Abstract
Amyotrophic lateral sclerosis (ALS) is a devastating paralytic disorder caused by dysfunction and degeneration of motor neurons starting in adulthood. Most of our knowledge about the pathophysiological mechanisms of ALS comes from transgenic mice models that emulate a subgroup of familial ALS cases (FALS), with mutations in the gene encoding superoxide dismutase (SOD1). In the more than 15 years since these mice were generated, a large number of abnormal cellular mechanisms underlying motor neuron degeneration have been identified, but to date this effort has led to few improvements in therapy, and no cure. Here, we consider that this surfeit of mechanisms is best interpreted by current insights that suggest a very early initiation of pathology in motor neurons, followed by a diversity of secondary cascades and compensatory mechanisms that mask symptoms for decades, until trauma and/or aging overloads their protective function. This view thus posits that adultonset ALS is the consequence of processes initiated during early development. In fact, motor neurons in neonatal mutant SOD mice display important alterations in their intrinsic electrical properties, synaptic inputs and morphology that are accompanied by subtle behavioral abnormalities. We consider evidence that human mutant SOD1 protein in neonatal hSOD1G93A mice instigates motor neuron degeneration by increasing persistent sodium currents and excitability, in turn altering synaptic circuits that control excessive motor neuron firing and leads to excitotoxicity. We also discuss how therapies that are aimed at suppressing abnormal neuronal activity might effectively mitigate or prevent the onset of irreversible neuronal damage in adulthood.
Keywords: ALS, SYNAPSE, EXCITOTOXICITY, PATHOLOGY, COMPENSATION, SODIUM CHANNELS
Patients with amyotrophic lateral sclerosis (ALS), Alzheimer’s disease (AD), Parkinson’s disease (PD), Huntington’s disease (HD), and other neurodegenerative disorders typically start to display clinical symptoms during adulthood. Groundbreaking genetic studies in humans, rodents, Caenorhabditis elegans, Drosophila, and yeast have identified mutations in genes such as SOD1, FUS/TLS and TDP-43 (for ALS), APP and presenilin (for AD), parkin and alpha-synuclein (for PD), and huntingtin (for HD), as being responsible for causing these diseases in adults, even though the mutation is present throughout life. Despite remarkable advances in understanding the diseases, no mechanism-based cures are currently available. This unfortunate situation is mainly due to the fact that the primary target(s) of the mutant proteins are not known, in part because early and ubiquitous expression of the aberrant genes in the developing CNS leads to many secondary effects and triggers compensatory mechanisms that mask the primary pathological event. Thus, although the primary disease process might be present throughout life, onset of symptoms coincides with the saturation of protection and compensation mechanisms, either by accumulation of dysfunction or via other pathogenic events such as trauma and environmental factors, which may be aggravated by aging [DeKosky and Marek, 2003; Palop et al., 2006]. To enable identification of mutant gene-driven mechanisms that trigger the cascade of events that culminate in degeneration and death later in life, a first step is to establish exactly when the neuropathology is initiated. The fact that the expression of the disease-causative proteins starts during embryonic stages raises the intriguing possibility that pathological changes in patients with adult-onset neurodegenerative disorders are triggered much earlier—in humans, such alterations may occur decades, and in mouse models months—prior to the manifestation of symptoms.
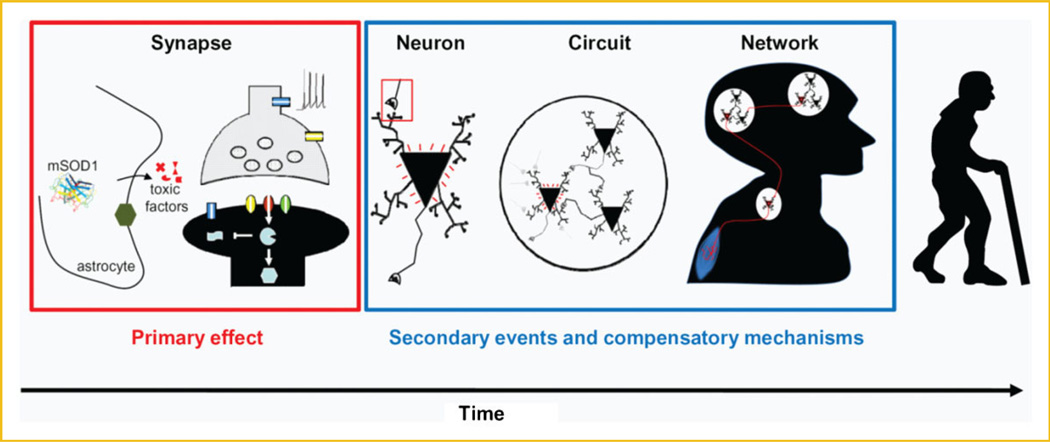
Recent support for this contention derives from findings that alterations are found in many CNS regions in pre-symptomatic transgenic mouse models (and even in pre-symptomatic human patients) of ALS (see below), AD [Santos et al., 2010], PK [Obeso et al., 2010], and HD [Raymond et al., 2011]. Based on evidence that is discussed more fully below, we propose a model (Fig. 1) in which mutant SOD1 initiates synaptic pathogenesis very early in development, causing alterations in neural circuits and network activity, and resulting in a vicious cycle that leads to neurological impairment. The onset of symptoms of ALS in humans may take years and even decades following these early events, and may become apparent only when compensatory mechanisms breaks down. Thus, it is imperative that the primary target(s) of disease-causing proteins be identified, which can then be used to establish pre-symptomatic diagnostic tools, and to develop novel therapies that can effectively prevent the onset of irreversible neuronal damage.
Fig. 1.
Model of how mutant SOD1 may underlie the etiology of ALS. Subtle synaptic dysfunction may be initiated by mutant SOD1 during development: either at early postnatal stages, or even during embryogenesis, when expression of wild-type and mutant SOD1 is first detected. Although this primary event causes little pathogenesis by itself, it may initiate a diversity of secondary cascades and compensatory mechanisms, which will influence the development and functioning of neuronal circuits and networks. Gradually (and in a vicious cycle) neuronal function is lost; however, during this early period—which can span a period of months in mouse models and decades in humans— symptoms have not yet manifested. Onset of disease may become apparent when compensatory mechanisms saturate and/or break down, either by accumulation of dysfunction or via other pathogenic events, such as trauma and environmental factors that may be aggravated by age. The mechanism(s) by which mutant SOD1 causes synaptic dysfunction is unknown, however, it could relate to the fact that ALS is at least partially a non-cell autonomous disease, and that expression of mutant SOD1 in astrocytes can induce the release of toxic factors (see text for more details).
SOD1 MUTATIONS AND FAMILIAL ALS
ALS is a fatal paralytic disorder caused by the progressive dysfunction and degeneration of cranial, brainstem, and spinal cord motor neurons in adulthood that leads to death by respiratory failure within 3–5 years of diagnosis. At least 15 ALS-associated gene loci have been identified, although most of our knowledge of this disease is based on studies of a subgroup of familial (FALS) cases, all of which have their origin in mutations in the gene encoding superoxide dismutase (SOD1) [Pasinelli and Brown, 2006; Bento-Abreu et al., 2010]. SOD1 pathology, however, might be more widespread in ALS: recent studies in sporadic ALS (SALS) patients suggest that misfolded wild-type SOD1 is present in many cases and can acquire toxic properties, hereby inducing pathogenic mechanisms similar to mutant SOD1 [Bosco et al., 2010; Haidet-Phillips et al., 2011; Guareschi et al., 2012]. These findings, together with reports showing that different mutant SOD1 induce multiple misfolded conformations [Prudencio and Borchelt, 2011], might explain why more than 150 mutations (http://alsod.iop.kcl.ac.uk/), dispersed throughout the SOD1 sequence, all lead to the ALS phenotype.
