Abstract
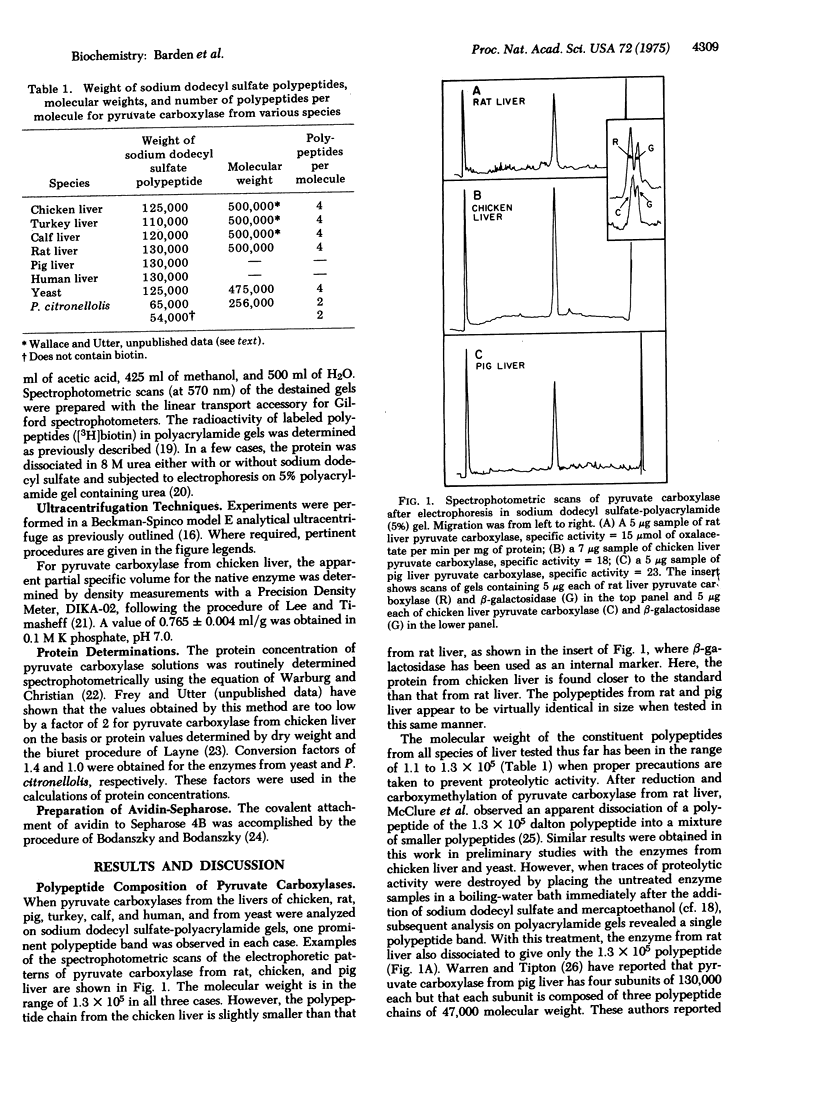
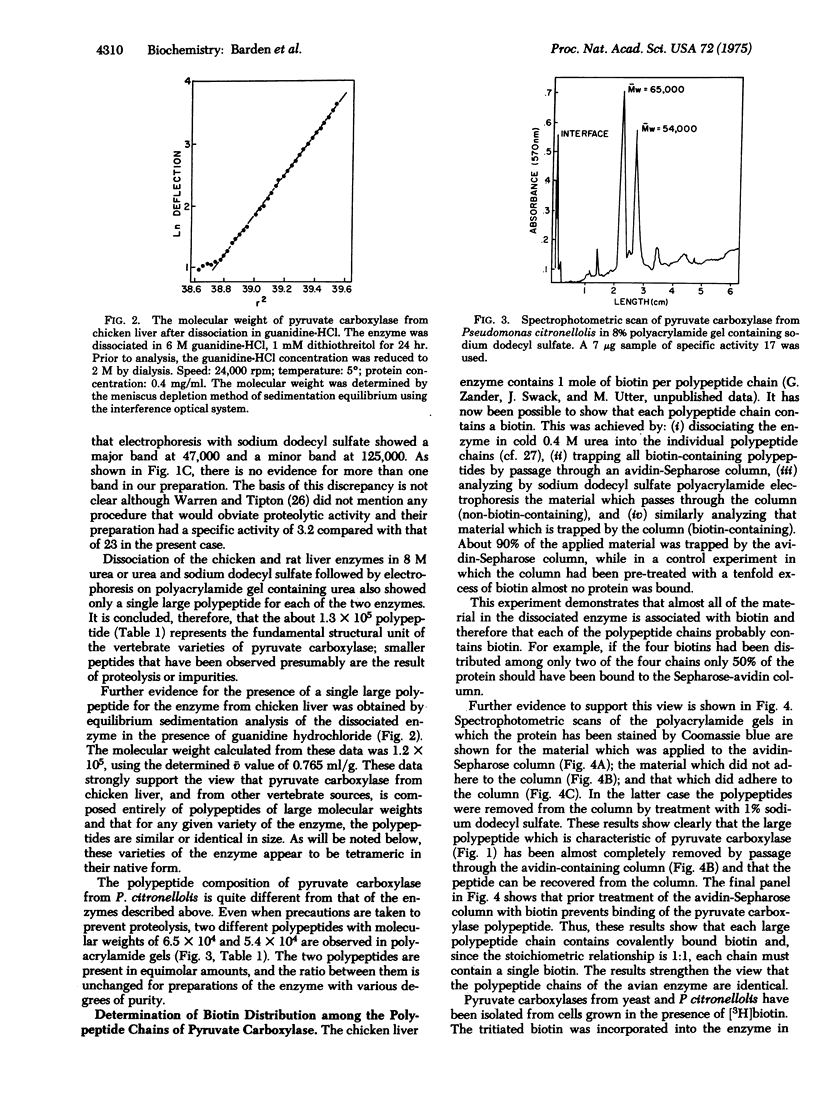
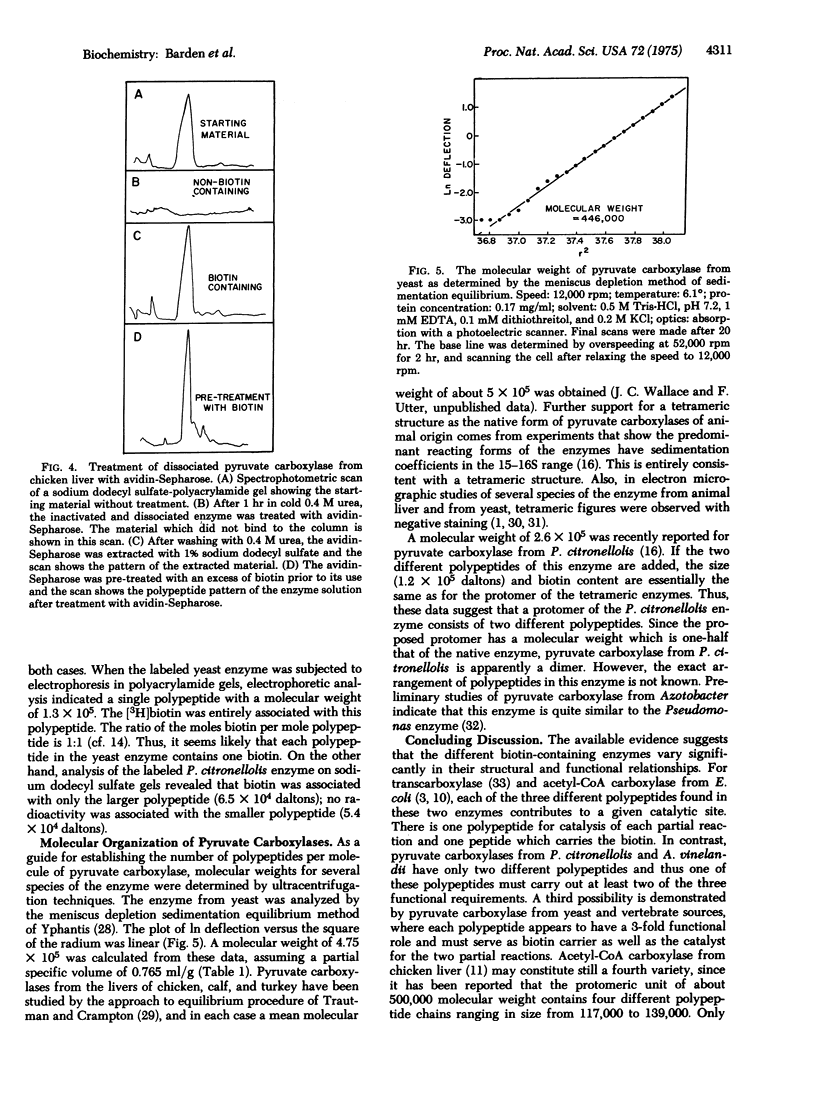
Varieties of pyruvate carboxylase [pyruvate: CO2 ligase (ADP-forming), EC 6.4.1.1] obtained from the livers of several species of vertebrates, including humans, all show the same basic structure. They are composed of large polypeptide chains of molecular weights ranging from 1.2 to 1.3 X 10(5) for the different varieties of the enzyme. The native form of the enzyme appears to be a tetramer with a molecular weight of about 5 X 10(5). In the case of pyruvate carboxylase from chicken liver each polypeptide chain contains a biotin moiety, thus supporting the thesis that the tetramer contains four identical polypeptide chains. Pyruvate carboxylase from yeast appears to be basically similar to those from the vertebrate species and has a tetrameric structure. Each protomer contains a single polypeptide chain with a molecular weitht of 1.25 X 10(5). In contrast, pyruvate carboxylase from two bacterial species, Pseudomonas citronellolis and Axotobacter vinelandii, appears to be a dimer with a molecular weight (2.5 X 10(5)) about half that of the animal and yeast species. As a further difference, each of the protomers of the bacterial enzymes contain two polypeptides of 6.5 and 5.4 X 10(5) molecular weight in case of the Pseudomonas enzyme. The larger of the two polypeptides contains the biotin moiety. The functional units of the bacterial enzyme thus appear to contain two polypeptides while that of the liver and yeast enzymes is made up of a single chain. Neither of these arrangements corresponds with those of other biotin enzymes whose structure has been extensively studied (acetyl-CoA carboxylases from liver or Excherichia coli, and transcarboxylase from Propionibacterium).
