Abstract
Loss of immune function and increased hematopoietic disease are among the most clinically significant consequences of aging. Hematopoietic stem cells (HSCs) from mice lacking aryl hydrocarbon receptor (AhR) have high rates of cell division. Studies were designed to test the hypothesis that aging AhR-null allele (AhR-KO) mice develop premature HSC exhaustion, and changes leading to hematological disease. Compared to wild-type, aging AhR-KO mice showed a decreased survival rate, splenomegaly, increased circulating white blood cells, hematopoietic cell accumulation in tissues, and anemia. Analysis of bone marrow indicated increased numbers of stem/progenitor and lineage-committed cells, but decreased erythroid progenitors. There was also decreased self-renewal capacity of HSCs determined by competitive repopulation and serial transplantation. HSCs also showed increased levels of reactive oxygen species (ROS), Ki-67, and γ-H2A.X, but decreased p16Ink4a. Splenic cells from aging KO mice had abnormal expression of genes, including Gata-1, Sh2d3c, Gfi-1, p21, and c-myc, involved in trafficking and associated with leukemia. HSCs from AhR-KO mice had gene changes related to HSC maintenance and consistent with phenotype observed. The most prominent gene changes (overexpression of Srpk2, Creb1, Hes1, mtor, pdp1) have been associated with HSC hyperproliferation, leukemia, and accelerated aging. Pathway analyses also indicated an enrichment of genes associated with oxidative stress, acute myelogenous leukemia, aging, and heat shock response, and the β-catenin/Wnt pathways. These data indicate that loss of AhR and associated changes in multiple signaling pathways promote premature HSC exhaustion and development of a myeloproliferative disorder. They also implicate a critical role of the AhR in the regulation of HSCs.
Introduction
In response to stress, hematopoietic stem cells (HSCs) can undergo extensive proliferation and expansion. However, under normal homeostatic conditions, HSCs are mostly quiescent to prevent premature exhaustion and limit their susceptibility to genetic alterations; thus, preserving the capability for long-term multi-lineage generation. The processes that regulate quiescence, self-renewal, aging, and senescence are not well understood. However, it is clear that age-related changes take place and these changes contribute to disease processes that occur at a greater frequency with age. It is not surprising that age is the single best predictor for the development of cancer; loss of immune function, and increased incidence of certain leukemias and myelodysplastic syndromes are some of the most clinically significant consequences of aging [1].
As immune system aging occurs in mice, there is a skewing of lineages toward the myeloid at the expense of lymphoid lineages, and aged HSCs regenerate bone marrow (BM) incompletely after hematopoietic stress [2]. It is likely that a complex combination of both intrinsic and environmental niche factors mediate age-associated changes in HSCs. Exposure to DNA-damaging and other agents that may generate reactive oxygen species (ROS) affect HSC function and hematopoietic aging [3]. Mice deficient in pathways responsible for DNA maintenance and repair show phenotypes that resemble aging [4]. The modified expression of several molecules, for example, Rb, p21Cip1, p27Kip1, and p16INK4a, directly coupled to cell-cycle progression and control mechanisms, has been shown to promote altered HSC quiescence and premature aging and senescence [2,5]. Similarly, a prolonged increased proportion of actively cycling HSCs appears to correlate with increased incidence of leukemia, lymphoma, and myelodysplastic syndrome in old age [6].
The aryl hydrocarbon receptor (AhR) is a basic helix-loop-helix transcription factor originally identified as mediating the toxicity of a large group of xenobiotics. Due in part to the ability of these xenobiotic ligands to have potent and persistent effects on the immune system in experimental animal models [7], there has been much work to define a physiological role of the AhR and possible relationships to human disease. Exposure to environmental AhR ligands, such as polychlorinated biphenyls and dioxins, has been linked with increased incidence of lymphoma and leukemia [8,9]. Altered expression and activity of the AhR has been described in human leukemia and lymphoma cells [10,11]. Studies have suggested a critical role of the AhR in the development of T-cell populations that have important functions in the control of autoimmune disease [12,13]. AhR antagonists are being investigated for their ability to promote the expansion of human HSCs for potential use in BM transplants [14]. These latter studies are consistent with other investigations suggesting a role of the AhR in the regulation of HSCs [15–18]. However, the mechanisms and actual functional significance remain unclear.
We have shown that HSCs from young adult AhR-null allele (AhR-KO) mice have inherently high rates of cell division [17]. These data, along with others [11,14] are consistent with a hypothesis that the AhR has an important role as a negative regulator of proliferation by promoting HSCs to remain quiescent. Based on these findings, we further hypothesize that prolonged loss of AhR may result in sensitivity to stress conditions, premature exhaustion of HSCs and/or aberrant changes in HSCs leading to hematologic disease. Together, the data reported here indicate that the AhR is a key transcription factor controlling signaling pathways involved in the regulation of HSCs and their protection from premature aging and hematopoietic stress.
Materials and Methods
Mice and cell preparations
C57BL/6J AhR-KO mice [19], were obtained from C. Bradfield and maintained as a breeding colony in the animal care facility at the University of Rochester. C57BL/6J Wild-type (WT) mice were used as experimental controls. Handling and experimental procedures were performed in accordance with University of Rochester approved protocols. Femur and tibia BM from female mice were harvested as previously reported [17].
5-FU treatments
The AhR-KO and WT mice were injected with a single dose of 5-fluorouracil (5-FU, 150 mg/kg; Sigma) intraperitoneally. Peripheral blood samples were collected at intervals from retroorbital plexus and hematological analysis was done using Heska HemaTrue Hematology Analyzer (Heska Corporation). Other groups of mice were treated with 5-FU and euthanized after 6 days for p16Ink4a and ROS assays.
Colony-forming unit assays
Colony Forming Assays were performed using MethoCult™ media (StemCell Technologies) according to manufacturer's protocols. HPP-CFCs were quantified and the Days 8 and 12 colony-forming unit (CFU)-Spleen assays (CFU-Sd8 and CFU-Sd12) was performed as previously described [16].
In vivo phenotyping experiments
Mice were euthanized at various ages by CO2 asphyxiation. Marrow was harvested as reported [16,17] and lineage depleted before immunophenotyping Lin− cells. HSC-enriched populations were defined as Lin−/Sca-1+/c-kit+ (LSK) using c-Kit APC (clone 2B8), Sca-1 PE (clone E13-161.7), and Streptavidin Per-CP (for staining of Lin+ cells). For analysis of lineage positive (Lin+) cells, the following antibodies (BD Biosciences) were used: CD3 FITC (clone 17A2), B220 PerCP-Cy5.5 (clone RA3-6B2), Mac-1 APC (clone M1/70), Gr-1 APC-Cy7 (clone RB6-8C5) and Ter-119 PE (clone Ly-76). Cells were prepared as previously described [17,20] and analysis (50,000 events per sample) performed on a BD FACSCalibur™ flow cytometer and using CellQuest software. The most primitive populations were defined as HSC, long-term HSC (LT-HSC), short-term HSC (ST-HSC), multi-potential progenitors, common lymphoid progenitors, and common myeloid progenitors using surface marker combinations previously reported [16]. Lin− cells were isolated and stained with antibodies Flt3 PE-Cy5 (clone A2F10), IL-7R PE-Cy7 (clone A7R34), Thy1.2 V450 (clone 53-2.1), FcR PE (clone 93) from eBiosciences and Sca-1 APC (clone E13-161.7), cKit APC-Cy7 (clone 2B8), CD34 FITC (clone RAM34) from BD Biosciences. The percentage of erythroid progenitors in BM cells were measured as double-positive Ter119 APC (clone TER119) and CD71 FITC (clone C2) cells. Erythroblast subpopulations were quantified as proerythroblasts (Ter119medCD71high), basophilic erythroblasts (Ter119highCD71high), polychromatophilic erythroblasts (Ter119highCD71med), and orthochromatophilic erythroblasts (Ter119highCD71low). Analyses of these cells were performed using a LSRII (BD Biosciences) flow cytometer and FlowJo software. For ROS measurement, Lin- cells were cultured with DCFDA (2′,7′-dichlorofluorescein diacetate) using DCFDA Cellular ROS Detection Assay Kit following manufacturer's protocol (Abcam, Inc.). After DCFDA staining, cells were stained for anti Sca-1-V450 and c-Kit-PE-Cy7 antibody (Lin-Sca-1+c-Kit). In separate experiments, Lin-cells were stained for Lin-Sca-1+c-Kit and fixed and permiabilized using BD Cytofix/Cytoperm kit and following manufacturer's protocol (BD Pharmingen) and stained separately for Ki-67 (anti-Ki-67- Alexa Fluor 700; BD Pharmingen), p16Ink4a (anti-p16-Alexa Fluor 488; Santa Cruz Biotechnology) and γ-H2A.X [anti-phospho-Histone H2A.X (Ser139)-FITC; Millipore].
