Abstract
The current global obesity pandemic is the leading cause for the soaring rates of metabolic diseases, especially diabetes, cardiovascular disease, hypertension and non-alcoholic hepatosteatosis. Efforts devoted to find cures for obesity and associated disorders in the past two decades have prompted intensive interest in adipocyte biology, and have led to major advances in the mechanistic understanding of adipose tissue as an essential endocrine organ. Adipose tissue secretes an array of hormones (adipokines) that signal key organs to maintain metabolic homeostasis, and their dysfunction has been causally linked to a wide range of metabolic diseases. In addition, obesity induces production of inflammatory cytokines (often referred to together with adipokines as adipocytokines) and infiltration of immune cells into adipose tissue, which creates a state of chronic low-grade inflammation. Metabolic inflammation has been increasingly recognized as a unifying mechanism linking obesity to a broad spectrum of pathological conditions. This review focuses on classic examples of adipocytokines that have helped to form the basis of the endocrine and inflammatory roles of adipose tissue, and it also details a few newly characterized adipocytokines that provide fresh insights into adipose biology. Studies of adipocytokines in clinical settings and their therapeutic potential are also discussed.
Introduction
In the past two decades, the world has seen a sustained increase in obesity, and the levels of overweight and obese persons worldwide have reached epidemic proportions (Finucane, et al. 2011). It is well established that obesity induces all major metabolic disorders, especially diabetes, cardiovascular disease, hypertension and fatty liver disease (Eckel, et al. 2005). Mounting evidence also links obesity to a growing list of debilitating disorders including neurodegenerative disease, airway disorders, and cancer, all of which contribute to the staggering morbidity and mortality associated with obesity. Aimed at developing effective therapies for obesity and its associated disorders, scientists worldwide have intensified their efforts to elucidate the pathophysiological mechanisms by which obesity induces or amplifies its major adverse consequences. The concept of an adipocytokine was developed in this process and dysfunction of adipocytokine pathways has been recognized as a key etiological factor of obesity-induced disorders. Furthermore, the rational manipulation of adipocytokines is becoming a promising avenue of therapy for obesity and associated metabolic abnormalities.
Endocrine function of adipose tissue and adipokines
Obesity is the expansion of white adipose tissue (WAT), the most effective lipid storage organ in the body. In obese subjects, white adipocytes in WAT have increased release of free fatty acids through lipolysis process leading to elevated serum fatty acid levels. This overflow of lipids from obese adipose depots has been considered a key reason for obesity-associated insulin resistance and hepatosteatosis for several decades (Randle, et al. 1963; Samuel, et al. 2010). But fatty acids in this setting have often been considered as a whole, and studies examining the distinct impact of individual lipid species have provided intriguing insights into the specificities of adipose-secreted lipids (Cao, et al. 2008). In 1994, leptin was identified as an adipose secreted hormone (adipokine) that exhibits potent anorexic effects, and this finding re-defined WAT as an endocrine organ (Zhang, et al. 1994). In the following two decades, several more adipokines were identified as critical regulators of systemic lipid and glucose homeostasis, and the list continues to grow (Fig. 1). Adipokines mediate the crosstalk between adipose tissue and other key metabolic organs, especially the liver, muscle, and pancreas, as well as the central nerve system (Rosen and Spiegelman 2006). Consistent with this notion, dysfunctions in adipokine pathways often result in impaired organ communications and metabolic abnormalities in multiple tissues thereby constituting a critical pathological component in the development of metabolic disease (Trujillo and Scherer 2006).
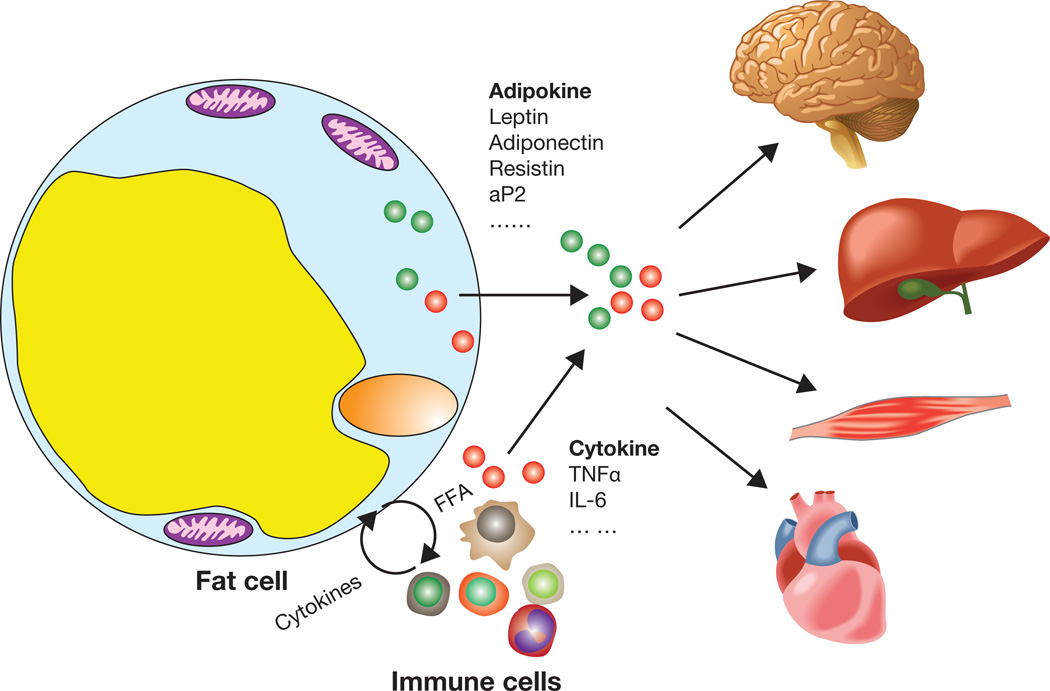
Figure 1. Adipocytokines and metabolic inflammation in adipose tissue.
Adipocokines derived from adipose tissue are the results of intertwined interaction between adipocytes and immune cells that infiltrate adipose tissue. Adipocytokines mediate crosstalk among different cell populations within adipose tissue and also travel to remote organs to regulate systemic energy metabolism. The level and action of adipocytokines are often altered in obese subjects, which contributes to obesity-induced disorders.
Metabolic inflammation and adipocytokines
In 1993, Hotamisligil and colleagues showed that adipose tissue in obese mice secretes TNFα, a proinflammatory cytokine typically produced by immune cells, and also demonstrated that adipocyte-derived TNFα plays a direct role in obesity-induced insulin resistance (Hotamisligil, et al. 1993). This was the first functional link between obesity and inflammation, and over the years it has evolved into the concept of metabolic inflammation (Fig. 1), which has been widely accepted as an important mechanistic connection between obesity and its complications (Hotamisligil 2006). After TNFα, it was demonstrated that adipose tissue produces an array of cytokines and chemokines such as IL-6 and MCP-1, which either positively or negatively regulate systemic glucose and lipid metabolism. Interestingly some adipokines also exhibit features of cytokines or regulate inflammatory responses, and so these two groups of adipose-derived factors are often collectively referred to “adipocytokines” (Fig. 1). In 2003 two studies simultaneously reported that obesity induces macrophage infiltration of adipose tissue in both mice and humans (Weisberg, et al. 2003; Xu, et al. 2003b), which not only provided an explanation for the source of adipose-derived cytokines but also demonstrated for the first time the close juxtaposition between immune and metabolic cells in a metabolic organ. Adipose-resident macrophages are classified into two very distinct subtypes, M1, or classically activated, and M2, or alternatively activated. M1 macrophages secrete proinflammatory cytokines such as TNFα and IL-6, produce iNOS and ROS, and cause insulin resistance. M2 macrophages produce IL-10 and IL-1 receptor antagonists and arginase-1 and have been implicated in tissue remodeling (Gordon 2003). Obesity causes a shift of macrophage subtypes in adipose tissue from M2 to M1 activation, leading to increased levels of proinflammatory cytokines and reactive oxygen species (ROS), which induce insulin resistance (Lumeng, et al. 2007). Meanwhile, the loss of certain beneficial effects associated with M2 macrophages might also contribute to the metabolic deterioration in obesity. For examples, M2 macrophages produce catecholamines that sustain adaptive thermogenesis (Nguyen, et al. 2011), and lipolysis during fasting recruits macrophages that buffer local lipid increase and protect adipose function (Kosteli, et al. 2010). Following macrophages, nearly every major type of immune cell has been identified in adipose tissue in recent years (Feuerer, et al. 2009; Liu, et al. 2009; Winer, et al. 2009; Wu, et al. 2011) and is actively involved in the endocrine function of adipose tissue in systemic metabolic regulation. Furthermore, the close physical and signaling interactions between immune and metabolic cells also exist in all major metabolic organs of obese subjects especially the liver, muscle and pancreas, indicating that metabolic inflammation is a universal feature and a pathological basis for obesity-induced metabolic dysfunction.