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barden R. E., Fung C. H., Utter M. F., Scrutton M. C. Pyruvate carboxylase from chicken liver. Steady state kinetic studies indicate a "two-site" ping-pong mechanism. J Biol Chem. 1972 Feb 25;247(4):1323–1333. [PubMed] [Google Scholar]
- Bodanszky A., Bodanszky M. Sepharose-avidin column for the binding of biotin or biotin-containing peptides. Experientia. 1970 Mar 15;26(3):327–327. doi: 10.1007/BF01900128. [DOI] [PubMed] [Google Scholar]
- Guchhait R. B., Zwergel E. E., Lane M. D. Acetyl coenzyme A carboxylase. Subunit structure of the protomeric form of the avian liver enzyme. J Biol Chem. 1974 Aug 10;249(15):4776–4780. [PubMed] [Google Scholar]
- Irias J. J., Olmsted M. R., Utter M. F. Pyruvate carboxylase. Reversible inactivation by cold. Biochemistry. 1969 Dec;8(12):5136–5148. doi: 10.1021/bi00840a068. [DOI] [PubMed] [Google Scholar]
- Lee J. C., Frigon R. P., Timasheff S. N. The chemical characterization of calf brain microtubule protein subunits. J Biol Chem. 1973 Oct 25;248(20):7253–7262. [PubMed] [Google Scholar]
- Lee J. C., Timasheff S. N. Partial specific volumes and interactions with solvent components of proteins in guanidine hydrochloride. Biochemistry. 1974 Jan 15;13(2):257–265. doi: 10.1021/bi00699a005. [DOI] [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Cleland W. W. Rat liver pyruvate carboxylase. 3. Isotopic exchange studies of the first partial reaction. J Biol Chem. 1971 Jun 10;246(11):3584–3590. [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Kneifel H. P. Rat liver pyruvate carboxylase. I. Preparation, properties, and cation specificity. J Biol Chem. 1971 Jun 10;246(11):3569–3578. [PubMed] [Google Scholar]