SOD1 is a cytosolic metalloenzyme of 153 amino acids ubiquitously expressed in all mammalian cells. It catalyzes the dismutation (conversion) of toxic superoxide anion to hydrogen peroxide (H2O2); this latter molecule is further detoxified to water and oxygen by glutathione peroxidase and catalase [Beckman et al., 2001]. Dominant inheritance of mutant SOD1, lack of symptoms in SOD1 knockout mice, and absence of a correlation between the enzymatic activity of the different mutant proteins and motor neuron toxicity, together indicate that mutations in this protein cause ALS through a gain-of-toxic-function [Cleveland and Rothstein, 2001; Pasinelli and Brown, 2006]. The discovery of the gene mutations has also led to the generation of transgenic mice that overexpress mutant SOD1 protein, and consequently develop a motor neuron disease that closely resembles human ALS. In 1994, Gurney and colleagues generated the first transgenic ALS mouse model by expressing high levels of human SOD1 that contained a substitution of glycine to alanine at amino acid position 93 (hSOD1G93A) driven by its endogenous human SOD1 promoter; these mice become paralyzed in one or more limbs and die of respiratory failure at 4–5 months of age [Gurney et al., 1994; Bellingham, 2011], despite the fact that the mutation has little effect on SOD’s enzyme activity. In the more than 15 years since the development of the Gurney model, the high expressor mutant hSOD1G93A transgenic mouse has been extensively studied—but disappointingly, we do not yet understand the exact molecular mechanisms that underlie mutant SOD1-mediated motor neuron degeneration. As a result, current therapies (e.g., riluzole; see below) rely more on alleviating symptoms rather than on modifying or curing the disease.
SOD1 AND THE HYPOTHESIS OF GLUTAMATE-INDUCED EXCITOTOXICITY
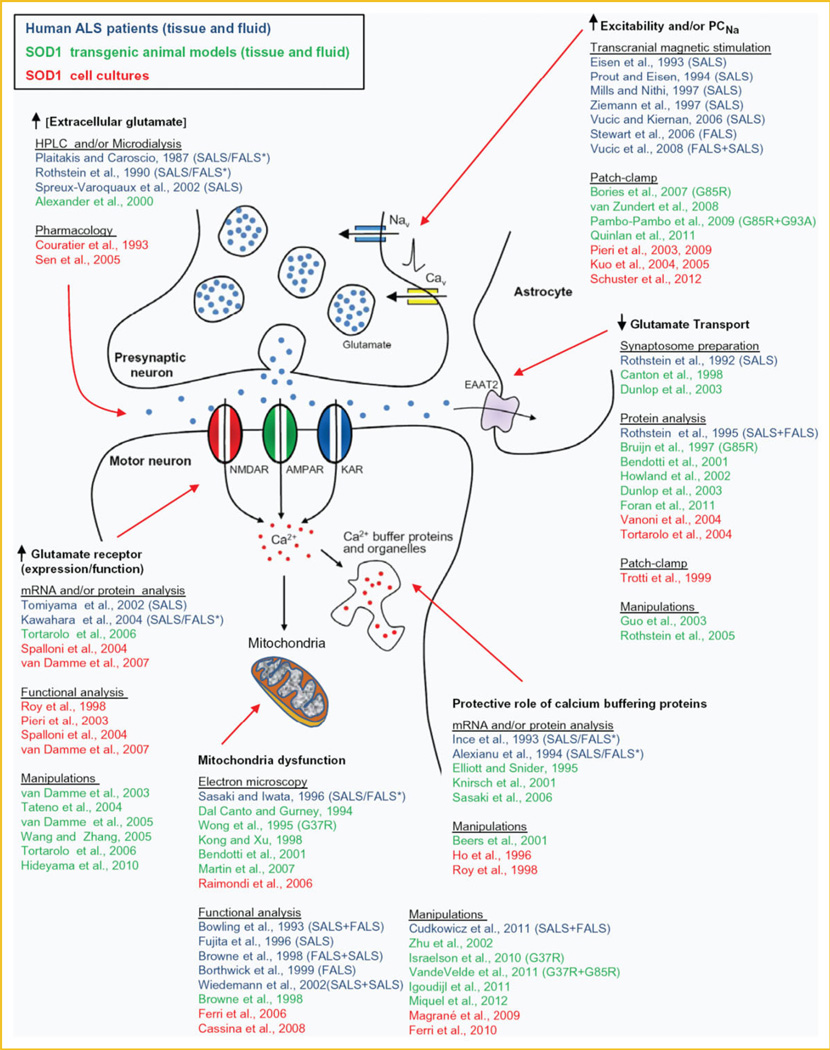
Understanding the cellular pathophysiological processes that underlie motor neuron dysfunction in ALS is an essential prerequisite for developing mechanism-based therapies for ALS. In vivo and in vitro studies with mutant hSOD1 transgenic mice reveal potential pathological changes in motor neurons that include excitotoxicity, hyperexcitability, disturbed calcium homeostasis, mitochondrial dysfunction, SOD1 aggregation, cytoskeletal disruption, deficits in axonal transport, activation of cell death signals, and oxidative stress [Cleveland and Rothstein, 2001; Pasinelli and Brown, 2006; Ilieva et al., 2009; Grosskreutz et al., 2010]. Whether these pathological events reflect the activity of a single unifying signaling cascade, or of multiple pathways that act in parallel to compromise the viability of motor neurons, is not yet clear. Based on decades of research, however, we can assert that glutamate-induced excitotoxicity is likely a node of convergence for multiple signaling cascades. The increased glutamate-induced excitotoxicity observed in ALS patients and in mouse models of ALS may be the result of increased glutamate release because enhanced Nav channel activity (hyperexcitability), decreased uptake of extrasynaptic glutamate by reduced EAAT2/GLT1 transporter activity/number, and/or alterations in synaptic glutamate receptor number and Ca2+ permeability (Fig. 2 and Supplementary information for references indicated in this figure). In turn, glutamate excitotoxicity increases the Ca2+ load of motor neurons and interneurons. The Ca2+ buffering capacity of motor neurons is limited, and relies heavily on mitochondrial uptake; thus excess Ca2+ might further alter the function of mitochondria in motor neurons, which could already be affected by abnormal accumulations of mutated SOD1 aggregates. Mitochondrial dysfunction is likely a following central event causing energetic and metabolic failures that trigger cellular stress responses, affect motor neuron axon function (including transmission at the neuromuscular junction) and, eventually lead to apoptosis cascades and cell death (Fig. 2) [for reviews see Bento-Abreu et al., 2010; Grosskreutz et al., 2010; Carrì and Cozzolino, 2011].
Fig. 2.
Evidence for the hypothesis that glutamate-induced excitotoxicity underlies the pathology in ALS. Schematic diagram to show how a variety of alterations in pre-synaptic neurons, post-synaptic neurons or glial cells might result in excessive Ca2+ entry into motor neurons. Citations listed on the diagram refer to reports that document the cellular pathophysiological processes underlying glutamate-induced excitotoxicity by analyzing fluid and tissue samples from either ALS patients (in blue) or SOD1 ALS mice models (in green). Results from cell culture-based SOD1 ALS models are also shown (in red). Studies are from high-expressor SOD1 G93A unless stated.* indicates that the SOD mutation was not identified in the FAIS patients. We have tried to acknowledge as many original studies as possible and apologize for those we have omitted. Reference list is shown in the supplementary data.
EARLY PATHOGENIC PROCESSES IN MOTOR NEURONS AND DISEASE PROGRESSION
Many alterations in pre-synaptic neurons, post-synaptic neurons, or glial cells that might contribute to glutamate-induced excitotoxicity in ALS have been described (Fig. 2). A fundamental question concerns the manner in which mutated SOD1 induces glutamate excitotoxicity: whether this is an early event associated with primary pathology (Fig. 1), or whether it is a late event linked to disease onset?
