Abstract
Background
Conflicting evidence links malnutrition to the reduced efficacy of rotavirus vaccines in developing countries, where diarrhea and undernutrition remain leading causes of child deaths. Here, we adapted mouse models of rotavirus vaccination (rhesus rotavirus, RRV), rotavirus infection (EDIM), and protein-energy malnutrition (PEM) to test the hypothesis that undernutrition reduces rotavirus vaccine immunogenicity and efficacy.
Methods
We randomized wild type Balb/C dams with 3-day-old pups to a control diet (CD) or an isocaloric, multideficient regional basic diet (RBD) that produces PEM. At 3 weeks of age, we weaned CD and RBD pups to their dams’ diet and subrandomized weanlings to receive a single dose of either live oral rotavirus vaccine (RRV) or PBS. At 6 weeks of age, we orally challenged all groups with murine rotavirus (EDIM). Serum and stool specimens were collected before and after RRV and EDIM administration to measure viral shedding and antibody responses by ELISA.
Results
RBD pups and weanlings exhibited significant failure to thrive compared to age-matched CD mice (P<.0001). RRV vaccination induced higher levels of serum anti-RV IgA responses in RBD vs. CD mice (P<.0001). Vaccination protected CD and RBD mice equally against EDIM infection, as measured by viral shedding. In unvaccinated RBD mice, EDIM shedding peaked 1 day earlier (P<.05), however we detected no effects of undernutrition on viral clearance nor of infection on bodyweight. EDIM infection provoked higher anti-RV serum IgA levels in RBD vs. CD mice, regardless of vaccination (P<.0001). Last, RRV vaccination mitigated stool IgA responses to EDIM more in CD vs. RBD mice (P<.0001).
Conclusions
Despite modulated IgA responses to vaccination and infection, undernutrition does not impair rotavirus vaccine efficacy nor exacerbate infection in this mouse model of protein-energy malnutrition. Alternative models are needed to elucidate host-pathogen factors undermining rotavirus vaccine effectiveness in high-risk global settings.
Keywords: rotavirus, malnutrition, tropical barrier, rhesus rotavirus, epizootic diarrhea of infant mice, environmental enteropathy
INTRODUCTION
Rotavirus is the most common cause of severe diarrhea in children under 5 years of age and the leading cause of diarrheal deaths worldwide. [1–5]. Over 90% of global child deaths from rotavirus occur in low-income countries, predominantly in Asia and Africa [4, 6]. The increased mortality in these settings is generally attributed to an unacceptably high prevalence of child undernutrition and limited access to medical care [7, 8].
Rotavirus immunization has emerged as a key component of global strategies to reduce childhood deaths from diarrhea [9]. The two currently available rotavirus vaccines (Rotarix™ and RotaTeq™) produce high rates of seroconversion (85–98%) and protection against severe gastroenteritis (85–89%) in the United States and Europe [10]; however, they do not provide an equal measure of protection in the developing world [11, 12]. For example, mean seroconversion for Rotarix™ is 75% in lower-middle and 63% in low-income countries and was only 57% in Malawi, prompting the question as to what extent will rotavirus vaccines work where they are needed most [10, 13, 14]. Subsequent reports by Zaman et al. and Armah et al. of rotavirus vaccine trials in Asia and sub-Saharan Africa found efficacy against severe diarrhea to be only 48.3% and 39.3%, respectively [15, 16].
The decreased efficacy of live oral vaccines in developing countries—a phenomenon known as the “tropical barrier”—is constrained to neither rotavirus nor the tropics [2, 6, 11, 17–20]. Host determinants of the tropical barrier are still unknown, however defects in innate and adaptive immunity due to high rates of child undernutrition, inadequate levels of sanitation and hygiene, tropical/environmental enteropathy, and natural selection for resistance to enteric pathogens have all been proposed to play an important role [6, 21–28].
