Abstract
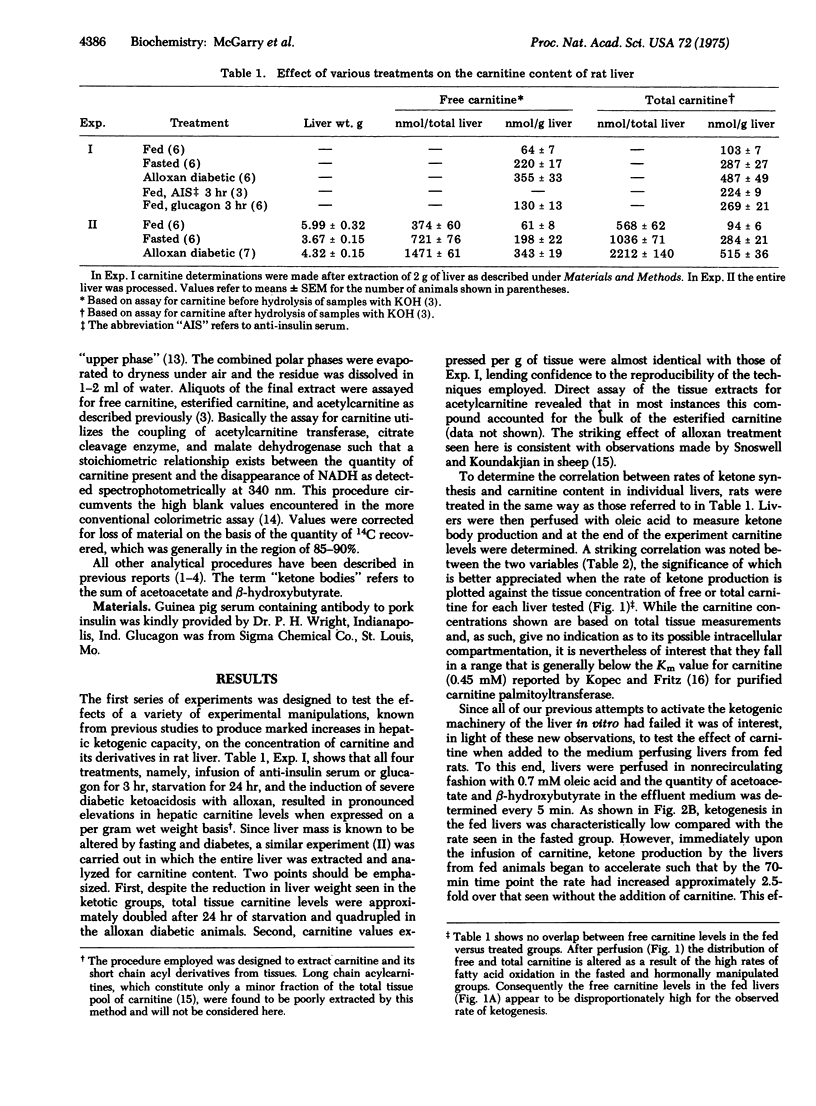
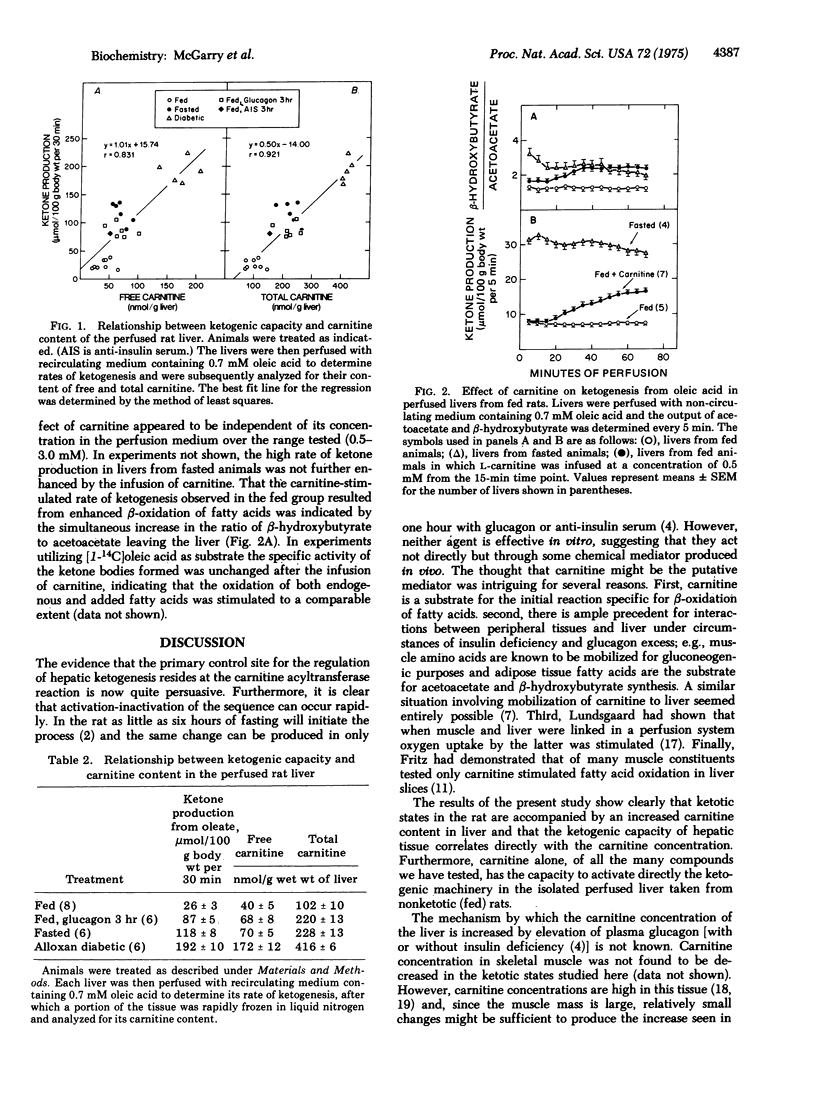
The enhancement of long-chain fatty acid oxidation and ketogenesis in the perfused rat liver, whether induced acutely by treatment of fed animals with anti-insulin serum or glucagon, or over the longer term by starvation or the induction of alloxan diabetes, was found to ba accompanied by a proportional elevation in the tissue carnitine content. Moreover, when added to the medium perfusing livers from fed rats, carnitine stimulated ketogenesis from oleic acid. The findings suggest that the increased fatty acid flux through the carnitine acyltransferase (carnitine palmitoyl-transferase; palmitoyl-CoA:L-carnitine O-palmitoyltransferase; EC 2.3.1.21) reaction brought about by glucagon excess, with or without insulin deficiency, is mediated, at least in part, by elevation in the liver carnitine concentration.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bohmer T. Tissue levels of activated fatty acids (acylcarnitines) and the regulation of fatty acid metabo.ism. Biochim Biophys Acta. 1967 Oct 2;144(2):259–270. [PubMed] [Google Scholar]
- Brosnan J. T., Kopec B., Fritz I. B. The localization of carnitine palmitoyltransferase on the inner membrane of bovine liver mitochondria. J Biol Chem. 1973 Jun 10;248(11):4075–4082. [PubMed] [Google Scholar]
- Exton J. H., Corbin J. G., Park C. R. Control of gluconeogenesis in liver. IV. Differential effects of fatty acids and glucagon on ketogenesis and gluconeogenesis in the perfused rat liver. J Biol Chem. 1969 Aug 10;244(15):4095–4102. [PubMed] [Google Scholar]
- FOLCH J., LEES M., SLOANE STANLEY G. H. A simple method for the isolation and purification of total lipides from animal tissues. J Biol Chem. 1957 May;226(1):497–509. [PubMed] [Google Scholar]