There are a number of potential underlying causes for obesity-induced adipose inflammation. Adipose tissue expansion in the development of obesity can cause hypoxia which induce compensatory angiogenesis. Macrophages are recruited to the site to facilitate the vascularization process (Pang, et al. 2008). Similar function of immune cells was also demonstrated in other metabolic tissues such as liver where Kupffer cell-secreted TNF-a and IL-6 in mouse liver are required for efficient liver regeneration (Abshagen, et al. 2007). Infiltrated macrophages in adipose tissues have also been proposed to be a mechanism to remove apoptotic cells (Cinti, et al. 2005; Strissel, et al. 2007). In addition, endotoxemia associated with altered gut permeability and obesity might potentiate adipose inflammation (Cani, et al. 2007). Whereas accumulating evidence supports an overall negative effect of adipose inflammation on energy metabolism, it should bear in mind that not all metabolic inflammation is detrimental to metabolic homeostasis. Inflammation associated with adipose expansion or repair might be necessary for the body to adapt to the excess energy and maintain metabolic homeostasis (Ye and McGuinness 2013). In the same vein, certain cytokines stimulate energy expenditure and reduce food intake which might help curtail obesity (Ye and Keller 2010). Therefore, the metabolic outcomes of adipose inflammation should always be considered in the context of their physiological underpinnings, and more studies are needed to fully understand the extent and mechanism of beneficial inflammatory responses associated with different stages of obesity.
Key adipocytokines in metabolic regulation and obesity-induced metabolic disorders
Leptin
Leptin was identified through positional cloning by Friedman and colleagues in 1994 (Zhang et al. 1994), and is one of most potent adipocytokines in metabolic regulation. Leptin regulates body weight by signaling nutritional status to other organs especially the hypothalamus, which produces neuropeptides and neurotransmitters that modulate food intake and energy expenditure (Friedman and Halaas 1998). Leptin also has anti-diabetic effects independent of its regulation of body weight and energy intake (Kamohara, et al. 1997). Leptin regulates hepatic lipogenesis by suppressing the expression of key enzymes in the fatty acid synthesis pathway (Cohen, et al. 2002) and enhances muscle fatty acid oxidation by activating a critical energy sensor 5’ AMP-activated protein kinase (AMPK) (Minokoshi, et al. 2002).
At the signaling level, leptin activates the leptin receptor, which has multiple splicing isoforms, although the long isoform mediates all known leptin actions (Lee, et al. 1996). There are multiple pathways downstream of the leptin receptor, each of which mediates different aspects of leptin activities (St-Pierre and Tremblay 2012). The main signaling branch of leptin is the Janus kinase (JAK)/signal transducer and activator of the transcription (STAT) (JAK-STAT) pathway, which regulates expression of anorexic neuropeptides (Baumann, et al. 1996). This pathway is essential for leptin regulation of energy balance but not its effects on reproduction (Bates, et al. 2003). The anti-diabetic effect of leptin is mediated by centrally activating the phosphatidylinositol-3-kinase (PI3K)/AKT pathway that stimulates insulin sensitivity in peripheral tissues (Morton, et al. 2005).
In light of the significance of metabolic inflammation in the pathogenesis of metabolic disease, it is worth mentioning that leptin bears striking similarity to cytokines and modulates immune responses (De Rosa, et al. 2007). Leptin is structurally similar to Class I helical cytokines and shares the same JAK-STAT pathway downstream of its receptor. Leptin expression can be induced by endotoxin or cytokine TNFα (Grunfeld, et al. 1996). Conversely, leptin increases thymic secretion of acute-phase reactants and TNFα and promotes T helper 1 cell differentiation (La Cava and Matarese 2004). Leptin acts on T cell, macrophages and other immune cells to stimulate the production of a wide spectrum of cytokines (La Cava and Matarese 2004). In light of the role of several cytokines in enhancing energy expenditure and suppressing food intake (Ye and Keller 2010), this proinflammatory action of leptin might contribute to its overall effects in body weight regulation. Interestingly, inflammation induced by metabolic stress also negatively regulates leptin signaling in a manner similar to insulin receptor signaling (Zhang, et al. 2008). In addition, leptin has been implicated in a number of immune dysfunctions. For examples, leptin is able to reverse starvation-induced immunosuppression (Lord, et al. 1998) and has been proposed to be a metabolic link to multiple sclerosis (Matarese, et al. 2010).
Despite the thorough understanding of leptin actions and numerous attempts to target leptin for obesity and metabolic disorders (Coppari and Bjorbaek 2012), leptin’s clinical applications have been very limited. Leptin has been used to treat genetically obese subjects carrying leptin mutations but such mutations are extremely rare (Farooqi, et al. 1999). Leptin is largely ineffective for treating regular obese patients due to leptin resistance caused by hyperleptinaemia, and leptin administration into these individuals does not generate anorexic effects (Heymsfield, et al. 1999). Leptin has been successfully used to treat insulin resistance and hepatic steatosis in patients with congenital severe lipodystrophy who have very low levels of circulating leptin (Oral, et al. 2002; Petersen, et al. 2002). With increased mechanistic understanding of leptin resistance (St-Pierre and Tremblay 2012), it is still possible that approaches to enhance leptin sensitivity could help revive some of stalled attempts to target leptin for anti-obesity and anti-diabetic therapies.
Adiponectin
Several research groups identified adiponectin almost simultaneously as an abundantly secreted adipokine (Hu, et al. 1996; Maeda, et al. 1996; Nakano, et al. 1996; Scherer, et al. 1995). Recombinant adiponectin can enhance insulin action and partially reverse insulin resistance in obese mice (Berg, et al. 2001; Yamauchi, et al. 2001). Consistently, multiple groups have reported that adiponectin-deficient mice develop insulin resistance associated with high level of TNFα in adipose tissue and reduced responsiveness to PPARλ (Maeda, et al. 2002; Nawrocki, et al. 2006) although an independently generated adiponectin knockout mouse line has no change in insulin sensitivity (Ma, et al. 2002). Adiponectin has also been reported to have antiatherogenic effects (Funahashi, et al. 1999; Ouchi, et al. 1999). In addition, adiponectin exhibits cardioprotective activity in ischemic heart disease through AMPK and cyclooxygenase 2 (Cox-2) pathways (Shibata, et al. 2005).
Adiponectin signaling is mediated by two adiponectin receptors, AdipoR1 and AdipoR2 (Yamauchi, et al. 2003). AdipoR1 is ubiquitously expressed whereas adipoR2 is enriched in liver tissue. Knockout of adipoR1 and adipoR2 abrogates adiponectin binding and causes lipid accumulation, inflammation and insulin resistance (Yamauchi, et al. 2007). Activation of adipoR1 in liver and muscle tissues increases AMPK activity, which mediate the insulin sensitizing effect of adiponectin and also enhances fatty acid oxidation (Yamauchi, et al. 2002). The adipoR2 pathway in the liver increases PPARα and expression of its target genes, which also results in increased fatty acid oxidation (Yamauchi et al. 2007). Recently it was reported that a variety of downstream effects of the adiponectin receptor are mediated by ceramidase activity associated with adipoR1 and R2 (Holland, et al. 2011). Adiponectin also has anti-inflammatory effects that contribute to its protective role against metabolic stress in obesity. Adiponectin suppresses TNFα production in obese mice (Xu, et al. 2003a), and adiponectin-deficient mice have high levels of TNFα in adipose tissue (Maeda, et al. 2002). Low levels of plasma adiponectin are associated with C-reactive protein in humans (Ouchi, et al. 2003). Adiponectin enhances the clearance of apoptotic cells by facilitating their opsonization and uptake by macrophages (Takemura, et al. 2007). Some of the anti-atherogenic effects of adiponectin are also mediated by its role in suppressing inflammatory responses. Adiponectin inhibits nuclear factor-κB (NFκB) activity and its downstream adhesion molecules leading to reduced monocyte adhesion to endothelial cells (Okamoto, et al. 2002; Ouchi et al. 1999). In addition, adiponectin confers vascular-protective activities by suppressing endothelial cell apoptosis (Kobayashi, et al. 2004).
Clinical observations support the idea that plasma adiponectin levels are associated with obesity-induced disorders, especially diabetes. Plasma adiponectin levels are decreased in type 2 diabetic patients, and higher adiponectin levels are associated with low diabetes risk (Li, et al. 2009). Adiponectin levels are also negatively associated with adiposity and fasting glucose (Ryo, et al. 2004). A multi-ethnic meta-analysis of a large cohort also demonstrated that numerous genetic loci associated with adiponectin levels influence risk of insulin resistance and type 2 diabetes (Dastani, et al. 2012). Currently, several strategies to boost adiponectin levels or adiponectin receptor activities are being explored for the treatment of obesity-induced inflammation and insulin resistance (Yamauchi and Kadowaki 2008).
TNFα
TNFα was the first cytokine identified in the adipose tissue of obese mice, marking the start of the metabolic inflammation concept (Hotamisligil et al. 1993). The direct involvement of TNFα in obesity-induced insulin resistance was confirmed by observations that TNFα treatment interferes with insulin signaling and blocks insulin actions (Hotamisligil, et al. 1994). Mice lacking the functions of TNFα or its receptors are protected from obesity-induced insulin resistance and hyperglycemia (Uysal, et al. 1998; Uysal, et al. 1997). It was initially thought that adipose-derived TNFα was produced mainly by adipocytes but the parallel trend of macrophage infiltration and TNFα expression in adipose tissue of obese mice suggests that a significant portion of the adipose TNFα pool might be derived from macrophages and other immune cells. Interesting, free fatty acid (FFA) strongly stimulates TNFα production in macrophages (Nguyen, et al. 2005) and in turn, TNFα stimulates lipolysis to increase fatty acid release from adipocytes (Wang, et al. 2008). This FFA-cytokine cycle suggests that metabolic inflammation, once started, can use this self-perpetuating mechanism to further its inhibitory effects on insulin signaling and energy metabolism. In addition, TNFα directly stimulates hepatic lipogenesis in vivo (Feingold and Grunfeld 1987), and adipose-derived TNFα is also a major mechanistic link between obesity and cancer (Park, et al. 2010).