- McClure W. R., Lardy H. A., Wagner M., Cleland W. W. Rat liver pyruvate carboxylase. II. Kinetic studies of the forward reaction. J Biol Chem. 1971 Jun 10;246(11):3579–3583. [PubMed] [Google Scholar]
- Moss J., Lane M. D. The biotin-dependent enzymes. Adv Enzymol Relat Areas Mol Biol. 1971;35:321–442. doi: 10.1002/9780470122808.ch7. [DOI] [PubMed] [Google Scholar]
- Nakashima K., Rudolph F. B., Wakabayashi T., Lardy H. A. Rat liver pyruvate carboxylase. V. Reversible dissociation by chloride salts of monovalent cations. J Biol Chem. 1975 Jan 10;250(1):331–336. [PubMed] [Google Scholar]
- Northrop D. B. Transcarboxylase. VI. Kinetic analysis of the reaction mechanism. J Biol Chem. 1969 Nov 10;244(21):5808–5819. [PubMed] [Google Scholar]
- Northrop D. B., Wood H. G. Transcarboxylase. VII. Exchange reactions and kinetics of oxalate inhibition. J Biol Chem. 1969 Nov 10;244(21):5820–5827. [PubMed] [Google Scholar]
- Pringle J. R. The molecular weight of the undegraded polypeptide chain of yeast hexokinase. Biochem Biophys Res Commun. 1970 Apr 8;39(1):46–52. doi: 10.1016/0006-291x(70)90755-2. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C. Pyruvate carboxylase from chicken liver: effects of sulfate and other anions on catalytic activity and structural parameters. Arch Biochem Biophys. 1972 Jun;150(2):636–647. doi: 10.1016/0003-9861(72)90084-7. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C., Taylor B. L. Isolation and characterization of pyruvate carboxylase from Azotobacter vinelandii OP. Arch Biochem Biophys. 1974 Oct;164(2):641–654. doi: 10.1016/0003-9861(74)90076-9. [DOI] [PubMed] [Google Scholar]