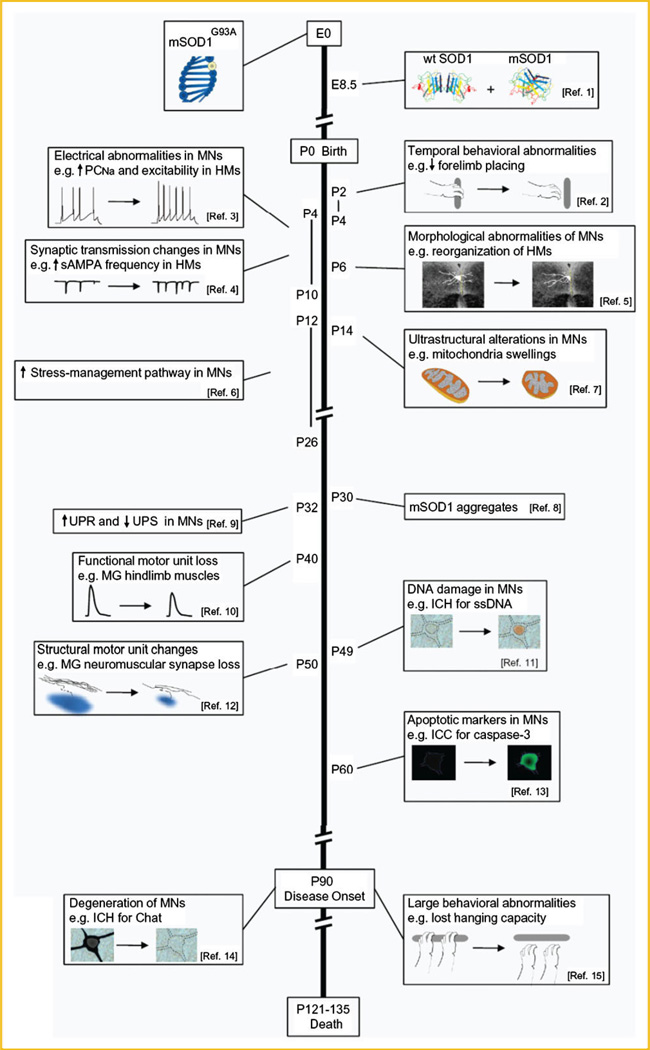
To reveal when the earliest deficits occur in ALS mouse models, studies must focus on early pre-symptomatic stages, and use techniques that are sensitive enough to determine subtle changes in individual neuronal subtypes. Using single cell-based techniques such as electrophysiological recordings, electron microscopy, immunohistochemical techniques, and functional as well as structural studies of motor units, many abnormalities in vulnerable motor neurons are detected in ALS mouse models weeks and even months before disease onset, this being defined by overt weakness and motor neuron death [Chiu et al., 1995]. In fact, and as indicated in the established time-line shown in Figure 3, in the high-expresser hSOD1G93A mouse (in contrast to wild-type and high-expresser hSOD1WT mouse) alterations in electrophysiological properties and synaptic circuits are detected already during the first days after birth. For example, use of acute slice preparations made from brains of P4–P10 mice reveals that hypoglossal brainstem motor neurons of the hSOD1G93A model display increased repetitive firing and persistent inwards currents (PICs) mediated by voltage-sensitive Na+ channels (PCNa) [van Zundert et al., 2008]. Analysis of young motor neurons in acute spinal cord slice preparations of hSOD1G93A mice also shows an enhanced PCNa from P0 through P12; moreover, from P6 through 12, the PCNa is accompanied by significantly larger PICs that are mediated by Ca2+ (PCCa), which are likely regulated by L-type Cav1.3 channels [Quinlan et al., 2011]. In addition, brainstem neurons from P4–10 hSOD1G93A mice display increases in spontaneous synaptic activity, mediated via excitatory glutamatergic AMPA/kainate receptors, and also via inhibitory GABAA/glycine receptors, suggesting an augmented activity in networks that drive motor neurons, which can result in enhanced and disorganized motor neuron bursting activity [Jiang et al., 2009]. Brainstem motor neurons in neonatal hSOD1G93A mice display also NMDA receptor currents with fast decay times, typically seen when receptor subunit composition changes from NR2B-rich (large calcium influx) to NR2A-rich (reduced calcium influx) [van Zundert et al., 2008]; such a subunit change could occur as a consequence of early activity-driven maturation, and might slow down activity-induced toxicity. Altered expression of NMDA receptor subunits can also influence the dendritic architecture of neurons [Sepulveda et al., 2010]; in support of this idea, precocious dendritic remodeling has been reported for hypoglossal motor neurons in the hSOD1G93A mice [van Zundert et al., 2008]. Subtle neuromotor abnormalities are also detected in these mice, likely as a consequence of neuronal circuitry alterations: forelimb placing and righting responses are transiently delayed between P2 and P4, but then recover [van Zundert et al., 2008]. Additional behavioral tests of labyrinth function and vibrissa sensibility were normal, highlighting that only specific motor functions are impaired in ALS.
Fig. 3.
Time-line of pathological and clinical changes in the high-expressor hSOD1G93A line of transgenic mice. The most important and earliest abnormalities reported for the hSOD1G93A transgenic mouse model are shown. Months before motor neurons degenerate and clinical symptoms appear (P90), widespread and early onset of pathological abnormalities are detected in this ALS mouse model. See references stated here and text for additional information. (Ref. 1 SOD1 expression is detected from embryonic (E) day 8.5 (E8.5) [Yon et al., 2008]). (Ref. 2 Temporal behavioral abnormalities during early postnatal (P) development: P2–4 mSOD1G93A mice show reduced forelimb placing and righting capacities [van Zundert et al., 2008]. Similar reversible sensorimotor alterations are observed for neonatal (P1–P7) hSOD1G85R mice [Amendola et al., 2004]). (Ref. 3 Electrical abnormalities in hSOD1G93A motor neurons (MNs) at P4–P10; hypoglossal motor neurons (HMs) display increased excitability and PCNa [van Zundert et al., 2008]. hSOD1G93A spinal cord motor neurons also possess enhanced PCNa (P0–P12)and PCCa(P6–P12) [Quinlan et al., 2011]. In addition, early (P6–P10) abnormal changes in electrical properties, including in excitability, are detected in the low-expressor hSOD1G93A transgenic line and the hSOD1G85R mutant mice [Bories et al., 2007; Pambo-Pambo et al., 2009]). (Ref. 4 Synaptic transmission mediated by AMPA, NMDA and glycine-receptors is altered for HMs in P4–10 hSOD1G93A mice [van Zundert et al., 2008]). (Ref. 5 Morphological abnormalities of motor neurons of hSOD1G93A mice at P6; precocious remodeling of HMs [van Zundert et al., 2008]. Early (P6–P10) abnormal dendritic branching is also observed for hSOD1G85R transgenic mice [Amendola and Durand, 2008]). (Ref. 6 Genes involved in stress-related pathways are transiently upregulated between P12 and P26, in vulnerable hSOD1G93A spinal motor neurons [Saxena et al., 2009]). (Ref. 7 Ultrastructural alterations of motor neurons in mSOD1G93A mice, starting at P14 (2 weeks); mitochondria swellings and small vacuoles are present in distal dendrites and in the cell bodies of spinal cord motor neurons [Bendotti et al., 2001]). (Ref. 8 mutant SOD1 aggregates in spinal motor neurons of hSOD1G93A mice at P30 [Johnston et al., 2000]). (Ref. 9 Genes involved in UPR and in the ubiquitin proteasome system (UPS) are up- and down-regulated, respectively, in vulnerable P32 spinal motor neurons of hSOD1G93A mice [Saxena et al., 2009]). (Ref. 10 Functional loss of motor unit for fast-twitch hind-limb muscles (medial gastrocnemius [MG]) is detected starting at P40in hSOD1G93A mice [Hegedusetal., 2007]). (Ref. 11 DNA damage (e.g., immunohistochemistry (ICH) with antibodies recognizing single-stranded breaks) is detected in motor neurons of hSOD1G93A mice, starting at P49 (7 weeks) [Martin et al., 2007]). (Ref. 12 Structural changes in motor units of hSOD1G93A mice starting at P50; loss of MG neuromuscular synapses and prominent vacuolation in nerve terminals [Frey et al., 2000]). [Ref. 13 Markers of apoptosis (including caspases 1 and 3) in motor neurons are detected as of P60 in spinal cord motor neurons of hSOD1G93A mice [Li et al., 2000]. See Martin et al., [2007] for discussion on apoptotic-necrotic hybrid forms of motor neuron death in ALS]. (Ref. 14 Degeneration of motor neurons (e.g., those that display choline acetyltransferas (Chat)-positive IRR)of hSOD1G93A mice starts at ∼P90 [Chiu et al., 1995]). (Ref. 15 Clinical symptoms (e.g., small tremors, weight lose) [Chiu et al., 1995] and more prominent behavioral abnormalities (e.g., decline in hanging test and rotarod performance) of hSOD1G93A mice starts at ∼P90). See also additional reviews for deficits during development in other mutant SOD1 mice, including the low-expressor hSOD1G93A, hSOD1G85R, and hSOD1G127X mice [Durand et al., 2006; Elbasiouny et al., 2010b; Quinlan, 2011].