To date, few clinical studies have investigated the impact of undernutrition on rotavirus vaccine efficacy. Linhares and colleagues found that undernourished Brazilian children were less protected from rotavirus and all-cause diarrhea following administration of low-dose RotaShield™ vaccine [29]. A more recent multi-country analysis by Perez-Schael et al. found that Rotarix™ protected children against rotavirus infection regardless of nutritional status [30]. Lastly, a prospective cohort study of the effects of undernutrition and environmental enteropathy on rotavirus and polio vaccine efficacy is currently underway in Bangladesh [www.providestudy.org]. To complement these clinical studies, we tested the effects of rhesus rotavirus (RRV) vaccine and murine rotavirus (EDIM) challenge responses in our recently described murine model of undernutrition with features of environmental enteropathy [31–32].
Formulated by Teodósio and colleagues to reflect dietary deficiencies of impoverished populations in northeastern Brazil with protein energy malnutrition (PEM), the regional basic diet (RBD) is a research diet moderately deficient in fat, protein, and minerals that induces PEM in rodents [33]. We have recently shown that a semi-purified RBD produces failure to thrive, small intestinal mucosal atrophy and gut barrier dysfunction in mice [31]. We hypothesized that undernutrition caused by the regional basic diet would impair the efficacy of oral rotavirus immunization and that undernutrition exacerbates rotavirus infection in weanling mice. Here we report that: 1) Despite altered antibody responses following murine rotavirus EDIM challenge, oral rotavirus vaccination appears to adequately protect undernourished mice against shedding of rotavirus, 2) In undernourished mice, anti-rotavirus IgA levels are altered in both immunized and unimmunized mice following EDIM challenge, and 3) Unimmunized, undernourished mice produce lower levels of anti-rotavirus IgG in response to EDIM infection.
METHODS
Viruses
The rhesus rotavirus (RRV) strain used in this study was obtained from Dr. Harry Greenberg (Stanford University, Palo Alto, CA). The murine rotavirus strain EDIM was originally obtained from M. Collins (Microbiological Associates, Bethesda, MD). Both viruses were passaged in the African green monkey kidney MA-104 cell line. Viruses were titered in this same cell line using a fluorescent focus assay as previously described [34].
Murine Model of Undernutrition
Timed pregnant BALB/c mice were purchased from Harlan Laboratories (Indianapolis, IN). All mice were housed in microisolation cages and shown to be rotavirus-negative by serology prior to use. Adoptions were set up to allocate 6 to 7 pups per cage. Fourteen dams of 3-day-old pups were randomized to an ad lib purified control diet (Control: 15% fat, 20% protein, 65% CHO) or an isocaloric regional basic diet (RBD: 5% fat, 7% protein, 88% CHO) to induce weanling undernutrition, as previously described [29]. Both diets were irradiated prior to administration. Beginning on day of life (DOL) 3, mice were weighed every three days. On DOL 21 pups were weaned to their dams’ diet (3–4 mice per cage) and body weights were recorded weekly. All animal procedures were conducted in accordance with the Cincinnati Children’s Hospital Research Foundation Institutional Animal Care and Use Committee.
RRV Vaccination
On DOL 21, 86 weanlings received a single dose (1.0×107 ffu/ml) of RRV by oral gavage (vaccine) or PBS sham. To determine shedding of RRV, two fecal pellets were collected by massage from each mouse individually at days 2, 3, and 4 after immunization and kept in 1 ml of Earle’s balanced salt solution (EBSS). Samples were stored frozen until analyzed, at which time they were homogenized and centrifuged to remove debris. Three weeks later, animals were bled from the orbital sinus and stool was collected for antibody analysis. Serum samples were centrifuged 10 min at 400×g and the sera was stored at −20°C. Rotavirus antigen and antibody levels, including total and rotavirus specific levels, were then quantified using ELISA as described previously described [35]
EDIM challenge and detection of viral shedding and antibodies
Three weeks following vaccination (week 6), all pups were challenged by oral gavage with murine rotavirus EDIM strain at a dose of 4 × 104 FFU (focus-forming units), or 105 shedding dose 50. To measure rotavirus shedding, two fecal pellets were collected from each mouse each day for 7 days following EDIM challenge and processed as described above. Serum and two fecal pellets were collected immediately prior to challenge (week 6) for analysis of pre-EDIM antibody titers and again at week 9 for analysis of post-EDIM titers. We did not test sera for viremia.