TNFα exerts its effects through two distinct receptors, p55 and p75, which further activate JNK1 and inhibit IκB kinase(IKK)/NFκB pathways (Baud and Karin 2001). JNK1 can directly inhibit insulin signaling by phosphorylating insulin receptor substrate-1 (IRS1) on serine residues (Aguirre, et al. 2002) and can also potentiate fatty acid-induced cytokine production (Nguyen et al., 2005). Consistent with these observations, JNK1 knockout mice are protected from obesity and insulin resistance (Hirosumi, et al. 2002). IKK can also directly inhibit IRS-1 function through serine phosphorylation in a manner similar to JNK1 (Gao, et al. 2002) and also activate NFκB to produce inflammatory cytokines both in metabolic organs and myeloid cells. It has been demonstrated that systemic or selective inhibition of IKK in either hepatocytes or myeloid cells improves glucose metabolism in mice (Arkan, et al. 2005; Cai, et al. 2005; Yuan, et al. 2001). TNFα also induces the expression of suppressor of cytokine signaling 3 (SOCS3), which inhibits insulin signaling by increasing ubiquitin-mediated insulin receptor substrate-1 (IRS-1) and IRS-2 degradation (Emanuelli, et al. 2001; Rui, et al. 2002). Recently, a report demonstrates that TNFα increase leptin receptor expression raising an interesting possibility that TNFα might enhance leptin action (Gan, et al. 2012) although the physiological relevance of this connection needs to be confirmed in an in vivo setting.
Numerous studies in humans have demonstrated strong associations between circulating TNFα and insulin resistance (Hivert, et al. 2008) or other obesity-associated metabolic complications (Berg and Scherer 2005). However, attempts to block TNFα function in patients have not yet produced consistent metabolic outcomes. For example, neutralization of TNFα with an engineered antibody did not improve insulin sensitivity in type 2 diabetes patients (Ofei, et al. 1996) whereas blockade of TNFα in patients with rheumatoid arthritis or psoriasis indeed improved their insulin resistance (Gonzalez-Gay, et al. 2006; Lo, et al. 2007). Considering the wide spectrum of inflammatory cytokines that are elevated in obesity, targeting TNFα alone might not have sufficient efficacy to improve systemic metabolic responses and might need to be considered in the context of managing the overall metabolic inflammation.
Resistin
Resistin was initially identified in a screen for adipocyte genes that are suppressed by insulin-sensitizing drugs in rodents (Steppan, et al. 2001). Depletion of circulating resistin by a neutralizing antibody improves insulin action in obese mice suggesting that resistin is an adipokine linking obesity to insulin resistance (Steppan et al. 2001). Subsequently, it was shown that resistin knockout mice on a high-fat diet have improved glucose metabolism mainly due to reduced glucose production in the liver (Banerjee, et al. 2004). Resistin also increases the expressions of cytokines and adhesion molecules in murine vascular endothelial cells and contributes to atherogenesis (Burnett, et al. 2005). Resistin circulates in two distinct assembly states, which exhibit differential activities in metabolic regulation (Patel, et al. 2004). However, the relevance of resistin to human disease is complicated by the fact that rodent resistin is produced in adipocytes and human resistin is produced mostly in macrophages. Human and rodent resistin only shares 59% identity at the amino acid level which is relatively low compared to other hormones (Ghosh, et al. 2003). But interestingly, human resistin, when expressed in mouse macrophages, also induces insulin resistance (Qatanani, et al. 2009) suggesting that human and mouse resistin might have similar function despite their different sites of production.
In humans, experimental endotoxemia induced elevated resistin and produced an insulin-resistant state (Lehrke, et al. 2004). Epidemiological studies have associated elevated circulating resistin with increased risk for type 2 diabetes, inflammatory markers, myocardial infarcation and atherosclerosis (Burnett, et al. 2006; Burnett et al. 2005; Chen, et al. 2009; Heidemann, et al. 2008; Reilly, et al. 2005). These studies support the idea that resistin levels could serve as an informative marker for metabolic disease in humans, and it will be of great interest to determine the therapeutic potential of resistin inhibition in future studies.
IL-6
IL-6 is one of the major pro-inflammatory cytokines whose expression level increases in the adipose tissue of obese mice and patients, but its role in glucose metabolism has not been fully resolved. IL-6 depletion in obese mice with a neutralizing antibody improves hepatic insulin action (Klover, et al. 2005) while chronic infusion of IL-6 causes insulin resistance in the liver of mice (Klover, et al. 2003). Conversely, mice with targeted ablation of IL-6 develop obesity and insulin resistance which can be reversed by centrally delivered exogenous IL-6 (Wallenius, et al. 2002) suggesting that IL-6 is required for the maintenance of whole body glucose metabolism and metabolic homeostasis. An independently generated IL-6 targeted mutation mouse line, however, does not develop obesity or insulin resistance and only exhibits elevated glucose level in a glucose tolerance test (Di Gregorio, et al. 2004). In a mouse model with adipose-specific ablation of JNK1, increased secretion of IL-6 was proposed to be the primary reason for systemic insulin resistance (Sabio, et al. 2008). There are several potential explanations for the seemingly contradictory data regarding IL-6 in insulin action and glucose metabolism. Effects of acute versus chronic treatments need to be differentiated and dose and site of action of IL-6 need to be carefully considered. In addition, IL-6 produced by different organs might also contribute to its complex effects on metabolic regulation.
During exercise, IL-6 is mainly released from working skeletal muscle. IL-6 release from contracting skeletal muscle might mediate the beneficial effects associated with exercise, including increased glucose uptake and fatty acid oxidation (Febbraio and Pedersen 2002). It appears that activation of AMPK by IL-6 mediates these effects (Al-Khalili, et al. 2006). In addition, transgenically expressed human IL-6 in mice increases leptin sensitivity and prevents diet-induced obesity (Sadagurski, et al. 2010). However, the function of muscle-derived IL-6 might also vary depending on its context. In a mouse model with muscle-specific disruption of PPARλ coactivator 1alpha (PGC-1alpha), muscle-secreted IL-6 causes impaired insulin production from pancreatic islets and glucose intolerance (Handschin, et al. 2007).
In patient studies, increased serum IL-6 correlates with obesity and insulin resistance (Bastard, et al. 2002; Spranger, et al. 2003; Vozarova, et al. 2001). The IL-6 174G>C single nucleotide polymorphism (SNP) is associated with insulin resistance and metabolic syndrome (Fernandez-Real, et al. 2000; Stephens, et al. 2007). However, the mechanism of action of IL-6 in human metabolism needs to be further studied to understand the therapeutic potential of IL-6, partly due to the fact that there is low similarity between human and mouse IL-6, and thus information generated from mouse studies cannot be readily applied to humans. To add to the complexity of IL6 signaling in human metabolism, two reports showed that monoclonal antibody against the IL-6 receptor, Tocilizumab, either increases or has no effects on insulin sensitivity in patients with rheumatoid arthritis (Ogata, et al. 2011; Schultz, et al. 2010; Ye and McGuinness 2013).
Rbp4
Rbp4 is a transport protein for retinol in systemic circulation, and is mainly produced by the liver but also expressed in white adipocytes. Rbp4 was first characterized as an adipokine based on the finding that Rbp4 is highly secreted from adipose tissue of Glut4-deficient mice and contributes to insulin resistance in this mouse model (Yang, et al. 2005). In humans, higher Rbp4 levels are also associated with insulin resistance in obese and diabetic subjects (Graham, et al. 2006). In addition, a number of Rbp4 SNPs have been identified and several of them are associated with increased risk for type 2 diabetes (Kovacs, et al. 2007; Munkhtulga, et al. 2007). However, several reports failed to detect association of Rbp4 with insulin resistance (Promintzer, et al. 2007; von Eynatten, et al. 2007; Yao-Borengasser, et al. 2007), potentially due to shortcomings in the methodologies for quantifying Rbp4 levels (Kotnik, et al. 2011). In general, clinical studies in children and adolescents have been more consistent in supporting a role for Rbp4 in obesity and insulin resistance, suggesting that Rbp4 might be more involved in the early stages of metabolic syndrome (Kotnik et al. 2011).