Further evidence for abnormalities during early postnatal development in ALS is reported for the hSOD1G85R and the low-expressor hSOD1G93A mouse models, in which alterations in neuronal excitability, reflex responses, and dendritic branching of lumbar motor neurons were also observed [Amendola et al., 2004; Durand et al., 2006; Bories et al., 2007; Amendola and Durand, 2008; Pambo-Pambo et al., 2009; Filipchuk and Durand, 2012]. Note, however, that despite similar alterations in dendritic branching the reported alterations on neuronal excitability in the two ALS model systems are not identical: neonatal motor neurons expressing hSOD1G85R show hypo-excitability [Bories et al., 2007; Pambo-Pambo et al., 2009], whereas those expressing SOD1G93A (either low- or high-expressors, in slice preparations or cultures) display hyper-excitability [Kuo et al., 2005; van Zundert et al., 2008; Pambo-Pambo et al., 2009; Pieri et al., 2009]. Many factors (e.g., type of SOD1 mutant, different time course of the disease in each model, type of recordings, or type of neuronal preparations studied) may contribute to the discrepancies between the excitability changes in the different SOD1 models: here we underscore, however, the results of recent computer modeling studies, which suggest that alterations in morphology (increased dendrite surface) and membrane biophysical properties (decrease in membrane resistivity, Rm) of hSOD1G85R mice motor neurons are both necessary to explain decreased input resistances (Ri) and their hypo-excitability, offsetting the presence of increased PICs [Amendola and Durand, 2008; Elbasiouny et al., 2010a]. In contrast, neonatal hSOD1G93A motor neurons display small changes, if any, in Ri [van Zundert et al., 2008; Quinlan et al., 2011]. A possible lack of changes in Rm and the fact that most dendrite surface is added at some distance from the cell body (where Ri is measured), suggest that the increase in firing response to somatic injected current in hSOD1G93A motor neurons (hyper-excitability) is dominated by an increase of PCNa [van Zundert et al., 2008]. An important question that has not yet been addressed is whether the functional and structural alterations that lead to changes in excitability are causal or adaptive in nature. In the context of the first possibility, note that most of the electrical and morphological alterations observed following the expression of mutant SOD1 are normally seen in more mature wild-type motor neurons, raising the possibility that pathology could be, at least in part, the result of accelerated aging [see also Discussion in Quinlan et al., 2011]. Independent of the motor neuron type of excitability, however, the observed abnormalities in function, neuronal structure, and behavior in the different transgenic SOD1 mouse models collectively argue that the pathological synaptic circuit alterations in the adult-onset disease ALS are actually initiated very early during development.
Early maturation of other pathways has also been documented in the ALS mouse models. Thus, ER stress-management pathways are upregulated as early as P12 in the more vulnerable SOD1G93A motor neuron subpopulations [Saxena et al., 2009], and coincide with the earliest ultrastructural evidence of mitochondria pathology [Bendotti et al., 2001]; both changes are evident just after the detected alterations in electrophysiological properties. In addition, upregulation of unfolded protein response (UPR)-related genes and proteins initiates at P32 and peaks at P38 [Saxena et al., 2009]. The ER stress responses are likely responsible for the formation of aggregates of mutant SOD1 observed starting to be detectable at P30 in spinal motor neurons [Johnston et al., 2000]. Soon after, SOD1G93A spinal motor neurons display DNA damage (P49) [Martin et al., 2007] and start to express apoptotic markers (P60) [Li et al., 2000]. In parallel, functional and structural changes in motor units are reported from P40 to P50 in mSOD1G93A mice [Frey et al., 2000; Hegedus et al., 2007], with fast motor units exhibiting pathological changes earlier than slow motor units. Collateral sprouts from slow motor axons transiently reoccupy vacated neuromuscular junctions, providing some compensation that may allow the animal to maintain muscle strength until clear signs of disease progression are evident in the majority of motor units [Frey et al., 2000; Hegedus et al., 2007]. Finally, at ∼P90, limb weakness can be clearly demonstrated by the loss of grip strength in the hanging wire test [Chiu et al., 1995].
In summary, the earliest changes in the hSOD1G93A model are characterized by hyper-activity and alterations in protein metabolism. Both precede the expression of functional deficits in the motor unit, which are the immediate cause of paresis. This pathology is eventually followed by induction of apoptosis/necrosis pathways and motor neuron cell death. Whether the early processes are independent, synergistic or interact in complex ways is not yet fully understood. In addition, it is important to dissect which processes are cell-autonomous and which mechanisms receive contribution from other cells. In particular, increases in motor neuron activity can be strongly influences by other cells. First, glial cells are important modulators of synaptic function and deficits in glial-mediated glutamate clearance might be a major contributor to disease [Bento-Abreu et al., 2010]. Second, the pre-motor interneuronal networks show alterations that could modify the balance of excitation and inhibition resulting in an excess of excitatory drive. It is likely that neurons other than motor neurons are also hyper-excitable. In fact, electrophysiological recordings from P10 to P12 interneurons of the superior colliculus (SC) from neonatal hSOD1 G93A mice showed increases in PCNa similar to vulnerable brainstem motor neurons [van Zundert et al., 2008]. Cortical circuit hyper-excitability is also present in ALS patients, even before the onset of motor symptoms, and in pre-symptomatic animal models [Vucic et al., 2008; Kiernan and Petri, 2012; and Fig. 2 for additional reference]. Similarly, excessive network excitability has been observed using in vitro spinal cord preparations [Jiang et al., 2009] and decreasing excitatory synaptic activity in VGLUT2 mutants has been shown to be partially protective of motor neuron degeneration [Wootz et al., 2010].
Excessive excitatory drive might be exacerbated by decreases in inhibition and there are some suggestions that motor neuron vulnerability is correlated with the composition and strength of inhibitory synapses [Lorenzo et al., 2006]. In particular, the recurrent inhibitory feedback circuit mediated by Renshaw cells and that controls the level of motor neuron firing is altered just before disease onset [F.J.A. and B.v.Z. unpublished work]. In this circuit, motor axon intraspinal collaterals establish synapses that excite interneurons known as Renshaw cells and these in turn inhibit the same motor neurons through a feedback loop [Alvarez and Fyffe, 2007]. Degenerative changes of motor axon synapses on Renshaw cells starting at 60–80 days in the hSOD1 G93A model results in the break-down of this circuit resulting in the loss of an important inhibitory control for motor neuron excessive firing [F.J.A. and B.v.Z. unpublished work]. Thus, motor axon synaptic pathology closely correlates temporally both centrally (inside the spinal cord) and in the periphery (at the neuromuscular junction) and its timing is suggestive that it is a contributor to the initiation of the last phase of motor neuron degeneration and eventual death. In addition, there might be more subtle changes that directly decrease inhibitory glycinergic (but not GABAergic) synaptic strength in general, even from the earliest stages of synaptogenesis [Chang and Martin, 2011], and there is also evidence that premotor inhibitory interneurons show stress markers and synaptic deficits around disease onset [Hossaini et al., 2011]. The loss of inhibition from feedback control circuits, and possibly also other inhibitory inputs, reduces the capacity of spinal circuits to counteract excessive motor neuron hyper-excitability.
POSSIBLE CELLULAR AND MOLECULAR MECHANISMS THAT UNDERLIE EARLY HYPER-EXCITABILITY IN ALS
We argue that very early increases in Na+ and Ca2+-mediated PICs could play a pivotal role in initiating the cascade of events responsible for hyper-excitability leading to glutamate excitotoxicity.
WHAT IS THE PHYSIOLOGICAL FUNCTION OF PICs?
Although PICs are only a small fraction of the total current (e.g., 1– 5% in the case of PCNa), they can profoundly affect neuron and network behavior. The prevailing view is that because PCNa is generated by the same Nav channels that produce the typical fast transient sodium current (TNa), and because PCNa can be activated close to the cell’s resting potential, small increases in this current can enhance intrinsic excitability, alter spike initiation, and amplify the firing rate [Crill, 1996; Goldin, 2003; Elbasiouny et al., 2010b]. In addition, the excitatory synaptic inputs received by a given neuron can be greatly amplified and prolonged by PCNa and PCCa, thereby impacting the neuron’s output by increasing its firing rates in response to synaptic modulation [Elbasiouny et al., 2010b].
HOW CAN INCREASED PICs DURING NEONATAL DEVELOPMENT BE PATHOLOGICAL IN ALS?
