Abstract
Background: Serum thyrotropin (TSH) concentration and thyroid autoimmunity may be of prognostic importance in differentiated thyroid cancer (DTC). Preoperative serum TSH level has been associated with higher DTC stage in cross-sectional studies; data are contradictory on the significance of thyroid autoimmunity at the time of diagnosis.
Objective: We sought to assess whether preoperative serum TSH and perioperative antithyroglobulin antibodies (TgAb) were associated with thyroid cancer stage and outcome in DTC patients followed by the National Thyroid Cancer Treatment Cooperative Study, a large multicenter thyroid cancer registry.
Methods: Patients registered after 1996 with available preoperative serum TSH (n=617; the TSH cohort) or perioperative TgAb status (n=1770; the TgAb cohort) were analyzed for tumor stage, persistent disease, recurrence, and overall survival (OS; median follow-up, 5.5 years). Parametric tests assessed log-transformed TSH, and categorical variables were tested with chi square. Disease-free survival (DFS) and OS was assessed with Cox models.
Results: Geometric mean serum TSH levels were higher in patients with higher-stage disease (Stage III/IV=1.48 vs. 1.02 mU/L for Stages I/II; p=0.006). The relationship persisted in those aged ≥45 years after adjusting for sex (p=0.01). Gross extrathyroidal extension (p=0.03) and presence of cervical lymph node metastases (p=0.003) were also significantly associated with higher serum TSH. Disease recurrence and all-cause mortality occurred in 37 and 38 TSH cohort patients respectively, which limited the power for survival analysis. Positive TgAb was associated with lower stage on univariate analysis (positive TgAb in 23.4% vs. 17.8% of Stage I/II vs. III/IV patients, respectively; p=0.01), although the relationship lost significance when adjusting for age and sex (p=0.34). Perioperative TgAb was not an independent predictor of DFS (hazard ratio=1.12 [95% confidence interval=0.74–1.69]) or OS (hazard ratio=0.98 [95% confidence interval=0.56–1.72]).
Conclusions: Preoperative serum TSH level is associated with higher DTC stage, gross extrathyroidal extension, and neck node metastases. Perioperative TgAb is not an independent predictor of DTC prognosis. A larger cohort is required to assess whether preoperative serum TSH level predicts recurrence or mortality.
Introduction
Although most patients diagnosed with differentiated thyroid cancer (DTC) have a good prognosis, a significant proportion of patients have persistent or recurrent disease, and a minority will die from thyroid cancer. Accurate risk stratification is needed to determine which patients will benefit from aggressive therapy and monitoring, but current systems fail to account for a significant proportion of patients' adverse disease outcomes (1,2). Therefore, novel baseline prognostic factors that could be added to risk stratification algorithms are required.
Two potential prognostic factors are serum thyrotropin (TSH) and thyroid autoimmunity. TSH is the major growth factor and regulator of the thyroid. Serum TSH concentration is directly associated with the risk of cancer in a thyroid nodule (3,4), and TSH suppression improves prognosis of high-risk DTC patients (5–7). However, it is less clear whether TSH is associated with stage of disease and other prognostic surrogates (3). Antithyroglobulin antibody (TgAb) and antithyroid peroxidase antibody (TPOAb) are serologic markers of autoimmune thyroid disease. Elevated TgAb is present in approximately 25% of thyroid cancer patients, and persistent or reemerging TgAb can signal recurrent disease (8). Whether thyroid autoimmunity also plays a role in DTC pathogenesis or whether autoimmunity is actually protective (9,10) remains uncertain.
In order to assess the prognostic significance of laboratory measures of serum TSH concentration and TgAb status at the time of patients' thyroid cancer diagnosis, we analyzed prospective data from The National Thyroid Cancer Treatment Cooperative Study (NTCTCS), a large nonrandomized thyroid cancer registry.
Methods
Registry protocol and data collection
The data collection and analytical methods of the NTCTCS have been described elsewhere (6,11–16). Briefly, 11 North American centers are current members, with registration beginning in January 1987 and continuing through to 2011. New patients were registered within 3 months of their initial surgery. Institutional review boards (IRB) of contributing centers approved the study, and ongoing oversight of the project occurs through the University of Texas M.D. Anderson Cancer Center Institutional Review Board, where the central database is currently maintained. As of the beginning of 2011, 4808 patients have been included in the database, representing 31,876 person-years of follow-up.
Management of patients was nonrandomized and solely at the discretion of their treating physicians on the basis of perceived best practice and clinical need, independent of registry participation. Prespecified baseline demographic, clinical, histologic, and radiologic data were entered into a PC-based clinical data management system locally (Medlog, v2000-2, Incline Village, NV) and transmitted to the central registry database. Clinical status, investigations, and treatments were updated on a yearly basis.