Sfrp5
Secreted frizzled-related protein 5 (Sfrp5) was recently identified as an anti-inflammatory adipocytokine (Ouchi, et al. 2010). Sfrp5 is highly expressed in adipose tissue of lean mice but downregulated in obese mice. Targeted mutation of Sfrp5 in mice caused insulin resistance, glucose intolerance and hepatosteatosis when the animals were fed a high fat diet (Ouchi et al. 2010). Mechanistically, Sfrp5 activates JNK1 through noncanonical Wnt signaling to increase the levels of inflammatory cytokines and block insulin action (Ouchi et al. 2010). However, a second independently generated Sfrp5 mutation mouse line was reported to have different phenotypes, and accordingly the authors proposed a very different mechanism of actions for Sfrp5. In this study, Sfrp5-deficient mice were resistant to diet-induced obesity due to enhanced mitochondrial activities (Mori, et al. 2012). Sfrp5 deficiency increased the expression of PGC1 and mitochondrial transcription factor A (Tfam) leading to increased mitochondrial biogenesis. Lack of Sfrp5 also stimulated mitochondrial respiration and gene expression through Wnt3a activity (Mori et al. 2012). The cause of these discrepancies is unclear. Human studies regarding Sfrp5 in metabolic disease have also given rise to conflicting data (Carstensen, et al. 2013; Hu, et al. 2013). Regardless, further studies about the function of Sfrp5 in metabolic regulation could provide important insights into adipose biology. Sfrp5 regulates multiple Wnt proteins that play a crucial role in adipogenesis (Cristancho and Lazar 2011). Dissecting the Sfrp5/Wnt network in adipose tissue could also help to explain the autocrine/paracrine mechanism of metabolic inflammation, which is still poorly understood.
aP2, a lipid-activated adipocytokine
The identification of aP2 as a lipid-activated adipokine is a surprising and exciting finding considering it has been extensively studied for over two decades as an essential intracellular regulator of lipid metabolism and inflammation in metabolic disease. AP2 is a member of fatty acid binding protein (FABP) family and was initially thought to be exclusively expressed in adipocytes. In fact, the aP2 promoter has been widely used to specially drive transgene expression in adipose tissue. AP2-deficient mice have normal adiposity and gain more weight than controls when put on high-fat diet but were partially protected from obesity-induced insulin resistance (Hotamisligil, et al. 1996). The mild effect of aP2 deficiency could be due to the up-regulation of mal1, a related FABP (Maeda, et al. 2005). Therefore mice deficient in both FABPs were produced to study the full impact of adipose FABP deficiency. The double knockout mice have reduced adiposity, enhanced insulin sensitivity and reduced hepatosteatosis (Maeda et al. 2005). It appears that some of the beneficial effects of FABP deficiency are mediated by robust up-regulation of the fatty acid species palmitoleate (C16:1n7) in adipose tissue and its secretion into circulation (Fig. 2). Palmitoleate enhances insulin action in the muscle and suppresses de novo lipogenesis in the liver (Cao et al. 2008).
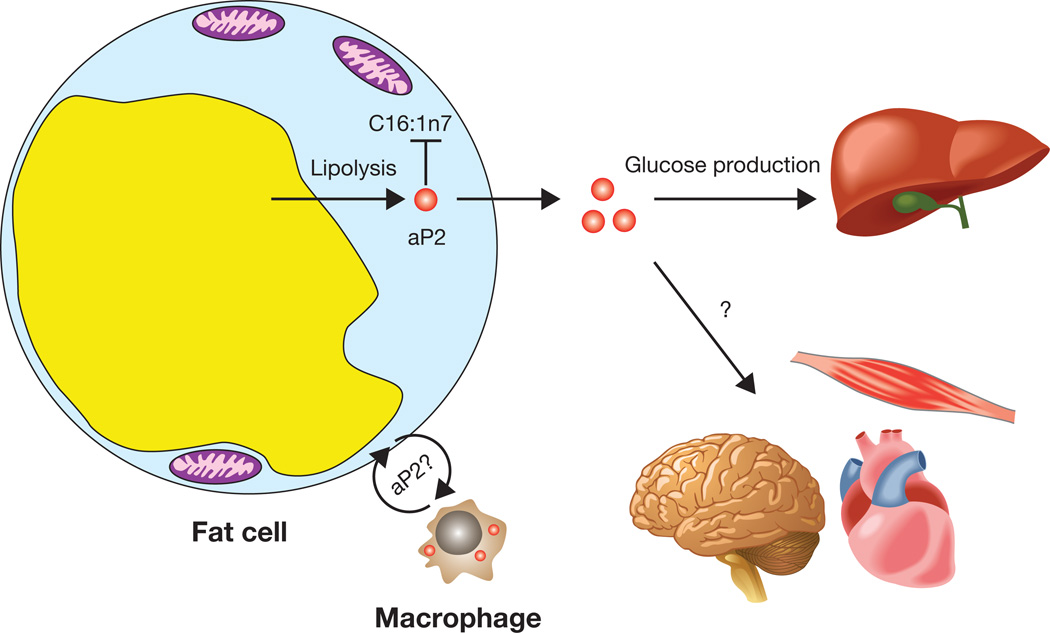
Figure 2. aP2 as a lipid-activated adipokine.
aP2 is secreted from adipocytes through a process that is regulated by fasting and lipolysis. Circulating aP2 acts on liver tissue to stimulate the gluconeogenic program and enhance hepatic glucose production. Other potential functions of aP2 in local adipose-macrophage interaction and on other metabolic organs warrant further investigation.
Yet the molecular mechanism for the pronounced reduction in gluconeogenesis in FABP-deficient mice remained elusive until it was found that aP2 is in fact actively secreted from adipocytes to control liver glucose metabolism (Cao, et al. 2013) (Fig. 2). Secretion of aP2 from adipocytes is regulated by lipolysis, which might be the reason that circulating aP2 levels are markedly elevated in obesity. Recombinant aP2 stimulates hepatic glucose production whereas neutralization of secreted aP2 reduces glucose production and corrects the diabetic phenotype of obese mice (Cao et al. 2013).
aP2 is the first adipokine whose secretion is strongly regulated by lipolysis-released fatty acids, suggesting that aP2 might function as a lipid sensor in adipocytes and might also carry specific lipids in plasma to specific organs or cells. Therefore, like other well-studied adipocytokines, it is conceivable that secreted aP2 could potentially act on other key organs such as the central nerve system or heart to regulate other aspects of metabolic homeostasis (Fig. 2) and these questions need to be addressed in future studies. Another interesting question is whether secreted aP2 is also involved in metabolic inflammation. Despite long having been considered an adipocyte-specific protein, aP2 was found to be expressed in macrophages (Makowski, et al. 2001) and can be quickly induced by endotoxin (Kazemi, et al. 2005). Mice with aP2 deficiency in macrophages are protected from atherosclerosis partly because of activated PPARγ and reduced inflammatory responses (Makowski, et al. 2005). The proinflammatory action of aP2 was also demonstrated in an asthma mouse model in which aP2 deficiency protects mice from airway inflammation (Shum, et al. 2006). It will be interesting to investigate whether aP2 is also secreted from macrophages and whether secreted aP2 regulates inflammatory responses in metabolic diseases (Fig. 2).
Accumulating evidence suggests that circulating aP2 is implicated in human metabolic syndrome. Plasma aP2 levels are closely associated with obesity and metabolic syndrome in cohorts of multiple ethnicities (Simon, et al. 2009; Stejskal and Karpisek 2006; Xu, et al. 2006). In addition, circulating aP2 has also been linked to carotid atherosclerosis in humans (Yeung, et al. 2007) and non-alcoholic fatty liver disease (NAFLD)(Koh, et al. 2009) . In NFALD patients, elevated plasma aP2 levels independently predict inflammation and fibrosis (Milner, et al. 2009). Neutralizing secreted aP2 robustly improves glucose metabolism (Cao et al. 2013), indicating that plasma aP2 could constitute a potential therapeutic target for diabetes, NFALD and cardiovascular disease.
Conclusion and future perspective
There is overwhelming evidence that adipocytokines play a pivotal role in metabolic homeostasis of healthy subjects, and that deficiencies in these factors, caused by excess adiposity and adipocyte dysfunction, are a central component in the pathogenesis of the constellation of diseases surrounding obesity. Therefore it will be fruitful to fully define the adipose secretome using improved technologies, which will no doubt lead to the discovery of novel adipocytokines and novel functions of adipose tissue. Identifying receptors for existing adipocytokines and mapping their downstream signaling pathways, especially in the context of metabolic disorders, is another area of research that could generate fresh therapeutic targets for managing adipocytokines to treat metabolic diseases. Due to the intertwined nature of metabolic and immune cells in major metabolic organs, further mechanistic investigations are required to understand how adipocytokines integrate metabolic and inflammatory responses in each site and the pathological significance of these responses in metabolic disorders. It is particularly important to differentiate the detrimental effects of metabolic inflammation inflicted by nutritional stress and those beneficial ones underlying the physiological tissue expansion when designing anti-inflammation therapies for metabolic disorders (Ye and McGuinness 2013). Following the example of adipocytokines, numerous muscle- and hepatocyte-secreted hormones (myokine and hepatokine) have been identified as essential metabolic regulators. Therefore it is very likely that a comprehensive endocrine network of organ communications in nutrient sensing and metabolic homeostasis could be established in the foreseeable future. Such a blueprint of organ crosstalk would have far-reaching impact on the development of effective therapies against obesity and metabolic disease.
Acknowledgements
Haiming Cao is funded by the Division of Intramural Research of the National Heart Lung and Blood Institute (HL006103-02) of the NIH, USA. I apologize for not being able to cite all worthy papers owing to space limitation.
Footnotes
Declaration of interest
The author declares that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review.