Statistical Methods
All statistical analyses were performed using the statistical software package GraphPad Prism, version 5. A two-sample t test was used when two groups were compared. ANOVA was used when more than two groups were compared, with Bonferroni corrections for multiple comparisons of anti-rotavirus and total antibody corrected immunoglobulin levels. Mann-Whitney U and Kruskal-Wallis tests were used compare data sets with non-parametric data as determined by a D’Agostino-Pearson normality test. Two-sided P values less than the Bonferroni-corrected values were considered statistically significant.
RESULTS
The regional basic diet (RBD) produces failure to thrive in pups and weanlings
We randomized dams of 3-day-old litters to a purified control diet (CD: 15% fat, 20% protein, 65% CHO, N=7) or an isocaloric regional basic diet (RBD: 5% fat, 7% protein, 88% CHO, N=7) formulated to induce protein energy malnutrition (Figure 1). All pups of RBD dams showed reduced weight (Figure 2A) by DOL 9 compared to pups of CD dams and remained underweight at the time of both RRV inoculation and EDIM challenge (Figure 2B; P<.0001 by RM ANOVA). RBD dams lost weight relative to CD dams as early as pup DOL 9 and continued to lose weight until weaning (data not shown).
Figure 1. Experimental design for comparing rotavirus immunization (RRV) and challenge (EDIM) responses in mice undernourished by a multideficient regional basic diet (RBD).
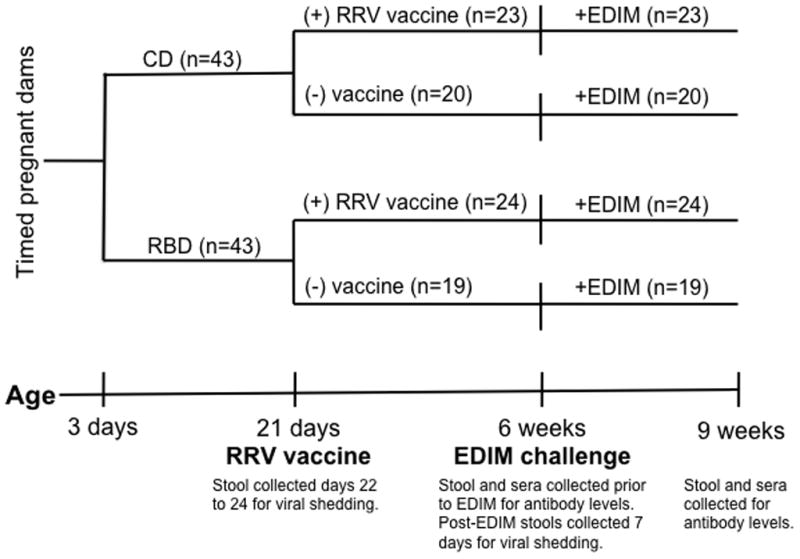
We randomized dams of 3-day-old litters to a purified control diet (CD: 15% fat, 20% protein, 65% CHO, N=7) or an isocaloric, regional basic diet (RBD: 5% fat, 7% protein, 88% CHO, N=7) formulated to induce protein-energy malnutrition. At 3 weeks of age, we weaned CD and RBD pups to their dams’ diet and subrandomized weanlings to receive a single dose of either live oral rotavirus vaccine (RRV) or PBS. At 6 weeks of age, we orally challenged all groups with murine rotavirus (EDIM). Serum and stool specimens were collected before and after RRV and EDIM administration to measure viral shedding and antibody responses by ELISA.
Figure 2. Regional Basic Diet (RBD) produces failure to thrive in suckling and weanling pups.