Hematological, histopathological and immunohistochemical analyses
Peripheral blood was obtained from the retro-orbital plexus and processed as previously described [16]. RBC and WBC counts and morphology-based differential WBC counts were analyzed in 3-, 12-, and 24-month old mice. Tissue sections were prepared and stained with hematoxylin and eosin for histological evaluation or for immunohistochemical analysis as previously described [17]. Slides were incubated with primary antibody (Ki-67, B220, CD3), secondary antibody conjugated with horseradish peroxidase, stained with 3,3′-diaminobenzidine (DAB), and counterstained with Meyers's hematoxylin.
Competitive repopulation unit assay and serial BM transplantation assay
The competitive repopulation unit (CRU) assay is used to analyze the frequency of donor HSCs able to repopulate recipient BM. This was done using a limiting dilution competitive repopulation assay with slight modification [20]. Five doses (2, 1, 0.5, 0.1, and 0.05×106) of BM cells from WT or AhR-KO mice (CD45.2+) along with 2×105 BM CD45.1+ competitor cells were prepared. Recipient CD45.1+ mice were irradiated (550+550 rads, 4 h apart) and 8 mice per dose of BM cells from both WT and AhR-KO were injected intravenously. After 20 weeks of transplantation, BM cells were isolated. These cells were stained with antibodies [CD45.1 APC (A20) and CD45.2 FITC (104)] (BD Biosciences) and were analyzed using a FACS Canto (BD Biosciences) flow cytometer. The presence of more than 1% cells from donor origin (CD45.2+) was considered as positive engraftment. The number of mice negative for reconstitution was recorded and frequency of HSCs was analyzed using Poisson statistics [21]. Animals unable to survive up to the end of the experimental period were excluded from the analysis.
For the serial transplantation assay, BM cells were isolated from 24-month old mice (CD45.2+ cells), mixed with competitor CD45.1+ cells at a ratio of 1:1 (0.5×106 BM cells each), and injected into the tail vein of irradiated (see paragraph above) recipient CD45.1+ mice. After 6 weeks, BM cells (1×106) were harvested from primary recipients and injected into irradiated CD45.1+ secondary recipient mice. This procedure was the same for the tertiary transplant. BM cells from primary, secondary, and tertiary CD45.1+ recipients were analyzed for cells of donor (CD45.2+) and recipient (CD45.1+) origin. The level of engraftment (% CD45.2+ cells) was determined after 6 weeks of transplantation at every stage.
Real-time PCR array analysis
Quantitative mRNA expression analyses were done using the RT2-PCR array for Mouse Inflammatory Cytokines and Receptors (PAMM-011; SABiosciences Corporation). Spleen samples were snap-frozen in liquid nitrogen. RNA was isolated using RNeasy Mini/Micro Kit (Qiagen), and quantified using a spectrophotometer (ND-1000; NanoDrop Technologies). cDNA from 0.5 μg of DNAse-treated RNA was prepared according to manufacturer's protocol. The RT2 Real Time™ SYBR Green PCR Master Mix (SA Biosciences) was used with the PCR array in a BioRad iCycler. Expression of mRNA for each gene was normalized using the expression of Hprt, Gapdh, and Actin b. Values from AhR-KO mice and WT mice were compared according to the 2−ΔΔCT method. Results were considered significant when relative mRNA expression was two-fold higher or lower than the WT control. Selected gene changes were validated by RT-qPCR using gene specific primers/probe (TaqMan Assay; Life Technologies).
Quantitative real-time PCR assay
RNA for real-time PCR was isolated using TRIzol reagent (Life Technologies) and quantified. First strand cDNA was produced following the protocol of SuperScriptII First Strand cDNA Synthesis system (Invitrogen) using 2.5–5 μg total RNA and oligo-dT primers. cDNA samples were phenol/chloroform extracted and ethanol precipitated. Primers were from Applied Biosciences. Real-time quantitative PCR was performed using TaqMan PCR primers and MasterMix (Applied Biosystems). Real-time detection (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/scd) and analysis were performed using the BioRad iCycler and data were analyzed using the standard curve method (ABI Prism 7700 Sequence Detection System User Bulletin #2). Samples were run in triplicate and normalized to Gapdh RNA.
Sorting and microarray analysis of Lin-CD48-CD150+ (SLAM+) cells
Cells for microarray analyses were obtained by laser-assisted sorting of lineage depleted cells, prepared as previously described [20], and stained with fluorochrome conjugated antibodies against Sca-1 (V450 Clone D7; BD Pharmingen), cKit (PeCy7 Clone 2B8; BD Pharmingen), CD34 (AF700 Clone Ram34; eBiosciences), CD48 (FITC Clone Hm48-1; BD Pharmingen), and CD150 (APC Clone 459911; R&D Systems). Cells were sorted into RNAlater RNA Stabilization Reagent and placed at −80°C for submission to the URMC Functional Genomics Center. Total RNA was isolated from sorted SLAM+ LT-HSCs from young adult mice using an RNeasy Mini Kit (Qiagen) and microarray analysis was performed using Genechip Mouse Gene 2.0 ST Array (Affymetrix) at the Functional Genomics Center, University of Rochester. The Iterplier algorithm was used to generate background-subtracted, quantile-normalized signals from the raw microarray data. These signals were used to compute mean expression ratios (KO/WT) and P values (two-tailed t-tests) with Microsoft Excel. Transcripts with a mean difference in expression of at least 1.5-fold and P<0.05 were examined for network/pathway associations by Ingenuity Pathway Analysis (IPA, Ingenuity Systems), which is based on a proprietary database. Top physiological functions were ascribed to the set of genes observed to be altered. The microarray data can be accessed through the Gene Expression Omnibus accession no. GSE46976. Microarray data were also tested for gene set enrichment analysis (GSEA, detailed in supporting information).
Statistical analyses
Unless otherwise specified, results were analyzed and plotted using GraphPad Prism (GraphPad, Inc). When appropriate, two-tailed Student's t-test was used to analyze statistical significance. A P-value of<0.05 was considered to be statistically significant. A right-tailed Fisher exact test was used to calculate a P-value to determine the probability that each biological function or disease assigned to the data set by IPA is due to chance alone.