Reference
- Abshagen K, Eipel C, Kalff JC, Menger MD, Vollmar B. Loss of NF-kappaB activation in Kupffer cell-depleted mice impairs liver regeneration after partial hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2007;292:G1570–G1577. doi: 10.1152/ajpgi.00399.2006. [DOI] [PubMed] [Google Scholar]
- Aguirre V, Werner ED, Giraud J, Lee YH, Shoelson SE, White MF. Phosphorylation of Ser307 in insulin receptor substrate-1 blocks interactions with the insulin receptor and inhibits insulin action. J Biol Chem. 2002;277:1531–1537. doi: 10.1074/jbc.M101521200. [DOI] [PubMed] [Google Scholar]
- Al-Khalili L, Bouzakri K, Glund S, Lonnqvist F, Koistinen HA, Krook A. Signaling specificity of interleukin-6 action on glucose and lipid metabolism in skeletal muscle. Mol Endocrinol. 2006;20:3364–3375. doi: 10.1210/me.2005-0490. [DOI] [PubMed] [Google Scholar]
- Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- Banerjee RR, Rangwala SM, Shapiro JS, Rich AS, Rhoades B, Qi Y, Wang J, Rajala MW, Pocai A, Scherer PE, et al. Regulation of fasted blood glucose by resistin. Science. 2004;303:1195–1198. doi: 10.1126/science.1092341. [DOI] [PubMed] [Google Scholar]
- Bastard JP, Maachi M, Van Nhieu JT, Jardel C, Bruckert E, Grimaldi A, Robert JJ, Capeau J, Hainque B. Adipose tissue IL-6 content correlates with resistance to insulin activation of glucose uptake both in vivo and in vitro. J Clin Endocrinol Metab. 2002;87:2084–2089. doi: 10.1210/jcem.87.5.8450. [DOI] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, et al. STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Baud V, Karin M. Signal transduction by tumor necrosis factor and its relatives. Trends Cell Biol. 2001;11:372–377. doi: 10.1016/s0962-8924(01)02064-5. [DOI] [PubMed] [Google Scholar]
- Baumann H, Morella KK, White DW, Dembski M, Bailon PS, Kim H, Lai CF, Tartaglia LA. The full-length leptin receptor has signaling capabilities of interleukin 6-type cytokine receptors. Proc Natl Acad Sci U S A. 1996;93:8374–8378. doi: 10.1073/pnas.93.16.8374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AH, Combs TP, Du X, Brownlee M, Scherer PE. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. 2001;7:947–953. doi: 10.1038/90992. [DOI] [PubMed] [Google Scholar]
- Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Burnett MS, Devaney JM, Adenika RJ, Lindsay R, Howard BV. Cross-sectional associations of resistin, coronary heart disease, and insulin resistance. J Clin Endocrinol Metab. 2006;91:64–68. doi: 10.1210/jc.2005-1653. [DOI] [PubMed] [Google Scholar]
- Burnett MS, Lee CW, Kinnaird TD, Stabile E, Durrani S, Dullum MK, Devaney JM, Fishman C, Stamou S, Canos D, et al. The potential role of resistin in atherogenesis. Atherosclerosis. 2005;182:241–248. doi: 10.1016/j.atherosclerosis.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, Neyrinck AM, Fava F, Tuohy KM, Chabo C, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- Cao H, Gerhold K, Mayers JR, Wiest MM, Watkins SM, Hotamisligil GS. Identification of a lipokine, a lipid hormone linking adipose tissue to systemic metabolism. Cell. 2008;134:933–944. doi: 10.1016/j.cell.2008.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao H, Sekiya M, Ertunc ME, Burak MF, Mayers JR, White A, Inouye K, Rickey LM, Ercal BC, Furuhashi M, et al. Adipocyte lipid chaperone AP2 is a secreted adipokine regulating hepatic glucose production. Cell Metab. 2013;17:768–778. doi: 10.1016/j.cmet.2013.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carstensen M, Herder C, Kempf K, Erlund I, Martin S, Koenig W, Sundvall J, Bidel S, Kuha S, Roden M, et al. Sfrp5 correlates with insulin resistance and oxidative stress. Eur J Clin Invest. 2013;43:350–357. doi: 10.1111/eci.12052. [DOI] [PubMed] [Google Scholar]
- Chen BH, Song Y, Ding EL, Roberts CK, Manson JE, Rifai N, Buring JE, Gaziano JM, Liu S. Circulating levels of resistin and risk of type 2 diabetes in men and women: results from two prospective cohorts. Diabetes Care. 2009;32:329–334. doi: 10.2337/dc08-1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cinti S, Mitchell G, Barbatelli G, Murano I, Ceresi E, Faloia E, Wang S, Fortier M, Greenberg AS, Obin MS. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- Cohen P, Miyazaki M, Socci ND, Hagge-Greenberg A, Liedtke W, Soukas AA, Sharma R, Hudgins LC, Ntambi JM, Friedman JM. Role for stearoyl-CoA desaturase-1 in leptin-mediated weight loss. Science. 2002;297:240–243. doi: 10.1126/science.1071527. [DOI] [PubMed] [Google Scholar]
- Coppari R, Bjorbaek C. Leptin revisited: its mechanism of action and potential for treating diabetes. Nat Rev Drug Discov. 2012;11:692–708. doi: 10.1038/nrd3757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cristancho AG, Lazar MA. Forming functional fat: a growing understanding of adipocyte differentiation. Nat Rev Mol Cell Biol. 2011;12:722–734. doi: 10.1038/nrm3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dastani Z, Hivert MF, Timpson N, Perry JR, Yuan X, Scott RA, Henneman P, Heid IM, Kizer JR, Lyytikainen LP, et al. Novel loci for adiponectin levels and their influence on type 2 diabetes and metabolic traits: a multi-ethnic meta-analysis of 45,891 individuals. PLoS Genet. 2012;8:e1002607. doi: 10.1371/journal.pgen.1002607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rosa V, Procaccini C, Cali G, Pirozzi G, Fontana S, Zappacosta S, La Cava A, Matarese G. A key role of leptin in the control of regulatory T cell proliferation. Immunity. 2007;26:241–255. doi: 10.1016/j.immuni.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Di Gregorio GB, Hensley L, Lu T, Ranganathan G, Kern PA. Lipid and carbohydrate metabolism in mice with a targeted mutation in the IL-6 gene: absence of development of age-related obesity. Am J Physiol Endocrinol Metab. 2004;287:E182–E187. doi: 10.1152/ajpendo.00189.2003. [DOI] [PubMed] [Google Scholar]
- Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- Emanuelli B, Peraldi P, Filloux C, Chavey C, Freidinger K, Hilton DJ, Hotamisligil GS, Van Obberghen E. SOCS-3 inhibits insulin signaling and is up-regulated in response to tumor necrosis factor-alpha in the adipose tissue of obese mice. J Biol Chem. 2001;276:47944–47949. doi: 10.1074/jbc.M104602200. [DOI] [PubMed] [Google Scholar]
- Farooqi IS, Jebb SA, Langmack G, Lawrence E, Cheetham CH, Prentice AM, Hughes IA, McCamish MA, O’Rahilly S. Effects of recombinant leptin therapy in a child with congenital leptin deficiency. N Engl J Med. 1999;341:879–884. doi: 10.1056/NEJM199909163411204. [DOI] [PubMed] [Google Scholar]
- Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–1347. doi: 10.1096/fj.01-0876rev. [DOI] [PubMed] [Google Scholar]
- Feingold KR, Grunfeld C. Tumor necrosis factor-alpha stimulates hepatic lipogenesis in the rat in vivo. J Clin Invest. 1987;80:184–190. doi: 10.1172/JCI113046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez-Real JM, Broch M, Vendrell J, Gutierrez C, Casamitjana R, Pugeat M, Richart C, Ricart W. Interleukin-6 gene polymorphism and insulin sensitivity. Diabetes. 2000;49:517–520. doi: 10.2337/diabetes.49.3.517. [DOI] [PubMed] [Google Scholar]
- Feuerer M, Herrero L, Cipolletta D, Naaz A, Wong J, Nayer A, Lee J, Goldfine AB, Benoist C, Shoelson S, et al. Lean, but not obese, fat is enriched for a unique population of regulatory T cells that affect metabolic parameters. Nat Med. 2009;15:930–939. doi: 10.1038/nm.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finucane MM, Stevens GA, Cowan MJ, Danaei G, Lin JK, Paciorek CJ, Singh GM, Gutierrez HR, Lu Y, Bahalim AN, et al. National, regional, and global trends in body-mass index since 1980: systematic analysis of health examination surveys and epidemiological studies with 960 country-years and 9.1 million participants. Lancet. 2011;377:557–567. doi: 10.1016/S0140-6736(10)62037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman JM, Halaas JL. Leptin and the regulation of body weight in mammals. Nature. 1998;395:763–770. doi: 10.1038/27376. [DOI] [PubMed] [Google Scholar]
- Funahashi T, Nakamura T, Shimomura I, Maeda K, Kuriyama H, Takahashi M, Arita Y, Kihara S, Matsuzawa Y. Role of adipocytokines on the pathogenesis of atherosclerosis in visceral obesity. Intern Med. 1999;38:202–206. doi: 10.2169/internalmedicine.38.202. [DOI] [PubMed] [Google Scholar]