Presurgical serum TSH was measured using a second- or third-generation assay and the result recorded in mU/L. TgAb were classified as either positive or negative, based on institutional reference ranges. Histologic subtype was abstracted from pathology reports. Co-existing benign thyroid diagnoses, preoperative levothyroxine use, preoperative thyroid scans, TPOAb status, and TSH receptor antibody status were not recorded. Disease stage was classified using a unique registry staging system (Table 1) (12). Conversion to the latest American Joint Committee on Cancer (7th edition) stage (17) is not possible because tumor size was recorded categorically and central versus lateral cervical node metastases were not differentiated in data collection. All thyroid cancer-related treatment was recorded. Individual investigators assessed and recorded the presence of baseline residual disease and recurrence. Where possible, the causes of death were reviewed and mortality data confirmed through the Social Security Death Index.
Table 1.
NTCTCS Staging Classification
| |
Papillary carcinoma |
Follicular carcinoma |
||
|---|---|---|---|---|
| Age <45 | Age ≥45 | Age <45 | Age ≥45 | |
| Primary tumor size | ||||
| <1 cm |
I |
I |
I |
II |
| 1–4 cm |
I |
II |
I |
III |
| >4 cm |
II |
III |
II |
III |
| Primary tumor description | ||||
| Microscopic multifocal |
I |
II |
I |
III |
| Macroscopic multifocal or macroscopic tumor capsule invasion |
I |
II |
II |
III |
| Microscopic extraglandular invasion |
I |
II |
I |
III |
| Macroscopic extraglandular invasion |
II |
III |
II |
III |
| Poor differentiation |
n/a |
n/a |
III |
III |
| Metastases | ||||
| Cervical lymph node metastases |
I |
III |
I |
III |
| Extracervical metastases | III | IV | III | IV |
The final staging classification is the highest of the individual Primary tumor size, Primary tumor description, and Metastases scores.
NTCTCS, The National Thyroid Cancer Treatment Cooperative Study.
Eligibility criteria
We assessed patients with DTC (papillary, follicular, or Hürthle cell carcinomas) who had available presurgical serum TSH and/or perioperative TgAb (TgAb available within 3 months of diagnosis). These data were not available for patients enrolled prior to 1996.
Statistical analysis
For the cross-sectional analysis, thyroid cancer stage (high-risk=Stages III/IV vs. low-risk=Stages I/II) was analyzed with respect to serum TSH and TgAb status. Serum TSH was assessed as both a continuous (assessing geometric mean, tested with t-tests for crude analyses and via regression models stratified by age and adjusted by sex) and a categorical variable (tested with Cochrane–Armitage test for trend). Cut points for the four TSH categories were determined as follows: cut points for low serum TSH and high serum TSH were prespecified at 0.4 mU/L and 4.5 mU/L respectively; patients with serum TSH within this range were divided at the median serum TSH, which was 1.5 mU/L. Analyses were also performed comparing TSH levels in relation to tumor size, invasion, and metastases (both nodal and distant). We used logistic regression to assess the association between TgAb (present or absent), adjusted for age (≥45 vs. <45 years, and ≥55 vs. <55 years) and sex.
Survival analysis was performed via log-rank tests and Cox proportional hazards models for outcomes of disease-free survival (DFS) and overall survival (OS). Serum TSH concentration was assessed both continuously and categorically. Where event numbers were sufficient, age, sex, and ethnicity were included in the models as potential confounders. The proportional hazards assumption in Cox models was checked by assessment for time interactions.
Results
Eligible subjects and comparison with full NTCTCS cohort
Of 3318 patients registered in the NTCTCS since 1996, serum TSH was measured preoperatively in 617 patients, and TgAb was measured perioperatively (preoperatively or within 3 months of surgery) in 1701 patients (Table 2). Both the TSH and TgAb cohorts had a median follow-up time of 5.5 years. There were slightly higher proportions of females and Caucasians among the group with available TSH information (Table 2). Those with TgAb information had a slightly higher prevalence of previous radiation exposure (Table 2). Otherwise, there were no differences between patients with available TSH/TgAb information and those without. Among 182 patients with available pre- and postoperative (within 3 months) TgAb status, there was a 93% concordance of these results, with the 12 discordant positive results divided equally between pre- and postoperative periods.
Table 2.