Results
Aging AhR-KO mice exhibit physical and pathological signs characteristic of a myeloproliferative disorder
Our previous studies observed that HSCs from young adult AhR-KO mice are inherently hyperproliferative as determined by cell cycle analysis, BrdU incorporation, and expansion under ex vivo conditions. Consistent with these data, young AhR-KO mice have increased numbers of HSCs with long-term repopulation potential defined by CRU and competitive engraftment in irradiated animals [17]. Similarly, there was a robust recovery of BM and lineage cells in AhR-KO animals after induction of short-term stress with a single dosage of 5-FU; as expected, the greater cycling rates of HSCs increased the initial sensitivity to BM cell loss immediately after injection (Supplementary Fig. S1A). However, during these investigations we observed an increase in ROS and p16INK4a content in HSC-enriched LSK cells from AhR-KO mice treated with 5-FU (Supplementary Fig. S1B, C). Together, these data suggested that AhR loss-induced increased cycling of HSCs does not deter the ability of HSCs to functionally respond to short-term stress conditions. However, this characteristic may confer increased sensitivity to long-term consequences of dysfunction associated with premature HSC exhaustion. To explore this, we examined the phenotypic and functional hematopoietic, and in particular HSC, characteristics in aging AhR-KO mice.
AhR-KO mice had a similar survival rate as WT mice until about 15 months of age (Supplementary Fig. S2). By 24 months, only 33% of the KO mice survived as opposed to more than 78% of WT mice.
There was profound splenomegaly in 24-month-old mice with spleen weights and cellularity being over 2- to 4-fold greater than age-matched WT controls (data not shown). Histological examination of the spleen revealed a decrease in the number and area of periarteriolar lymphatic sheath along with extensive lymphocytic infiltration in the red pulp area (Supplementary Fig. S3A, B). Increased Ki-67 antibody staining in spleens from KO mice (Supplementary Fig. S3C, D) indicated an increased number of proliferating cells. Hematopoietic cell infiltration and/or proliferation disrupted the architecture of other tissues, for example, liver, lung, kidney, and bone (Supplementary Fig. S3E–L).
Aging AhR-KO mice develop a phenotype of increased circulating white blood cells but decreased red blood cells
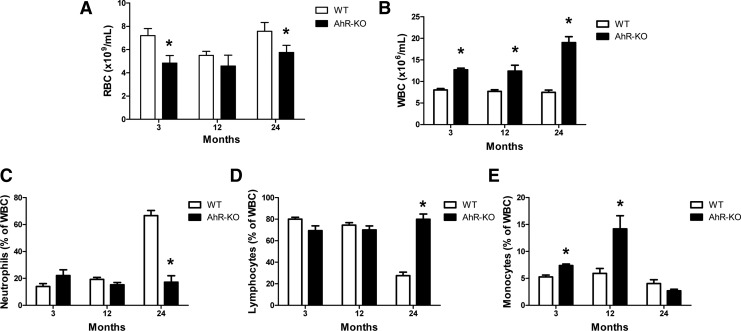
As early as 3 months of age, AhR-KO mice exhibited elevated WBC counts but decreased RBC counts in peripheral blood (Fig. 1A, B). At 24 months, and consistent with the increased presence of hematopoietic cells in tissues, the number of WBC in AhR-KO mice was over 2.5-fold that in control animals. At 24 months of age, control WT mice showed a “myeloid shift,” an expected age-dependent increase in the relative percent of neutrophils and a decrease in the relative percentage of lymphocytes (Fig. 1C, D). Monocytes were significantly higher at 3 and 12 months in AhR-KO mice, but no difference from WT was observed at 24 months (Fig. 1E). However, the above shift was not observed in AhR-KO; the number of myeloid and lymphoid cells remained high throughout all months examined mainly due to the increased total number of WBC without substantial changes in the relative percentages. Overall, these data suggest an AhR-dependent loss in the ability to regulate numbers of immune cells, as well as the age-dependent skewing that normally occurs.
FIG. 1.
Aging aryl hydrocarbon receptor (AhR)-KO mice have abnormal changes in peripheral blood cell numbers. Blood was drawn from the retro-orbital plexus of wild type (WT) and AhR-KO mice and analyzed for total red (A) and white (B) blood cell counts. Blood film slides were stained with Wright-Giemsa stain for differential counting of WBC (C–E). Data are shown for 3-, 12-, and 24-month old mice. Data are mean±S.D., n=4–6 mice/group. *Values significantly different from WT control (P<0.05).
Loss of AhR produces long lasting alterations in BM and the stem cell/progenitor compartment
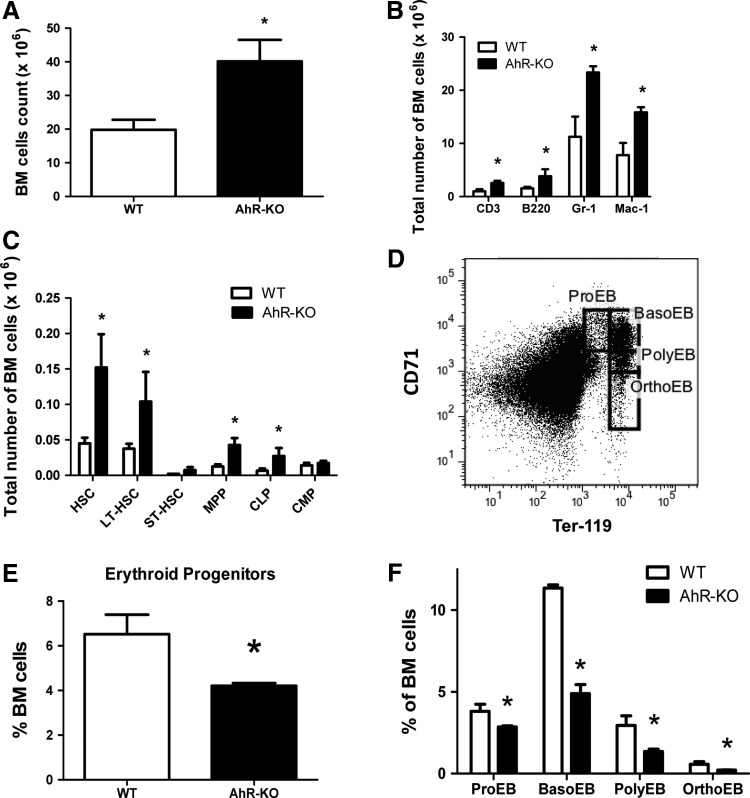
Our previous analyses of 6–8 week-old AhR-KO mice indicated both phenotypic and functional changes in BM hematopoietic precursors as indicated by increased number of lineage-restricted progenitors, increased numbers of LSK cells, and altered trafficking of these cells after transplant [17]. At 24 months, consistent with the histopathology, the number of BM cells in KO mice was about double that in WT animals (Fig. 2A). Phenotypic analysis indicated an overall increase in the number of progenitors, as well as lineage-committed cells (Fig. 2B, C). Similar increases in the total number of primitive progenitors, that is, LSK and LT-HSC, were observed in both 3 and 12 month-old AhR-KO mice (not shown). Notably, consistent with the mild anemia observed, there were significant decreases in the percentages of erythroid progenitors in BM of 24-month old AhR-KO mice (Fig. 2D–F). However, AhR-KO mice may not have a substantial difference in total number of erythroid progenitors, if the increased total number of BM cells is taken into consideration (Fig. 2A–E). Nevertheless, there was still a significant decrease in RBC number in peripheral blood in AhR-KO mice (Fig. 1A) that may be due to defective differentiation and maturation of erythroid progenitors (Fig. 2E, F).
FIG. 2.
Twenty-four month old AhR-KO mice have increased numbers of bone marrow (BM) cells and primitive progenitor cells. BM cells were counted (A) and analyzed by flow cytometry to define lineage-committed cells (B) and primitive progenitors (C). Data are mean of total cells in BM±S.D (n=4). Erythroid differentiation within BM was further analyzed using Ter119 and CD71 antibodies, according to the scheme shown in (D). (E) Erythroid progenitors (Ter119+CD71+), and (F) proerythroblasts (Ter119medCD71high) basophilic erythroblasts (Ter119highCD71high), polychromatophilic erythroblasts (Ter119highCD71med) and orthochromatophilic erythroblasts (Ter119highCD71low) were identified. Data are mean±S.D. *Values significantly different from control (P<0.05).