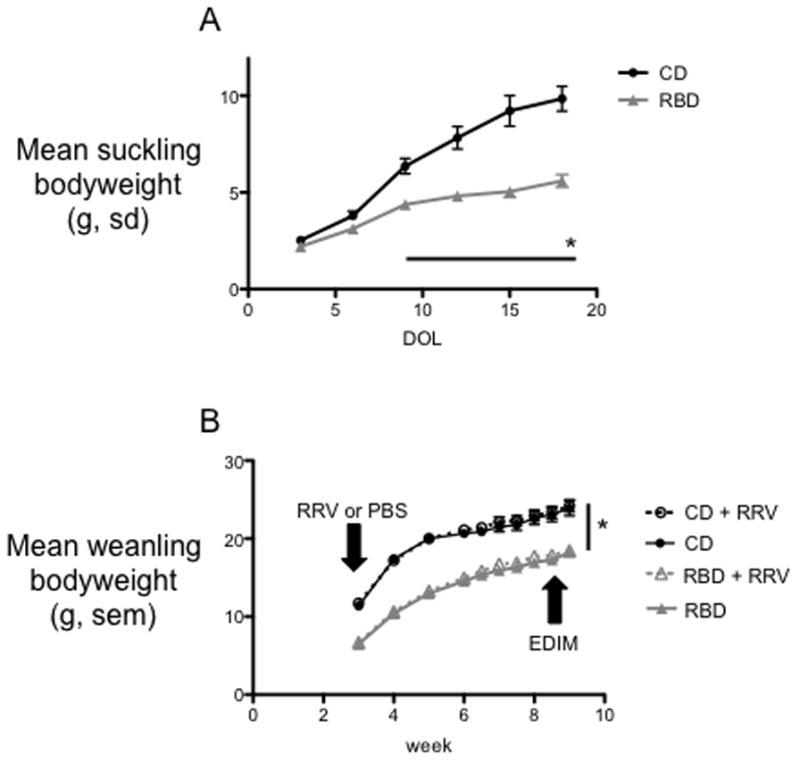
A) Mean bodyweight of suckling pups, day of life 3 to 21, whose dams received a control diet (CD) or RBD. Pups of RBD dams diet failed to thrive compared to pups of CD dams (*P<.0001 by RM ANOVA). N = 43 mice per group. Error bars represent standard deviations. B) Pups were weaned to their dams’ diet on DOL 21. RBD mice remained underweight relative to age-matched controls (*P<.0001 by RM ANOVA). Mouse weights were influenced neither by vaccination nor EDIM challenge. N= 19 to 24 mice per group. Error bars represent standard error.
Serum and stool antibody responses to RRV immunization in RBD vs. CD mice
To determine the effects of undernutrition on mouse responses to rotavirus vaccination, 22-day-old RBD and CD weanlings were immunized with either RRV (1.0×107 ffu/ml, N=47) or PBS (N= 39) by oral gavage. RRV shedding was detectable in only 1 of 23 and 2 of 24 vaccinated CD and RBD mice, respectively. In separate experiments, we tested a 3-fold higher dose of RRV (3.0×107 ffu/ml) and detected viral shedding in 50% of all mice, regardless of nutritional status (data not shown). To prevent over-immunization and masking potential effects of undernutrition on RRV− protection, we chose to perform our study with the original (1.0×107 ffu/ml) RRV dose.
Comparing the response to RRV vaccine in RBD vs. CD animals by antibody levels obtained at week 6 (just prior to EDIM challenge) revealed that both anti-RV IgG and sera anti-RV IgA were increased in RBD mice relative to CD mice (Figure 3A and 3B), however this difference was not significant when correcting for increases in total IgG and total sera IgA in RBD mice (Figures 3D and 3E). We detected no difference in anti-RV stool IgA between CD and RBD mice (Figure 3C); however, total stool IgA was decreased in RBD mice relative to CD mice (2208±188mg/ml vs. 5155±425mg/ml; P<0.0001) (Figure 3F). Total serum IgA was increased in RBD mice compared to controls in both vaccinated and unvaccinated groups (4772±609mg/ml vs. 2812±265mg/ml, P<0.01; 4248±279mg/ml vs. 2403±208mg/ml, P<0.05; Figure 3E).
