Abstract
Aims: Glutathione peroxidase (GPX) mimic ebselen and superoxide dismutase (SOD) mimic copper diisopropylsalicylate (CuDIPs) were used to rescue impaired glucose-stimulated insulin secretion (GSIS) in islets of GPX1 and(or) SOD1-knockout mice. Results: Ebselen improved GSIS in islets of all four tested genotypes. The rescue in the GPX1 knockout resulted from a coordinated transcriptional regulation of four key GSIS regulators and was mediated by the peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α)-mediated signaling pathways. In contrast, CuDIPs improved GSIS only in the SOD1 knockout and suppressed gene expression of the PGC-1α pathway. Innovation: Islets from the GPX1 and(or) SOD1 knockout mice provided metabolically controlled intracellular hydrogen peroxide (H2O2) and superoxide conditions for the present study to avoid confounding effects. Bioinformatics analyses of gene promoters and expression profiles guided the search for upstream signaling pathways to link the ebselen-initiated H2O2 scavenging to downstream key events of GSIS. The RNA interference was applied to prove PGC-1α as the main mediator for that link. Conclusion: Our study revealed a novel metabolic use and clinical potential of ebselen in rescuing GSIS in the GPX1-deficient islets and mice, along with distinct differences between the GPX and SOD mimics in this regard. These findings highlight the necessities and opportunities of discretional applications of various antioxidant enzyme mimics in treating insulin secretion disorders. Rebound Track: This work was rejected during standard peer review and rescued by Rebound Peer Review (Antioxid Redox Signal 16: 293–296, 2012) with the following serving as open reviewers: Regina Brigelius-Flohe, Vadim Gladyshev, Dexing Hou, and Holger Steinbrenner. Antioxid. Redox Signal. 20, 191–203.
Introduction
Type 2 diabetes is one of the most prevalent chronic diseases worldwide. While insulin resistance was regarded as a hallmark of this disease, defective insulin secretion has recently been recognized as the main culprit (2). Being a “privilege” of aerobic organisms, oxidative stress is implicated in glucose-stimulated insulin secretion (GSIS) of pancreatic islet β-cells (50). Thus, the relatively low expression of antioxidant enzymes in islets (39) may not only render them susceptible to oxidative insults, but also provide a necessary metabolic condition for their sensitive responses to reactive oxygen species (ROS)-mediated signaling in GSIS (49). In fact, hydrogen peroxide (H2O2) functions as an essential second messenger (19) in initiating and regulating GSIS (49, 50), and it demonstrates concentration-dependent dual effects on insulin signaling and other metabolic processes (29, 51).
Innovation.
Knockout of antioxidant enzymes glutathione peroxidase 1 (GPX1) and superoxide dismutase 1 (SOD1) alone or together impaired glucose-stimulated insulin secretion (GSIS), but the molecular mechanism and signaling pathway remain unclear. Using islets isolated from the GPX1 and (or) SOD1 knockout mice, we have demonstrated that the GPX mimic ebselen rescued impaired GSIS in the GPX1 knockout islets and mice via regulating glucokinase, glucose transporter type 2, pancreatic and duodenal homeobox 1, and uncoupling protein 2 by activating peroxisome proliferator-activated receptor gamma coactivator 1 alpha-mediated antioxidant response element/glucocorticoid receptor signaling pathways. In contrast, the SOD mimic copper diisopropylsalicylates showed different roles and mechanisms in regulating GSIS. Our results revealed a novel metabolic effect of ebselen in promoting GSIS, and provided a new strategy to treat disorders related to insulin secretion.
Se-dependent glutathione peroxidase-1 (GPX1) and Cu,Zn-superoxide dismutase (SOD1) represent two major intracellular antioxidant enzymes that can modulate intracellular H2O2 status. Despite low GPX1 and SOD1 activities in islets (only 2% and 29% of that in liver, respectively) (39), we have found that knockout of GPX1 (GPX1 knockout [GKO]) and SOD1 (SOD1 knockout [SKO]) alone or in combination (double knockout of GPX1 and SOD1 [dKO]) caused substantial impairment of GSIS (68). Consistently, a Gpx1 gene variant (C198T) lowering the enzyme activity was identified in the South Indian population, which resulted in increased incidences of type 2 diabetes (C/T, 1.4-fold and T/T, 1.8-fold) (54). Intriguingly, the overt phenotypes of GSIS impairments in the GKO and SKO mice were similar (68), although GPX1 catalyzes H2O2 breakdown whereas SOD1 catalyzes H2O2 formation. It is puzzling that the presumed opposite effects of these two knockouts on islet intracellular H2O2 production might have induced seemingly similar biochemical and signaling regulation of GSIS. The biochemical regulation of GSIS depends on four key proteins: glucose transporter type 2 (GLUT2), glucokinase (GK), pancreatic and duodenal homeobox 1 (PDX1), and uncoupling protein 2 (UCP2) (30). However, it remains unclear whether the impacts of GKO and SKO on GSIS were mediated by altering functional expressions of these proteins in a coordinated fashion. Although we previously observed changes of PDX1 and UCP2 (68) in the whole pancreas of these knockout mice, systematic responses of GK, GLUT2, PDX1, and UCP2 in their islets have not been studied. More importantly, there is no information on the signaling cascade and molecular mechanism to link these knockout—initiated islet intracellular ROS changes to the responses of these four proteins and the observed GSIS phenotypes.
The gene promoter regions of GK, GLUT2, PDX1, and UCP2 may share common domains that bind transcriptional factors involved in signaling pathways related to the question described earlier (45). The first is the peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC-1α)-mediated antioxidant response element (ARE) signaling pathway. While PGC-1α is involved in responses of various genes to redox regulation (62) and regulates GSIS in human islets (40), two of its gene variants are associated with increased risks of type 2 diabetes in the Indian population (6, 73). The second is the glucocorticoid receptor (GR) pathway that is negatively regulated by ROS or H2O2 in inflammation and immune responses (31). Polymorphisms of gr are associated with type 2 diabetes with low insulin levels and attenuated first phase of GSIS in women (65). The third is the wingless-type MMTV integration site (Wnt) pathway that is positively regulated by ROS or H2O2 (70). Elevated oxidation status by selenium (Se) deficiency (33) or GPX3 knockout (4) activated the Wnt pathway in mice. This pathway activated gk gene transcription and insulin secretion in isolated islets of C57BL/6 mice (56), and has at least four variants of TCF7L2 that are associated with increased risks of type 2 diabetes (63). The fourth is the nuclear factor of activated T-cells (NFAT) pathway that stimulates gk, glut2, and pdx1 expression and insulin secretion in β-cell (24). This pathway in mouse C141 cells is activated by superoxide, but inhibited by H2O2 (32). Since many members in these four signaling pathways are ROS responsive and involved in GSIS, and can bind to domains in the gene promoter regions of GLUT2, GK, PDX1, and UCP2, it is fascinating for us to explore whether GKO, SKO, and dKO initiated their impacts on GSIS via these pathways.
The GPX mimic ebselen has been shown to protect against oxidative injuries (15), suppress (14) the diazinon-induced hyperglycemia in rats, and decrease islet UCP2 (69). The SOD mimic copper diisopropylsalicylate (CuDIPs) attenuated the streptozotocin (STZ)-induced diabetes in rats (20) and restored the suppressed foxa2 expression in the SKO islets (68). As an essential micronutrient, Se is a component of 25 human selenoproteins (36) that are involved in antioxidant defense (10, 11) and regulation of β-cells (61). Either overexpression or deficiency of selenoproteins disturbed glucose homeostasis in mice (37). It has been reported that Se functioned as an insulin mimetic in isolated adipocytes (17) and STZ-induced diabetic rodents (5). Despite all these well-documented features, little is known on the roles and mechanisms of ebselen, CuDIPs, and Se in regulating GSIS. Due to the specific inactivation of the target GPX1 and(or) SOD1, islets isolated from the GKO, SKO, and dKO mice are ideal experimental models for such studies. Therefore, our objectives were (i) to determine whether ebselen, CuDIPs, and sodium selenite could rescue the impaired GSIS in GKO, SKO, and dKO islets and(or) mice; (ii) to reveal whether these yet-illustrated GSIS rescues were mediated by regulating islet GK, GLUT2, PDX1, and UCP2 expressions and functions; and (iii) to elucidate whether the ultimate control of this regulation was initiated by the mimics-restored ROS-responsive signaling via the ARE, GR, Wnt, and NFAT pathways.
Rebound Track.
This work was rejected during standard peer review and rescued by Rebound Peer Review (Antioxid Redox Signal 16: 293–296, 2012) with the following serving as open reviewers: Regina Brigelius-Flohe, Vadim Gladyshev, Dexing Hou, and Holger Steinbrenner. The comments by these reviewers supporting the rescue are listed below:
Regina Breigelius-Flohe (flohe@dife.de): I am a qualified reviewer (per Antioxid Redox Signal 16:293-296) and move to rescue this manuscript that was rejected during the regular peer review process after reviewing all versions of the manuscript and detailed reviewer comments.