BM progenitor cells were assessed for their functional differentiation potential. There were varied and age-dependent differences in CFU-preB, CFU-GM, CFU-G, CFU-M, and HPP-CFC (Supplementary Fig. 4B–D), although all of these, except CFU-M, were significantly increased at 24 months in the BM from KO mice. Consistent with anemia and the decreased number of erythroid progenitors observed in AhR deficient animals, there were also decreases in BFU-E and CFU-E at all ages examined (Supplementary Fig. S4A). A significant decrease in CFU-Sday8 (Supplementary Fig. S5), an in vivo assay of myeloerythroid progenitors, is also consistent with these findings.
These data indicate that long-term loss of AhR results in the uncontrolled ability to produce hematopoietic cells of most, if not all, nonerythroid lineages at the expense of erythroid cells.
HSCs from aging AhR-KO mice have decreased competitive repopulation ability
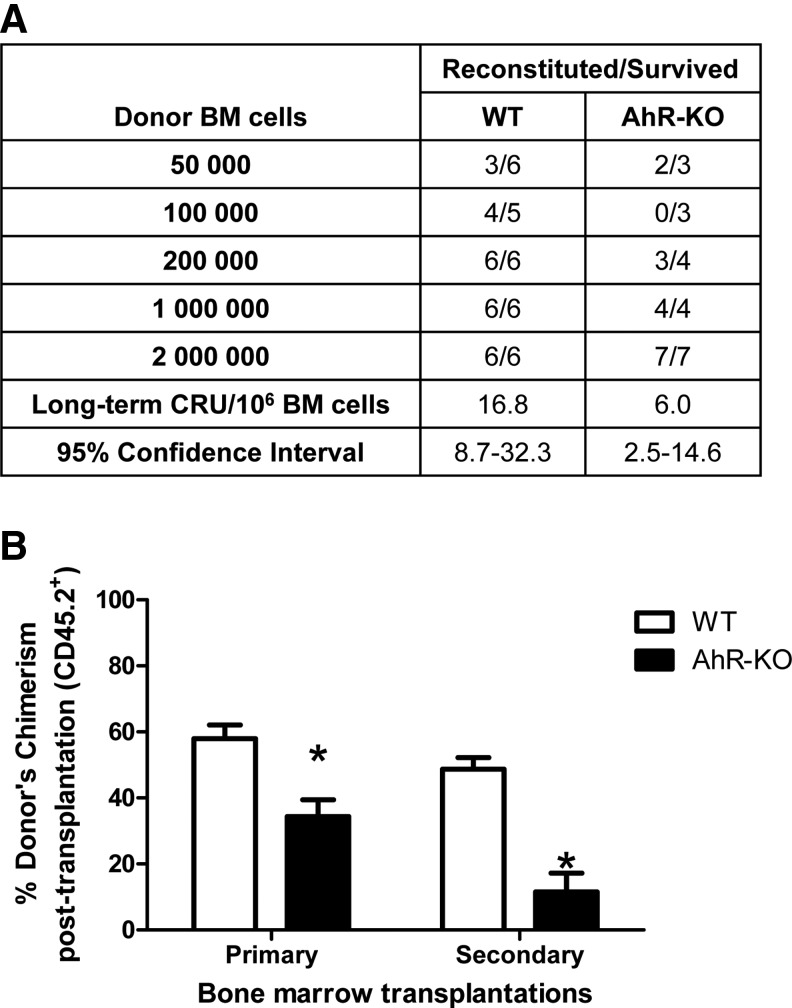
An increased pool of HSCs that is maintained throughout the lifetime of AhR-KO mice suggests that the peripheral accumulation of hematopoietic cells is largely a consequence of disruption and lack of regulation within the stem/progenitor cell compartment. This is consistent with our findings showing loss of quiescence and increased cycling of HSCs lacking AhR [17]. If this persists throughout the lifetime of these mice, as the data suggest, then we would expect BM, and particularly HSCs, to undergo early hematopoietic aging. To test this, we performed a limiting dilution competitive repopulation assay to determine the self-renewal capability of HSCs from 24-month old AhR-KO mice. The CRU frequency in the BM of AhR-KO mice was significantly decreased, indicating that HSCs from these animals have lower repopulation potential (Fig. 3A). To test this further, we performed serial transplants of HSCs. The serial repopulation ability of BM from AhR-KO mice was dramatically reduced in primary and secondary recipients (Fig. 3B). The tertiary recipient showed further reduced repopulation ability and only 2 mice transplanted with AhR-KO cells survived (data not shown).
FIG. 3.
Twenty-four month old AhR-KO mouse BM cells have decreased long-term competitive repopulation units (CRU) and competitive repopulation ability. (A) Five separate doses of donor CD45.2+ BM cells (2, 1, 0.5, 0.1, and 0.05×106) from WT or AhR-KO mice along with 2×105 BM cells from competitive donor mice (CD45.1+) were injected into irradiated CD45.1+ mice (8 per cell dose per group). Recipient BM cells were analyzed after 20 weeks. (B) Donor BM cells from 24-month old WT and AhR-KO mice were mixed with competitor (CD45.1+) cells at a ratio of 1:1 (0.5×106 cells each) and injected into irradiated CD45.1+ recipients (6 each for WT and KO). Subsequent serial transplant of BM cells harvested after 6 weeks of transplantation were performed as described in Materials and Methods. BM cells were analyzed for cells of donor (CD45.2+) and recipient origin (CD45.1+) at each stage of transplantation. Data are presented as mean±S.D. *Values significantly different from control (P<0.05).
LSK cells from aging AhR-KO mice have changes in intracellular levels of Ki67, ROS, p16Ink4a, and γ-H2A.X
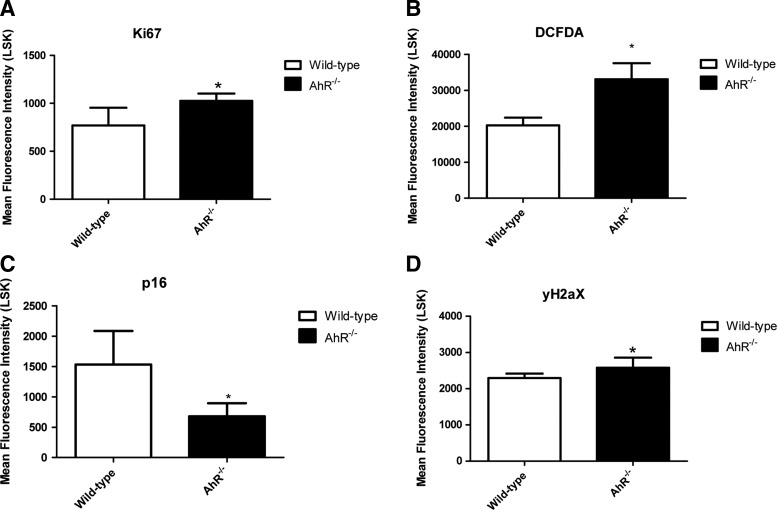
LSK cells from aging AhR-KO mice were found to have increased Ki67 staining, indicating increased proliferation (Fig. 4A). Consistent with what others have observed in BM cells from young adult AhR-KO mice [18], LSK cells from aging AhR-KO animals have significantly elevated ROS levels (Fig. 4B). Activation of the INK4a locus contributes to cell aging and exhaustion, and elevation of p16Ink4a has been suggested to be a marker of aging and senescent HSCs that may escape apoptosis and accumulate DNA damage [22]. In contrast to what we observed in younger KO mice treated with 5-FU (Supplementary Fig. S1), the p16Ink4a levels were significantly lower in LSK cells from aging AhR-KO mice (Fig. 4C). A small but significant increase in γ-H2A.X level was also observed in LSK cells of aging AhR-KO mice (Fig. 4D) indicating an increase in DNA damage.