- Gan L, Guo K, Cremona ML, McGraw TE, Leibel RL, Zhang Y. TNF-alpha up-regulates protein level and cell surface expression of the leptin receptor by stimulating its export via a PKC-dependent mechanism. Endocrinology. 2012;153:5821–5833. doi: 10.1210/en.2012-1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Z, Hwang D, Bataille F, Lefevre M, York D, Quon MJ, Ye J. Serine phosphorylation of insulin receptor substrate 1 by inhibitor kappa B kinase complex. J Biol Chem. 2002;277:48115–48121. doi: 10.1074/jbc.M209459200. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Singh AK, Aruna B, Mukhopadhyay S, Ehtesham NZ. The genomic organization of mouse resistin reveals major differences from the human resistin: functional implications. Gene. 2003;305:27–34. doi: 10.1016/s0378-1119(02)01213-1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, Llorca J. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24:83–86. [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Graham TE, Yang Q, Bluher M, Hammarstedt A, Ciaraldi TP, Henry RR, Wason CJ, Oberbach A, Jansson PA, Smith U, et al. Retinol-binding protein 4 and insulin resistance in lean, obese, and diabetic subjects. N Engl J Med. 2006;354:2552–2563. doi: 10.1056/NEJMoa054862. [DOI] [PubMed] [Google Scholar]
- Grunfeld C, Zhao C, Fuller J, Pollack A, Moser A, Friedman J, Feingold KR. Endotoxin and cytokines induce expression of leptin, the ob gene product, in hamsters. J Clin Invest. 1996;97:2152–2157. doi: 10.1172/JCI118653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handschin C, Choi CS, Chin S, Kim S, Kawamori D, Kurpad AJ, Neubauer N, Hu J, Mootha VK, Kim YB, et al. Abnormal glucose homeostasis in skeletal muscle-specific PGC-1alpha knockout mice reveals skeletal muscle-pancreatic beta cell crosstalk. J Clin Invest. 2007;117:3463–3474. doi: 10.1172/JCI31785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidemann C, Sun Q, van Dam RM, Meigs JB, Zhang C, Tworoger SS, Mantzoros CS, Hu FB. Total and high-molecular-weight adiponectin and resistin in relation to the risk for type 2 diabetes in women. Ann Intern Med. 2008;149:307–316. doi: 10.7326/0003-4819-149-5-200809020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heymsfield SB, Greenberg AS, Fujioka K, Dixon RM, Kushner R, Hunt T, Lubina JA, Patane J, Self B, Hunt P, et al. Recombinant leptin for weight loss in obese and lean adults: a randomized, controlled, dose-escalation trial. JAMA. 1999;282:1568–1575. doi: 10.1001/jama.282.16.1568. [DOI] [PubMed] [Google Scholar]
- Hirosumi J, Tuncman G, Chang L, Gorgun CZ, Uysal KT, Maeda K, Karin M, Hotamisligil GS. A central role for JNK in obesity and insulin resistance. Nature. 2002;420:333–336. doi: 10.1038/nature01137. [DOI] [PubMed] [Google Scholar]
- Hivert MF, Sullivan LM, Fox CS, Nathan DM, D’Agostino RB, Sr., Wilson PW, Meigs JB. Associations of adiponectin, resistin, and tumor necrosis factor-alpha with insulin resistance. J Clin Endocrinol Metab. 2008;93:3165–3172. doi: 10.1210/jc.2008-0425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland WL, Miller RA, Wang ZV, Sun K, Barth BM, Bui HH, Davis KE, Bikman BT, Halberg N, Rutkowski JM, et al. Receptor-mediated activation of ceramidase activity initiates the pleiotropic actions of adiponectin. Nat Med. 2011;17:55–63. doi: 10.1038/nm.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Johnson RS, Distel RJ, Ellis R, Papaioannou VE, Spiegelman BM. Uncoupling of obesity from insulin resistance through a targeted mutation in aP2, the adipocyte fatty acid binding protein. Science. 1996;274:1377–1379. doi: 10.1126/science.274.5291.1377. [DOI] [PubMed] [Google Scholar]
- Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci U S A. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hu E, Liang P, Spiegelman BM. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J Biol Chem. 1996;271:10697–10703. doi: 10.1074/jbc.271.18.10697. [DOI] [PubMed] [Google Scholar]
- Hu W, Li L, Yang M, Luo X, Ran W, Liu D, Xiong Z, Liu H, Yang G. Circulating Sfrp5 is a signature of obesity-related metabolic disorders and is regulated by glucose and liraglutide in humans. J Clin Endocrinol Metab. 2013;98:290–298. doi: 10.1210/jc.2012-2466. [DOI] [PubMed] [Google Scholar]
- Kamohara S, Burcelin R, Halaas JL, Friedman JM, Charron MJ. Acute stimulation of glucose metabolism in mice by leptin treatment. Nature. 1997;389:374–377. doi: 10.1038/38717. [DOI] [PubMed] [Google Scholar]
- Kazemi MR, McDonald CM, Shigenaga JK, Grunfeld C, Feingold KR. Adipocyte fatty acid-binding protein expression and lipid accumulation are increased during activation of murine macrophages by toll-like receptor agonists. Arterioscler Thromb Vasc Biol. 2005;25:1220–1224. doi: 10.1161/01.ATV.0000159163.52632.1b. [DOI] [PubMed] [Google Scholar]
- Klover PJ, Clementi AH, Mooney RA. Interleukin-6 depletion selectively improves hepatic insulin action in obesity. Endocrinology. 2005;146:3417–3427. doi: 10.1210/en.2004-1468. [DOI] [PubMed] [Google Scholar]
- Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52:2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Ouchi N, Kihara S, Walsh K, Kumada M, Abe Y, Funahashi T, Matsuzawa Y. Selective suppression of endothelial cell apoptosis by the high molecular weight form of adiponectin. Circ Res. 2004;94:e27–e31. doi: 10.1161/01.RES.0000119921.86460.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh JH, Shin YG, Nam SM, Lee MY, Chung CH, Shin JY. Serum adipocyte fatty acid-binding protein levels are associated with nonalcoholic fatty liver disease in type 2 diabetic patients. Diabetes Care. 2009;32:147–152. doi: 10.2337/dc08-1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosteli A, Sugaru E, Haemmerle G, Martin JF, Lei J, Zechner R, Ferrante AW., Jr Weight loss and lipolysis promote a dynamic immune response in murine adipose tissue. J Clin Invest. 2010;120:3466–3479. doi: 10.1172/JCI42845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotnik P, Fischer-Posovszky P, Wabitsch M. RBP4: a controversial adipokine. Eur J Endocrinol. 2011;165:703–711. doi: 10.1530/EJE-11-0431. [DOI] [PubMed] [Google Scholar]
- Kovacs P, Geyer M, Berndt J, Kloting N, Graham TE, Bottcher Y, Enigk B, Tonjes A, Schleinitz D, Schon MR, et al. Effects of genetic variation in the human retinol binding protein-4 gene (RBP4) on insulin resistance and fat depot-specific mRNA expression. Diabetes. 2007;56:3095–3100. doi: 10.2337/db06-1647. [DOI] [PubMed] [Google Scholar]
- La Cava A, Matarese G. The weight of leptin in immunity. Nat Rev Immunol. 2004;4:371–379. doi: 10.1038/nri1350. [DOI] [PubMed] [Google Scholar]
- Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379:632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- Lehrke M, Reilly MP, Millington SC, Iqbal N, Rader DJ, Lazar MA. An inflammatory cascade leading to hyperresistinemia in humans. PLoS Med. 2004;1:e45. doi: 10.1371/journal.pmed.0010045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Shin HJ, Ding EL, van Dam RM. Adiponectin levels and risk of type 2 diabetes: a systematic review and meta-analysis. JAMA. 2009;302:179–188. doi: 10.1001/jama.2009.976. [DOI] [PubMed] [Google Scholar]
- Liu J, Divoux A, Sun J, Zhang J, Clement K, Glickman JN, Sukhova GK, Wolters PJ, Du J, Gorgun CZ, et al. Genetic deficiency and pharmacological stabilization of mast cells reduce diet-induced obesity and diabetes in mice. Nat Med. 2009;15:940–945. doi: 10.1038/nm.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo J, Bernstein LE, Canavan B, Torriani M, Jackson MB, Ahima RS, Grinspoon SK. Effects of TNF-alpha neutralization on adipocytokines and skeletal muscle adiposity in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2007;293:E102–E109. doi: 10.1152/ajpendo.00089.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord GM, Matarese G, Howard JK, Baker RJ, Bloom SR, Lechler RI. Leptin modulates the T-cell immune response and reverses starvation-induced immunosuppression. Nature. 1998;394:897–901. doi: 10.1038/29795. [DOI] [PubMed] [Google Scholar]
- Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest. 2007;117:175–184. doi: 10.1172/JCI29881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda K, Cao H, Kono K, Gorgun CZ, Furuhashi M, Uysal KT, Cao Q, Atsumi G, Malone H, Krishnan B, et al. Adipocyte/macrophage fatty acid binding proteins control integrated metabolic responses in obesity and diabetes. Cell Metab. 2005;1:107–119. doi: 10.1016/j.cmet.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Maeda K, Okubo K, Shimomura I, Funahashi T, Matsuzawa Y, Matsubara K. cDNA cloning and expression of a novel adipose specific collagen-like factor, apM1 (AdiPose Most abundant Gene transcript 1) Biochem Biophys Res Commun. 1996;221:286–289. doi: 10.1006/bbrc.1996.0587. [DOI] [PubMed] [Google Scholar]