Figure 3. ELISA quantification of serum and stool anti-rotavirus and total serum antibodies before rotavirus (EDIM) challenge.
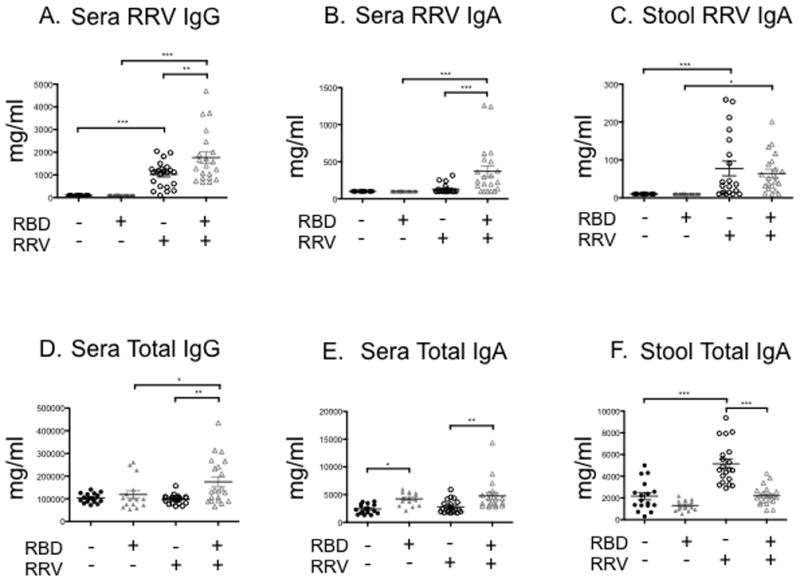
Unvaccinated control diet (RBD−, RRV−, solid black circles) and unvaccinated regional basic diet (RBD+, RRV−, solid grey triangles) weanling mice were vaccinated with placebo on DOL 21. Vaccinated control diet (RBD−, RRV+, open black circles) and vaccinated regional basic diet (RBD+, RRV+, open grey triangles) weanling mice were vaccinated with RRV DOL 21. A) Sera anti-RV IgG, B) sera anti-RV IgA, C) stool anti-RV IgA, D) sera total IgG, E) sera total IgA, and F) stool total IgA. Serum and stool were collected one day before EDIM challenge (week 6). N = 19 to 24 mice per group. Error bars represent standard error. (***P<0.001, **P<0.01, *P<0.05)
Rotavirus vaccine protects undernourished and nourished mice equally against EDIM
To determine the extent to which undernutrition influences protection from EDIM infection and viral replication in immunized vs. unimmunized and nourished vs. undernourished mice, we challenged all 4 experimental groups with murine rotavirus (EDIM) by oral gavage at 6 weeks of age and collected stool for 7 days immediately post-challenge. Rotavirus vaccine was highly efficacious in both nourished and undernourished mice. As shown in Figure 4, we observed a significant reduction in virus shedding in RRV-immunized RBD and CD mice compared to unimmunized controls. In unimmunized mice, peak intensity of infection occurred 1 day earlier in the RBD group (Figure 4). Day 2 after EDIM challenge, viral shedding was 1917±487ng/ml for control mice and 5018±622ng/ml for RBD mice (P<0.001) while on Day 3, viral shedding was 4708±580ng/ml for control mice and 2361±374/ml for RBD mice (P<0.01).
Figure 4. The regional basic diet (RBD) accelerates rotavirus shedding in undernourished mice but does not impair rotavirus vaccine-acquired immunity.