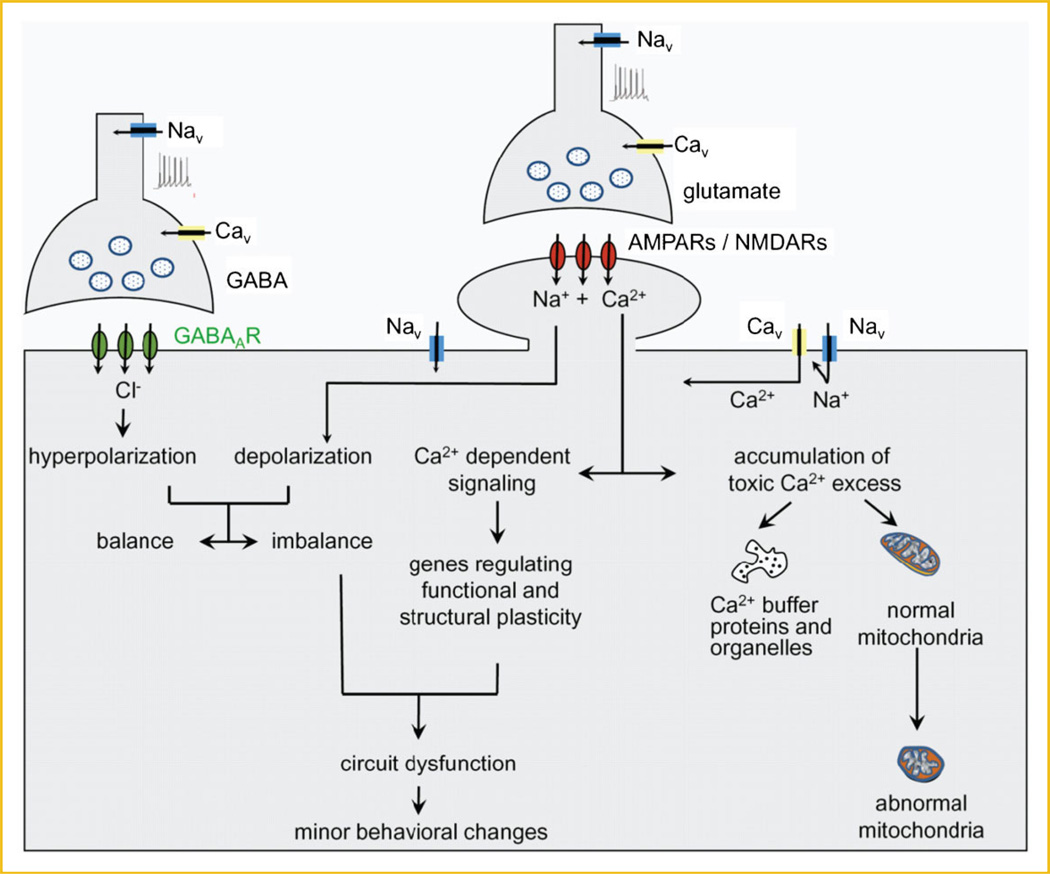
The increased PCNa and PCCa detected in motor neurons and interneurons of the spinal cord and brainstem in slice preparations made from neonatal mSOD1G93A mice is accompanied by alterations in the excitability, synaptic inputs, and morphology of the neurons [van Zundert et al., 2008; Quinlan et al., 2011; Fig. 3]. Importantly, these abnormal functional and structural alterations are also linked to minor behavioral symptoms in mSOD1G93A neonates [van Zundert et al., 2008]. Based on these findings, and on the fact that PICs largely influence synaptic inputs and firing rates, we hypothesize that, during neonatal development of mutant SOD1 mice, increases in Na+ and Ca2+ PICs in motor neurons as well as in non-motor neurons significantly enhance intrinsic and extrinsic (network) excitability, amplify and prolong synaptic inputs, and increase firing rates (Fig. 4). It is also interesting that recurrent inhibition through Renshaw cells is a major modulator of PIC-mediated amplification of synaptic inputs and firing [Bui et al., 2008]. Therefore, the failure of this circuit before disease onset would exacerbate pathology resulting from excessive PIC activity already established during early development. The post-synaptic and pre-synaptic membrane depolarization induced by influxes through Nav channels (PCNa) and Cav1.3 channels (PCCa), will also cause sustained Ca2+ (and Na+) influxes through other receptors/ channels, such as NMDA- and AMPA-receptors and other Cav channels, hereby directly contributing to excitotoxicity. Because motor neurons express low levels of Ca2+-binding proteins (calbindin-D28K, parvalbumin), Ca2+ influx in these cells would have to be mainly buffered by mitochondria: while these organelles do have a limited capacity for buffering of Ca2+ that aids in maintaining mitochondrial function, prolonged Ca2+ overload would structurally and functionally damage mitochondria, and lead to a deficit in energy production and apoptotic and/or necrotic cell death cascades. Failure to control hyper-excitability and excitotoxicity in the hSOD1G93A mice leads ultimately to motor neuron degeneration and death 4–4.5 months later.
Fig. 4.
Model of how PCNa might induce synaptic dysfunction in pre-symptomatic neonatal/juvenile hSOD1G93A transgenic mice. Based on the alterations in electrophysiological properties and synaptic circuits that are detected in motor neurons and interneurons of hSOD1G93A mice during the first days after birth, we propose a model in which small increases in PCNa mediated by Nav channels and PCCa mediated by Cav channels (likely Cav1.3) may significantly enhance neuronal excitability and increase synaptic transmission, leading to sustained toxic influxes of and Ca2+ (and Na+) through NMDA- and AMPA-receptors, and through Cav channels. In motor neurons, the limited expression of Ca2+ buffering proteins obligates the mitochondria to perform the buffering task, leading to mitochondrial dysfunction and damage. Excess Ca2+ also induces expression of plasticity-related genes that may, together with the imbalance between hyper-polarization and depolarization, disrupt local circuitry and networks, induce minor behavioral changes. The underlying mechanism(s) responsible for the increased PCNa has not been determined, but could be related to the ROS/RNS produced by astrocytes that express mutations in SOD1 (not shown).
HOW WOULD mSOD1 INFLUENCE THE FUNCTION OF NaV CHANNELS AND PCNa?
Regulation of PCNa has been well studied: it is widely believed that this current can be modulated by several mechanisms, including the alpha and beta subunits of Nav channels (PCNa is greater for Nav1.1 and Nav1.6 as compared to Nav1.2 and Nav1.3), several protein kinases, and different types of reactive oxygen and nitrogen species (ROS/ RNS) [Franceschetti et al., 2000; Hammarström and Gage, 2000; Goldin, 2003; Kassmann et al., 2008; Nani et al., 2010]. Of particular interest to ALS are biochemical studies that indicate that mutant SOD1, either alone or by binding to Rac1/NADPH-oxidase (Nox), can paradoxically generate ; together with nitric oxide (NO), the can produce peroxynitrite (ONOO−) [Harraz et al., 2008; see also Beckman et al., 2001 and Cleveland and Rothstein, 2001 for discussion on several hypotheses concerning the production of different ROS/RNS by mutant SOD1]. Evidence for the harmful production of Nox-derived was obtained in vivo in both human SALS and the SOD1 G93A transgenic mouse model [Wu et al., 2006; Harraz et al., 2008]. Deleting the Nox protein gp91phox increased the lifespan and reduced neurodegeneration in SOD1G93A transgenic mice [Wu et al., 2006]. Moreover, treatment of hSOD G93A transgenic mice with the Nox inhibitor apocynin (starting at P14) markedly slows disease progression (from approximately 100 to 200 days), and resulted in a large increase in life span (from 125 to 238 days) [Harraz et al., 2008].
In vitro studies also show that SOD1G93A-expressing astrocytes and microglial cells can produce toxic ROS/RNS that kills wild-type motor neurons [Cassina et al., 2008; Harraz et al., 2008]. Similar results are obtained when microglial cells are incubated with extracellular SOD1G93A protein [Zhao et al., 2010]. These findings are in line with the fact that ALS is at least partially a non-cell autonomous disease, and that expression of mutant SOD1 in cultured astrocytes can induce the release of toxic factors [Di Giorgio et al., 2007; Nagai et al., 2007; Haidet-Phillips et al., 2011; reviewed in Ilieva et al., 2009]. It might be interesting to undertake an electrophysiological analysis aimed at determining which specific ROS/RNS is released by ALS astrocytes and capable of activating Nav channels, and thereby increase PCNa and motor neuron excitability.
Undoubtedly, the hSOD1 G93A -generated ROS/RNS would have many targets besides Nav channels. Several studies provide compelling evidence that a number of proteins, and even RNA, are modified by ROS/RNS in ALS patients and in transgenic mouse models [Wu et al., 2006; Chang et al., 2008]. However, when compared to the functional alterations in the Nav channels, the protein and RNA modifications may cause less immediate damage. For example, ROS/RNS can actually decrease the function of many Ca2+ permeable channels/receptors, including the NMDA receptors [Bodhinathan et al., 2010], AMPA receptors [Plested and Mayer, 2009] and L-type Cav channels [Li et al., 2007]—this is accomplished either directly by altering their redox state, or indirectly by changing the oxidative state of their signaling or scaffolding proteins, which therefore could “protect” neurons (at least temporally) from toxic Ca2+ influxes.
CONSEQUENCES OF EARLY ABNORMAL NEURONAL ACTIVITY ON THERAPEUTIC PROSPECTS
Findings on the outcome of abnormal neuronal activity that are reviewed below increase our understanding about the molecular-pathophysiological underpinnings of ALS. Here, we present these observations within the framework of two questions that are fundamental for the development of meaningful therapies: (i) Is abnormal neuronal activity also a key event in SALS? and (ii) Why are Nav channel inhibitors not more effective in delaying onset of ALS symptoms and in enhancing motor neuron survival?