The present study is the logical follow-up of a previous one (Wang et al., Antioxid Redox Signal 14:391–401, 2011) in which the impairment of glucose homeostasis by knockdown of GPX1 (GKO) or/and SOD1 (SKO and dKO) was shown. The effect was particularly high in islets of SKO mice. The focus in the presently paper is laid on the capability of ebselen to rescue the effects of GPX1 deletion on glucose-stimulated insulin secretion (GSIS) and the expression of enzymes crucial for GSIS as underlying mechanisms. Ebselen up-regulated GK, GLUT2 and PDX1 and down-regulated UCP2 in islets of GKO mice at mRNA and protein level by lowering ‘ROS’ levels. Genomics and bioinformatic analyses revealed that the regulation was mediated by PGC-1α in ARE and GR pathways. PGC-1α siRNA experiments confirmed this finding. Thus, pathways regulating insulin secretion (and insulin signaling) are responsive to redox regulation. Also in GKO mice, the SOD mimic CuDIPs exerted effects on gene expression opposite to those of ebselen. This shows that GPX and SOD, two enzymes considered as ‘antioxidant’, differently affect redox-sensitive pathways depending on which ‘ROS’ is produced or eliminated, respectively. This is of great importance not only for insulin signaling as here described, but also and more importantly for understanding redox regulation in general. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
Holger Steinbrenner (Holger.Steinbrenner@uni-duesseldorf.de): I am a qualified reviewer (per Antioxid Redox Signal 16:293-296) and move to rescue this manuscript that was rejected during the regular peer review process after reviewing all versions of the manuscript and detailed reviewer comments. The authors investigated the capability of GPX and SOD mimics to stimulate glucose-induced insulin secretion (GSIS) in islets of wild-type mice and three different transgenic models (GPX1, SOD1 and double GPX1/SOD1 knock-out mice). This paper is very timely, as it contributes to an ongoing discussion on the role of reactive oxygen species and the interference of antioxidants in GSIS. The present paper continues recently published work of the group of Xingen Lei but its major findings are novel and innovative. It demonstrates convincingly that the GPX mimic ebselen stimulates GSIS in islets of wild-type mice and may improve the impaired GSIS in islets from GKO and SKO mice. In contrast, the SOD mimic CuDIPs was effective to rescue GSIS only in islets from SKO mice. The study includes a strong mechanistic part, as the authors elucidated the ebselen- and CuDIPs-mediated regulation of β-cell proteins involved in GSIS as well as the underlying signaling pathways. The results emphasize the need to better understand the effects of antioxidants targeting different ROS (hydrogen peroxide, superoxide) at different cellular sites in β-cells. Moreover, a clinical perspective is highlighted to use ebselen (or possibly other selenoorganic compounds with GPX activity but better solubility in water) for treatment of type 2 diabetes mellitus. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
De-Xing Hou (k8469751@kadai.jp): I am a qualified reviewer (per Antioxid Redox Signal 16:293-296) and move to rescue this manuscript that was rejected during the regular peer review process after reviewing all versions of the manuscript and detailed reviewer comments.
Authors used multiple transgenic mouse models, including GPX1 knockout (GKO), double knockout of GPX1 and SOD1 (dKO), SOD1 knockout (SKO), to investigate the role of ebselen and copper diisopropylsalicylate (CuDIPs) on insulin secretion in murine islets. In general, this is an interesting and well-designed study although there are some weaknesses in signaling analysis. Specifically, the data revealed that ebselen could rescue impaired GSIS in the GPX1 knockout islets and mice via regulating GK, GLUT2, PDX1, and UCP2. These results will shed new light on the metabolic role and therapeutic potential of ebselen in this field. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
Vadim Gladyshev (vgladyshev@rics.bwh.harvard.edu): I am a qualified reviewer (per Antioxid Redox Signal 16:293-296) and move to rescue this manuscript that was rejected during the regular peer review process after reviewing all versions of the manuscript and detailed reviewer comments. The manuscript by Wang et al. describes a novel finding that ebselen, a selenium-containing compound with significant peroxidase activity, can rescue insulin secretion from the islets deficient in glutathione peroxidase 1. In the opinion of this reviewer, the data for this main finding are convincing, and they add to the potential use of ebselen under conditions of glutathione peroxidase deficiency (and possibly selenium deficiency, too). The paper also strengthens the previous findings regarding the role of GPX1 status in insulin secretion. Another strength is the identification of the PCG-1α pathway as the pathway affected by ebselen. Therefore, in the interest of science, I take full responsibility to rescue this work from rejection.
Results
Effects of ebselen, CuDIPs, and Se on GSIS of islets
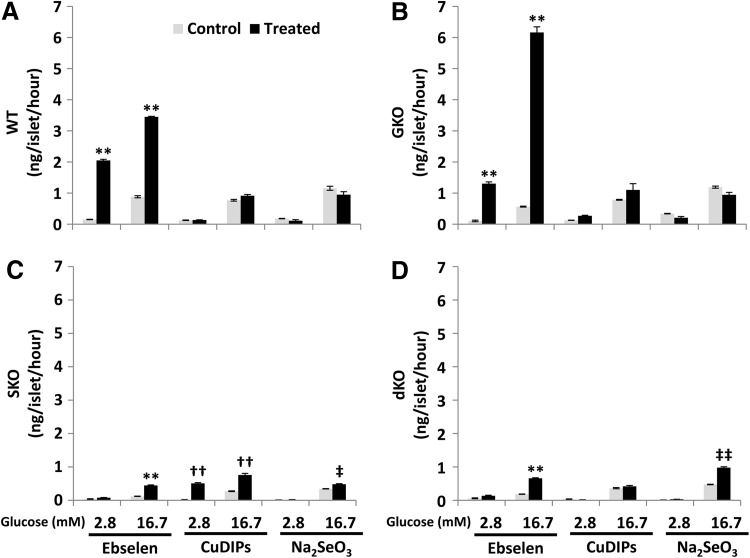
Ebselen, CuDIPs, and Se were used as the GPX mimic, SOD mimic, and compound incorporated as selenocysteine into the active center of GPX, respectively, and incubated with islets isolated from the GKO, SKO, and dKO mice, along with the wild type (WT), to rescue their impaired GSIS. Ebselen enhanced (p<0.01) GSIS of islets of all four genotypes treated with 16.7 mM glucose (Fig. 1A–D), compared with their respective controls. Notably, such enhancement was so strong in the GKO islets that their GSIS even exceeded the WT level. The ebselen treatment also elevated (p<0.01) in the baseline insulin secretions of WT and GKO islets (at 2.8 mM glucose). In contrast, CuDIPs promoted GSIS and the baseline insulin secretion only in the SKO islets (p<0.01). Meanwhile, Na2SeO3 elevated islet GSIS of SKO (p<0.05) and dKO (p<0.01).
FIG. 1.
Impacts of the GPX and SOD mimics and inorganic selenium on insulin secretion by islets. The islets were isolated from WT (A), GKO (B), SKO (C), and dKO (D) mice and pre-treated with ebselen (50 μM in DMSO), CuDIPs (10 μM in ethanol), and sodium selenite (100 nM in saline) or the respective solvent controls for 5 h. The 16.7 mM glucose stimulation increased insulin secretion (p < 0.05 versus 2.8 mM glucose) in all treatment groups. **p < 0.01 versus DMSO solvent control; ††p < 0.01 versus ethanol solvent control; ‡p < 0.05, ‡‡p < 0.01 versus saline control. Values are means ± SEM (n = 3). CuDIPs, copper diisopropylsalicylate; dKO, double knockout of GPX1 and SOD1; DMSO, dimethyl sulfoxide; GKO, GPX1 knockout; GPX, glutathione peroxidase; GSIS, glucose-stimulated insulin secretion; SEM, standard error of means; SKO, SOD1 knockout; SOD, superoxide dismutase; WT, wild type.
Responses of GK, GLUT2, PDX1, UCP2, lactate dehydrogenase, glutathione reductase, and ROS
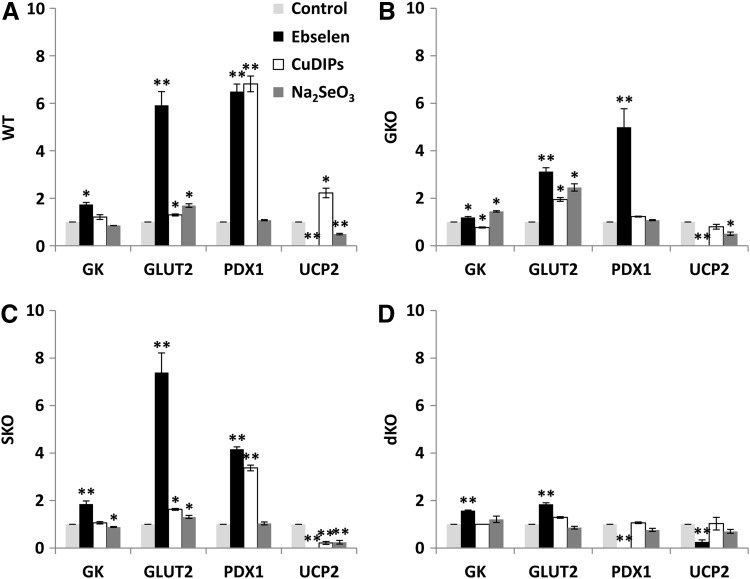
To explore biochemical mechanisms for the GSIS rescues observed earlier by ebselen, CuDIPs, and Se, we determined their effects on the activity or protein levels of four key regulators (GK, GLUT2, PDX1, and UCP2) of GSIS. Ebselen elevated (p<0.05) GK activity by 74%, 20%, 86%, and 58% and GLUT2 by 4.9-fold, 2.1-fold, 6.4-fold, and 85%, respectively, in the WT, GKO, SKO, and dKO islets (Fig. 2A–D and Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/ars), compared with their respective controls. Meanwhile, ebselen diminished (p<0.01) islet UCP2 in all genotypes. While ebselen elevated (p<0.01) PDX1 in WT (5.5-fold), GKO (4.0-fold), and SKO (3.2-fold) islets, it actually blocked (p<0.01) the same protein in the dKO islets. In the SKO islets, CuDIPs elevated (p<0.05) GLUT2 and PDX1 by 63% and 2.4-fold, respectively, but decreased (p<0.01) UCP2 by 78%. While CuDIPs increased (p<0.05) GLUT2, PDX1, and UCP2 in the WT islets (Fig. 2A), it caused opposite changes in GK activity (decrease) and GLUT2 (increase) in the GKO islets (Fig. 2B). Compared with the controls, the Na2SeO3 treatment increased (p<0.05) GLUT2 by 31% to 1.5-fold, but decreased (p<0.05) UCP2 by 49–75%, respectively, in the WT, GKO, and SKO islets. The same treatment increased (p<0.05) GK activity in the GKO islets, but decreased (p<0.05) that in the SKO islets. Neither CuDIPs nor Na2SeO3 affected any of these four proteins in the dKO islets (Fig. 2D).