Comparison of Thyrotropin and Antithyroglobulin Antibodies Cohorts with the Overall NTCTCS Population
| Parameter | Overall differentiated thyroid cancer cohort since 1996 | Preoperative TSH cohort | Perioperative TgAb cohort |
|---|---|---|---|
| Number of patients (n) |
3308 |
617 |
1701 |
| Percent female (%) |
72.2 |
76.3a |
72.7 |
| Age, years (mean±SD) |
45.6±15.3 |
46.6±15.3 |
46.0±15.4 |
| Histology (%) | |||
| Papillary |
89.0 |
90.4 |
88.7 |
| Follicular |
6.8 |
7.6 |
7.8 |
| Hürthle cell |
4.2 |
2.0 |
3.5 |
| Race/ethnicity (%) | |||
| Caucasian |
83.6 |
74.8b |
83.2 |
| Asian |
5.1 |
7.6 |
5.4 |
| Hispanic |
4.9 |
11.0 |
6.2 |
| Black |
4.4 |
5.3 |
4.4 |
| Other |
2.0 |
1.5 |
0.8 |
| History of prior radiation exposure (%) |
3.6 |
4.9c |
4.3d |
| NTCTCS Stage (%) | |||
| I |
44.1 |
45.7 |
42.7 |
| II |
27.8 |
24.4 |
28.2 |
| III |
24.0 |
25.2 |
25.3 |
| IV |
4.1 |
4.7 |
3.8 |
| Outcomes (n) | |||
| Disease present at entry |
385 |
82 |
211 |
| Dated recurrence |
298 |
37 |
169 |
| Death |
178 |
38 |
84 |
| Disease-related death | 58 | 13 | 30 |
Comparisons were made between those with TSH/TgAb values available, and those with missing data. The statistical differences were found to be significant (ap=0.01, bp=0.01) or borderline significant (cp=0.053) compared to patients with missing TSH values, and significant (dp=0.03) compared to patients with missing TgAb status.
TSH, thyrotropin; TgAb, antithyroglobulin antibodies.
Serum TSH concentration
Cross-sectional analysis
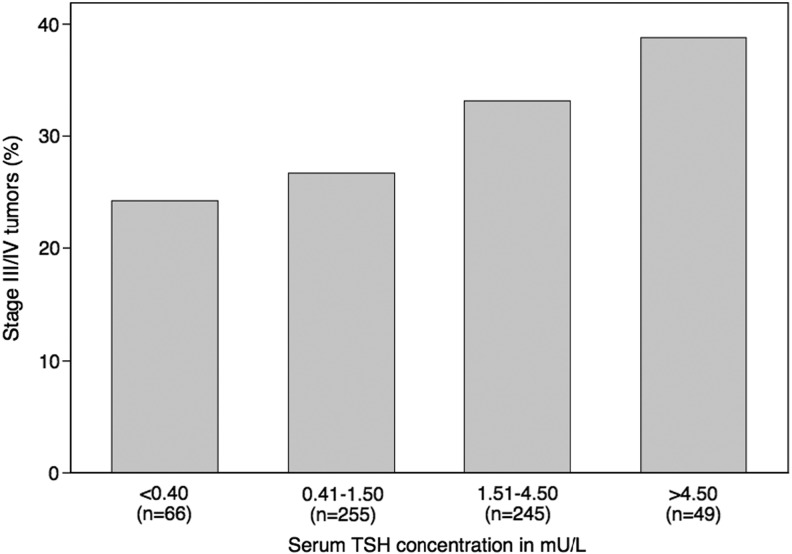
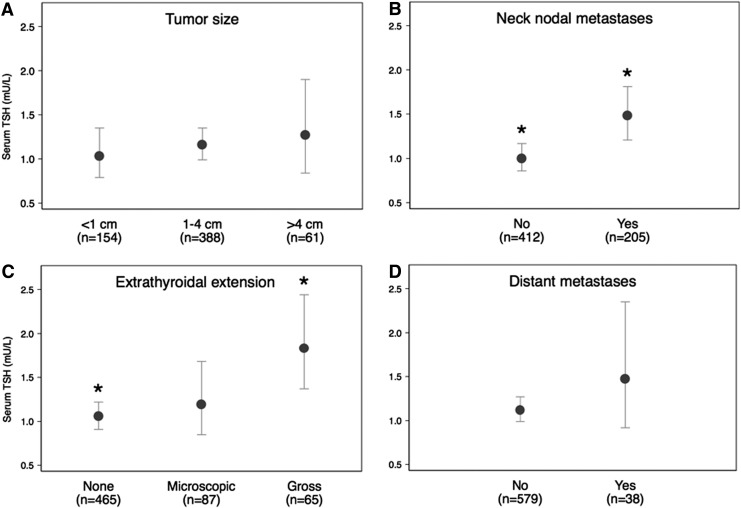
The geometric mean for serum TSH was higher in patients with high-risk versus low-risk disease (1.48 mU/L vs. 1.02 mU/L; p=0.006). When considered categorically, higher serum TSH concentration was associated with high-risk disease (Fig. 1; ptrend=0.03). The relationship persisted in those aged ≥45 years after adjusting for sex (Table 3). Only 16 patients aged <45 years were classified as having high-risk disease. The difference in geometric mean for serum TSH in this group was similar between high- and low-risk patients to those aged ≥45 years, but this analysis did not reach statistical significance, likely because of low numbers (Table 3). The results did not change when using an alternate age cut point of 55 years (not shown). Serum TSH level was also significantly associated with the presence of gross extrathyroidal extension (p=0.03) and cervical nodal metastases (p=0.003), but neither tumor size (p=0.63) nor the presence of distant metastases (p=0.31; Fig. 2).