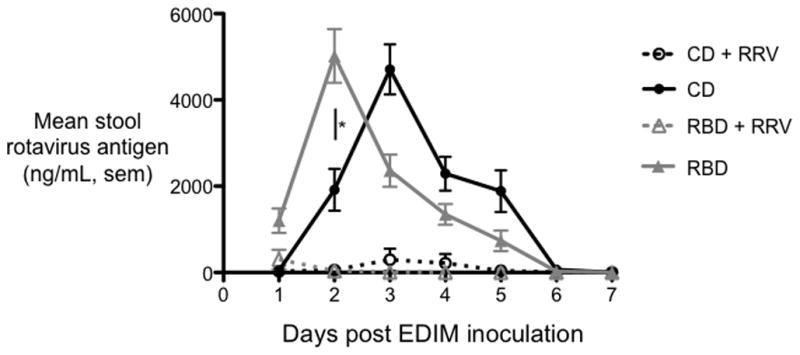
ELISA measurements of stool rotavirus antigen following EDIM challenge at age 9 weeks in undernourished (RBD, triangles) and nourished (control diet (CD), circles) mice, unvaccinated (dotted lines) or vaccinated (solid lines) at age 3 weeks with RRV. Peak rotavirus shedding occurred 1 day earlier in undernourished, unvaccinated mice (P<.05). N = 19 to 24 mice per group. Error bars represent standard error.
Serum and stool antibody responses to EDIM challenge in undernourished mice
We detected no differences in titers of anti-RV serum IgG, anti-RV stool IgA, total serum IgG and total serum IgA following EDIM challenge in unvaccinated RBD and CD mice (Figure 5A, 5C, 5D, and 5F). Moreover, we found no differences in levels of anti-RV serum IgG and anti-RV stool IgA between vaccinated RBD and CD mice (Figure 5A and 5C). In contrast, both immunized and unimmunized RBD mice exhibited significantly higher mean anti-RV serum IgA relative to nourished controls (P<.0001 by ANOVA, Figure 5B). Unvaccinated RBD mice showed significant increases in total serum IgA (Figure 5E, P<0.01). Furthermore, in immunized RBD mice a higher percentage of rotavirus stool IgA was specific for RV following EDIM challenge relative to nourished controls (mean of 23% vs. 9%; P<0.001 by ANOVA corrected for total IgA).
Figure 5. ELISA quantification of serum and stool anti-rotavirus and total serum antibodies following rotavirus (EDIM) challenge.
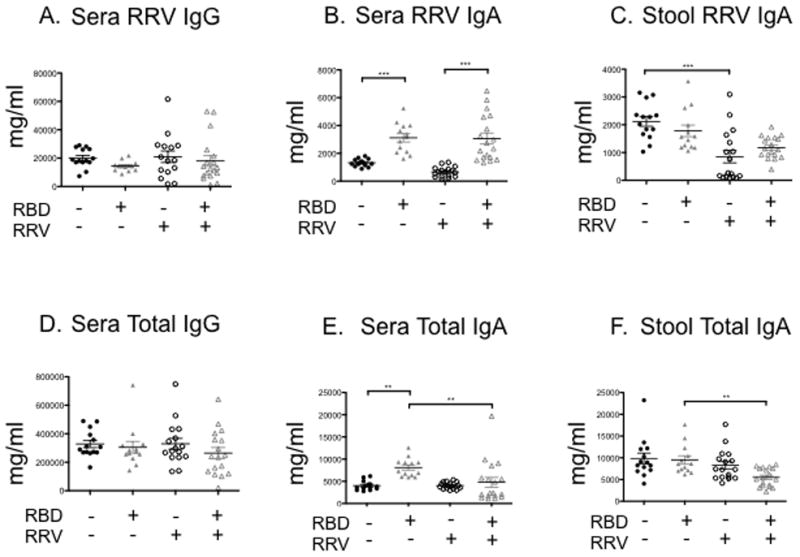
Unvaccinated control diet (RBD−, RRV−, solid black circles) and unvaccinated regional basic diet (RBD+, RRV−, solid grey triangles) weanling mice were vaccinated with placebo on DOL 21. Vaccinated control diet (RBD−, RRV+, open black circles) and vaccinated regional basic diet (RBD+, RRV+, open grey triangles) weanling mice were vaccinated with RRV DOL 21. A) Sera anti-RV IgG, B) sera anti-RV IgA, C) stool anti-RV IgA, D) sera total IgG, E) sera total IgA, and F) stool total IgA. Serum and stool were collected post-EDIM challenge (week 9). N = 19 to 24 mice per group. Error bars represent standard error.