FIG. 4.
Lin−/Sca-1+/c-kit+ (LSK) cells from aging KO mice have altered levels of reactive oxygen species (ROS) (DCFDA staining), Ki-67, p16Ink4a and γ-H2A.X. Lin- cells from 1.5 year old mice (n=5–8) were isolated and used for separate experiments for each endpoint as described in Material and Methods. Stained Lin- cells were gated for LSK cells as shown in Supplementary Fig. S6, and mean fluorescence intensity (MFI) profiles of individual antibody staining were generated (A–D). MFI data for LSK cells from separate groups were generated and presented as mean±S.D (A–D). *Values significantly different from control (P<0.05).
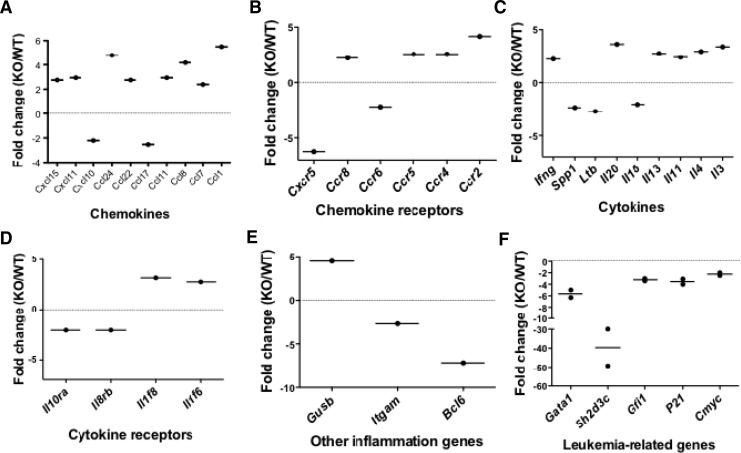
Splenocytes from aging AhR-KO mice have abnormal expression of several leukemia-associated genes, as well as those involved in development and trafficking
The above studies indicate that premature hematopoietic aging occurs in mice that are AhR deficient. These data along with the observed splenomegaly, increased Ki67 antibody staining, and that the splenomegaly can be transplanted into irradiated animals using HSCs/progenitors from KO mice [17], suggests that AhR deficiency promotes an uncontrolled growth and/or accumulation of cells in the hematopoietic compartment. RT-PCR arrays were used to analyze the mRNA expression profile in splenocytes from 24 month-old KO mice. These analyses showed a greater than two-fold increase in 21 genes and two-fold decrease in 11 genes associated with the development and trafficking (Fig. 5A–E). We also observed a significant downregulation of the leukemia-associated genes Gata-1, Sh2d3C, Gfi-1, p21, and c-Myc (Fig. 5F). We previously reported that young adult AhR-KO mice, also exhibiting splenomegaly, had an increase in spleen cells expressing B220(+) and Mac-1(+) [17]. However, the relative percentage of B220(+) and Mac-1(+) cells in spleen did not change dramatically between WT and AhR-KO mice. Nevertheless, we did not perform a phenotypic analysis of spleen cells in 2-year old AhR-KO mice, and it is possible that some changes in cellular populations/subpopulations, may be responsible, at least in part, for these gene differences.
FIG. 5.
Splenic cells from 24-month old AhR-KO mice have changes in gene expression. Quantitative real-time PCR Arrays were used for mRNA analyses of inflammatory chemokines (A), chemokine receptors (B), cytokine (C), cytokine receptors (D), and other inflammatory genes (E). Data were obtained from RNA pooled from three WT and KO spleens. Quantitative real-time PCR was used for analyses of mRNA expression of leukemia-associated genes (F). The dots represent data from one independent experiment using pooled mRNA from three WT and KO spleens. The averages of two independent experiments are represented as lines. Fold changes are expressed relative to WT mice, using GAPDH as endogenous control.
SLAM+ (LSK CD34- CD48- CD150+) BM cells from young AhR-KO mice have changes in global gene expression profiles
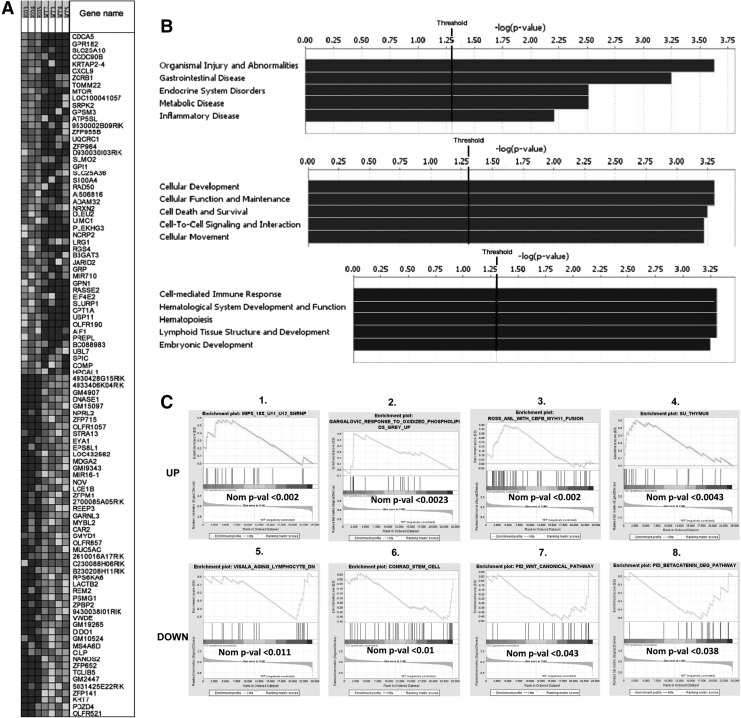
We sought to determine gene changes in HSCs lacking AhR that may promote premature exhaustion. The global gene expression profile of SLAM+ cells from young adult KO and WT mice were examined. Significant changes in a total of 673 genes were observed in AhR-KO HSCs compared to WT controls. The top 50 differential global gene changes are represented in a hierarchical clustering are shown in Fig. 6A. The detailed differential gene expression profiles of top 20 genes are presented in Supplementary Tables S2 and S3. HSCs from AhR-KO mice had changes in genes within Gene Ontology categories related to cellular development, function and maintenance, cell death and survival, cell to cell signaling and interactions, cellular movement, hematological system development and function, as well as lymphoid tissue development and hematopoiesis (Fig. 6B). There was a significant upregulation of genes associated with leukemia and abnormal proliferation (Srpk2, Mir170, Creb1), increased levels of y-H2A.X and proliferation and aging (Rad50), and aging-associated decline in functions of HSCs (mTOR). Significant downregulation of Stra13 was also observed. Overexpression of Stra13 has been shown to protect cells from oxidative stress, while downregulation increases sensitivity [23]. These gene changes support our findings of increased staining of Ki-67, DCFDA, and y-H2A.X in LSK cells of AhR-KO mice and myeloproliferative-like pathology. Changes in expression of Bcl6 and Ccl1 in spleen and mTor and Stra13 in SLAM+ BM cells were validated by RT-PCR (Supplementary Fig. S7). Using GSEA, the up- and downregulated genes in SLAM+ cells were compared with published gene sets representing different pathways and conditions. It is notable that gene sets showing significant enrichment (Fig. 6C) included those that are regulated by oxidative stress, acute myelogenous leukemia, aging and heat shock response, and the β-catenin/Wnt pathways. The latter are particularly interesting given the demonstrated importance of these signaling pathways in HSC regulation and aging. The detailed GSEA comparative reports are shown in Supplementary Tables S4–S11.
