Abstract
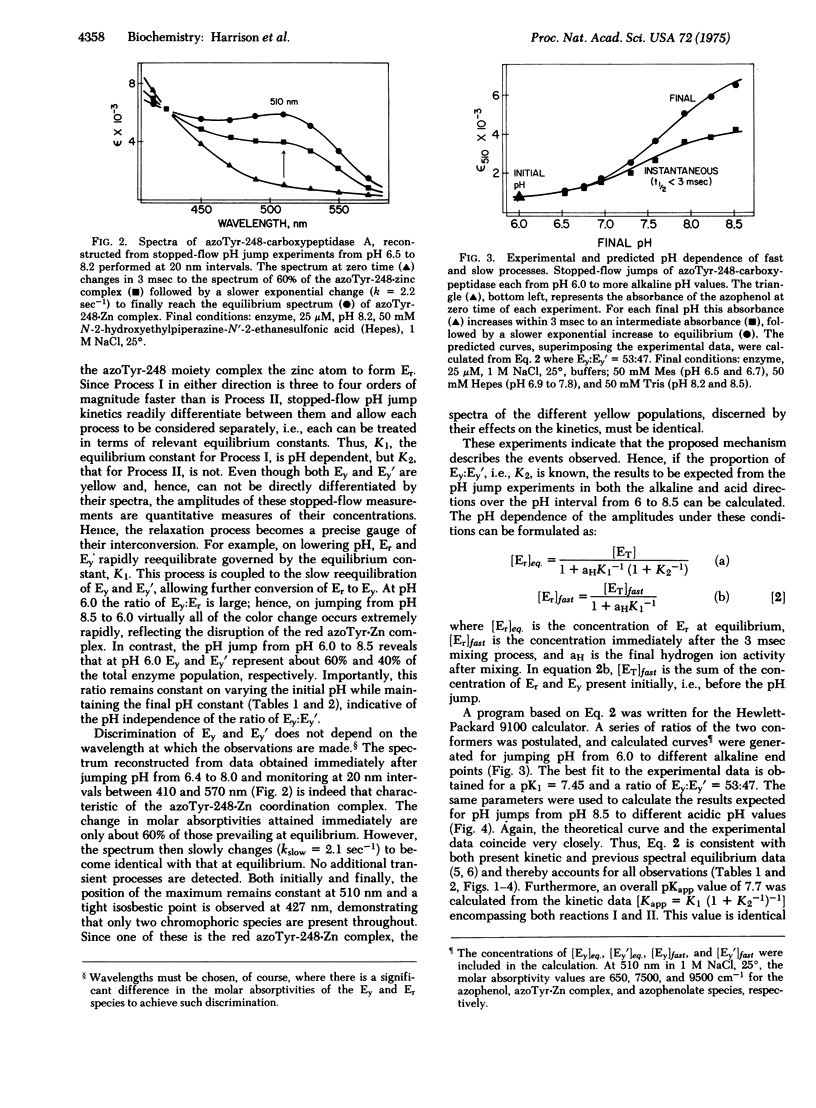
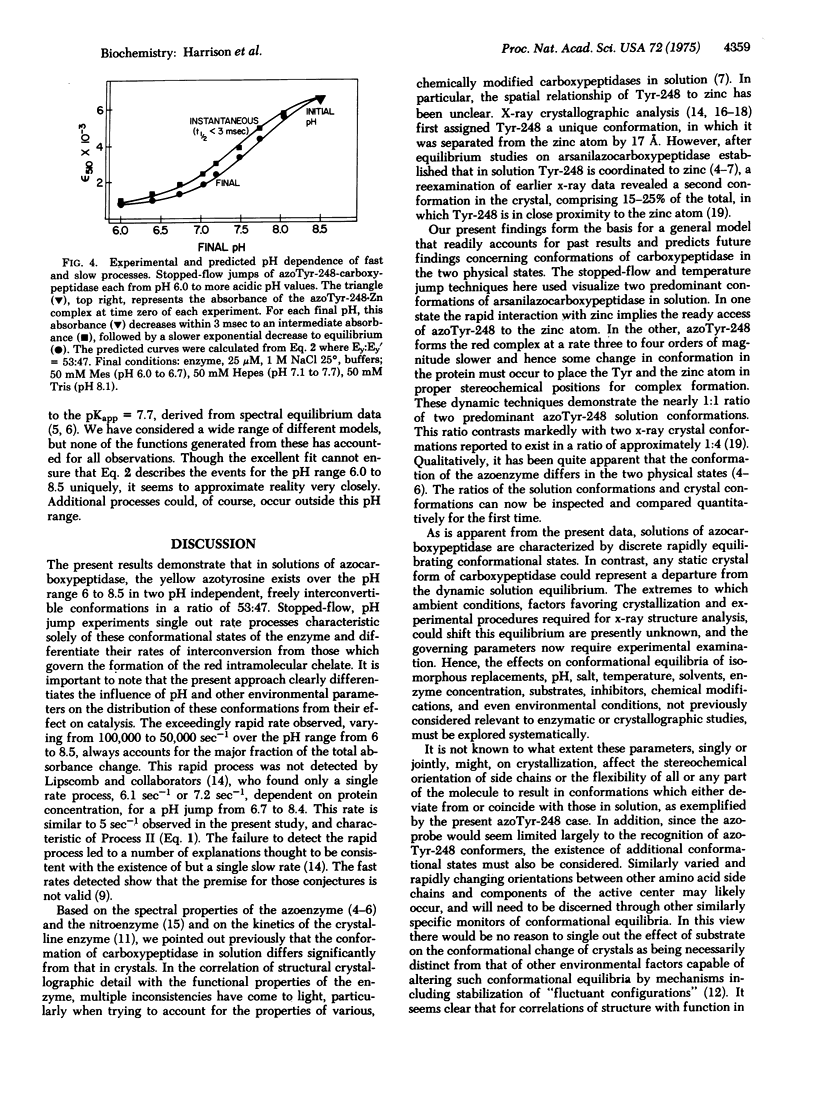
The red azoTyr-248-Zn complex of arnilazocarboxypeptidase, previously used to demonstrate differences in conformation of the enzyme in crystals and in solution, has now provided means to detect multiple conformations of the enzyme in solution by stopped-flow pH and temperature jump experiments. These studies identify two distinct processes. Er + H+ in equilibrium Ey (I), is the extremely rapid, Kfast about 10(5) sec-1, pH dependent dissociation of the metal complex. Ey in equilibrium Ey' (II), is much slower, Kslow about 5 sec-1, pH independent interconversion of two distinct populations of protein molecules, Ey and Ey', in which the yellow azo-Tyr-248 is different conformations. These two conformations can be differentiated readily by stopped-flow pH-jump experiments, since I is three to four orders of magnitude faster than II. Mathematical expressions derived from this mechanism accurately predict all observations over the pH range from 6.0 to 8.5. In a previous stopped-flow pH-jump experiment, Lipcomb and coworkers [Quiocho, F. A., McMurray, C. H. & Lipcomb, W. H. (1972), Proc. Nat. Acad. Sci. USA 69, 2850-2854] recognized only a single process with a rate constant of about 6 sec-1, but not the major, very rapid rate observed here. The failure to detect this fast process led to the postulation of a number of explanations intended to account for the detection of only a single, slow rate. The present observations show that the premise for those conjectures is not valid. The azoprobe reveals the existence of rapidly interconvertible substructures of carboxypeptidase A, and the results support the view that in solution, enzymes can adopt multiple, readily interconvertible and related conformations which could then either facilitate or impede catalysis. In crystals, rearrangement of molecular structure could be severely impaired or restricted, and crystallization