21.Myint PK, Luben RN, Welch AA, Bingham SA, Wareham NJ, Khaw KT. Plasma vitamin C concentrations predict risk of incident stroke over 10 y in 20 649 participants of the European Prospective Investigation into Cancer Norfolk prospective population study. Am J Clin Nutr. 2008; 87:64-69 [DOI] [PubMed] [Google Scholar]
- 22.Yokoyama T, Date C, Kokubo Y, Yoshiike N, Matsumura Y, Tanaka H. Serum vitamin C concentration was inversely associated with subsequent 20‐year incidence of stroke in a Japanese rural community. The Shibata study. Stroke. 2000; 31:2287-2294 [DOI] [PubMed] [Google Scholar]
- 23.DerSimonian R, Laird N. Meta‐analysis in clinical trials. Control Clin Trials. 1986; 7:177-188 [DOI] [PubMed] [Google Scholar]
- 24.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose‐response data, with applications to meta‐analysis. Am J Epidemiol. 1992; 135:1301-1309 [DOI] [PubMed] [Google Scholar]
- 25.Orsini N, Bellocco R, Greenland S. Generalized least squares for trend estimation of summarized dose‐respose data. Stata J. 2006; 6:40-57 [Google Scholar]
- 26.Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. Meta‐analysis for linear and nonlinear dose‐response relations: examples, an evaluation of approximations, and software. Am J Epidemiol. 2012; 175:66-73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Stat Med. 2002; 21:1539-1558 [DOI] [PubMed] [Google Scholar]
- 28.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ. 1997; 315:629-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee DH, Folsom AR, Harnack L, Halliwell B, Jacobs DR., Jr Does supplemental vitamin C increase cardiovascular disease risk in women with diabetes? Am J Clin Nutr. 2004; 80:1194-1200 [DOI] [PubMed] [Google Scholar]
- 30.Eichholzer M, Stahelin HB, Gey KF. Inverse correlation between essential antioxidants in plasma and subsequent risk to develop cancer, ischemic heart disease and stroke respectively: 12‐year follow‐up of the Prospective Basel Study. EXS. 1992; 62:398-410 [DOI] [PubMed] [Google Scholar]
- 31.Watkins ML, Erickson JD, Thun MJ, Mulinare J, Heath CW., Jr Multivitamin use and mortality in a large prospective study. Am J Epidemiol. 2000; 152:149-162 [DOI] [PubMed] [Google Scholar]
- 32.Witztum JL. The oxidation hypothesis of atherosclerosis. Lancet. 1994; 344:793-795 [DOI] [PubMed] [Google Scholar]
- 33.Cherubini A, Vigna GB, Zuliani G, Ruggiero C, Senin U, Fellin R. Role of antioxidants in atherosclerosis: epidemiological and clinical update. Curr Pharm Des. 2005; 11:2017-2032 [DOI] [PubMed] [Google Scholar]
- 34.Fairfield KM, Fletcher RH. Vitamins for chronic disease prevention in adults: scientific review. JAMA. 2002; 287:3116-3126 [DOI] [PubMed] [Google Scholar]
- 35.Padayatty SJ, Katz A, Wang Y, Eck P, Kwon O, Lee JH, Chen S, Corpe C, Dutta A, Dutta SK, Levine M. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003; 22:18-35 [DOI] [PubMed] [Google Scholar]
- 36.McColl BW, Allan SM, Rothwell NJ. Systemic inflammation and stroke: aetiology, pathology and targets for therapy. Biochem Soc Trans. 2007; 35:1163-1165 [DOI] [PubMed] [Google Scholar]
- 37.Wannamethee SG, Lowe GD, Rumley A, Bruckdorfer KR, Whincup PH. Associations of vitamin C status, fruit and vegetable intakes, and markers of inflammation and hemostasis. Am J Clin Nutr. 2006; 83:567-574‐ [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Cook NR, Albert CM, Van Denburgh M, Manson JE. Effects of vitamins C and E and beta‐carotene on the risk of type 2 diabetes in women at high risk of cardiovascular disease: a randomized controlled trial. Am J Clin Nutr. 2009; 90:429-437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Goodman M, Bostick RM, Kucuk O, Jones DP. Clinical trials of antioxidants as cancer prevention agents: past, present, and future. Free Radic Biol Med. 2011; 51:1068-1084 [DOI] [PubMed] [Google Scholar]
- 40. MRC/BHF Heart Protection Study of antioxidant vitamin supplementation in 20,536 high‐risk individuals: a randomised placebo‐controlled trial. Lancet. 2002; 360:23-33 [DOI] [PubMed] [Google Scholar]
- 41.Cook NR, Albert CM, Gaziano JM, Zaharris E, MacFadyen J, Danielson E, Buring JE, Manson JE. A randomized factorial trial of vitamins C and E and beta carotene in the secondary prevention of cardiovascular events in women: results from the Women's Antioxidant Cardiovascular Study. Arch Intern Med. 2007; 167:1610-1618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD, Johnson LL, Gail MH, Dong ZW, Yu B, Mark SD, Taylor PR. Total and cancer mortality after supplementation with vitamins and minerals: follow‐up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009; 101:507-518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sesso HD, Buring JE, Christen WG, Kurth T, Belanger C, MacFadyen J, Bubes V, Manson JE, Glynn RJ, Gaziano JM. Vitamins E and C in the prevention of cardiovascular disease in men: the Physicians' Health Study II randomized controlled trial. JAMA. 2008; 300:2123-2133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine M, Wang Y, Padayatty SJ, Morrow J. A new recommended dietary allowance of vitamin C for healthy young women. Proc Natl Acad Sci USA. 2001; 98:9842-9846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Michels KB, Welch AA, Luben R, Bingham SA, Day NE. Measurement of fruit and vegetable consumption with diet questionnaires and implications for analyses and interpretation. Am J Epidemiol. 2005; 161:987-994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frei B, Birlouez‐Aragon I, Lykkesfeldt J. Authors' perspective: what is the optimum intake of vitamin C in humans? Crit Rev Food Sci Nutr. 2012; 52:815-829 [DOI] [PubMed] [Google Scholar]
- 47.Bevan S, Porteous L, Sitzer M, Markus HS. Phosphodiesterase 4D gene, ischemic stroke, and asymptomatic carotid atherosclerosis. Stroke. 2005; 36:949-953 [DOI] [PubMed] [Google Scholar]