FIG. 6.
Global gene microarray of SLAM+ cells from AhR-KO mice showed significant changes in gene expression profiles in comparison to WT mice. (A) Heat map of gene expression of the top 50 gene changed genes in AhR-KO and WT mice. (B) Enrichment score for Gene Ontology categories in the set of differentially expressed genes indicate that AhR-KO mice have significant changes in genes regulating hematopoiesis, hematopoietic development and other cellular functions. (C) Genes up- and downregulated in sorted SLAM+ cells from AhR-KO versus WT and were further compared with published gene sets using gene set enrichment analysis (GSEA). Upregulated (top row) and downregulated (bottom row). The genes differentially regulated in Hematopoietic stem cells (HSCs) from AhR-KO mice were enriched for genes in the following published gene sets: genes in the human 18S U11/U12 snRNP not found in the U2-dependent spliceosome (Fig. C1), genes regulated by the exposure of cultured aortic endothelial cells to oxidized phospholipids (Fig. C2), genes representing an expression signature for acute myelogenous leukemia (Fig. C3), genes differentially regulated in human thymus compared to other human tissues (Fig. C4), those genes differentially changed in human peripheral lymphocytes by aging and heat shock response (Fig. C5), genes involved in the regulation of adult germline stem cells (Fig. C6), genes in the canonical Wnt pathway (Fig. C7), and genes in the canonical pathway for β-catenin degradation (Fig. C8) [See References in Supplementary Information section: Gene expression Analysis].
Discussion
The AhR is known for its ability to mediate the toxicity and carcinogenicity of a family of environmental contaminants, the most potent being 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Increased incidence of leukemia and lymphoma has been associated with exposure of humans to TCDD and related xenobiotics [8,9], and TCDD is classified as a human carcinogen. Tumors develop in diverse tissues after chronic exposure of rodents to TCDD [24], and the in utero exposure of mice to polycyclic aromatic hydrocarbons produces lymphoma in an AhR-dependent manner [25]. However, loss of AhR has also been associated with increased tumorigenesis [24], and several studies have suggested that AhR functions as a tumor suppressor gene [26]. Silencing of the Ahr gene by hypermethylation was reported in cases of human acute lymphoblastic leukemia [11].
The data reported here demonstrate that lack of AhR expression results in long-lasting effects in the hematopoietic compartment that are characterized by increased proliferation and accumulation of hematopoietic cells, and premature HSC aging. All of the observations, including hyperproliferation of HSCs [17], decreased CRU, decreased competitive ability of HSCs in serial transplants, anemia, abnormal tissue accumulation of hematopoietic cells, as well as decreased survival are consistent with this. Overall, these data, and others [11,14–18], implicate a critical role of the AhR in the regulation of HSCs, and more specifically the balance between quiescence and proliferation. It appears that lack of AhR expression and/or activity allows HSCs to escape from quiescence, lose control over signals that limit proliferation and differentiation, and this ultimately results in premature stem cell aging. As aging occurs, all of these events increase the risk for development of myeloproliferative disease and cancer. It has been shown that loss of quiescence can lead to HSC engraftment defects, HSC exhaustion, and eventually to the development of myeloproliferative disease and leukemia [27]. Since the increased proportion of actively cycling stem cells correlates with increased incidence of leukemia, lymphoma and myelodysplastic syndrome in old age [1,2], it is tempting to speculate that dysregulation of AhR may be one factor contributing to the development of these diseases in humans. A previous report indicated an earlier onset of neoplasms, particularly lymphomas, in AhR-KO mice [18]. Furthermore, the AhR is active in regulating the expansion of human HSCs [14].
Previous investigations indicate a genetic regulation of HSC exhaustion in mice, and a role of HSC exhaustion in the aging process [28]. Furthermore, there appears to be a negative correlation between the mean lifespan of various mouse strains and the replication rates of HSC [29]. Notably, a grouping of some mouse strains correlated with differences in longevity [30] also corresponds to different Ahr polymorphisms. For example, the C57BL/6 and 129Sv strains are characterized by low HSC cycling rates and long lifespan, and also possess a high affinity AhR protein (ie, high affinity for TCDD). Other strains, DBA/2 and AKR, which are characterized by high cycling rates and shorter lifespan, possess a low affinity AhR [29–31]. Here and elsewhere we show that lack of AhR expression in C57BL/6 mice results in increased and uncontrolled HSC cycling [17], shorter lifespan, and eventually the development of premature HSC aging. It remains to be determined whether the relationships among Ahr genotype, HSC cycling, lifespan, and development of hematopoietic disease are causal.
Previous studies showing that HSCs/progenitors from AhR-KO mice proliferate more rapidly ex vivo compared to cells from control littermates [17] are consistent with altered characteristics being dependent on lack of AhR presence in these cells and not on the niche environment in the global KO mouse. However, it is unclear whether these HSC characteristics are due to inherent changes in HSC signaling pathways and/or programmed changes in cellular characteristics and/or sensitivity to signals in the HSC AhR-deficient environment that are also responsible for the maintenance of stem cell function. For example, altered TGF-β and retinoic acid levels have been reported in AhR-KO mice, and a role of these factors has been established in normal and malignant hematopoiesis [32,33]. Additional studies with targeted AhR deletions should help to address the relative contributions of altered signaling pathways in HSCs and the BM niche. Nevertheless, our data clearly demonstrate a molecular phenotype in hematopoietic cells from aging AhR-KO mice that is conducive to premature exhaustion and development of hematopoietic disease. The mRNA analyses of splenic cells revealed modified expression of many genes, for example, Ccl1, IL-8rb/Cxcr2, and Bcl6, that affect the development, proliferation and trafficking of these cells. Besides being a potent chemoattractant for monocytes and lymphocytes and playing a major role in inflammatory diseases, Ccl1 is an antiapoptotic factor supporting the survival of human lymphoma cells [34]. IL-8rb is known to be involved in lymphocyte homing and trafficking, and IL-8rb-KO mice develop splenomegaly [35]. Bcl6 is a proto-oncoprotein that can bind and repress several oncogenes, including the antiapoptotic genes Bcl2, Mcl1, and Bcl2a1 [36]. This, in part, along with the increased expression of Ccl1, may contribute to the observed increased accumulation and survival of hematopoietic cells. Importantly, we also observed the altered expression of several genes (Gfi-1, S2d3c, Gata-1, c-myc, and p21) whose modified expression is associated with altered HSC self-renewal and differentiation, HSC exhaustion, as well as leukemia and/or predisposition to leukemia [37–39].
HSCs from aging AhR-KO mice demonstrated molecular changes associated with premature exhaustion. A marked increase in DCFDA staining in progenitors/HSCs from aged KO mice (Fig. 4) indicates a loss of control of ROS levels. Delicate control of ROS is crucial for HSC maintenance, and prolonged elevation of ROS is associated with DNA damage, loss of quiescence, alterations in cell cycle, aging, and the development of a myeloproliferative disorder [40], all of which we observed in AhR-KO animals. An unexpected finding was the decreased expression of p16Ink4a in KO HSCs. The expression of this protein in most cells is increased with age and this is thought to confer protection against growth defects and cancer by inducing senescence in response to the lifetime accumulation of stressors, such as DNA damage and ROS [22]. However, there is a reported decreased expression with age in human acute myeloid leukemia and the suggestion that the suppression of defense mechanisms that protect older cells against cellular damage may facilitate oncogenesis [41]. As such, our finding of a somewhat “paradoxical” decrease in p16Ink4a expression in HSCs from AhR-KO mice is actually consistent with the phenotype we observed, as well as the reported early and increased incidence of neoplasms in these animals [18].