might single out either active or inactive conformations. In the latter case, such crystals would have greatly reduced activities and markedly altered catalytic behavior, as is observed for carboxypeptidase A. In combination with detailed kinetic analysis of crystals, conformational analysis in solution should be a valuable guide to discern enzyme mechanisms and select crystals for x-ray structure analysis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Benedetti E., Goodman M. Conformational aspects of polypeptide structure. 28. Side-chain Cotton effect from poly-L-p-(2'-hydroxy-5'-methylphenylazo) phenylalanine. Biochemistry. 1968 Dec;7(12):4242–4247. doi: 10.1021/bi00852a015. [DOI] [PubMed] [Google Scholar]
- French T. C., Yu N. T., Auld D. S. Relaxation spectra of proteinases. Isomerizations of carboxypeptidase A (Cox) and (Anson). Biochemistry. 1974 Jul 2;13(14):2877–2882. doi: 10.1021/bi00711a016. [DOI] [PubMed] [Google Scholar]
- Harrison L. W., Auld D. S., Vallee B. L. Intramolecular arsanilazotyrosine-248-Zn complex of carboxypeptidase A: a monitor of catalytic events. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3930–3933. doi: 10.1073/pnas.72.10.3930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Conformations of arsanilazotyrosine-248 carboxypeptidase A alpha, beta, gamma, comparison of crystals and solution. Proc Natl Acad Sci U S A. 1973 Jul;70(7):2006–2010. doi: 10.1073/pnas.70.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Differences between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1971 Oct;68(10):2532–2535. doi: 10.1073/pnas.68.10.2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen J. T., Vallee B. L. Environment and conformation dependent sensitivity of the arsanilazotyrosine-248 carboxypeptidase A chromophore. Biochemistry. 1975 Feb 25;14(4):649–660. doi: 10.1021/bi00675a001. [DOI] [PubMed] [Google Scholar]