FIG. 2.
Impacts of the GPX and SOD mimics and inorganic selenium on protein production or function of key GSIS regulators in islets. The islets were isolated from WT (A), GKO (B), SKO (C), and dKO (D) mice and pre-treated with the mimics or selenium for 5 h. Thereafter, the islets were stimulated by 16.7 mM glucose for 1 h. GK is shown as enzyme activity (mU/mg protein), and GLUT2, PDX1, and UCP2 are shown as relative protein production by integrated optical densitometry. *p < 0.05, **p < 0.01 versus respective solvent control. Values are means ± SEM (n = 5). GK, glucokinase; GLUT2, glucose transporter type 2; PDX1, pancreatic and duodenal homeobox 1; UCP2, uncoupling protein 2.
Impacts of the GKO and ebselen on islet intracellular H2O2 status were indirectly assessed by a non-specific ROS probe (2′,7′-dichlorofluorescin diacetate [DCFH-DA], Supplementary Fig. S2A). Ebselen decreased the intracellular ROS by 74% in the GKO islets and removed their initial genotype difference from the WT islets. However, the ebselen treatment showed no effect on the activities of two thiol-containing enzymes: lactate dehydrogenase (LDH) and glutathione reductase (GSR) in the WT and GKO islets (Supplementary Fig. S2B, C).
Signaling mapping for GSIS regulation by ebselen
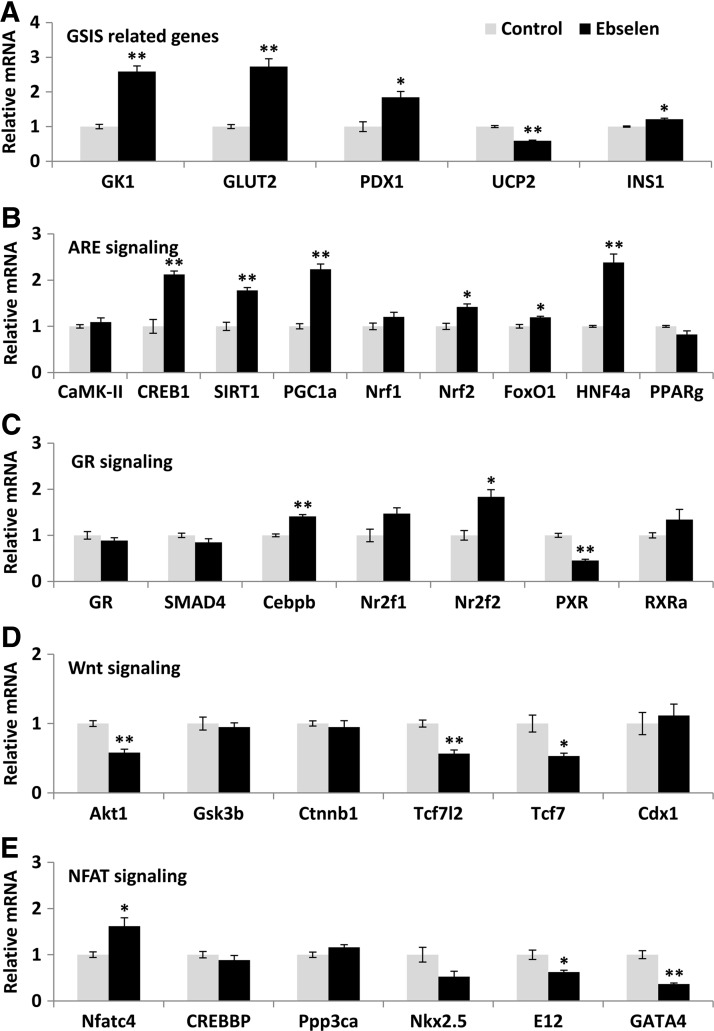
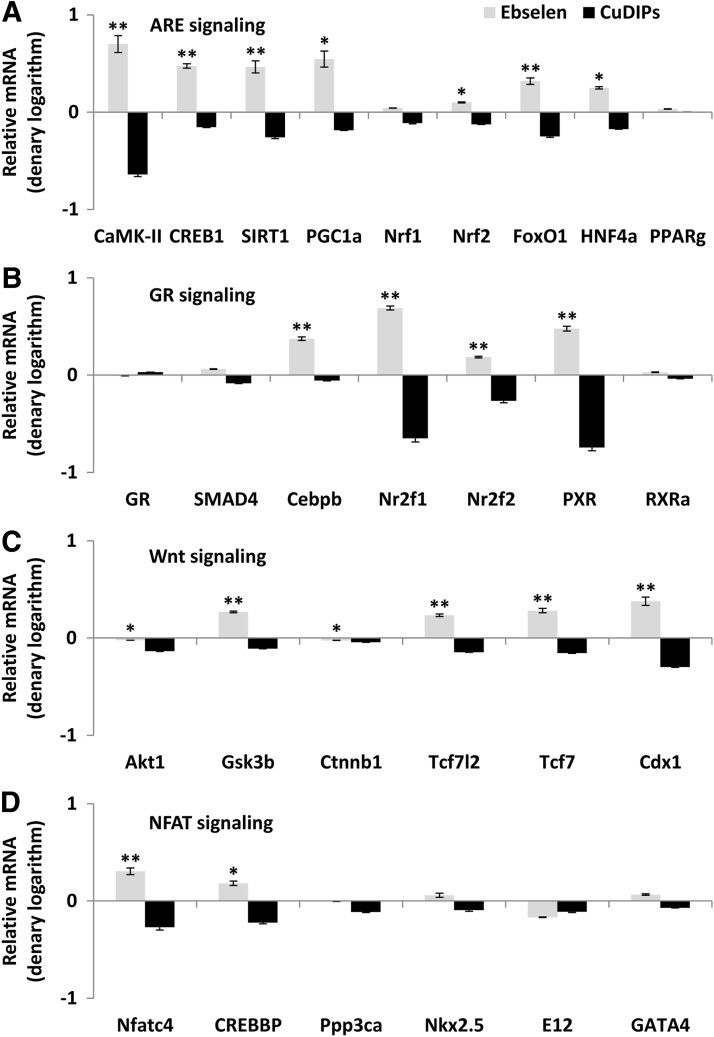
To reveal whether the illustrated effects of ebselen on islet GK, GLUT2, PDX1, and UCP2 were mediated by transcriptional regulation, we determined their mRNA responses to ebselen in the GKO islets by quantitative real-time polymerase chain reaction (Q-PCR). Since their mRNA changes resembled those of their protein responses (Fig. 3A), we proposed a transcriptional regulation for this cascade and analyzed their gene promoter regions to search for shared binding domains that might mediate the regulation. After 1000 bp upstream genomic sequences of each gene had been retrieved from EMBL database (http://ensembl.org/), the sequences were submitted to the Transcription Element Search System (TESS) database (http://cbil.upenn.edu/tess) for transcriptional factor binding domain prediction. The common binding domains of these four genes with p<0.05 were submitted to Beta Cell Biology Consortium (BCBC) Gene Interactions Databases (http://genomics.betacell.org/gbco/home.jsp) for gene relationship comparison. The predicted transcriptional factor binding domains and their related proteins were submitted to Kyoto Encyclopedia of Genes and Genomes (KEGG) database (http://genome.jp/kegg/) for signaling pathway mapping. This bioinformatics approach identified the PGC-1α-mediated ARE and other three signaling pathways (GR, Wnt, and NFAT, Supplementary Fig. S3). Subsequent Q-PCR analysis showed that six out of the nine genes in the PGC-1α-mediated ARE pathway (Fig. 3B) and two out of the seven genes in the GR pathway (Fig. 3C) were up-regulated by ebselen. Meanwhile, three out the six genes in the Wnt pathway (Fig. 3D) and two out of the six genes in the NFAT pathway (Fig. 3E) were down-regulated by ebselen. After the dKO islets had been treated with ebselen, many genes in these four pathways were up-regulated (p<0.05) compared with the controls (Fig. 4). In contrast, CuDIPs suppressed (p<0.05) expressions of these genes in the dKO islets.
FIG. 3.
Impacts of the GPX mimic on gene expression of the key regulators of GSIS and their potential upstream signal proteins in islets of the GKO mice. The GSIS-related (A), the ARE pathway (B), the GR pathway (C), the Wnt pathway (D), and the NFAT pathway (E). The islets were pre-treated with ebselen for 5 h and then stimulated by 16.7 mM glucose for 1 h. *p < 0.05, **p < 0.01 versus control. Values are means ± SEM (n = 6). ARE, antioxidant response element; GR, glucocorticoid receptor; NFAT, nuclear factor of activated T-cells; Wnt, wingless-type MMTV integration site.
FIG. 4.
Comparative impacts of the GPX and SOD mimics on gene expression of the potential upstream signal proteins of the key regulators of GSIS in islets of the dKO mice. The ARE pathway (A), the GR pathway (B), the Wnt pathway (C), and the NFAT pathway (D). The islets were pre-treated with the mimics for 5 h and then stimulated by 16.7 mM glucose for 1 h. Data are presented as denary logarithm values (stimulation and inhibition are shown as positive and negative values, respectively). *p < 0.05, **p < 0.01 ebselen versus CuDIPs group. Values are means ± SEM (n = 6).