DISCUSSION
In this first ever study of effects of weanling undernutrition on immune responses to both rotavirus immunization (RRV) and challenge (EDIM) we find that oral rotavirus vaccination adequately protects mice against EDIM despite altered antibody responses to vaccination and challenge. In addition, we show that serum anti-rotavirus IgA levels are elevated in both immunized and unimmunized undernourished mice following EDIM infection. We further demonstrate that unimmunized, undernourished mice shed rotavirus more rapidly than unimmunized, nourished mice. Strikingly, we find that in immunized RBD mice anti-RV stool IgA makes up a higher percentage of the total stool IgA compared to CD mice, both pre- and post-EDIM challenge.
Similar to secondary analyses of clinical trial data conducted by Parez-Schael et al., we found that malnutrition alone does not impair the efficacy of rotavirus immunization [30]. The strengths of our laboratory study design allowed us to examine undernutrition, rotavirus immunization, and rotavirus infection, alone and in combination, with appropriate controls for age and diet. One potential limitation of our study design is that we did not pair feed mice. Pair feeding of control mice, instead of ad libitum access to an isocaloric control diet, would have further strengthened our design by controlling for potential effects of amount of rations consumed.
We predicted that undernourished mice would be more susceptible to rotavirus replication and have more severe disease, however this was clearly not the case. As previously observed by Offor et al in malnourished suckling mice [36], we found accelerated rotavirus shedding in undernourished mice, however both undernourished and nourished animals were able to clear rotavirus effectively. These later results stand in contrast to findings by Guerrant and colleagues that report more severe disease and exacerbation of malnutrition when undernourished mice are infected with Cryptosporidium [37], Giardia [38] and enteroaggregative E. coli [39]. Of note, by choosing to challenge adult mice, our models were better designed to examine rotavirus infection and shedding rather than frank diarrhea—a response limited to EDIM infection of young mice. Additional host factors that might account for the divergence of our findings from other published mouse models of malnutrition and gut infection include mouse strain and the method by which undernutrition is induced, e.g. caloric restriction vs. multideficient diets vs. timed separations of pups from dams.
To our knowledge, the “vicious cycle” of diarrhea and undernutrition has not yet been definitively recapitulated in rodent models of viral diarrhea. In addition, the findings of our mouse study parallel results of a large case-control study of diarrhea hospitalizations in Bangladesh, which found that children admitted with rotavirus-positive diarrhea had better nutritional status than children admitted for parasitic or bacteria-associated diarrheal illnesses [40]. Another recent mouse study also found that underweight mice had one less day of diarrhea as compared to their normal-weight and overweight counterparts [41]. The current animal data, together with previously published clinical findings, suggest that undernutrition may indeed be an important risk factor for initial or even repeat rotavirus infections, but that mild-to-moderate malnutrition is not a significant contributor to the severity of rotavirus infections.
When nourished and undernourished mice were vaccinated with RRV, we found no group differences in viral clearance following EDIM challenge; however, we did detect group differences in serum and stool antibody responses. Lower levels of total stool IgA in RBD vaccinated mice compared to CD mice might be explained by a deficiency of mucosal IgA production or transport secondary to a delay in maturation of the secretory IgA system due to protein malnutrition, as reported by Green and Heyworth [42]. Our finding of increased serum IgA and IgG in RBD-fed mice is also supported by the work of Neumann et al. who described increases in these immunoglobulins in malnourished children [43]. We have previously described intestinal barrier defects in mice fed the regional basic diet that parallel those seen in children with environmental enteropathy, hence gut-to-blood bacterial translocation leading to a systemic immune response and elevations in serum immunoglobulins may explain our current findings [31].