FIG. 1.
Proportion of patients with Stage III/IV differentiated thyroid cancer according to serum thyrotropin (TSH) category; ptrend=0.03. Total n for this analysis is 615 because two patients aged ≥45 with otherwise Stage I/II tumors had missing tumor size data.
Table 3.
Relationship of Thyroid Stage to Serum Thyrotropin, Adjusting for Age and Sex
| Stage | Geometric mean serum TSH (mU/L) [CI] | p |
|---|---|---|
| Age <45 years | ||
| I/II (n=275) |
1.12 [0.90, 1.39] |
|
| III (n=16) |
1.67 [0.79, 3.54] |
0.29 |
| Age ≥45 years | ||
| I/II (n=156) |
0.95 [0.71, 1.28] |
|
| III/IV (n=168) | 1.52 [1.18, 1.95] | 0.01 |
Under the NTCTCS staging system, patients <45 years of age with distant metastases are classified as having Stage III disease. CI, 95% confidence interval.
FIG. 2.
Relationship of prognostic markers of differentiated thyroid cancer (DTC) to serum TSH concentration. Prognostic markers assessed were tumor size (A), neck nodal metastases (B), extrathyroidal extension (C), and distant metastases (D). The center circles represent geometric means for serum TSH, and whiskers represent confidence intervals. *Statistically significant differences between groups (Bonferroni-corrected for extrathyroidal extension).
Longitudinal outcomes analysis
Among those with TSH information, 38 patients died from all causes. Among the 499 patients without residual disease at baseline, 37 patients had timed recurrences recorded. Using categorical serum TSH and assessing with the log-rank test, no relationship was seen with DFS (p=0.93) or OS (p=0.48). Adjusted analyses could not be performed due to low event numbers. Continuous serum TSH analyses did not satisfy modeling assumptions and thus results are not reported.
Serum TgAb status
Cross-sectional analysis
On crude analysis, positive TgAb was found in 17.8% of Stage III/IV patients versus 23.4% of Stage I/II patients (odds ratio=0.71 [95% confidence interval=0.54–0.93]). However, when age and sex were included in the models, the association was attenuated and was no longer statistically significance (odds ratio=0.85 [95% confidence interval=0.60–1.20]). Altering the age cut point did not alter the findings (not shown). TgAb status was not significantly associated with serum TSH concentration (geometric mean of 1.27 mU/L in TgAb positive patients vs. 1.00 mU/L in TgAb negative patients; n=469; p=0.20).
Longitudinal outcomes analysis
Among patients with TgAb information, 84 died of all causes. Recurrence occurred in 151 patients out of 1149 patients recorded as being free of disease at baseline. TgAb status was not associated with DFS or OS in either univariate or adjusted analyses (Table 4). Altering the age cut point for the analysis again did not alter the results (not shown).
Table 4.
Survival Analysis for Antithyroglobulin Antibody Status
| |
DFS |
OS |
||
|---|---|---|---|---|
| TgAb status | Crude HR [CI] | Adjusted HR [CI] | Crude HR [CI] | Adjusted HR [CI] |
| Negative |
1 |
1 |
1 |
1 |
| Positive | 1.11 [0.76, 1.61] | 1.12 [0.74, 1.69] | 0.70 [0.40, 1.28] | 0.98 [0.56, 1.72] |
HR, hazard ratio; DFS, disease-free survival; OS, overall survival.
Discussion
In this population of prospectively studied thyroid cancer patients, we have found that a higher serum TSH concentration at diagnosis is associated with higher thyroid cancer stage. Proportionally, patients with gross extrathyroidal extension and lymph node metastasis had higher serum TSH concentrations. Assessment of long-term outcomes associated with at-diagnosis serum TSH concentration is not possible due to the low number of events at this follow-up.
Our analysis confirms and extends the majority of data showing that higher serum TSH concentrations at the time of diagnosis are associated with higher thyroid cancer stage (3,18–22), versus a minority of studies that show no relationship (23,24), utilizing the largest data set assessed thus far in the literature. It is also in accord with clinical observations that suppressing serum TSH in high-risk patients improves prognosis (5–7). Extrathyroidal extension (20,21) and lymph node metastases (19,21) have been reported to be associated with serum TSH concentration in some studies, consistent with our analysis. We found that higher serum TSH is associated with gross extrathyroidal extension, indicating more aggressive behavior. As in other studies (20,21), serum TSH concentration and tumor size were not associated in our data. This latter observation makes the possibility of reverse causation less likely, that is, that tumors with extrathyroidal extension replace so much normal thyroid tissue that they cause an elevated serum TSH. The best cross-sectional evidence that serum TSH is associated with tumors of high biologic aggressiveness would be a finding that tumors with distant metastasis have higher serum TSH. While our data are suggestive of this (Fig. 2D), there are too few patients with metastatic disease in the TSH cohort to demonstrate a statistically significant association.