PGC-1α as a main mediator for the upstream signaling regulation
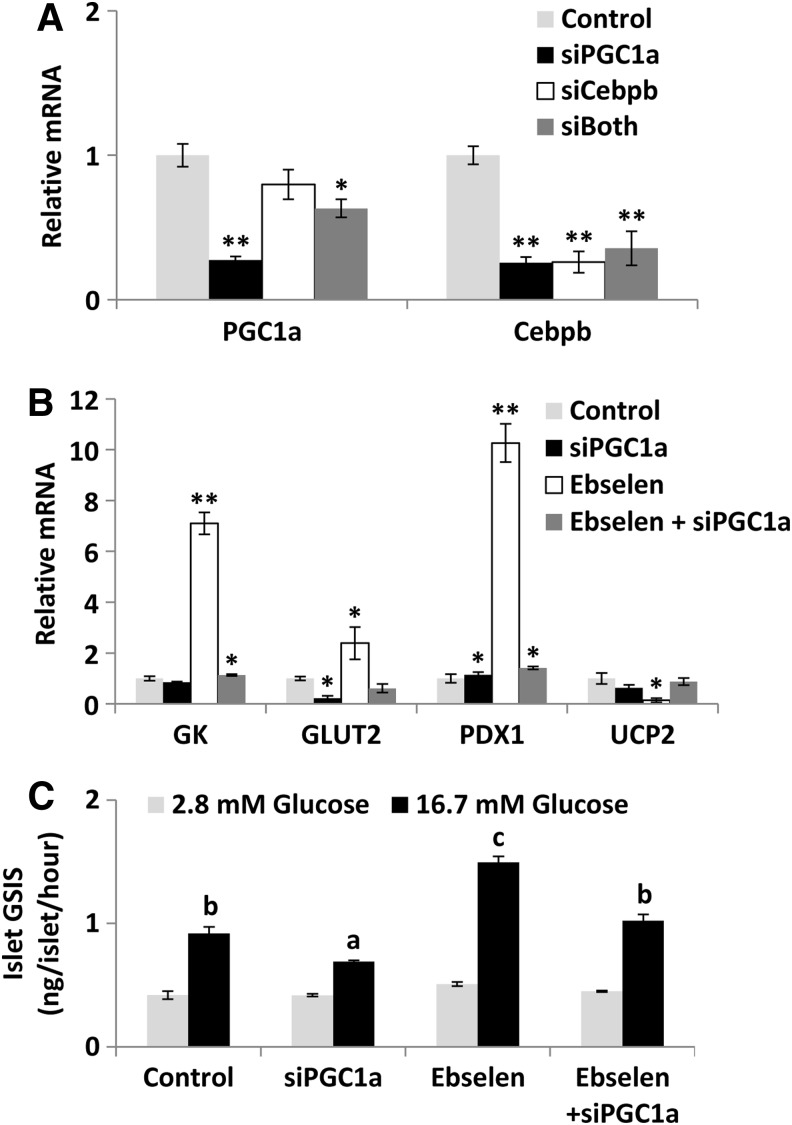
The overall positive responses of the ARE and GR pathway genes to the ebselen treatment led us to project PGC-1α (the central effector of the ARE pathway) and CCAAT/enhancer binding protein (C/EBP), beta (C/EBPβ, the main effector of the GR pathway) as the primary mediators for the upstream signaling regulation of GSIS by ebselen. Subsequently, we applied small interfering RNA (siRNA) to knock down these two genes in islets to assess their relative importance in the event. The pgc-1α siRNA suppressed the gene expression of both pgc-1α and c/ebpβ by more than 70% (p<0.01), whereas the c/ebpβ siRNA decreased (p<0.01) only its own expression (Fig. 5A). The double siRNA did not produce suppression of either gene further than the single treatment. Most striking, knockdown of pgc-1α blocked the ebselen-induced up-regulation of gk, glut2, and pdx1 mRNA levels; down-regulation of ucp2 mRNA level (Fig. 5B); and GSIS rescue (Fig. 5C) in the GKO islets.
FIG. 5.
Blocking the GPX mimic-mediated GSIS rescue in islets of the GKO mice by suppressing target mediator genes. Knock down of pgc-1α and c/ebpβ gene expression (A), mRNA responses of the key regulators of GSIS (B), and responses of the ebselen-mediated GSIS rescue (C). *p < 0.05, **p < 0.01 versus control in (A) and (B), and means with different letters a, b, and c, p < 0.05 in (C). Values are means ±SEM (n = 6). c/ebpβ, CCAAT/enhancer binding protein (C/EBP), beta; pgc-1α, proxisome proliferator-activated receptor gamma coactivator 1 alpha; siRNA, small interfering RNA.
Rescue of GSIS in the GKO mice by ebselen
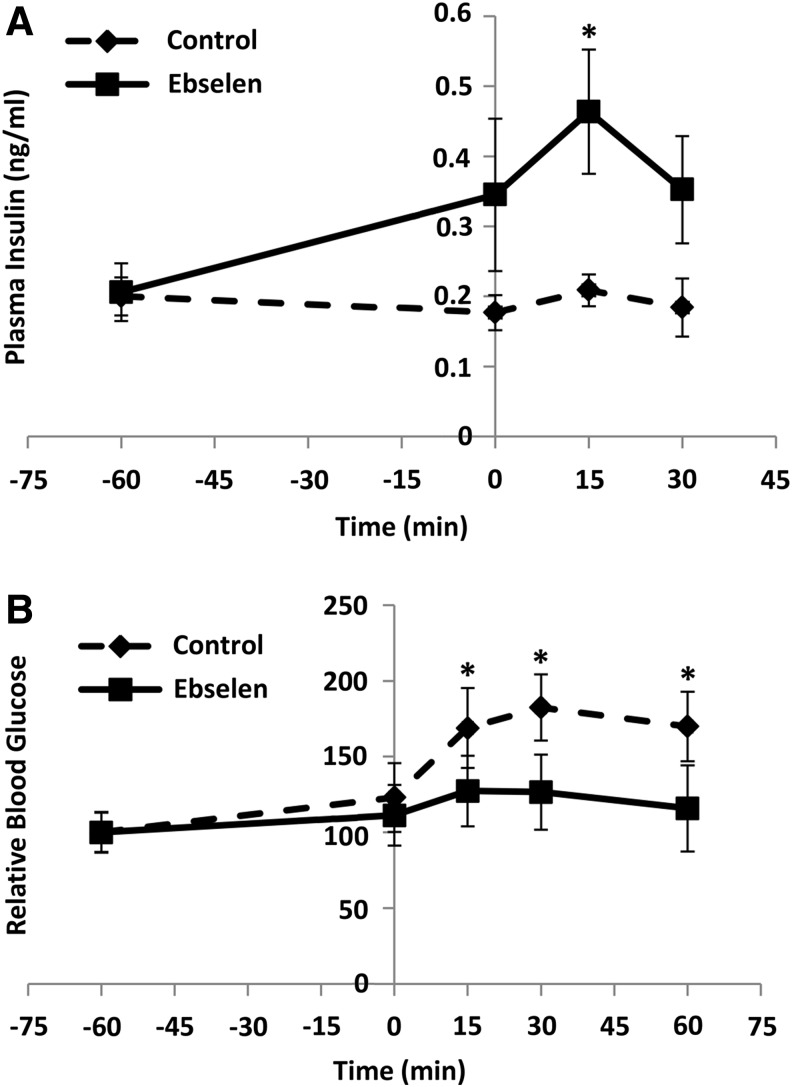
To determine whether the observed rescue of GSIS in GKO islets by ebselen was reproducible at physiological conditions and whether ebselen caused any “off-target” toxicity, we gave the fasted GKO mice an intraperitoneal (i.p.) injection of ebselen at 1 h before the GSIS test. The injection elevated their plasma insulin concentrations at 0 min (baseline) by 95%, 15 min by 1.2-fold (p<0.05), and 30 min by 91% after the glucose challenge (Fig. 6A). Consequently, glucose clearance was improved by 17%, 18%, and 21% (p<0.05) at 15, 30, and 60 min, respectively, by the ebselen injection (Fig. 6B). The injection produced no differences from the control in activities of plasma alanine aminotransferase (ALT) and alkaline phosphatase (AKP) or hepatic LDH and GSR (Supplementary Fig. S4).
FIG. 6.
The in vivo rescue of insulin secretion and glucose tolerance by the GPX mimic in the GKO mice. GSIS (A) and GTT (B). The ebselen (50 mg/kg) was injected (i.p.) into fasting (overnight for 8 h) GKO mice at 1 h before the glucose challenge (1 g/kg), and DMSO was injected as the solvent control. GTT data are presented as relative values (fasting blood glucose before ebselen injection was defined as 100). *p < 0.05 versus control. Values are means ± SEM (n = 6). GTT, glucose tolerance test.
Discussion
The most exciting, novel finding of the present study was that the GPX mimic ebselen (57), at a relatively low dose (14), rescued GSIS in the GKO islets and mice. Despite its recognition as the GPX mimic in 1984 (47) and subsequent extensive research on its protections against ROS, ischemic damage, and inflammation (15), only a couple of studies have explored its involvement in insulin synthesis (16) or hypoglycemic effect (14). Unprecedentedly, our study provides the direct evidence for a novel metabolic effect of ebselen in promoting GSIS. Due to the recently discovered association between the GPX1 mutation and increased risk of type 2 diabetes (54), our findings offer a potentially new therapy of insulin secretion defects that are associated with diabetes and hyperglycemia (41). Despite its lipophilic property, ebselen was prepared in 4% w/v hydroxypropyl-β-cyclodextrin for an i.p. injection to C57BL/6 mice (58) or was directly suspended in water for oral administration to humans (72). The ebselen that was delivered in both ways was absorbed to blood and even crossed the blood–brain barrier (58, 72). Since ebselen is included in the National Institutes of Health Clinical Collection (3), it has a history of use in human clinical trials and known safety profiles (www.nihchinicalcollection.com). Meanwhile, the ebselen doses used in the present study produced no “off-target” toxicity (1) or side effect on functions of thiol-containing enzymes (42) in islets or the liver. However, the long-term effectiveness of ebselen in promoting GSIS and the potential risk of over-stimulation (46) associated with the treatment should be checked under physiological conditions (52). Possible cross-talks between the pancreas and other tissues induced by the ebselen treatment (12), and its global effect on the whole body ROS status (34) should also be evaluated.