- FRITZ I. The effect of muscle extracts on the oxidation of palmitic acid by liver slices and homogenates. Acta Physiol Scand. 1955 Oct 12;34(4):367–385. doi: 10.1111/j.1748-1716.1955.tb01256.x. [DOI] [PubMed] [Google Scholar]
- Fritz I. B. An hypothesis concerning the role of carnitine in the control of interrelations between fatty acid and carbohydrate metabolism. Perspect Biol Med. 1967 Summer;10(4):643–677. doi: 10.1353/pbm.1967.0019. [DOI] [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Comparison of properties of carnitine palmitoyltransferase I with those of carnitine palmitoyltransferase II, and preparation of antibodies to carnitine palmitoyltransferases. J Biol Chem. 1973 Jun 10;248(11):4069–4074. [PubMed] [Google Scholar]
- Kopec B., Fritz I. B. Properties of a purified carnitine palmitoyltransferase, and evidence for the existence of other carnitine acyltransferases. Can J Biochem. 1971 Aug;49(8):941–948. doi: 10.1139/o71-136. [DOI] [PubMed] [Google Scholar]
- LUNDSGAARD E. Observations on a factor determining the metabolic rate of the liver. Biochim Biophys Acta. 1950 Jan;4(1-3):322–329. doi: 10.1016/0006-3002(50)90038-2. [DOI] [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. ENZYMOLOGICAL DETERMINATION OF FREE CARNITINE CONCENTRATIONS IN RAT TISSUES. J Lipid Res. 1964 Apr;5:184–187. [PubMed] [Google Scholar]
- MARQUIS N. R., FRITZ I. B. THE DISTRIBUTION OF CARNITINE, ACETYLCARNITINE, AND CARNITINE ACETYLTRANSFERASE IN RAT TISSUES. J Biol Chem. 1965 May;240:2193–2196. [PubMed] [Google Scholar]
- Mallette L. E., Exton J. H., Park Effects of glucagon on amino acid transport and utilization in the perfused rat liver. J Biol Chem. 1969 Oct 25;244(20):5724–5728. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The metabolism of (minus)-octanoylcarnitine in perfused livers from fed and fasted rats. Evidence for a possible regulatory role of carnitine acyltransferase in the control of ketogenesis. J Biol Chem. 1974 Dec 25;249(24):7984–7990. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from octanoic acid. The role of the tricarboxylic acid cycle and fatty acid synthesis. J Biol Chem. 1971 Feb 25;246(4):1149–1159. [PubMed] [Google Scholar]
- McGarry J. D., Foster D. W. The regulation of ketogenesis from oleic acid and the influence of antiketogenic agents. J Biol Chem. 1971 Oct 25;246(20):6247–6253. [PubMed] [Google Scholar]
- McGarry J. D., Meier J. M., Foster D. W. The effects of starvation and refeeding on carbohydrate and lipid metabolism in vivo and in the perfused rat liver. The relationship between fatty acid oxidation and esterification in the regulation of ketogenesis. J Biol Chem. 1973 Jan 10;248(1):270–278. [PubMed] [Google Scholar]
- McGarry J., Wright P. H., Foster D. W. Hormonal control of ketogenesis. Rapid activation of hepatic ketogenic capacity in fed rats by anti-insulin serum and glucagon. J Clin Invest. 1975 Jun;55(6):1202–1209. doi: 10.1172/JCI108038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raskin P., McGarry J. D., Foster D. W. Independence of cholesterol and fatty acid biosynthesis from cyclic adenosine monophosphate concentration in the perfused rat liver. J Biol Chem. 1974 Oct 10;249(19):6029–6032. [PubMed] [Google Scholar]
- Snoswell A. M., Koundakjian P. P. Relationships between carnitine and coenzyme A esters in tissues of normal and alloxan-diabetic sheep. Biochem J. 1972 Mar;127(1):133–141. doi: 10.1042/bj1270133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H. Alpha- and beta-cell interrelationships in health and disease. Metabolism. 1974 Jun;23(6):581–593. doi: 10.1016/0026-0495(74)90086-9. [DOI] [PubMed] [Google Scholar]