Additional analyses indicated that AhR-KO mice develop genomic changes at an early age that likely lead or contribute to the phenotypic changes observed in aging AhR-KO mice. Microarray global gene analysis of SLAM+ cells of young adult AhR-KO mice showed significant alterations in many genes, that is, Srpk2, Rad50, mTOR, Creb1, Hes1, Pdp-1, and Stra13, related to various aspects of HSC maintenance. The most prominent gene changes (overexpression of Srpk2, Creb1, Hes1) have been associated with HSC hyperproliferation and leukemia [42–44]. mTOR is a critical transcription factor that coordinates responses to a variety of signals, such as insulin-receptor activation or nutrient, amino acid, and oxygen availability. Upregulation of mTOR is known to accelerate the aging process and increases susceptibility to aging associated diseases [45]. Pdp-1 is a negative modulator of the insulin/IGF-1 signaling pathway and in coordination with TGFβ regulates longevity, development and metabolism [46]. Stra13 has been shown to protect cells from oxidative damage [23]. Notably, we observed a 3.5-fold downregulation of Stra13 in HSCs from aged AhR-KO animals consistent with the finding of enhanced ROS accumulation in LSK cells. We also observed a significant increase in Rad50 mRNA, the product of which is involved in double-strand break repair [47]. The combination of substantially increased ROS and also increased Rad50 expression may have contributed to the relatively small, but significant, increase in γ-H2A.X in LSK cells from KO mice.
All of the above data are consistent with the interpretation that a lifetime of altered signaling pathways in HSCs lacking AhR results in functional changes that affect the proliferation, cell death, and/or accumulation of hematopoietic cells ultimately leading to the premature exhaustion, and a myeloproliferative disorder.
More work is clearly needed to define these relationships, as well as dissect the signaling pathways directly regulated by the AhR that determine HSC characteristics and function. Notably, of the 65 genes significantly changed in young AhR-KO mice (with a fold-change in expression of >1.5 and a P-value<0.01), 31% (21/65) (including Plekhg3, Creb1, and Stra13) possess putative AhR response elements. A very recent study has been done to analyze regulators of HSC-specific gene expression and the proximal promoters of 322 HSC-enriched genes. Four motifs were identified as putative binding sites for transcription factors (EGR1, SOX4, AhR and STAT1) that are important in the control of genes playing a critical role in HSCs [48]. This study supports our hypothesis that the AhR plays a very important role in HSC functions. Ultimately, however, the cellular and molecular changes that we describe here and elsewhere [20] suggest a complex crosstalk between the AhR and pathways associated with HSC quiescence and proliferation. The persistence of changes induced by AhR dysregulation may elicit other degenerative modifications, for example, epigenetic and/or genetic, in hematopoietic cell regulatory pathways (eg, β-catenin/Wnt; see Fig. 6) that alter their ability to sense their microenvironment, alter trafficking behavior, and promote hematological disease. As such, the AhR appears to be a key cofactor in the regulation of HSCs and their protection from long-term hematopoietic stress, premature aging, and age-related disease.
Supplementary Material
Acknowledgments
The authors thank Dr. Ellen Henry for critical reading of the manuscript. This work was supported by NIH Center Grant ES01247, Training Grant ES07026, and Grants ES04862 and ES016606.
Author Disclosure Statement
The authors have declared that no conflict of interest exists.
References
- 1.Dorshkind K. and Swain S. (2009). Age-associated declines in the immune system development and function: causes, consequences, and treatment. Curr Opin Immunol 21:404–407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sudo K, Ema H, Morita Y. and Nakauchi H. (2000). Age-related characteristics of murine hematopoietic stem cells. J Exp Med 192:1273–1280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yahata Y, Takanashi T, Muguruma Y, Ibrahim AA, Matsuzawa H, Uno T, Sheng Y, Onizuka M, Ito M, Kato S. and Ando K. (2011). Accumulation of oxidative DNA damage restricts the self-renewal capacity of human hematopoietic stem cells. Blood 118:2941–2950 [DOI] [PubMed] [Google Scholar]
- 4.Rossi DJ, Bryder D, Seita J, Nussenzweig A, Hoeijmakers J. and Weissman IL. (2007). Deficiencies in DNA damage repair limit the function of hematopoietic stem cells with age. Nature 447:725–729 [DOI] [PubMed] [Google Scholar]
- 5.Li J. (2011). Quiescence regulators for hematopoietic stem cell. Exp Hematol 39:511–520 [DOI] [PubMed] [Google Scholar]
- 6.Morrison SJ, Prowse KR, HO P. and Weissman IL. (1996). Telomerase activity in hematopoietic cells is associated with self-renewal potential. Immunity 5:207–216 [DOI] [PubMed] [Google Scholar]
- 7.Esser C, Rannug A. and Stockinger B. (2009). The aryl hydrocarbon receptor in immunity. Trends Immunol 30:447–454 [DOI] [PubMed] [Google Scholar]
- 8.Bertazzi PA, Consonni D, Bachetti S, Rubagotti M, Baccarelli A, Zocchetti C. and Pesatori AC. (2001). Health effects of dioxin exposure: a 20-years mortality study. Am J Epidemiol 153:1031–1044 [DOI] [PubMed] [Google Scholar]
- 9.Viel JF, Arveux P, Baverel J. and Cahn JY. (2000). Soft-tissue sarcoma and non-Hodgkin's lymphoma clusters around a municipal solid waste incinerator with high dioxin emission levels. Am J Epidemiol 152:13–19 [DOI] [PubMed] [Google Scholar]
- 10.Hayashibara T, Yamada Y, Mori N, Harasawa H, Sugahara K, Miyanishi T, Kamihira S. and Tomonaga M. (2003). Possible involvement of aryl hydrocarbon receptor (AhR) in adult T-cell leukemia (ALT) leukemogenesia: constitutive activation of AhR in ALT. Biochem Biophys Res Commun 300:128–134 [DOI] [PubMed] [Google Scholar]
- 11.Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Rupero S, Esteller M. and Fernandez-Salguero PM. (2006). The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis 27:1099–1104 [DOI] [PubMed] [Google Scholar]
- 12.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M. and Weiner HL. (2008). Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature 453:65–71 [DOI] [PubMed] [Google Scholar]
- 13.Veldhoen M, Herata K, Westendorf AM, Buer J, Dumoutier L, Renauld JC. and Stockinger B. (2008). The aryl hydrocarbon receptor links TH17-cell-mediated autoimmunity to environmental toxins. Nature 453:106–109 [DOI] [PubMed] [Google Scholar]
- 14.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, et al. (2010). Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietc stem cells. Science 329:1345–1348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sakai R, Kajiume T, Inoue H, Kanno R, Miyazaki M, Ninomiya Y. and Kanno M. (2003). TCDD treatment eliminates the long-term reconstitution activity of hematopoietic stem cells. Toxicol Sci 72:84–91 [DOI] [PubMed] [Google Scholar]
- 16.Singh KP, Wyman A, Casado FL, Garrett RW. and Gasiewicz TA. (2009). Treatment of mice with the Ah receptor agonist and human carcinogen dioxin results in altered numbers and function of hematopoietic stem cells. Carcinogenesis 30:11–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singh KP, Garrett RW, Casado FL. and Gasiewicz TA. (2011). Aryl hydrocarbon receptor-null allele mice have hematopoietic stem/progenitor cells with abnormal characteristics and functions. Stem Cells Dev 20:769–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hirabayashi Y. and Inoue T. (2009). Aryl hydrocarbon receptor biology and xenobiotic responses in hematopoietic progenitor cells. Biochem Pharmacol 77:521–535 [DOI] [PubMed] [Google Scholar]