Ebselen rescued GSIS in the GKO islets by up-regulating GK, GLUT2, and PDX1, and down-regulating UCP2. Two strong evidences supported that this rescue was executed by ebselen via ROS scavenging instead of simply being an Se carrier. The first evidence was the 74% decrease of intracellular ROS level in the ebselen-treated GKO islets compared with the control. The second evidence was the different effects of sodium selenite and ebselen on the four GSIS regulators or GSIS itself in the GKO islets. In fact, the potential of ebselen as an Se carrier or transporter was questioned by a recent study due to the lack of stimulation of GPX activity or selenoprotein P expression in HepG2 cells (27). By taking four consecutive steps of genomics and bioinformatics analyses into consideration, we have revealed that the regulation of ebselen on the four key regulators of GSIS took place at transcription and was mediated by PGC-1α via the ARE and(or) GR pathways. In the first step, the Q-PCR analysis depicted parallel responses of mRNA and protein levels of GK, GLUT2, PDX1, and UCP2 in the GKO islets to ebselen, and suggested transcriptional regulation as the action site of ebselen. In the second step, four signaling pathways (ARE, GR, Wnt, and NFAT) were identified as the potential mediator for the initial action of ebselen by analyzing gene promoters of the four key regulators of GSIS. Indeed, these pathways are highly responsive to redox regulation (31, 32, 62, 70) and involved in transcriptional regulation of the key regulators of GSIS (8, 24, 59, 65). In the third step, the candidate pathways (ARE and GR) and mediators (PGC-1α and C/EBPβ) were chosen based on their gene expression responsiveness to ebselen. In the final step, we applied siRNA to prove that PGC-1α, indeed, served as the main mediator to link the ebselen-initiated intracellular ROS decrease to the downstream gene expression of gk, glut2, pdx1, and ucp2 for the GSIS rescue in the GKO islets.
There are both scientific and clinical significances to elucidate the novel role of PGC-1α in initiating the positive effect of the GPX mimic ebselen on GSIS. This reveals not only a new signaling pathway to explain how GPX1 and(or) ebselen regulate insulin secretion but also a new potential therapeutic target to treat insulin secretion disorders (26). Expression and function of PGC-1α is highly responsive to ROS (62). Down-regulation of PGC-1α decreased GSIS and(or) insulin production in both human and rat islets (40). Two of its gene variants were associated with increased risks of type 2 diabetes in the Indian population (6, 73). As a transcriptional co-activator for peroxisome proliferator activated receptors and LXR (53), it may play a dual role in GSIS on the metabolic conditions (22, 25, 64). It is also interesting to note that PGC-1α activates gene expression of GPX1 and several other antioxidant enzymes (60). Thus, the ebselen-mediated activation of PGC-1α (71) may constitute a positive feedback between GPX1 and PGC-1α. However, potential roles of other redox-sensitive or ARE-related factors such as nuclear factor (erythroid-derived 2)-like 2 (Nrf2) (and nuclear respiratory factor 1 [Nrf1]) (62) in the ebselen-induced cascade events should also be considered. In fact, Nrf2 was a downstream target molecule of PGC-1α in the pathway scheme derived from the present study, and its mRNA was up-regulated by ebselen in the GKO islets. Thus, the roles of these two proteins were cooperative or coordinated in activating the cascade. In the future, plasmid reporter assays and/or chromatin immune-precipitation analyses should be conducted to discern the true initiation of transcription of GK, GLUT2, PDX1, and UCP2 by PGC-1α, Nrf2, and other factors in the ebselen-mediated GSIS rescue.
Another significant finding of the present study was that the GPX and SOD mimics demonstrated distinctly different regulation of signal transduction and gene expression related to GSIS. For many years, many antioxidants such as these mimics have been perceived to be the same “pathway” or “family” members for ROS scavenging (7). Using the GKO, SKO, and dKO islets, we were able to metabolically control the intracellular H2O2 and superoxide status (68) without confounding factors as encountered in conventional models (38, 46, 66), for comparing the functions and mechanisms of the GPX and SOD mimics in regulating islet GSIS. Overall, these two mimics depicted at least three distinctly different features. First, ebselen promoted GSIS in all four genotypes, whereas CuDIPs helped only SKO islets. Second, ebselen caused consistent up-regulation of GK and GLUT2 and down-regulation of UCP2 in islets of the four genotypes; while CuDIPs produced an opposite effect on UCP2 between the WT and SKO islets. As an uncoupler of respiration and oxidative phosphorylation, UCP2 is activated by endogenously generated superoxide (35) and is a negative regulator of insulin secretion in β-cells (74). Thus, down-regulation of UCP2 in all genotypes by ebselen and in SKO by CuDIPs was consistent with their effects on GSIS. However, these two mimics exhibited an opposite effect on UCP2 in the WT islets (CuDIPs showed no effect on GSIS in the WT islets). Lastly, gene expression of the four identified pathways related to GSIS was largely suppressed by CuDIPs, but promoted by ebselen in the dKO islets. This helps explain why ebselen but not CuDIPs promoted GSIS in the dKO islets. Comparatively, these impacts of ebselen and CuDIPs on islet GSIS and related pathways were in line with those of GKO and SKO on hepatotoxicity of acetaminophen (38), femoral mechanical characteristics (67), islet β-cell mass and insulin synthesis (68), and hepatic energy metabolism (66). Although antioxidants are often perceived to be beneficial to islet β-cell function and survival, clinical applications remain controversial or restricted to a narrow window of therapeutic dosage (7). Our finding highlights the importance of discretionary use of antioxidants in clinical treatment of GSIS to avoid harmful effects based on reciprocal results of seemingly “similar” antioxidant compounds. Furthermore, altering GPX1 and SOD1 activities might be applied to manipulate or restore intracellular H2O2 and superoxide balance in islets as a new or more effective strategy to treat insulin secretion disorders, in comparison with the insulin-centric therapy of insulin stimulators or analogues (55).
However, it seems puzzling that the increased H2O2 by GKO impaired GSIS and the removal of H2O2 by ebselen increased GSIS, whereas the blocking of enzymatic H2O2 production from superoxide by SKO also impaired GSIS and the recovery of H2O2 production by CuDIPs in the SKO islets rescued GSIS. This again underscores the complexity of the ROS regulation on GSIS, and may be explained by the concentration-dependent dual effect of H2O2 (29). Although excessive H2O2 could suppress GSIS (50), a minimal amount of H2O2 from the glucose metabolism is an essential signaling molecule for triggering GSIS (49). Thus, the SKO islets might lack sufficient H2O2 or appropriate ratios of H2O2 to superoxide to initiate GSIS, and the CuDIPs treatment rescued this function by restoring H2O2 generation. However, this notion could not explain the positive effect of ebselen on GSIS in the SKO islets. Likewise, sodium selenite improved GSIS in the SKO and dKO islets, but not in the WT or GKO islets. This might be due to a low SOD1-like catalytic activity of selenite in transforming superoxide to H2O2 (18) in the SKO and dKO islets. However, selenite did not affect GSIS of the WT and GKO islets, which was different from that reported in rat islets and min6 cells (13). Since GPX1 and ebselen were supposed to reduce both H2O2 and organic hydroperoxides (43), the latter were likely involved in the impaired GSIS in GKO mice and the rescue by ebselen. Although lipid profiles (total cholesterol, total triglyceride, and nonesterified fatty acid) were not different between the GKO and WT mice (66), the role of organic hydroperoxides in the ebselen-rescued GSIS needs further research. In addition, ebselen stimulated insulin secretion in the WT and GKO islets at 2.8 mM glucose, and CuDIPs did that in the SKO islets. Although low glucose level at 2.8 mM often inhibits insulin secretion by other stimuli, for example, glucagon-like peptide 1 and glucose-dependent insulinotropic polypeptide (23), certain therapeutic insulin secretagogues such as glibenclamide elevated islet insulin secretion at both basal (1 mM) and high (15 mM) glucose levels (26). Likewise, GK activators also increased beta cell cytosolic calcium and insulin secretion at 1 mM glucose (44). Despite this, stimulation of insulin secretion by ebselen and CuDIPs in the respective genotypes was still stronger at 16.7 than 2.8 mM glucose in the present study.
In summary, we have demonstrated a novel metabolic role and therapeutic potential of the GPX mimic ebselen (57) in rescuing GSIS in the GKO islets and mice. This rescue constituted coordinated regulation of GK, GLUT2, PDX1, and UCP2 by activating the PGC-1α-mediated ARE and(or) GR signaling. In comparison, the SOD mimic CuDIPs exerted different impacts on GSIS and the related gene expression. Thus, our findings have clarified that GPX1 and SOD1, as two important intracellular antioxidant enzymes, function distinguishably in regulating insulin secretion. Most likely, evolution has selected unique signaling pathways for different antioxidant enzymes or compounds to precisely control insulin secretion in response to complicated metabolic conditions. Clinically, these unique features of different antioxidant enzymes or mimics may be used to fine-tune islet ROS status and the related signaling for effective treatments of insulin secretion and diabetic disorders.
Materials and Methods
Mouse models, animal care, and in vivo study
Our mouse experiments were approved by the Institutional Animal Care and Use Committee at Cornell University and were conducted in accordance with National Institutes of Health guidelines for animal care. The GKO, SKO, and their dKO mice were derived from the C57BL/6 line. Deletions of gpx1 and sod1 genes in respective genotypes were verified by PCR analysis using tail DNA as templates and enzyme activity assays of various tissues (38). All experimental mice were 3–6 month-old males, weaned at 3 weeks of age, reared in plastic cages in an animal room at a constant temperature (22°C) with a 12 h light–dark cycle, and were given free access to a Torula yeast and sucrose-based diet added with 0.3 mg Se/kg (as sodium selenite) (68) and distilled water.