- Maeda N, Shimomura I, Kishida K, Nishizawa H, Matsuda M, Nagaretani H, Furuyama N, Kondo H, Takahashi M, Arita Y, et al. Diet-induced insulin resistance in mice lacking adiponectin/ACRP30. Nat Med. 2002;8:731–737. doi: 10.1038/nm724. [DOI] [PubMed] [Google Scholar]
- Makowski L, Boord JB, Maeda K, Babaev VR, Uysal KT, Morgan MA, Parker RA, Suttles J, Fazio S, Hotamisligil GS, et al. Lack of macrophage fatty-acid-binding protein aP2 protects mice deficient in apolipoprotein E against atherosclerosis. Nat Med. 2001;7:699–705. doi: 10.1038/89076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makowski L, Brittingham KC, Reynolds JM, Suttles J, Hotamisligil GS. The fatty acid-binding protein, aP2, coordinates macrophage cholesterol trafficking and inflammatory activity. Macrophage expression of aP2 impacts peroxisome proliferator-activated receptor gamma and IkappaB kinase activities. J Biol Chem. 2005;280:12888–12895. doi: 10.1074/jbc.M413788200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matarese G, Carrieri PB, Montella S, De Rosa V, La Cava A. Leptin as a metabolic link to multiple sclerosis. Nat Rev Neurol. 2010;6:455–461. doi: 10.1038/nrneurol.2010.89. [DOI] [PubMed] [Google Scholar]
- Milner KL, van der Poorten D, Xu A, Bugianesi E, Kench JG, Lam KS, Chisholm DJ, George J. Adipocyte fatty acid binding protein levels relate to inflammation and fibrosis in nonalcoholic fatty liver disease. Hepatology. 2009;49:1926–1934. doi: 10.1002/hep.22896. [DOI] [PubMed] [Google Scholar]
- Minokoshi Y, Kim YB, Peroni OD, Fryer LG, Muller C, Carling D, Kahn BB. Leptin stimulates fatty-acid oxidation by activating AMP-activated protein kinase. Nature. 2002;415:339–343. doi: 10.1038/415339a. [DOI] [PubMed] [Google Scholar]
- Mori H, Prestwich TC, Reid MA, Longo KA, Gerin I, Cawthorn WP, Susulic VS, Krishnan V, Greenfield A, Macdougald OA. Secreted frizzled-related protein 5 suppresses adipocyte mitochondrial metabolism through WNT inhibition. J Clin Invest. 2012;122:2405–2416. doi: 10.1172/JCI63604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton GJ, Gelling RW, Niswender KD, Morrison CD, Rhodes CJ, Schwartz MW. Leptin regulates insulin sensitivity via phosphatidylinositol-3-OH kinase signaling in mediobasal hypothalamic neurons. Cell Metab. 2005;2:411–420. doi: 10.1016/j.cmet.2005.10.009. [DOI] [PubMed] [Google Scholar]
- Munkhtulga L, Nakayama K, Utsumi N, Yanagisawa Y, Gotoh T, Omi T, Kumada M, Erdenebulgan B, Zolzaya K, Lkhagvasuren T, et al. Identification of a regulatory SNP in the retinol binding protein 4 gene associated with type 2 diabetes in Mongolia. Hum Genet. 2007;120:879–888. doi: 10.1007/s00439-006-0264-4. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Tobe T, Choi-Miura NH, Mazda T, Tomita M. Isolation and characterization of GBP28, a novel gelatin-binding protein purified from human plasma. J Biochem. 1996;120:803–812. doi: 10.1093/oxfordjournals.jbchem.a021483. [DOI] [PubMed] [Google Scholar]
- Nguyen KD, Qiu Y, Cui X, Goh YP, Mwangi J, David T, Mukundan L, Brombacher F, Locksley RM, Chawla A. Alternatively activated macrophages produce catecholamines to sustain adaptive thermogenesis. Nature. 2011;480:104–108. doi: 10.1038/nature10653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen MT, Satoh H, Favelyukis S, Babendure JL, Imamura T, Sbodio JI, Zalevsky J, Dahiyat BI, Chi NW, Olefsky JM. JNK and tumor necrosis factor-alpha mediate free fatty acid-induced insulin resistance in 3T3-L1 adipocytes. J Biol Chem. 2005;280:35361–35371. doi: 10.1074/jbc.M504611200. [DOI] [PubMed] [Google Scholar]
- Ofei F, Hurel S, Newkirk J, Sopwith M, Taylor R. Effects of an engineered human anti-TNF-alpha antibody (CDP571) on insulin sensitivity and glycemic control in patients with NIDDM. Diabetes. 1996;45:881–885. doi: 10.2337/diab.45.7.881. [DOI] [PubMed] [Google Scholar]
- Ogata A, Morishima A, Hirano T, Hishitani Y, Hagihara K, Shima Y, Narazaki M, Tanaka T. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70:1164–1165. doi: 10.1136/ard.2010.132845. [DOI] [PubMed] [Google Scholar]
- Okamoto Y, Kihara S, Ouchi N, Nishida M, Arita Y, Kumada M, Ohashi K, Sakai N, Shimomura I, Kobayashi H, et al. Adiponectin reduces atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2002;106:2767–2770. doi: 10.1161/01.cir.0000042707.50032.19. [DOI] [PubMed] [Google Scholar]
- Oral EA, Simha V, Ruiz E, Andewelt A, Premkumar A, Snell P, Wagner AJ, DePaoli AM, Reitman ML, Taylor SI, et al. Leptin-replacement therapy for lipodystrophy. N Engl J Med. 2002;346:570–578. doi: 10.1056/NEJMoa012437. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Higuchi A, Ohashi K, Oshima Y, Gokce N, Shibata R, Akasaki Y, Shimono A, Walsh K. Sfrp5 is an anti-inflammatory adipokine that modulates metabolic dysfunction in obesity. Science. 2010;329:454–457. doi: 10.1126/science.1188280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Arita Y, Maeda K, Kuriyama H, Okamoto Y, Hotta K, Nishida M, Takahashi M, Nakamura T, et al. Novel modulator for endothelial adhesion molecules: adipocyte-derived plasma protein adiponectin. Circulation. 1999;100:2473–2476. doi: 10.1161/01.cir.100.25.2473. [DOI] [PubMed] [Google Scholar]
- Ouchi N, Kihara S, Funahashi T, Nakamura T, Nishida M, Kumada M, Okamoto Y, Ohashi K, Nagaretani H, Kishida K, et al. Reciprocal association of C-reactive protein with adiponectin in blood stream and adipose tissue. Circulation. 2003;107:671–674. doi: 10.1161/01.cir.0000055188.83694.b3. [DOI] [PubMed] [Google Scholar]
- Pang C, Gao Z, Yin J, Zhang J, Jia W, Ye J. Macrophage infiltration into adipose tissue may promote angiogenesis for adipose tissue remodeling in obesity. Am J Physiol Endocrinol Metab. 2008;295:E313–E322. doi: 10.1152/ajpendo.90296.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park EJ, Lee JH, Yu GY, He G, Ali SR, Holzer RG, Osterreicher CH, Takahashi H, Karin M. Dietary and genetic obesity promote liver inflammation and tumorigenesis by enhancing IL-6 and TNF expression. Cell. 2010;140:197–208. doi: 10.1016/j.cell.2009.12.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Rajala MW, Rossetti L, Scherer PE, Shapiro L. Disulfide-dependent multimeric assembly of resistin family hormones. Science. 2004;304:1154–1158. doi: 10.1126/science.1093466. [DOI] [PubMed] [Google Scholar]
- Petersen KF, Oral EA, Dufour S, Befroy D, Ariyan C, Yu C, Cline GW, DePaoli AM, Taylor SI, Gorden P, et al. Leptin reverses insulin resistance and hepatic steatosis in patients with severe lipodystrophy. J Clin Invest. 2002;109:1345–1350. doi: 10.1172/JCI15001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Promintzer M, Krebs M, Todoric J, Luger A, Bischof MG, Nowotny P, Wagner O, Esterbauer H, Anderwald C. Insulin resistance is unrelated to circulating retinol binding protein and protein C inhibitor. J Clin Endocrinol Metab. 2007;92:4306–4312. doi: 10.1210/jc.2006-2522. [DOI] [PubMed] [Google Scholar]
- Qatanani M, Szwergold NR, Greaves DR, Ahima RS, Lazar MA. Macrophage-derived human resistin exacerbates adipose tissue inflammation and insulin resistance in mice. J Clin Invest. 2009;119:531–539. doi: 10.1172/JCI37273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randle PJ, Garland PB, Hales CN, Newsholme EA. The glucose fatty-acid cycle. Its role in insulin sensitivity and the metabolic disturbances of diabetes mellitus. Lancet. 1963;1:785–789. doi: 10.1016/s0140-6736(63)91500-9. [DOI] [PubMed] [Google Scholar]
- Reilly MP, Lehrke M, Wolfe ML, Rohatgi A, Lazar MA, Rader DJ. Resistin is an inflammatory marker of atherosclerosis in humans. Circulation. 2005;111:932–939. doi: 10.1161/01.CIR.0000155620.10387.43. [DOI] [PubMed] [Google Scholar]
- Rosen ED, Spiegelman BM. Adipocytes as regulators of energy balance and glucose homeostasis. Nature. 2006;444:847–853. doi: 10.1038/nature05483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- Ryo M, Nakamura T, Kihara S, Kumada M, Shibazaki S, Takahashi M, Nagai M, Matsuzawa Y, Funahashi T. Adiponectin as a biomarker of the metabolic syndrome. Circ J. 2004;68:975–981. doi: 10.1253/circj.68.975. [DOI] [PubMed] [Google Scholar]