CAN FAMILIAL AND SPORADIC ALS BOTH BE TRIGGERED BY A COMMON PATHOGENIC MECHANISM(S) THAT INVOLVES ABNORMAL NEURONAL ACTIVITY?
Patients with FALS and SALS present symptoms that are indistinguishable: we thus argue that disease progression in both types of patients has a common pathogenic mechanism. The most widely accepted argument that supports the contribution of glutamate excitotoxicity to the pathogenesis of ALS is based on the beneficial effects of riluzole: the drug extends the survival of patients with SALS and FALS by ∼3 months [Bellingham, 2011]. At the clinical level, mounting evidence also suggests that motor neuron hyperexcitability is a common pathophysiological feature in both types of ALS, and that such increased activity precedes clinical onset in FALS patients [Vucic et al., 2008; Kiernan and Petri, 2012 and see references listed in Fig. 2]. At the molecular level, recent studies with the use of a conformation-specific antibody (C4F6), which was generated specifically against the hSOD1 G93A mutant protein, is of particular interest: while the C4F6 antibody does not react with unmodified endogenous wild-type SOD1, it does recognize oxidized wild-type SOD1 (SOD1ox) [Urushitani et al., 2007; Bosco et al., 2010]. Furthermore, positive C4F6 staining on spinal cord tissue is observed in almost 50% of SALS cases tested [Bosco et al., 2010] and wild-type SOD1 is oxidized in SALS patients [Guareschi et al., 2012]. Together, these findings indicate that wild-type SOD1 can mimic the structural features of FALS SOD1 mutants, and can become pathogenic in SALS via non-heritable, post-translational modifications. We thus conclude that the SOD1-dependent pathogenic mechanisms—and also the comparable clinical symptoms (including hyperexcitability) shared by patients with sporadic as well as familial ALS—argue in favor of the possibility that suppression of abnormal neuronal activity early in life will prove beneficial in both forms of ALS.
WHY ARE NaV INHIBITORS NOT MORE EFFECTIVE IN DELAYING ONSET AND SURVIVAL?
Riluzole is thought to exert its beneficial effects by reducing glutamatergic synaptic activity, although it is precise mechanism(s)-of-action remain unclear because this drug can affect multiple targets [Bellingham, 2011]. Nevertheless, clinically relevant concentrations (1–2 µM in plasma) of riluzole are most effective in blocking Nav channels, thereby suppressing PCNa and neuronal excitability [Kuo et al., 2005; Theiss et al., 2007; Bellingham, 2011; Schuster et al., 2012]. Surprisingly, however, riluzole treatment starting at P30–P50 delays death in transgenic mSOD1G93A ALS mice by only 9–14 days [Bellingham, 2011]. This modest delay in survival after riluzole treatment is disappointing from a therapeutic and conceptual point of view, especially when considering that riluzole did not significantly affect disease onset. These studies need, however, to be considered in view that electrophysiological abnormalities that are likely targeted by riluzole are initiated much earlier (P4–P10) than the start of the drug treatment in these studies (P30–P50). Increased excitability and neuronal activity during neonatal development might induce a pathophysiological cascade of events that cannot be reversed by later riluzole treatment.
In addition to the timing of treatment, another concern is that the precise mechanisms, whereby mutant SOD1 is toxic to motor neurons are not fully defined and it is possible that other processes induced at early stages independently contribute to the eventual demise of motor neurons. For example, future studies should elucidate how early abnormal protein metabolism is related to hyper-excitability and whether these two early alterations constitute independent parallel pathways of cellular pathology with additive properties. One possibility is that a constellation of early changes is induced and each one has the capacity of causing motor dysfunction by itself given enough time. This might explain why most treatments targeting just a single mechanism (as riluzole) achieve at most a slight slowing of the pathology.
CONCLUSION
Detailed analyses of the mSOD1G93A mouse model during various stages of development have gradually revealed that pathologies in motor neurons and interneurons can be detected from the first postnatal days, much before the onset of behavioral symptoms. It is not clear whether the Nav channel-mediated abnormal neuronal activity and circuit alterations observed in neonatal mice are the first pathogenic processes or whether in fact the disease starts even earlier, during embryonic stages, when the expression of SOD1 begins. Identification of the primary pathogenic event in SOD1-ALS mice is imperative for the development of rational, mechanism-based therapies, and will likely yield important insights about the underlying mechanisms for disease onset in ALS that is induced by other mutations (e.g., TDP43 and FUS/TLS) as well as in sporadic ALS. Without such insights, even the development of symptomatic treatments for ALS patients is a challenge, because we do not know how secondary events and compensatory mechanisms are defining the course of pathogenic processes. Results of longitudinal studies on disease pathogenesis should also lead to the identification of specific biomarkers of temporal disease progress, which could result in more precise interventional treatments for specific disease stages. Longitudinal studies also have the potential to uncover the compensatory mechanisms that the body uses to mitigate the effects of degeneration of the vulnerable motor neurons. Such novel therapies should prolong the intrinsic compensatory mechanisms and significantly delay the suffering of ALS patients.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by ALS Therapy Alliance-CVS Pharmacy, no. N/A to B.v.Z., by Fondecyt, no. 1101012 to B.v.Z., by Conicyt, no. 24090204 to P.I., and by NIH, no. NS NS047357 to F.J.A.
Grant sponsor: ALS Therapy Alliance-CVS Pharmacy; Grant sponsor: Fondecyt; Grant number: 1101012; Grant sponsor: Conicyt; Grant number: 24090204; Grant sponsor: NIH; Grant number: NS NS047357.
Footnotes
Additional supporting information may be found in the online version of this article.
REFERENCES
- Alvarez FJ, Fyffe RE. The continuing case for the Renshaw cell. J Physiol. 2007;584:31–45. doi: 10.1113/jphysiol.2007.136200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amendola J, Durand J. Morphological differences between wild-type and transgenic superoxide dismutase 1 lumbar motoneurons in postnatal mice. J Comp Neurol. 2008;511:329–341. doi: 10.1002/cne.21818. [DOI] [PubMed] [Google Scholar]
- Amendola J, Verrier B, Roubertoux P, Durand J. Altered sensorimotor development in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2004;20:2822–2826. doi: 10.1111/j.1460-9568.2004.03745.x. [DOI] [PubMed] [Google Scholar]
- Beckman JS, Estévez AG, Crow JP, Barbeito L. Superoxide dismutase and the death of motoneurons in ALS. Trends Neurosci. 2001;24:S15–S20. doi: 10.1016/s0166-2236(00)01981-0. [DOI] [PubMed] [Google Scholar]
- Bellingham MC. A review of the neural mechanisms of action and clinical efficiency of riluzole in treating amyotrophic lateral sclerosis: What have we learned in the last decade? CNS Neurosc Ther. 2011;17:4–31. doi: 10.1111/j.1755-5949.2009.00116.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendotti C, Calvaresi N, Chiveri L, Prelle A, Moggio M, Braga M. Early vacuolization and mitochondrial damage in motor neurons of FALS mice are not associated with apoptosis or with changes in cytochrome oxidase histochemical reactivity. J Neurol Sci. 2001;191:25–33. doi: 10.1016/s0022-510x(01)00627-x. [DOI] [PubMed] [Google Scholar]
- Bento-Abreu A, Van Damme P, Van Den Bosch L, Robberecht W. The neurobiology of amyotrophic lateral sclerosis. Eur J Neurosci. 2010;31:2247–2265. doi: 10.1111/j.1460-9568.2010.07260.x. [DOI] [PubMed] [Google Scholar]