To examine the in vivo effect of ebselen on insulin secretion, fasting (overnight for 8 h) GKO mice (3 month-old male, n=6 per group) were given an i.p. injection of ebselen (at 50 mg/kg body weight) at 1 h before the glucose tolerance test (1 g of glucose/kg) and GSIS test (46). Ebselen was dissolved in dimethyl sulfoxide (DMSO) and injected with saline (1:1 by volume) together. The same volume of DMSO and saline was used as the solvent control. Blood glucose concentrations were measured by clipping tails and using the Glucometer Elite system (Bayer, Elkhart, IN). Plasma insulin concentrations were determined using a rat/mouse Insulin enzyme-linked immunosorbent assay kit with mouse insulin as standards (Crystal Chem, Downers Grove, IL).
In vitro experiments
All chemicals were purchased from Sigma (Saint Louis, MO) unless indicated otherwise. Detailed protocols, reagents, and instruments for islet isolation, culture, and insulin secretion were the same as previously described (69). Briefly, Langerhans' islets were isolated from the WT, GKO, SKO, and dKO mice using a standard procedure (21) with minor modifications. Isolated islets (50 per sample, n=3 per group) were recovered in RPMI 1640 (Gibco, Grand Island, NY) with 5.5 mM glucose and 10% fetal bovine serum for 2 h before treatment. For the islet experiments, ebselen, CuDIPs, and Se (sodium selenite) were used at 50, 10, and 0.1 μM and prepared in DMSO, ethanol, and saline, respectively. The same amounts of vehicle were used as the respective controls. The chemicals were removed after 5 h of incubation, and the islets were transferred to Krebs–Henseleit buffer for 30 min and then incubated for 60 min with 2.8 or 16.7 mM glucose in the same buffer. The supernatants were collected for insulin analysis, and the islets were used for mRNA, protein, and enzyme activity analyses.
Real-time Q-PCR and Western blot analyses
Total RNA was extracted from islets using Trizol reagent (Invitrogen, Carlsbad, CA). Reverse transcription was performed using Super Script III reverse transcriptase, RNaseOUT Ribonuclease Inhibitor, and Oligo(dT)12–18 (Invitrogen). The cDNA obtained from 1 μg of total RNA was used as a template for PCR amplification. Oligonucleotide primers were designed based on Genebank entries and IDT PrimerQuest. Primer sequences for the GSIS-related genes are described in Supplementary Table S1. Relative mRNA levels were determined by real-time Q-PCR (7900HT; Applied Biosystems, Foster City, CA) as previously described (48).
Islet samples used for Western blot analysis were homogenized in phosphate buffer (50 mM, pH 7.4) containing 0.1% Triton X-100 and protease inhibitor mixture (AEBSF, aprotinin, bestatin hydrochloride, E-64-[N-(transepoxysuccinyl)-l-leucine 4-guanidinobutylamide], leupeptin, and pepstatin A). The homogenates were centrifuged at 14,000 g for 10 min at 4°C. A total of 10 μg of protein per lane was subjected to Western blot analysis (46). After the gel electrophoresis, the separated proteins were transferred onto a protran BA85 nitrocellulose membrane (Schleicher Schuell Bioscience, Keene, NH). The membranes were incubated first with respective primary antibodies (rabbit anti-GLUT2, anti-PDX1, and anti-UCP2; Millipore, Billerica, MA), and next, the second antibody against rabbit immunoglobulin G (Bio-Rad, Hercules, CA). For loading and transfer normalization, the rabbit anti-β-actin antibody (Cell Signaling, Beverly, MA) was used. An enhanced chemiluminescent kit (Pierce, Rockford, IL) was used for detection of the band intensity.
Enzyme activity measures
Islet GK activity was assayed at 28°C by measuring absorbance increases of NADPH at 340 nm in a coupled enzyme system. One unit of GK activity was defined as the amount that catalyzes the formation of 1 μmol of glucose 6-phosphate per minute. Islet and liver LDH activities were measured using a kit from Sigma according to the manufacturer's instructions. Islet and liver GSR activities were measured as previously described (75), and one unit of activity was defined as 1 nmol of glutathione disulfide reduced per minute. Plasma ALT activity was assayed using a kit (Thermo Scientific, Waltham, MA) according to the manufacturer's instructions. Plasma AKP activity was measured by the hydrolysis of p-nitrophenol phosphate to p-nitrophenol, and the enzyme activity unit was defined as the amount of activity that releases 1 μmol of p-nitrophenol per minute at 30°C (9).
Intracellular ROS
Islet ROS levels were determined using the fluorescent probe, 2′,7′-dichlorofluorescin diacetate. Briefly, the treated islets were washed and incubated with DCFH-DA (20 μM) for 10 min at 37°C. After removal of the probe, cells were washed with pre-warmed phosphate-buffered saline. Fluorescence was monitored at 488 nm excitation and 525 nm emission wavelengths.
RNA interference
Silencer® Selected Pre-Designed siRNAs (Invitrogen) duplex sequences that specifically target pgc-1α [NM_008904.2] and c/ebpβ [NM_009883.3] were employed. The Silencer Selected Negative Control from Invitrogen was used as non-targeting scramble siRNA. The transfection was performed according to the manufacturer's recommendations with minor modifications. Briefly, islets were dispersed into single cells by mechanical shaking at 37°C for 3 min in 0.05% trypsin with 0.5 mM ethylenediaminetetraaceticacid (28). The enzymatic reaction was stopped by adding 10% bovine serum albumin. Cells were washed and recovered in full culture medium at 37°C for 2 h. Thereafter, islet cells were divided equally into 24-well plates in culture medium without antibiotics or fetal bovine serum. The siRNAs (20 nM) was transfected twice using Lipofectamine™ RNAiMAX (Invitrogen). The second transfection was performed 24 h after the first. The efficiency of siRNA was verified by real-time Q-PCR analysis of the target genes. After the second transfection, the islets were recovered in RPMI 1640 culture medium with 5.5 mM glucose and 10% fetal bovine serum for 2 h. Subsequently, the ebselen treatment and the GSIS test were performed as described earlier. The medium supernatants and the islets were collected for insulin and mRNA analyses, respectively.
Bioinformatics analyses of gene promoter sequences
Upstream (1000 bp) genomic sequences of gk, glut2, pdx1, and ucp2 were retrieved from EMBL database (http://ensembl.org/). The transcription factor binding sites were predicted using TESS database (http://cbil.upenn.edu/tess) and compared with related factors in beta cells in Gene Interactions Database from BCBC (http://genomics. betacell.org/gbco/home.jsp). The candidate transcription factors were then submitted to the KEGG pathway database, and were sorted into different signaling pathways.
Statistical analysis
Data were analyzed using SAS (release 6.11; SAS Institute, Cary, NC). Treatment effects were determined by one-way or two-way analysis of variance. Results are presented as mean±standard error of the mean, and significance level was set at p<0.05.
Supplementary Material
Abbreviations Used
- AKP
alkaline phosphatase
- Akt1
protein kinase B
- ALT
alanine aminotransferase
- ARE
antioxidant response element
- BCBC
Beta Cell Biology Consortium
- C/EBPβ
CCAAT/enhancer binding protein (C/EBP), beta
- Caln
protein phosphatase 3, catalytic subunit, alpha isoform (Ppp3ca)
- CaMK-II
calcium/calmodulin-dependent protein kinase II
- Cdx1
caudal type homeobox 1
- CREB1
cAMP responsive element binding protein 1
- CREBBP
CREB-binding protein (CBP)
- Ctnnb1
catenin (cadherin associated protein), beta 1
- CuDIPs
copper diisopropylsalicylate
- DCFH-DA
2′,7′-dichlorofluorescin diacetate
- dKO
double knockout of GPX1 and SOD1
- DMSO
dimethyl sulfoxide
- E12
transcription factor E2-alpha
- FoxO1
forkhead box O1
- GATA4
GATA binding protein 4
- GIP
glucose-dependent insulinotropic polypeptide
- GK
glucokinase
- GKO
GPX1 knockout
- GLP-1
glucagon-like peptide 1
- GLUT2
glucose transporter type 2
- GPX1
glutathione peroxidase 1
- GR
glucocorticoid receptor
- GSIS
glucose-stimulated insulin secretion
- GSK-3β
glycogen synthase kinase 3 beta
- GSR
glutathione reductase
- GTT
glucose tolerance test
- GWAS
genome wide association study
- H2O2
hydrogen peroxide
- HNF4a
hepatocyte nuclear factor 4, alpha
- HPRT
hypoxanthine guanine phosphoribosyl transferase
- INS1
preproinsulin 1
- KEGG
Kyoto Encyclopedia of Genes and Genomes
- Kir6.2
potassium inwardly rectifying channel, subfamily J, member 11
- LDH
lactate dehydrogenase
- NFAT
nuclear factor of activated T-cells
- Nfatc4
nuclear factor of activated T cells, cytoplasmic, calcineurin dependent 4
- Nkx2.5
NK2 transcription factor related, locus 5
- Nr2f1
nuclear receptor subfamily 2, group F, member 1
- Nr2f2
nuclear receptor subfamily 2, group F, member 2
- Nrf1
nuclear respiratory factor 1
- Nrf2
nuclear factor (erythroid-derived 2)-like 2
- PDX1
pancreatic and duodenal homeobox 1
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1 alpha
- PI3K
phosphoinositol-3-kinase
- PPARγ
peroxisome proliferator activated receptor gamma
- PXR
pregnane X receptor
- Q-PCR
quantitative real-time polymerase chain reaction
- ROS
reactive oxygen species
- RXR
retinoid X receptor
- Se
selenium
- SEM
standard error of the mean
- siRNA
small interfering RNA
- SIRT1
NAD-dependent deacetylase sirtuin-1
- SKO
SOD1 knockout
- SMAD4
SMAD family member 4
- SOD1
superoxide dismutase 1
- STZ
streptozotocin
- Tcf7
transcription factor 7, T cell specific
- Tcf7l2
transcription factor 7 like 2, T cell specific, HMG box
- TESS
transcription Element Search System
- UCP2
uncoupling protein 2
- Wnt
wingless-type MMTV integration site
- WT
wild type
Acknowledgment
This research was supported in part by NIH grant (DK53018).