- Sabio G, Das M, Mora A, Zhang Z, Jun JY, Ko HJ, Barrett T, Kim JK, Davis RJ. A stress signaling pathway in adipose tissue regulates hepatic insulin resistance. Science. 2008;322:1539–1543. doi: 10.1126/science.1160794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Norquay L, Farhang J, D’Aquino K, Copps K, White MF. Human IL6 enhances leptin action in mice. Diabetologia. 2010;53:525–535. doi: 10.1007/s00125-009-1580-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuel VT, Petersen KF, Shulman GI. Lipid-induced insulin resistance: unravelling the mechanism. Lancet. 2010;375:2267–2277. doi: 10.1016/S0140-6736(10)60408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer PE, Williams S, Fogliano M, Baldini G, Lodish HF. A novel serum protein similar to C1q, produced exclusively in adipocytes. J Biol Chem. 1995;270:26746–26749. doi: 10.1074/jbc.270.45.26746. [DOI] [PubMed] [Google Scholar]
- Schultz O, Oberhauser F, Saech J, Rubbert-Roth A, Hahn M, Krone W, Laudes M. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5:e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata R, Sato K, Pimentel DR, Takemura Y, Kihara S, Ohashi K, Funahashi T, Ouchi N, Walsh K. Adiponectin protects against myocardial ischemia-reperfusion injury through AMPK- and COX-2-dependent mechanisms. Nat Med. 2005;11:1096–1103. doi: 10.1038/nm1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum BO, Mackay CR, Gorgun CZ, Frost MJ, Kumar RK, Hotamisligil GS, Rolph MS. The adipocyte fatty acid-binding protein aP2 is required in allergic airway inflammation. J Clin Invest. 2006;116:2183–2192. doi: 10.1172/JCI24767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon I, Escote X, Vilarrasa N, Gomez J, Fernandez-Real JM, Megia A, Gutierrez C, Gallart L, Masdevall C, Vendrell J. Adipocyte fatty acid-binding protein as a determinant of insulin sensitivity in morbid-obese women. Obesity (Silver Spring) 2009;17:1124–1128. doi: 10.1038/oby.2008.665. [DOI] [PubMed] [Google Scholar]
- Spranger J, Kroke A, Mohlig M, Hoffmann K, Bergmann MM, Ristow M, Boeing H, Pfeiffer AF. Inflammatory cytokines and the risk to develop type 2 diabetes: results of the prospective population-based European Prospective Investigation into Cancer and Nutrition (EPIC)-Potsdam Study. Diabetes. 2003;52:812–817. doi: 10.2337/diabetes.52.3.812. [DOI] [PubMed] [Google Scholar]
- St-Pierre J, Tremblay ML. Modulation of leptin resistance by protein tyrosine phosphatases. Cell Metab. 2012;15:292–297. doi: 10.1016/j.cmet.2012.02.004. [DOI] [PubMed] [Google Scholar]
- Stejskal D, Karpisek M. Adipocyte fatty acid binding protein in a Caucasian population: a new marker of metabolic syndrome? Eur J Clin Invest. 2006;36:621–625. doi: 10.1111/j.1365-2362.2006.01696.x. [DOI] [PubMed] [Google Scholar]
- Stephens JW, Hurel SJ, Lowe GD, Rumley A, Humphries SE. Association between plasma IL-6, the IL6 −174G>C gene variant and the metabolic syndrome in type 2 diabetes mellitus. Mol Genet Metab. 2007;90:422–428. doi: 10.1016/j.ymgme.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- Strissel KJ, Stancheva Z, Miyoshi H, Perfield JW, 2nd, DeFuria J, Jick Z, Greenberg AS, Obin MS. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56:2910–2918. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- Takemura Y, Ouchi N, Shibata R, Aprahamian T, Kirber MT, Summer RS, Kihara S, Walsh K. Adiponectin modulates inflammatory reactions via calreticulin receptor-dependent clearance of early apoptotic bodies. J Clin Invest. 2007;117:375–386. doi: 10.1172/JCI29709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trujillo ME, Scherer PE. Adipose tissue-derived factors: impact on health and disease. Endocr Rev. 2006;27:762–778. doi: 10.1210/er.2006-0033. [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Hotamisligil GS. Functional analysis of tumor necrosis factor (TNF) receptors in TNF-alpha-mediated insulin resistance in genetic obesity. Endocrinology. 1998;139:4832–4838. doi: 10.1210/endo.139.12.6337. [DOI] [PubMed] [Google Scholar]
- Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- von Eynatten M, Lepper PM, Liu D, Lang K, Baumann M, Nawroth PP, Bierhaus A, Dugi KA, Heemann U, Allolio B, et al. Retinol-binding protein 4 is associated with components of the metabolic syndrome, but not with insulin resistance, in men with type 2 diabetes or coronary artery disease. Diabetologia. 2007;50:1930–1937. doi: 10.1007/s00125-007-0743-8. [DOI] [PubMed] [Google Scholar]
- Vozarova B, Weyer C, Hanson K, Tataranni PA, Bogardus C, Pratley RE. Circulating interleukin-6 in relation to adiposity, insulin action, and insulin secretion. Obes Res. 2001;9:414–417. doi: 10.1038/oby.2001.54. [DOI] [PubMed] [Google Scholar]
- Wallenius V, Wallenius K, Ahren B, Rudling M, Carlsten H, Dickson SL, Ohlsson C, Jansson JO. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8:75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- Wang S, Soni KG, Semache M, Casavant S, Fortier M, Pan L, Mitchell GA. Lipolysis and the integrated physiology of lipid energy metabolism. Mol Genet Metab. 2008;95:117–126. doi: 10.1016/j.ymgme.2008.06.012. [DOI] [PubMed] [Google Scholar]
- Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winer S, Chan Y, Paltser G, Truong D, Tsui H, Bahrami J, Dorfman R, Wang Y, Zielenski J, Mastronardi F, et al. Normalization of obesity-associated insulin resistance through immunotherapy. Nat Med. 2009;15:921–929. doi: 10.1038/nm.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM. Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis. Science. 2011;332:243–247. doi: 10.1126/science.1201475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wang Y, Keshaw H, Xu LY, Lam KS, Cooper GJ. The fat-derived hormone adiponectin alleviates alcoholic and nonalcoholic fatty liver diseases in mice. J Clin Invest. 2003a;112:91–100. doi: 10.1172/JCI17797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu A, Wang Y, Xu JY, Stejskal D, Tam S, Zhang J, Wat NM, Wong WK, Lam KS. Adipocyte fatty acid-binding protein is a plasma biomarker closely associated with obesity and metabolic syndrome. Clin Chem. 2006;52:405–413. doi: 10.1373/clinchem.2005.062463. [DOI] [PubMed] [Google Scholar]
- Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003b;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int J Obes (Lond) 2008;32(Suppl 7):S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Ito Y, Tsuchida A, Yokomizo T, Kita S, Sugiyama T, Miyagishi M, Hara K, Tsunoda M, et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature. 2003;423:762–769. doi: 10.1038/nature01705. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Minokoshi Y, Ito Y, Waki H, Uchida S, Yamashita S, Noda M, Kita S, Ueki K, et al. Adiponectin stimulates glucose utilization and fatty-acid oxidation by activating AMP-activated protein kinase. Nat Med. 2002;8:1288–1295. doi: 10.1038/nm788. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Kamon J, Waki H, Terauchi Y, Kubota N, Hara K, Mori Y, Ide T, Murakami K, Tsuboyama-Kasaoka N, et al. The fat-derived hormone adiponectin reverses insulin resistance associated with both lipoatrophy and obesity. Nat Med. 2001;7:941–946. doi: 10.1038/90984. [DOI] [PubMed] [Google Scholar]
- Yamauchi T, Nio Y, Maki T, Kobayashi M, Takazawa T, Iwabu M, Okada-Iwabu M, Kawamoto S, Kubota N, Kubota T, et al. Targeted disruption of AdipoR1 and AdipoR2 causes abrogation of adiponectin binding and metabolic actions. Nat Med. 2007;13:332–339. doi: 10.1038/nm1557. [DOI] [PubMed] [Google Scholar]
- Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- Yao-Borengasser A, Varma V, Bodles AM, Rasouli N, Phanavanh B, Lee MJ, Starks T, Kern LM, Spencer HJ, 3rd, Rashidi AA, et al. Retinol binding protein 4 expression in humans: relationship to insulin resistance, inflammation, and response to pioglitazone. J Clin Endocrinol Metab. 2007;92:2590–2597. doi: 10.1210/jc.2006-0816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, Keller JN. Regulation of energy metabolism by inflammation: a feedback response in obesity and calorie restriction. Aging (Albany NY) 2010;2:361–368. doi: 10.18632/aging.100155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye J, McGuinness OP. Inflammation during obesity is not all bad: evidence from animal and human studies. Am J Physiol Endocrinol Metab. 2013;304:E466–E477. doi: 10.1152/ajpendo.00266.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung DC, Xu A, Cheung CW, Wat NM, Yau MH, Fong CH, Chau MT, Lam KS. Serum adipocyte fatty acid-binding protein levels were independently associated with carotid atherosclerosis. Arterioscler Thromb Vasc Biol. 2007;27:1796–1802. doi: 10.1161/ATVBAHA.107.146274. [DOI] [PubMed] [Google Scholar]
- Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Proenca R, Maffei M, Barone M, Leopold L, Friedman JM. Positional cloning of the mouse obese gene and its human homologue. Nature. 1994;372:425–432. doi: 10.1038/372425a0. [DOI] [PubMed] [Google Scholar]