It is biologically plausible that higher TSH concentration could lead to more aggressive DTC by influencing signaling pathways implicated in adverse thyroid cancer prognosis. Recent mouse model data suggest that TSH signaling predisposes thyrocytes to Braf-induced transformation (25). In humans, BRAF mutation-positive papillary thyroid cancers are, in turn, associated with extrathyroidal extension, lymph-node metastasis, and higher-stage disease (26–28). TSH receptor activation also influences other cellular pathways (29), some of which are important in progressive follicular and anaplastic carcinomas. A recent study demonstrating the importance of Akt activation in tumor progression, invasion, and distant metastasis used a mouse model (PV mice) which features markedly elevated serum TSH levels (30). However, it is unclear if less extreme elevations in serum TSH concentrations would significantly influence the Akt pathway to produce more aggressive disease.
The implications of our study that higher serum TSH is associated with worse thyroid cancer prognosis may have clinical utility. Our findings suggest that those with elevated TSH at diagnosis may benefit from heightened surveillance. Furthermore, this information may enhance prognostic assessment at initial diagnosis; and guide aggressiveness of initial therapy as it relates to lymph node dissection. Current risk stratification systems fail to explain a significant proportion of the variation in risk of adverse outcomes (1,2), and thus additional prognostic markers are needed. For these potential applications to become reality, longitudinal outcome data need to show not only that serum TSH concentration is associated with thyroid cancer outcome, but also that serum TSH provides incremental prognostic information independent of current staging systems and established prognostic factors. Future NTCTCS analyses may be able to answer these questions.
Our analysis found no prognostic significance of baseline TgAb status once the effects of age and sex were considered. Whether or not autoimmune thyroiditis is associated with thyroid cancer prognosis is controversial (9,10). Several studies have reported improved prognosis associated with either diffuse thyroiditis (31,32) or clinical/antibody markers of autoimmunity (33), as distinguished from intratumoral lymphocyte infiltration. However, only one of these studies included a multivariate analysis (31). Other studies have not confirmed a protective effect from autoimmune thyroiditis after accounting for potential confounders (34,35). Our data suggest that perioperative TgAb status (as a marker of thyroid autoimmunity) is not an independent predictor of thyroid cancer stage or outcome. The univariate relationship observed between TgAb status and thyroid cancer stage was explained by the higher frequency of TgAb positivity in younger females, who collectively had earlier-stage disease. Our findings underscore the importance of assessing potential confounders in the analysis of autoimmunity in the prognosis of thyroid cancer.
Baseline TgAb status may be a nonspecific proxy of thyroid autoimmunity, and this could limit the value of speculating on the mechanistic implications of our results. Because TgAb assessment occurred perioperatively, it could represent a mixed population of patients with pre-existing autoimmunity and those manifesting an immune response to tumor. Two well-performed studies using multivariate analyses have suggested that intratumoral lymphocytic infiltration is associated with better prognosis (36,37). It is possible that pre-existing diffuse thyroid autoimmunity, as opposed to a specific immune response to tumor cells, could have different prognostic implications. Our data do not include information about lymphocytic infiltration on histology or TPOAb status because these variables were not included in registry data collection, nor does it permit knowledge of whether patients had pre-existing thyroid autoimmunity. However, the fact that TgAb status was not associated with serum TSH concentration in our study suggests either that a significant proportion of the TgAb positive patients did not have long-standing autoimmune thyroiditis, or that many were well treated with levothyroxine.
Several other potential limitations in our analysis need to be considered. While the current registry datasets for baseline TSH concentration and TgAb status are large compared to the literature, the TSH data set in particular had few recurrences and deaths (both all-cause and disease-specific mortality). Three factors likely contributed to this: (i) the small baseline sample size; (ii) the good prognosis of many thyroid cancer patients; and (iii) the relatively short median 5.5 years of follow-up. Future registry analyses may overcome these limitations. Data for serum TSH and TgAb status were also absent in a substantial proportion of patients, although those with available data appeared reasonably representative of the wider NTCTCS cohort. Thus, while selection bias is possible, there is no signal for this in the data. It is also possible that unmeasured confounding may have influenced our results. However, our analyses adjusted for major potential confounders, and other than prior treatment with levothyroxine, we are unaware of any obvious potential confounders that should have been included in our models. As discussed previously, interpretation of the TgAb data should be limited to the setting of TgAb positivity, rather than thyroid autoimmunity per se. Our data set also lacks information on whether thyroid autonomy or Graves' disease was the cause of patients' preoperative subnormal TSH levels. However, given that the proportion of high-risk patients increased through each higher serum TSH category, this missing information is unlikely to alter our interpretation of the data.