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Amacher DE. A toxicologist's guide to biomarkers of hepatic response. Hum Exp Toxicol 21: 253–262, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Ashcroft FM. and Rorsman P. Diabetes mellitus and the beta cell: the last ten years. Cell 148: 1160–1171, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin CP, Brady LS, Insel TR, and Collins FS. NIH molecular libraries initiative. Science 306: 1138–1139, 2004 [DOI] [PubMed] [Google Scholar]
- 4.Barrett CW, Ning W, Chen X, Smith JJ, Washington MK, Hill KE, Coburn LA, Peek RM, Chaturvedi R, Wilson KT, Burk RF, and Williams CS. Tumor suppressor function of the plasma glutathione peroxidase gpx3 in colitis-associated carcinoma. Cancer Res 73: 1245–1255, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Becker DJ, Reul B, Ozcelikay AT, Buchet JP, Henquin JC, and Brichard SM. Oral selenate improves glucose homeostasis and partly reverses abnormal expression of liver glycolytic and gluconeogenic enzymes in diabetic rats. Diabetologia 39: 3–11, 1996 [DOI] [PubMed] [Google Scholar]
- 6.Bhat A, Koul A, Rai E, Sharma S, Dhar MK, and Bamezai RN. PGC-1alpha Thr394Thr and Gly482Ser variants are significantly associated with T2DM in two North Indian populations: a replicate case-control study. Hum Genet 121: 609–614, 2007 [DOI] [PubMed] [Google Scholar]
- 7.Bisbal C, Lambert K, and Avignon A. Antioxidants and glucose metabolism disorders. Curr Opin Clin Nutr Metab Care 13: 439–446, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Bordonaro M. Role of Wnt signaling in the development of type 2 diabetes. Vitam Horm 80: 563–581, 2009 [DOI] [PubMed] [Google Scholar]
- 9.Bowers GN, Jr., and McComb RB. A continuous spectrophotometric method for measuring the activity of serum alkaline phosphatase. Clin Chem 12: 70–89, 1966 [PubMed] [Google Scholar]
- 10.Brigelius-Flohe R. Glutathione peroxidases and redox-regulated transcription factors. Biol Chem 387: 1329–1335, 2006 [DOI] [PubMed] [Google Scholar]
- 11.Brigelius-Flohe R. and Flohe L. Basic principles and emerging concepts in the redox control of transcription factors. Antioxid Redox Signal 15: 2335–2381, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cabou C. and Burcelin R. GLP-1, the gut-brain, and brain-periphery axes. Rev Diabet Stud 8: 418–431, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campbell SC, Aldibbiat A, Marriott CE, Landy C, Ali T, Ferris WF, Butler CS, Shaw JA, and Macfarlane WM. Selenium stimulates pancreatic beta-cell gene expression and enhances islet function. FEBS Lett 582: 2333–2337, 2008 [DOI] [PubMed] [Google Scholar]
- 14.Costa MD, Gai BM, Acker CI, Souza AC, Brandao R, and Nogueira CW. Ebselen reduces hyperglycemia temporarily-induced by diazinon: A compound with insulin-mimetic properties. Chem Biol Interact 197: 80–86, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Day BJ. Catalase and glutathione peroxidase mimics. Biochem Pharmacol 77: 285–296, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de-Mello MA, Flodstrom M, and Eizirik DL. Ebselen and cytokine-induced nitric oxide synthase expression in insulin-producing cells. Biochem Pharmacol 52: 1703–1709, 1996 [DOI] [PubMed] [Google Scholar]
- 17.Ezaki O. The insulin-like effects of selenate in rat adipocytes. J Biol Chem 265: 1124–1128, 1990 [PubMed] [Google Scholar]
- 18.Feroci G. and Fini A. Study of the antioxidant effect of several selenium and sulphur compounds. J Trace Elem Med Biol 12: 96–100, 1998 [DOI] [PubMed] [Google Scholar]
- 19.Forman HJ. Reactive oxygen species and alpha,beta-unsaturated aldehydes as second messengers in signal transduction. Ann N Y Acad Sci 1203: 35–44, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandy SE, Buse MG, Sorenson JR, and Crouch RK. Attenuation of streptozotocin diabetes with superoxide dismutase-like copper(II)(3,5-diisopropylsalicylate)2 in the rat. Diabetologia 24: 437–440, 1983 [DOI] [PubMed] [Google Scholar]
- 21.Gotoh M, Maki T, Kiyoizumi T, Satomi S, and Monaco AP. An improved method for isolation of mouse pancreatic islets. Transplantation 40: 437–438, 1985 [DOI] [PubMed] [Google Scholar]
- 22.Gremlich S, Nolan C, Roduit R, Burcelin R, Peyot ML, Delghingaro-Augusto V, Desvergne B, Michalik L, Prentki M, and Wahli W. Pancreatic islet adaptation to fasting is dependent on peroxisome proliferator-activated receptor alpha transcriptional up-regulation of fatty acid oxidation. Endocrinology 146: 375–382, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Gromada J, Holst JJ, and Rorsman P. Cellular regulation of islet hormone secretion by the incretin hormone glucagon-like peptide 1. Pflugers Arch 435: 583–594, 1998 [DOI] [PubMed] [Google Scholar]
- 24.Heit JJ, Apelqvist AA, Gu X, Winslow MM, Neilson JR, Crabtree GR, and Kim SK. Calcineurin/NFAT signalling regulates pancreatic beta-cell growth and function. Nature 443: 345–349, 2006 [DOI] [PubMed] [Google Scholar]
- 25.Helleboid-Chapman A, Helleboid S, Jakel H, Timmerman C, Sergheraert C, Pattou F, Fruchart-Najib J, and Fruchart JC. Glucose regulates LXRalpha subcellular localization and function in rat pancreatic beta-cells. Cell Res 16: 661–670, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Henquin JC. Pathways in beta-cell stimulus-secretion coupling as targets for therapeutic insulin secretagogues. Diabetes 53Suppl 3: S48–S58, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Hoefig CS, Renko K, Kohrle J, Birringer M, and Schomburg L. Comparison of different selenocompounds with respect to nutritional value vs. toxicity using liver cells in culture. J Nutr Biochem 22: 945–955, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Ianus A, Holz GG, Theise ND, and Hussain MA. In vivo derivation of glucose-competent pancreatic endocrine cells from bone marrow without evidence of cell fusion. J Clin Invest 111: 843–850, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iwakami S, Misu H, Takeda T, Sugimori M, Matsugo S, Kaneko S, and Takamura T. Concentration-dependent dual effects of hydrogen peroxide on insulin signal transduction in H4IIEC hepatocytes. PLoS One 6: e27401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jensen MV, Joseph JW, Ronnebaum SM, Burgess SC, Sherry AD, and Newgard CB. Metabolic cycling in control of glucose-stimulated insulin secretion. Am J Physiol Endocrinol Metab 295: E1287–E1297, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kao TC, Wu CH, and Yen GC. Glycyrrhizic acid and 18beta-glycyrrhetinic acid recover glucocorticoid resistance via PI3K-induced AP1, CRE and NFAT activation. Phytomedicine 20: 295–302, 2013 [DOI] [PubMed] [Google Scholar]
- 32.Ke Q, Li J, Ding J, Ding M, Wang L, Liu B, Costa M, and Huang C. Essential role of ROS-mediated NFAT activation in TNF-alpha induction by crystalline silica exposure. Am J Physiol Lung Cell Mol Physiol 291: L257–L264, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Kipp A, Banning A, van Schothorst EM, Meplan C, Schomburg L, Evelo C, Coort S, Gaj S, Keijer J, Hesketh J, and Brigelius-Flohe R. Four selenoproteins, protein biosynthesis, and Wnt signalling are particularly sensitive to limited selenium intake in mouse colon. Mol Nutr Food Res 53: 1561–1572, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Kohen R. and Nyska A. Oxidation of biological systems: oxidative stress phenomena, antioxidants, redox reactions, and methods for their quantification. Toxicol Pathol 30: 620–650, 2002 [DOI] [PubMed] [Google Scholar]
- 35.Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, and Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest 112: 1831–1842, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kryukov GV, Castellano S, Novoselov SV, Lobanov AV, Zehtab O, Guigo R, and Gladyshev VN. Characterization of mammalian selenoproteomes. Science 300: 1439–1443, 2003 [DOI] [PubMed] [Google Scholar]
- 37.Labunskyy VM, Lee BC, Handy DE, Loscalzo J, Hatfield DL, and Gladyshev VN. Both maximal expression of selenoproteins and selenoprotein deficiency can promote development of type 2 diabetes-like phenotype in mice. Antioxid Redox Signal 14: 2327–2336, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lei XG, Zhu JH, McClung JP, Aregullin M, and Roneker CA. Mice deficient in Cu,Zn-superoxide dismutase are resistant to acetaminophen toxicity. Biochem J 399: 455–461, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lenzen S, Drinkgern J, and Tiedge M. Low antioxidant enzyme gene expression in pancreatic islets compared with various other mouse tissues. Free Radic Biol Med 20: 463–466, 1996 [DOI] [PubMed] [Google Scholar]