- Lipscomb W. N. Enzymatic activities of carobxypeptidase A's in solution and in crystals. Proc Natl Acad Sci U S A. 1973 Dec;70(12):3797–3801. doi: 10.1073/pnas.70.12.3797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipscomb W. N., Hartsuck J. A., Reeke G. N., Jr, Quiocho F. A., Bethge P. H., Ludwig M. L., Steitz T. A., Muirhead H., Coppola J. C. The structure of carboxypeptidase A. VII. The 2.0-angstrom resolution studies of the enzyme and of its complex with glycyltyrosine, and mechanistic deductions. Brookhaven Symp Biol. 1968 Jun;21(1):24–90. [PubMed] [Google Scholar]
- Lipscomb W. N., Reeke G. N., Jr, Hartsuck J. A., Quiocho F. A., Bethge P. H. The structure of carboxypeptidase A. 8. Atomic interpretation at 0.2 nm resolution, a new study of the complex of glycyl-L-tyrosine with CPA, and mechanistic deductions. Philos Trans R Soc Lond B Biol Sci. 1970 Feb 12;257(813):177–214. doi: 10.1098/rstb.1970.0020. [DOI] [PubMed] [Google Scholar]
- Quiocho F. A., McMurray C. H., Lipscomb W. N. Similarities between the conformation of arsanilazotyrosine 248 of carboxypeptidase A in the crystalline state and in solution. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2850–2854. doi: 10.1073/pnas.69.10.2850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riordan J. F., Muszynska G. Differences between the conformations of nitrotyrosyl-248 carboxypeptidase A in the crystalline state and in solution. Biochem Biophys Res Commun. 1974 Mar 25;57(2):447–451. doi: 10.1016/0006-291x(74)90951-6. [DOI] [PubMed] [Google Scholar]
- Spilburg C. A., Bethune J. L., Vallee B. L. The physical state dependence of carboxypeptidase Aalpha and Agamma kinetics. Proc Natl Acad Sci U S A. 1974 Oct;71(10):3922–3926. doi: 10.1073/pnas.71.10.3922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallee B. L., Riordan J. F., Johansen J. T., Livingston D. M. Spectro-chemical probes for protein conformation and function. Cold Spring Harb Symp Quant Biol. 1972;36:517–531. doi: 10.1101/sqb.1972.036.01.066. [DOI] [PubMed] [Google Scholar]