- Scrutton M. C., Young M. R., Utter M. F. Pyruvate carboxylase from baker's yeast. The presence of bound zinc. J Biol Chem. 1970 Nov 25;245(22):6220–6227. [PubMed] [Google Scholar]
- Taylor B. L., Barden R. E., Utter M. F. Identification of the reacting form of pyruvate carboxylase. J Biol Chem. 1972 Nov 25;247(22):7383–7390. [PubMed] [Google Scholar]
- Taylor B. L., Routman S., Utter M. F. The control of the synthesis of pyruvate carboxylase in Pseudomonas citronellolis. Evience from double labeling studies. J Biol Chem. 1975 Mar 25;250(6):2376–2382. [PubMed] [Google Scholar]
- Utter M. F., Barden R. E., Taylor B. L. Pyruvate carboxylase: an evaluation of the relationships between structure and mechanism and between structure and catalytic activity. Adv Enzymol Relat Areas Mol Biol. 1975;42:1–72. doi: 10.1002/9780470122877.ch1. [DOI] [PubMed] [Google Scholar]
- Valentine R. C., Wrigley N. G., Scrutton M. C., Irias J. J., Utter M. F. Pyruvate carboxylase. 8. The subunit structure as examined by electron microscopy. Biochemistry. 1966 Oct;5(10):3111–3116. doi: 10.1021/bi00874a005. [DOI] [PubMed] [Google Scholar]
- Warren G. B., Tipton K. F. Pig liver pyruvate carboxylase. Purification, properties and cation specificity. Biochem J. 1974 May;139(2):297–310. doi: 10.1042/bj1390297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- YPHANTIS D. A. EQUILIBRIUM ULTRACENTRIFUGATION OF DILUTE SOLUTIONS. Biochemistry. 1964 Mar;3:297–317. doi: 10.1021/bi00891a003. [DOI] [PubMed] [Google Scholar]