In conclusion, this study adds weight to the hypothesis that higher serum TSH concentration is associated with more aggressive DTC. Definitive evidence for an association with long-term outcome may be forthcoming with future registry analyses. TgAb status was not an independent predictor of thyroid cancer stage or outcome. Because it remains possible that a specific immune response to tumor could be associated with a better prognosis, future studies should assess prognosis of thyroid cancers in patients with pre-existing autoimmune thyroid disease.
Acknowledgments
The NTCTCSG is supported in part by research grants from Genzyme Corporation and Pfizer, and by the University of Texas M.D. Anderson Cancer Center Support Grant (NCI Grant P30 CA016672). A Royal Australasian College of Physicians IMS Traveling Fellowship and a Cancer Council Queensland PhD scholarship supported D.S.A.M. We, as principal investigators at each NTCTCSG institution, thank the physicians and staff members who participated in the management and follow-up of these patients. We acknowledge the substantial contributions of the institutional research staff that collected and submitted the data, including Shehnaz Bana, Marge E. Ewertz, RN, and Beverly McLaughlin, RMA. We also appreciate the considerable assistance provided by Jeffrey Cui for the management of NTCTCSG's databases. Finally, we acknowledge the efforts of the numerous physicians and scientists whose contributions were critical in the early years of the registry.
Author Disclosure Statement
B.R.H. receives lecture fees from Genzyme Corp. J.M. is an employee of Genzyme Corp. D.S.R. has consulted for Genzyme Corp. and Novo Nordisk. D.L.S. receives research funding from Veracyte. S.I.S. consults for Bayer, Exelixis, Eisai, Pfizer, Eli Lilly, Novo Nordisk, Roche, and AstraZeneca, and is a member of the advisory board of Veracyte. D.S.A.M., D.S.C., P.W.L., K.B.A., J.D.B., H.G.F., J.J., M.C.S., and H.R.M. have no competing financial interests to declare.
References
- 1.Brierley JD, Panzarella T, Tsang RW, Gospodarowicz MK, O'Sullivan B.1997A comparison of different staging systems predictability of patient outcome. Thyroid carcinoma as an example. Cancer 79:2414–2423 [PubMed] [Google Scholar]
- 2.Lang BH, Chow SM, Lo CY, Law SC, Lam KY.2007Staging systems for papillary thyroid carcinoma: a study of 2 tertiary referral centers. Ann Surg 246:114–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLeod DS, Watters KF, Carpenter AD, Ladenson PW, Cooper DS, Ding EL.2012Thyrotropin and thyroid cancer diagnosis: a systematic review and dose-response meta-analysis. J Clin Endocrinol Metab 97:2682–2692 [DOI] [PubMed] [Google Scholar]
- 4.Fiore E, Vitti P.2012Serum TSH and risk of papillary thyroid cancer in nodular thyroid disease. J Clin Endocrinol Metab 97:1134–1145 [DOI] [PubMed] [Google Scholar]
- 5.Pujol P, Daures JP, Nsakala N, Baldet L, Bringer J, Jaffiol C.1996Degree of thyrotropin suppression as a prognostic determinant in differentiated thyroid cancer. J Clin Endocrinol Metab 81:4318–4323 [DOI] [PubMed] [Google Scholar]
- 6.Jonklaas J, Sarlis NJ, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis M, Maxon HR, Sherman SI.2006Outcomes of patients with differentiated thyroid carcinoma following initial therapy. Thyroid 16:1229–1242 [DOI] [PubMed] [Google Scholar]
- 7.Hovens GC, Stokkel MP, Kievit J, Corssmit EP, Pereira AM, Romijn JA, Smit JW.2007Associations of serum thyrotropin concentrations with recurrence and death in differentiated thyroid cancer. J Clin Endocrinol Metab 92:2610–2615 [DOI] [PubMed] [Google Scholar]
- 8.Spencer CA, Takeuchi M, Kazarosyan M, Wang CC, Guttler RB, Singer PA, Fatemi S, LoPresti JS, Nicoloff JT.1998Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 83:1121–1127 [DOI] [PubMed] [Google Scholar]
- 9.Feldt-Rasmussen U, Rasmussen AK.2010Autoimmunity in differentiated thyroid cancer: significance and related clinical problems. Hormones (Athens) 9:109–117 [DOI] [PubMed] [Google Scholar]