- 40.Ling C, Del Guerra S, Lupi R, Ronn T, Granhall C, Luthman H, Masiello P, Marchetti P, Groop L, and Del Prato S. Epigenetic regulation of PPARGC1A in human type 2 diabetic islets and effect on insulin secretion. Diabetologia 51: 615–622, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lubos E, Loscalzo J, and Handy DE. Glutathione peroxidase-1 in health and disease: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal 15: 1957–1997, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lugokenski TH, Muller LG, Taube PS, Rocha JB, and Pereira ME. Inhibitory effect of ebselen on lactate dehydrogenase activity from mammals: a comparative study with diphenyl diselenide and diphenyl ditelluride. Drug Chem Toxicol 34: 66–76, 2011 [DOI] [PubMed] [Google Scholar]
- 43.Maiorino M, Roveri A, Coassin M, and Ursini F. Kinetic mechanism and substrate specificity of glutathione peroxidase activity of ebselen (PZ51). Biochem Pharmacol 37: 2267–2271, 1988 [DOI] [PubMed] [Google Scholar]
- 44.Matschinsky FM. Assessing the potential of glucokinase activators in diabetes therapy. Nat Rev Drug Discov 8: 399–416, 2009 [DOI] [PubMed] [Google Scholar]
- 45.Mazzarelli JM, Brestelli J, Gorski RK, Liu J, Manduchi E, Pinney DF, Schug J, White P, Kaestner KH, and Stoeckert CJ, Jr., EPConDB: a web resource for gene expression related to pancreatic development, beta-cell function and diabetes. Nucleic Acids Res 35: D751–D755, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McClung JP, Roneker CA, Mu W, Lisk DJ, Langlais P, Liu F, and Lei XG. Development of insulin resistance and obesity in mice overexpressing cellular glutathione peroxidase. Proc Natl Acad Sci U S A 101: 8852–8857, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Muller A, Cadenas E, Graf P, and Sies H. A novel biologically active seleno-organic compound—I. Glutathione peroxidase-like activity in vitro and antioxidant capacity of PZ 51 (Ebselen). Biochem Pharmacol 33: 3235–3239, 1984 [DOI] [PubMed] [Google Scholar]
- 48.Pepper MP, Vatamaniuk MZ, Yan X, Roneker CA, and Lei XG. Impacts of dietary selenium deficiency on metabolic phenotypes of diet-restricted GPX1-overexpressing mice. Antioxid Redox Signal 14: 383–390, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pi J, Bai Y, Zhang Q, Wong V, Floering LM, Daniel K, Reece JM, Deeney JT, Andersen ME, Corkey BE, and Collins S. Reactive oxygen species as a signal in glucose-stimulated insulin secretion. Diabetes 56: 1783–1791, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Pi J, Zhang Q, Fu J, Woods CG, Hou Y, Corkey BE, Collins S, and Andersen ME. ROS signaling, oxidative stress and Nrf2 in pancreatic beta-cell function. Toxicol Appl Pharmacol 244: 77–83, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Piwkowska A, Rogacka D, Angielski S, and Jankowski M. Hydrogen peroxide induces activation of insulin signaling pathway via AMP-dependent kinase in podocytes. Biochem Biophys Res Commun 428: 167–172, 2012 [DOI] [PubMed] [Google Scholar]
- 52.Ponzani P. Long-term effectiveness and safety of liraglutide in clinical practice. Minerva Endocrinol 38: 103–112, 2013 [PubMed] [Google Scholar]
- 53.Puigserver P. and Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): transcriptional coactivator and metabolic regulator. Endocr Rev 24: 78–90, 2003 [DOI] [PubMed] [Google Scholar]
- 54.Ramprasath T, Murugan PS, Kalaiarasan E, Gomathi P, Rathinavel A, and Selvam GS. Genetic association of glutathione peroxidase-1 (GPx-1) and NAD(P)H:quinone oxidoreductase 1(NQO1) variants and their association of CAD in patients with type-2 diabetes. Mol Cell Biochem 361: 143–150, 2012 [DOI] [PubMed] [Google Scholar]
- 55.Robertson RP. Antioxidant drugs for treating beta-cell oxidative stress in type 2 diabetes: glucose-centric versus insulin-centric therapy. Discov Med 9: 132–137, 2010 [PubMed] [Google Scholar]
- 56.Schinner S, Ulgen F, Papewalis C, Schott M, Woelk A, Vidal-Puig A, and Scherbaum WA. Regulation of insulin secretion, glucokinase gene transcription and beta cell proliferation by adipocyte-derived Wnt signalling molecules. Diabetologia 51: 147–154, 2008 [DOI] [PubMed] [Google Scholar]
- 57.Sies H. Ebselen, a selenoorganic compound as glutathione peroxidase mimic. Free Radic Biol Med 14: 313–323, 1993 [DOI] [PubMed] [Google Scholar]
- 58.Singh N, Halliday AC, Thomas JM, Kuznetsova OV, Baldwin R, Woon EC, Aley PK, Antoniadou I, Sharp T, Vasudevan SR, and Churchill GC. A safe lithium mimetic for bipolar disorder. Nat Commun 4: 1332, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Soyal S, Krempler F, Oberkofler H, and Patsch W. PGC-1alpha: a potent transcriptional cofactor involved in the pathogenesis of type 2 diabetes. Diabetologia 49: 1477–1488, 2006 [DOI] [PubMed] [Google Scholar]
- 60.St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, and Spiegelman BM. Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408, 2006 [DOI] [PubMed] [Google Scholar]
- 61.Steinbrenner H, Hotze AL, Speckmann B, Pinto A, Sies H, Schott M, Ehlers M, Scherbaum WA, and Schinner S. Localization and regulation of pancreatic selenoprotein P. J Mol Endocrinol 50: 31–42, 2013 [DOI] [PubMed] [Google Scholar]
- 62.Tkachev VO, Menshchikova EB, and Zenkov NK. Mechanism of the Nrf2/Keap1/ARE signaling system. Biochemistry (Mosc) 76: 407–422, 2011 [DOI] [PubMed] [Google Scholar]
- 63.Tong Y, Lin Y, Zhang Y, Yang J, Liu H, and Zhang B. Association between TCF7L2 gene polymorphisms and susceptibility to type 2 diabetes mellitus: a large Human Genome Epidemiology (HuGE) review and meta-analysis. BMC Med Genet 10: 15, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tordjman K, Standley KN, Bernal-Mizrachi C, Leone TC, Coleman T, Kelly DP, and Semenkovich CF. PPARalpha suppresses insulin secretion and induces UCP2 in insulinoma cells. J Lipid Res 43: 936–943, 2002 [PubMed] [Google Scholar]
- 65.van Raalte DH, van Leeuwen N, Simonis-Bik AM, Nijpels G, van Haeften TW, Schafer SA, Boomsma DI, Kramer MH, Heine RJ, Maassen JA, Staiger H, Machicao F, Haring HU, Slagboom PE, Willemsen G, de Geus EJ, Dekker JM, Fritsche A, Eekhoff EM, Diamant M, and t Hart LM. Glucocorticoid receptor gene polymorphisms are associated with reduced first-phase glucose-stimulated insulin secretion and disposition index in women, but not in men. Diabet Med 29: e211–e216, 2012 [DOI] [PubMed] [Google Scholar]
- 66.Wang L, Jiang Z, and Lei XG. Knockout of SOD1 alters murine hepatic glycolysis, gluconeogenesis, and lipogenesis. Free Radic Biol Med 53: 1689–1696, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang X, Gillen EA, van der Meulen MC, and Lei XG. Knockouts of Se-glutathione peroxidase-1 and Cu,Zn superoxide dismutase exert different impacts on femoral mechanical performance of growing mice. Mol Nutr Food Res 52: 1334–1339, 2008 [DOI] [PubMed] [Google Scholar]
- 68.Wang X, Vatamaniuk MZ, Roneker CA, Pepper MP, Hu LG, Simmons RA, and Lei XG. Knockouts of SOD1 and GPX1 exert different impacts on murine islet function and pancreatic integrity. Antioxid Redox Signal 14: 391–401, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang XD, Vatamaniuk MZ, Wang SK, Roneker CA, Simmons RA, and Lei XG. Molecular mechanisms for hyperinsulinaemia induced by overproduction of selenium-dependent glutathione peroxidase-1 in mice. Diabetologia 51: 1515–1524, 2008 [DOI] [PubMed] [Google Scholar]
- 70.Wen JW, Hwang JT, and Kelly GM. Reactive oxygen species and Wnt signalling crosstalk patterns mouse extraembryonic endoderm. Cell Signal 24: 2337–2348, 2012 [DOI] [PubMed] [Google Scholar]
- 71.Xiao H. and Parkin KL. Induction of phase II enzyme activity by various selenium compounds. Nutr Cancer 55: 210–223, 2006 [DOI] [PubMed] [Google Scholar]
- 72.Yamaguchi T, Sano K, Takakura K, Saito I, Shinohara Y, Asano T, and Yasuhara H. Ebselen in acute ischemic stroke: a placebo-controlled, double-blind clinical trial. Ebselen Study Group. Stroke 29: 12–17, 1998 [DOI] [PubMed] [Google Scholar]
- 73.Yang Y, Mo X, Chen S, Lu X, and Gu D. Association of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PPARGC1A) gene polymorphisms and type 2 diabetes mellitus: a meta-analysis. Diabetes Metab Res Rev 27: 177–184, 2011 [DOI] [PubMed] [Google Scholar]
- 74.Zhang CY, Baffy G, Perret P, Krauss S, Peroni O, Grujic D, Hagen T, Vidal-Puig AJ, Boss O, Kim YB, Zheng XX, Wheeler MB, Shulman GI, Chan CB, and Lowell BB. Uncoupling protein-2 negatively regulates insulin secretion and is a major link between obesity, beta cell dysfunction, and type 2 diabetes. Cell 105: 745–755, 2001 [DOI] [PubMed] [Google Scholar]
- 75.Zhu JH. and Lei XG. Double null of selenium-glutathione peroxidase-1 and copper, zinc-superoxide dismutase enhances resistance of mouse primary hepatocytes to acetaminophen toxicity. Exp Biol Med (Maywood) 231: 545–552, 2006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.