- Bodhinathan K, Kumar A, Foster TC. Intracellular redox state alters NMDA receptor response during aging through Ca2+/calmodulin-dependent protein kinase II. J Neurosci. 2010;30:1914–1924. doi: 10.1523/JNEUROSCI.5485-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bories C, Amendola J, Lamotte d'Incamps B, Durand J. Early electrophysiological abnormalities in lumbar motoneurons in a transgenic mouse model of amyotrophic lateral sclerosis. Eur J Neurosci. 2007;25:451–459. doi: 10.1111/j.1460-9568.2007.05306.x. [DOI] [PubMed] [Google Scholar]
- Bosco DA, Morfini G, Karabacak NM, Song Y, Gros-Louis F, Pasinelli P, Goolsby H, Fontaine BA, Lemay N, McKenna-Yasek D, Frosch MP, Agar JN, Julien JP, Brady ST, Brown RH. Wild-type and mutant SOD1 share an aberrant conformation and a common pathogenic pathway in ALS. Nat Neurosci. 2010;13:1396–1403. doi: 10.1038/nn.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bui TV, Grande G, Rose PK. Multiple modes of amplification of synaptic inhibition to motoneurons by persistent inward currents. J Neurophysiol. 2008;99:571–582. doi: 10.1152/jn.00717.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrì MT, Cozzolino M. SOD1 and mitochondria in ALS: A dangerous liaison. J Bioenerg Biomembr. 2011;43:593–599. doi: 10.1007/s10863-011-9394-z. [DOI] [PubMed] [Google Scholar]
- Cassina P, Cassina A, Pehar M, Castellanos R, Gandelman M, de Leon A, Robinson KM, Mason RP, Beckman JS, Barbeito L, Radi R. Mitochondrial dysfunction in SOD1G93A–bearing astrocytes promotes motor neuron degeneration: Prevention by mitochondrial-targeted antioxidants. J Neurosci. 2008;28:4115–4122. doi: 10.1523/JNEUROSCI.5308-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Q, Martin LJ. Glycine receptor channels in spinal motoneurons are abnormal in a transgenic mouse model of amyotrophic lateral sclerosis. J Neurosci. 2011;31:2815–2827. doi: 10.1523/JNEUROSCI.2475-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, Kong Q, Shan X, Tian G, Ilieva H, Cleveland DW, Rothstein JD, Borchelt DR, Wong PC, Lin CL. Messenger RNA oxidation occurs early in disease pathogenesis and promotes motor neuron degeneration in ALS. PLoS ONE. 2008;3:e2849. doi: 10.1371/journal.pone.0002849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu AY, Zhai P, Dal Canto MC, Peters TM, Kwon YW, Prattis SM, Gurney ME. Age-dependent penetrance of disease in a transgenic mouse model of familial amyotrophic lateral sclerosis. Mol Cell Neurosci. 1995;6:349–362. doi: 10.1006/mcne.1995.1027. [DOI] [PubMed] [Google Scholar]
- Cleveland DW, Rothstein JD. From Charcot to Lou Gehrig: Deciphering selective motor neuron death in ALS. Nat Rev Neurosci. 2001;2:806–819. doi: 10.1038/35097565. [DOI] [PubMed] [Google Scholar]
- Crill WE. Persistent sodium current in mammalian central neurons. Annu Rev Physiol. 1996;58:349–362. doi: 10.1146/annurev.ph.58.030196.002025. [DOI] [PubMed] [Google Scholar]
- DeKosky ST, Marek K. Looking backward to move forward: Early detection of neurodegenerative disorders. Science. 2003;302:830–834. doi: 10.1126/science.1090349. [DOI] [PubMed] [Google Scholar]
- Di Giorgio FP, Carrasco MA, Siao MC, Maniatis T, Eggan K. Non-cell autonomous effect of glia on motor neurons in an embryonic stem cell-based ALS model. Nat Neurosci. 2007;10:608–614. doi: 10.1038/nn1885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durand J, Amendola J, Bories C, Lamotte d’Incamps B. Early abnormalities in transgenic mouse models of amyotrophic lateral sclerosis. J Physiol. 2006;99:211–220. doi: 10.1016/j.jphysparis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Elbasiouny SM, Amendola J, Durand J, Heckman CJ. Evidence from computer simulations for alterations in the membrane biophysical properties and dendritic processing of synaptic inputs in mutant superoxide dismutase-1 motoneurons. J Neurosci. 2010a;30:5544–5558. doi: 10.1523/JNEUROSCI.0434-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ElBasiouny SM, Schuster JE, Heckman CJ. Persistent inward currents in spinal motoneurons: Important for normal function but potentially harmful after spinal cord injury and in amyotrophic lateral sclerosis. Clin Neurophys. 2010b;121:1669–1679. doi: 10.1016/j.clinph.2009.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filipchuk AA, Durand J. Postnatal dendritic development in lumbar motoneurons in mutant superoxide dismutase 1 mouse model of amyotrophic lateral sclerosis. Neuroscience. 2012;209:144–154. doi: 10.1016/j.neuroscience.2012.01.046. [DOI] [PubMed] [Google Scholar]
- Franceschetti S, Taverna S, Sancini G, Panzica F, Lombardi R, Avanzini G. Protein kinase C-dependent modulation of Na+ currents increases the excitability of rat neocortical pyramidal neurones. J Physiol. 2000;528:291–304. doi: 10.1111/j.1469-7793.2000.00291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey D, Schneider C, Xu L, Borg J, Spooren W, Caroni P. Early and selective loss of neuromuscular synapse subtypes with low sprouting competence in motoneuron diseases. J Neurosci. 2000;20:2534–2542. doi: 10.1523/JNEUROSCI.20-07-02534.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AL. Mechanisms of sodium channel inactivation. Curr Opin Neurobiol. 2003;13:284–290. doi: 10.1016/s0959-4388(03)00065-5. [DOI] [PubMed] [Google Scholar]
- Grosskreutz J, Van Den Bosch L, Keller BU. Calcium dysregulation in amyotrophic lateral sclerosis. Cell Calcium. 2010;47:165–174. doi: 10.1016/j.ceca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Guareschi S, Cova E, Cereda C, Ceroni M, Donetti E, Bosco DA, Trotti D, Pasinelli P. An over-oxidized form of superoxide dismutase found in sporadic amyotrophic lateral sclerosis with bulbar onset shares a toxic mechanism with mutant SOD1. Proc Natl Acad Sci USA. 2012;109:5074–5079. doi: 10.1073/pnas.1115402109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX. Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science. 1994;264:1772–1775. doi: 10.1126/science.8209258. [DOI] [PubMed] [Google Scholar]
- Haidet-Phillips AM, Hester ME, Miranda CJ, Meyer K, Braun L, Frakes A, Song S, Likhite S, Murtha MJ, Foust KD, Rao M, Eagle A, Kammesheidt A, Christensen A, Mendell JR, Burghes AH, Kaspar BK. Astrocytes from familial and sporadic ALS patients are toxic to motor neurons. Nat Biotechnol. 2011;29:824–832. doi: 10.1038/nbt.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammarstro¨m AK, Gage PW. Oxygen-sensing persistent sodium channels in rat hippocampus. J Physiol. 2000;529:107–118. doi: 10.1111/j.1469-7793.2000.00107.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harraz MM, Marden JJ, Zhou W, Zhang Y, Williams A, Sharov VS, Nelson K, Luo M, Paulson H, Scho¨neich C, Engelhardt JF. SOD1 mutations disrupt redox-sensitive Rac regulation of NADPH oxidase in a familial ALS model. J Clin Invest. 2008;118:659–670. doi: 10.1172/JCI34060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hegedus J, Putman CT, Gordon T. Time course of preferential motor unit loss in the SOD1 G93A mouse model of amyotrophic lateral sclerosis. Neurobiol Dis. 2007;28:154–164. doi: 10.1016/j.nbd.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Hossaini M, Cano SC, van Dis V, Haasdijk ED, Hoogenraad CC, Holstege JC, Jaarsma D. Spinal inhibitory interneuron pathology follows motor neuron degeneration independent of glial mutant superoxide dismutase 1 expression in SOD1-ALS mice. J Neuropathol Exp Neurol. 2011;70:662–677. doi: 10.1097/NEN.0b013e31822581ac. [DOI] [PubMed] [Google Scholar]
- Ilieva H, Polymenidou M, Cleveland DW. Non-cell autonomous toxicity in neurodegenerative disorders: ALS and beyond. J Cell Biol. 2009;187:761–772. doi: 10.1083/jcb.200908164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang M, Schuster JE, Fu R, Siddique T, Heckman CJ. Progressive changes in synaptic inputs to motoneurons in adult sacral spinal cord of a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2009;29:15031–15038. doi: 10.1523/JNEUROSCI.0574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kassmann M, Hansel A, Leipold E, Birkenbeil J, Lu SQ, Hoshi T, Heinemann SH. Oxidation of multiple methionine residues impairs rapid sodium channel inactivation. Pflugers Arch. 2008;456:1085–1095. doi: 10.1007/s00424-008-0477-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiernan MC, Petri S. Hyperexcitability and amyotrophic lateral sclerosis. Neurology. 2012;78:1544–1545. doi: 10.1212/WNL.0b013e3182563c0a. [DOI] [PubMed] [Google Scholar]