- 10.Cunha LL, Ferreira RC, Marcello MA, Vassallo J, Ward LS.2011Clinical and pathologic implications of concurrent autoimmune thyroid disorders and papillary thyroid cancer. J Thyroid Res 2011:387062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cooper DS, Specker B, Ho M, Sperling M, Ladenson PW, Ross DS, Ain KB, Bigos ST, Brierley JD, Haugen BR, Klein I, Robbins J, Sherman SI, Taylor T, Maxon HR., 3rd1998Thyrotropin suppression and disease progression in patients with differentiated thyroid cancer: results from the National Thyroid Cancer Treatment Cooperative Registry. Thyroid 8:737–744 [DOI] [PubMed] [Google Scholar]
- 12.Sherman SI, Brierley JD, Sperling M, Ain KB, Bigos ST, Cooper DS, Haugen BR, Ho M, Klein I, Ladenson PW, Robbins J, Ross DS, Specker B, Taylor T, Maxon HR., 3rd1998Prospective multicenter study of thyroid carcinoma treatment: initial analysis of staging and outcome. National Thyroid Cancer Treatment Cooperative Study Registry Group. Cancer 83:1012–1021 [DOI] [PubMed] [Google Scholar]
- 13.Taylor T, Specker B, Robbins J, Sperling M, Ho M, Ain K, Bigos ST, Brierley J, Cooper D, Haugen B, Hay I, Hertzberg V, Klein I, Klein H, Ladenson P, Nishiyama R, Ross D, Sherman S, Maxon HR.1998Outcome after treatment of high-risk papillary and non-Hurthle-cell follicular thyroid carcinoma. Ann Intern Med 129:622–627 [DOI] [PubMed] [Google Scholar]
- 14.Ross DS, Litofsky D, Ain KB, Bigos T, Brierley JD, Cooper DS, Haugen BR, Jonklaas J, Ladenson PW, Magner J, Robbins J, Skarulis MC, Steward DL, Maxon HR, Sherman SI.2009Recurrence after treatment of micropapillary thyroid cancer. Thyroid 19:1043–1048 [DOI] [PubMed] [Google Scholar]
- 15.Jonklaas J, Cooper DS, Ain KB, Bigos T, Brierley JD, Haugen BR, Ladenson PW, Magner J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI.2010Radioiodine therapy in patients with stage I differentiated thyroid cancer. Thyroid 20:1423–1424 [DOI] [PubMed] [Google Scholar]
- 16.Jonklaas J, Nogueras-Gonzalez G, Munsell M, Litofsky D, Ain KB, Bigos ST, Brierley JD, Cooper DS, Haugen BR, Ladenson PW, Magner J, Robbins J, Ross DS, Skarulis MC, Steward DL, Maxon HR, Sherman SI.2012The impact of age and gender on papillary thyroid cancer survival. J Clin Endocrinol Metab 97:E878–E887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Joint Committee on Cancer 2010Thyroid In: Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. (eds) AJCC Cancer Staging Manual. Seventh edition. Springer, New York, pp 87–96 [Google Scholar]
- 18.Haymart MR, Repplinger DJ, Leverson GE, Elson DF, Sippel RS, Jaume JC, Chen H.2008Higher serum thyroid stimulating hormone level in thyroid nodule patients is associated with greater risks of differentiated thyroid cancer and advanced tumor stage. J Clin Endocrinol Metab 93:809–814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiore E, Rago T, Provenzale MA, Scutari M, Ugolini C, Basolo F, Di Coscio G, Berti P, Grasso L, Elisei R, Pinchera A, Vitti P.2009Lower levels of TSH are associated with a lower risk of papillary thyroid cancer in patients with thyroid nodular disease: thyroid autonomy may play a protective role. Endocr Relat Cancer 16:1251–1260 [DOI] [PubMed] [Google Scholar]
- 20.Haymart MR, Glinberg SL, Liu J, Sippel RS, Jaume JC, Chen H.2009Higher serum TSH in thyroid cancer patients occurs independent of age and correlates with extrathyroidal extension. Clin Endocrinol (Oxf) 71:434–439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim SS, Lee BJ, Lee JC, Song SH, Kim BH, Son SM, Kim IJ, Kim YK, Kang YH.2011Preoperative serum thyroid stimulating hormone levels in well-differentiated thyroid carcinoma is a predictive factor for lateral lymph node metastasis as well as extrathyroidal extension in Korean patients: a single-center experience. Endocrine 39:259–265 [DOI] [PubMed] [Google Scholar]
- 22.Jung E, Shon H.2011Association between serum TSH level and papillary thyroid microcarcinoma in Korean euthyroid patients. Thyroid 21:A93.(Abstract). [Google Scholar]