- 19.Schmidt JV, Su GH, Reddy JK, Simon MC. and Bradfield CA. (1996). Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A 93:6731–6736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Casado FL, Singh KP. and Gasiewicz TA. (2011). Aryl hydrocarbon receptor activation in hematopoietic stem and progenitor cells alters cell function and pathway-specific gene modulation reflecting changes in cellular trafficking and migration. Mol Pharmacol 80:673–682 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Purton LE. and Scadden DT. (2007). Limiting factors in murine hematopoietic stem cell assays. Stem Cell 1:263–270 [DOI] [PubMed] [Google Scholar]
- 22.Singer RAJ, Montecino-Rodrigues E, Witte ON. and Dorshkind K. (2008). Aging and cancer resistance in lymphoid progenitors are linked process conferred by p16Ink4a and Arf. Gene Dev 22:3115–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vercherat C, Chung TK, Yalcin S, Gulbagci N, Gopinadhan S, Ghaffari S. and Taneja R. (2009). Stra13 regulate oxidative stress mediated skeletal muscle degeneration. Human Mol Gen 18:4304–4316 [DOI] [PubMed] [Google Scholar]
- 24.Kawajiri K, Kobayashi Y, Ohtake F, Ikuta T, Matsushima Y, Mimura J, Pettersson S, Pollenz RS, Sakaki T, et al. (2009). Aryl hydrocarbon receptor suppresses intestinal carcinogenesis in ApcMin/+ mice with natural ligands. Proc Natl Acad Sci U S A 106:13481–13486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu Z, Loehr CV, Fischer KA, Louderback AM, Krueger SK, Dashwood RH, Kerkvliet NI, Pereira CB, Jennings-Gee JE, et al. (2006). In utero exposure of mice to dibenzo[a,l]pyrene produces lymphoma in the offspring: role of the aryl hydrocarbon receptor. Cancer Res 66:755–761 [DOI] [PubMed] [Google Scholar]
- 26.Fan Y, Boivin GP, Knudsen ES, Nebert DW, Xia Y. and Puga A. (2010). The aryl hydrocarbon receptor functions as a tumor suppressor of liver carcinogenesis. Cancer Res 70:212–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jude CD, Gaudet JJ, Speck NA. and Ernst P. (2008). Leukemia and hematopoietic stem cells balancing proliferation and quiescence. Cell Cycle 7:586–591 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan R, Astle CM, Chen J. and Harrison DE. (2005). Genetic regulation of hematopoietic stem cell exhaustion during development and growth. Exp Hematol 33:243–250 [DOI] [PubMed] [Google Scholar]
- 29.de Haan G, Nijhof W. and Van Zant G. (1997). Mouse strain-dependent changes in frequency and proliferation of hematopoietic stem cells during aging: correlation between lifespan and cycling activity. Blood 89:1543–1550 [PubMed] [Google Scholar]
- 30.Spindler SR, Koizumi A, Walford RL. and Mote PL. (1989). P1–450 and P3–450 gene expression and maximum life span in mice. Mut Res 219:89–94 [DOI] [PubMed] [Google Scholar]
- 31.Nebert DW, Brown DD, Towne DW. and Eisen HJ. (1984). Association of fertility, fitness and longevity with the murine Ah locus among (C57BL/6N) (C3H/HeN) recombinant inbred strain. Biol Reprod 30:363–373 [DOI] [PubMed] [Google Scholar]
- 32.Andreola F, Fernandez-Salguero PM, Chiantore MV, Petkovich MP, Gonzalez FJ. and De Luca LM. (1997). Aryl hydrocarbon receptor knockout mice (AHR-/-) exhibit liver retinoid accumulation and reduced retinoic acid metabolism. Cancer Res 57:2835–2838 [PubMed] [Google Scholar]
- 33.Roman AC, Carvajal-Gonzalez JM, Rico-Leo EM. and Fernandez-Salguero PM. (2009). Dioxin receptor deficiency impairs angiogenesis by a mechanism involving VEGF-A depletion in the endothelium and transforming growth factor-beta overexpression in the stroma. J Biol Chem 284:25135–25148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ruckes T, Saul D, Van Snick J, Hermine O. and Grassmann R. (2001). Autocrine antiapoptotic stimulation of cultured adult T-cell leukemia cells by overexpression of the chemokine I-309. Blood 98:1150–1159 [DOI] [PubMed] [Google Scholar]
- 35.Flaishon L, Hart G, Zelman E, Moussion C, Grabovsky V, Lapidot Tal G, Feigelson S, Margalit R, Harmelin A, et al. (2008). Anti-inflammatory effects of an inflammatory chemokine: CCL2 inhibits lymphocyte homing by modulation of CCL21-triggered integrin-mediated adhesions. Blood 112:5016–5025 [DOI] [PubMed] [Google Scholar]
- 36.Ci W, Polo JM, Cerchietti L, Shanknovich R, Wang L, Yang SN, Ye K, Farinha P, Horsman DE, et al. (2009). The BCL6 transcriptional program features repression of multiple oncogenes in primary B cells and is deregulated in DLBCL. Blood 113:5536–5548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sauvageau M, Miller M, Lemieux S, Lessard J, Herbert J. and Sauvageau G. (2008). Quantitative expression profiling guided by common retroviral insertion sites reveals novel and cell type specific cancer genes in leukemia. Blood 111:790–799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shimizu R, Kuroha T, Ohneda O, Pan X, Ohneda K, Takahashi S, Philipsen S. and Yamamoto M. (2004). Leukemogenesis caused by incapacitated GATA-1 function. Mol Cell Biol 24:10814–10825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Khandanpour C, Kosan C, Gaudreau MC, Duhrsen U, Hebert J, Zeng H. and Moroy T. (2011). Growth factor Independence 1 (Gfi1) protects hematopoietic stem cells against apoptosis but also prevents the development of a myeloproliferative-like disease. Stem Cells 29:376–385 [DOI] [PubMed] [Google Scholar]
- 40.Ergen AV. and Goodell MA. (2010). Mechanisms of hematopoietic stem cell aging. Exp Gerontol 45:286–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.de Jonge HJM, Woolthuis CM, de Bont ESJM. and Huls G. (2009). Paradoxical down-regulation of p16Ink4a mRNA with advancing age in acute myeloid leukemia. Aging 1:949–953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jang SW, Yang SJ, Ehlen A, Dong S, Khoury H, Chen J, Persson JL. and Ye K. (2008). Serine/Arginine protein-specific kinase 2 promotes leukemia cell proliferation by phosphorylating acinus and regulating cyclin A1. Cancer Res 68:4559–4570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudley DD, Wang HC. and Sun XH. (2009). Hes1 potentiates T cell lymphomagenesis by up-regulating a subset of notch target genes. PLoS One 4:e6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cheng JC, Kinjo K, Judelson DJ, Chang J, Wu WS, Schmid I, Shankar DB, Kasahara N, Stripecke R, et al. (2008). CREB is a critical regulator of normal hematopoiesis and leukemogenesis. Blood 111:1182–1192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johnson SC, Ravinovitch PS. and Kaeberlein M. (2013). mTOR is a key modulator of ageing and age-related disease. Nature 493:338–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Narasimhan SD, Yen K, Bansal A, Kwon ES, Padmanabhan S. and Tissenbaum HA. (2011). PDP1 link the TGF-β and IIS pathways to regulate longevity, development, and metabolism. PLoS Genet 7:e1001377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morales M, Liu Y, Laiakis EC, Morgan WF, Nimer SD. and Petrini JH. (2008). DNA damage signaling in hematopoietic cells: a role for Mre11 complex repair of topoisomerase lesions. Cancer Res 68:2186–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gazit R, Garrison BS, Rao TN, Shay T, Costello J, Ericson J, Kim F, Collins JJ, Regev A, Wagner AJ. and Rossi DJ. (2013). Transcriptome analysis identifies regulators of hematopoietic stem and progenitor cells. Stem Cell Rep 1:266–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.