- Kuo JJ, Siddique T, Fu R, Heckman CJ. Increased persistent Na(+) current and its effect on excitability in motoneurones cultured from mutant SOD1 mice. J Physiol. 2005;563:843–854. doi: 10.1113/jphysiol.2004.074138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Ona VO, Guégan C, Chen M, Jackson-Lewis V, Andrews LJ, Olszewski AJ, Stieg PE, Lee JP, Przedborski S, Friedlander RM. Functional role of caspase-1 and caspase-3 in an ALS transgenic mouse model. Science. 2000;288:335–339. doi: 10.1126/science.288.5464.335. [DOI] [PubMed] [Google Scholar]
- Li XM, Yang JM, Hu DH, Hou FQ, Zhao M, Zhu XH, Wang Y, LiJ G, Hu P, Chen L, Qin LN, Gao TM. Contribution of downregulation of l-type calcium currents to delayed neuronal death in rat hippocampus after global cerebral ischemia and reperfusion. J Neurosci. 2007;27:5249–5259. doi: 10.1523/JNEUROSCI.0802-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo LE, Barbe A, Portalier P, Fritschy JM, Bras H. Differential expression of GABAA and glycine receptors in ALS-resistant vs. ALS-vulnerable motoneurons: Possible implications for selective vulnerability of motoneurons. Eur J Neurosci. 2006;23:3161–3170. doi: 10.1111/j.1460-9568.2006.04863.x. [DOI] [PubMed] [Google Scholar]
- Martin LJ, Liu Z, Chen K, Price AC, Pan Y, Swaby JA, Golden WC. Motor neuron degeneration in amyotrophic lateral sclerosis mutant superoxide dismutase-1 transgenic mice: Mechanisms of mitochondriopathy and cell death. J Comp Neurol. 2007;500:20–46. doi: 10.1002/cne.21160. [DOI] [PubMed] [Google Scholar]
- Nagai M, Re DB, Nagata T, Chalazonitis A, Jessell TM, Wichterle H, Przedborski S. Astrocytes expressing ALS-linked mutated SOD1 release factors selectively toxic to motor neurons. Nat Neurosci. 2007;10:615–622. doi: 10.1038/nn1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nani F, Cifra A, Nistri A. Transient oxidative stress evokes early changes in the functional properties of neonatal rat hypoglossal motoneurons in vitro. Eur J Neurosci. 2010;31:951–966. doi: 10.1111/j.1460-9568.2010.07108.x. [DOI] [PubMed] [Google Scholar]
- Obeso JA, Rodriguez-Oroz MC, Goetz CG, Marin C, Kordower JH, Rodriguez M, Hirsch EC, Farrer M, Schapira AH, Halliday G. Missing pieces in the Parkinson’s disease puzzle. Nat Med. 2010;16:653–661. doi: 10.1038/nm.2165. [DOI] [PubMed] [Google Scholar]
- Palop JJ, Chin J, Mucke L. A network dysfunction perspective on neurodegenerative diseases. Nature. 2006;443:768–773. doi: 10.1038/nature05289. [DOI] [PubMed] [Google Scholar]
- Pambo-Pambo A, Durand J, Gueritaud JP. Early excitability changes in lumbar motoneurons of transgenic SOD1G85R and SOD1G(93A–Low) mice. J Neurophysiol. 2009;102:3627–3642. doi: 10.1152/jn.00482.2009. [DOI] [PubMed] [Google Scholar]
- Pasinelli P, Brown RH. Molecular biology of amyotrophic lateral sclerosis: Insights from genetics. Nat Rev Neurosci. 2006;7:710–723. doi: 10.1038/nrn1971. [DOI] [PubMed] [Google Scholar]
- Pieri M, Carunchio I, Curcio L, Mercuri NB, Zona C. Increased persistent sodium current determines cortical hyperexcitability in a genetic model of amyotrophic lateral sclerosis. Exp Neurol. 2009;215:368–379. doi: 10.1016/j.expneurol.2008.11.002. [DOI] [PubMed] [Google Scholar]
- Plested AJ, Mayer ML. AMPA receptor ligand binding domain mobility revealed by functional cross linking. J Neurosci. 2009;29:11912–11923. doi: 10.1523/JNEUROSCI.2971-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, Borchelt DR. Superoxide dismutase 1 encoding mutations linked to ALS adopts a spectrum of misfolded states. Mol Neurodegener. 2011;6:77. doi: 10.1186/1750-1326-6-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KA. Links between electrophysiological and molecular pathology of amyotrophic lateral sclerosis. Integr Comp Biol. 2011;51:913–925. doi: 10.1093/icb/icr116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan KA, Schuster JE, Fu R, Siddique T, Heckman CJ. Altered postnatal maturation of electrical properties in spinal motoneurons in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2011;589:2245–2260. doi: 10.1113/jphysiol.2010.200659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond LA, André VM, Cepeda C, Gladding CM, Milnerwood AJ, Levine MS. Pathophysiology of Huntington’s disease: Time-dependent alterations in synaptic and receptor function. Neuroscience. 2011;198:252–273. doi: 10.1016/j.neuroscience.2011.08.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santos SF, Pierrot N, Octave JN. Network excitability dysfunction in Alzheimer’s disease: Insights from in vitro and in vivo models. Rev Neurosci. 2010;21:153–171. doi: 10.1515/revneuro.2010.21.3.153. [DOI] [PubMed] [Google Scholar]
- Saxena S, Cabuy E, Caroni P. A role for motoneuron subtype-selective ER stress in disease manifestations of FALS mice. Nat Neurosci. 2009;12:627–636. doi: 10.1038/nn.2297. [DOI] [PubMed] [Google Scholar]
- Schuster JE, Fu R, Siddique T, Heckman CJ. Effect of prolonged riluzole exposure on cultured motoneurons in a mouse model of ALS. J Neurophysiol. 2012;107:484–492. doi: 10.1152/jn.00714.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sepulveda FJ, Bustos FJ, Inostroza E, Zuniga FA, Neve RL, Montecino M, van Zundert B. Differential roles of NMDA receptor subtypes NR2A and NR2B in dendritic branch development and requirement of RasGRF1. J Neurophysiol. 2010;103:1758–1770. doi: 10.1152/jn.00823.2009. [DOI] [PubMed] [Google Scholar]
- Theiss RD, Kuo JJ, Heckman CJ. Persistent inward currents in rat ventral horn neurones. J Physiol. 2007;580:507–522. doi: 10.1113/jphysiol.2006.124123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urushitani M, Ezzi SA, Julien JP. Therapeutic effects of immunization with mutant superoxide dismutase in mice models of amyotrophic lateral sclerosis. Proc Natl Acad Sci USA A. 2007;104:2495–2500. doi: 10.1073/pnas.0606201104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Zundert B, Peuscher MH, Hynynen M, Chen A, Neve RL, Brown RH, Jr, Constantine-Paton M, Bellingham MC. Neonatal neuronal circuitry shows hyperexcitable disturbance in a mouse model of the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J Neurosci. 2008;28:10864–10874. doi: 10.1523/JNEUROSCI.1340-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vucic S, Nicholson GA, Kiernan MC. Cortical hyperexcitability may precede the onset of familial amyotrophic lateral sclerosis. Brain. 2008;131:1540–1550. doi: 10.1093/brain/awn071. [DOI] [PubMed] [Google Scholar]
- Wootz H, Enjin A, Wallen-Mackenzie A, Lindholm D, Kullander K. Reduced VGLUT2 expression increases motor neuron viability in Sod1(G93A) mice. Neurobiol Dis. 2010;37:58–66. doi: 10.1016/j.nbd.2009.09.006. [DOI] [PubMed] [Google Scholar]
- Wu DC, Ré DB, Nagai M, Ischiropoulos H, Przedborski S. The inflammatory NADPH oxidase enzyme modulates motor neuron degeneration in amyotrophic lateral sclerosis mice. Proc Natl Acad Sci USA A. 2006;103:12132–12137. doi: 10.1073/pnas.0603670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yon JM, Baek IJ, Lee SR, Jin Y, Kim MR, Nahm SS, Kim JS, Ahn B, Lee BJ, Yun YW, Nam SY. The spatio-temporal expression pattern of cytoplasmic Cu/Zn superoxide dismutase (SOD1) mRNA during mouse embryogenesis. J Mol Histol. 2008;39:95–103. doi: 10.1007/s10735-007-9134-1. [DOI] [PubMed] [Google Scholar]
- Zhao W, Beers DR, Henkel JS, Zhang W, Urushitani M, Julien JP, Appel SH. Extracellular mutant SOD1 induces microglial-mediated motoneuron injury. Glia. 2010;58:231–243. doi: 10.1002/glia.20919. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.