- 23.Kim ES, Lim DJ, Baek KH, Lee JM, Kim MK, Kwon HS, Song KH, Kang MI, Cha BY, Lee KW, Son HY.2010Thyroglobulin antibody is associated with increased cancer risk in thyroid nodules. Thyroid 20:885–891 [DOI] [PubMed] [Google Scholar]
- 24.Ahn D, Sohn JH, Kim JH, Shin CM, Jeon JH, Park JY.2013Preoperative subclinical hypothyroidism in patients with papillary thyroid carcinoma. Am J Otolaryngol 34:312–219 [DOI] [PubMed] [Google Scholar]
- 25.Franco AT, Malaguarnera R, Refetoff S, Liao XH, Lundsmith E, Kimura S, Pritchard C, Marais R, Davies TF, Weinstein LS, Chen M, Rosen N, Ghossein R, Knauf JA, Fagin JA.2011Thyrotrophin receptor signaling dependence of Braf-induced thyroid tumor initiation in mice. Proc Natl Acad Sci USA 108:1615–1620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nikiforova MN, Kimura ET, Gandhi M, Biddinger PW, Knauf JA, Basolo F, Zhu Z, Giannini R, Salvatore G, Fusco A, Santoro M, Fagin JA, Nikiforov YE.2003BRAF mutations in thyroid tumors are restricted to papillary carcinomas and anaplastic or poorly differentiated carcinomas arising from papillary carcinomas. J Clin Endocrinol Metab 88:5399–5404 [DOI] [PubMed] [Google Scholar]
- 27.Basolo F, Torregrossa L, Giannini R, Miccoli M, Lupi C, Sensi E, Berti P, Elisei R, Vitti P, Baggiani A, Miccoli P.2010Correlation between the BRAF V600E mutation and tumor invasiveness in papillary thyroid carcinomas smaller than 20 millimeters: analysis of 1060 cases. J Clin Endocrinol Metab 95:4197–4205 [DOI] [PubMed] [Google Scholar]
- 28.Xing M, Westra WH, Tufano RP, Cohen Y, Rosenbaum E, Rhoden KJ, Carson KA, Vasko V, Larin A, Tallini G, Tolaney S, Holt EH, Hui P, Umbricht CB, Basaria S, Ewertz M, Tufaro AP, Califano JA, Ringel MD, Zeiger MA, Sidransky D, Ladenson PW.2005BRAF mutation predicts a poorer clinical prognosis for papillary thyroid cancer. J Clin Endocrinol Metab 90:6373–6379 [DOI] [PubMed] [Google Scholar]
- 29.Morshed SA, Latif R, Davies TF.2009Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology 150:519–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saji M, Narahara K, McCarty SK, Vasko VV, La Perle KM, Porter K, Jarjoura D, Lu C, Cheng SY, Ringel MD.2011Akt1 deficiency delays tumor progression, vascular invasion, and distant metastasis in a murine model of thyroid cancer. Oncogene 30:4307–4315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kashima K, Yokoyama S, Noguchi S, Murakami N, Yamashita H, Watanabe S, Uchino S, Toda M, Sasaki A, Daa T, Nakayama I.1998Chronic thyroiditis as a favorable prognostic factor in papillary thyroid carcinoma. Thyroid 8:197–202 [DOI] [PubMed] [Google Scholar]
- 32.Huang BY, Hseuh C, Chao TC, Lin KJ, Lin JD.2011Well-differentiated thyroid carcinoma with concomitant Hashimoto's thyroiditis present with less aggressive clinical stage and low recurrence. Endocr Pathol 22:144–149 [DOI] [PubMed] [Google Scholar]
- 33.Souza SL, Montalli Da Assumpção LV, Ward LS.2003Impact of previous thyroid autoimmune diseases on prognosis of patients with well-differentiated thyroid cancer. Thyroid 13:491–495 [DOI] [PubMed] [Google Scholar]
- 34.Kebebew E, Treseler PA, Ituarte PH, Clark OH.2001Coexisting chronic lymphocytic thyroiditis and papillary thyroid cancer revisited. World J Surg 25:632–637 [DOI] [PubMed] [Google Scholar]
- 35.Kim EY, Kim WG, Kim WB, Kim TY, Kim JM, Ryu JS, Hong SJ, Gong G, Shong YK.2009Coexistence of chronic lymphocytic thyroiditis is associated with lower recurrence rates in patients with papillary thyroid carcinoma. Clin Endocrinol (Oxf) 71:581–586 [DOI] [PubMed] [Google Scholar]
- 36.Matsubayashi S, Kawai K, Matsumoto Y, Mukuta T, Morita T, Hirai K, Matsuzuka F, Kakudoh K, Kuma K, Tamai H.1995The correlation between papillary thyroid carcinoma and lymphocytic infiltration in the thyroid gland. J Clin Endocrinol Metab 80:3421–3424 [DOI] [PubMed] [Google Scholar]
- 37.Loh KC, Greenspan FS, Dong F, Miller TR, Yeo PP.1999Influence of lymphocytic thyroiditis on the prognostic outcome of patients with papillary thyroid carcinoma. J Clin Endocrinol Metab 84:458–463 [DOI] [PubMed] [Google Scholar]