Three decades after the first trial of a live oral rotavirus vaccine candidate, rotavirus immunizations are now a key component of global strategies to reduce childhood deaths from diarrhea [9]. Although global malnutrition remains the most common cause of human immunodeficiency worldwide and is known to alter cellular mediated immunity, the complement system, and phagocytosis [44], malnutrition alone did not recapitulate the “tropical barrier” in our model. Alternative explanations for the tropical barrier—and strategies to optimize live oral vaccine response in the developing world—will require intensive additional study. Pre-clinical models of co-infection with other pathogens such as helminths [45], micronutrient deficiencies [46], small bowel bacterial overgrowth [20], maternal antibodies [47], and environmental enteropathy [18] all merit further consideration.
We conclude that rotavirus vaccination protects nourished and undernourished mice equally against rotavirus infection, despite significant differences in antibody responses to immunization and challenge. Further laboratory and clinical studies are urgently needed to elucidate host, pathogen, and environmental factors underlying the impaired efficacy of rotavirus vaccines in the developing world in order to continue to improve outcomes for the world’s most vulnerable children [48].
Highlights.
Weanling undernutrition alters serum and stool IgA responses to rhesus rotavirus vaccination and murine rotavirus infection
Undernutrition does not impair vaccine-acquired protection against rotavirus
Undernutrition accelerates murine rotavirus shedding but does not impair viral clearance
Acknowledgments
Funding: Supported by a Round 7 Grand Challenges Explorations Award from the Bill & Melinda Gates Foundation, an Independent Scientist in Global Health Award K02 from the Fogarty International Center/NIH, and Cincinnati Children’s Research Foundation.
Abbreviations
- RRV
rhesus rotavirus vaccine
- EDIM
epizootic diarrhea of infant mice
- RV
rotavirus
- ELISA
Footnotes
Disclosures: No conflicts of interest
Presented in part at the 2012 annual Meeting of the American Society of Tropical Medicine in Atlanta, GA and Hygiene and the 2013 Nutrition-Agriculture Convening at the Bill & Melinda Gates Foundation in Seattle, WA.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Smiley KL, McNeal MM, Basu M, Choi AH, Clements JD, Ward RL. Association of gamma interferon and interleukin-17 production in intestinal CD4+ T cells with protection against rotavirus shedding in mice intranasally immunized with VP6 and the adjuvant LT(R192G) Journal of virology. 2007;81:3740–8. doi: 10.1128/JVI.01877-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Glass RI, Bresee JS, Turcios R, Fischer TK, Parashar UD, Steele aD. Rotavirus vaccines targeting the developing world. Journal of Infectious Disease. 2005;192 (Suppl):S160–6. doi: 10.1086/431504. [DOI] [PubMed] [Google Scholar]
- 3.Tate JE, Burton AH, Boschi-Pinto C, Steele aD, Duque J, Parashar UD. 2008 Estimate of Worldwide Rotavirus-Associated Mortality in Children Younger Than 5 Years Before the Introduction of Universal Rotavirus Vaccination Programmes: a Systematic Review and Meta-Analysis. The Lancet Infectious Diseases. 2012;12:136–41. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- 4.Parashar U, Steele D, Neuzil K, et al. Progress with rotavirus vaccines: summary of the Tenth International Rotavirus Symposium. Expert Review of Vaccines. 2013;12:113–7. doi: 10.1586/erv.12.148. [DOI] [PubMed] [Google Scholar]
- 5.Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. The Lancet. 2013 doi: 10.1016/S0140-6736(13)60844-2. [DOI] [PubMed] [Google Scholar]
- 6.Cunliffe N, Nakagomi O. Introduction of rotavirus vaccines in developing countries: remaining challenges. Annals of tropical paediatrics. 2007;27:157–67. doi: 10.1179/146532807X220262. [DOI] [PubMed] [Google Scholar]
- 7.Salazar-Lindo E, Allen S, Brewster DR, et al. Intestinal infections and environmental enteropathy Working Group report of the second World Congress of Pediatric Gastroenterology, Hepatology, and Nutrition. Journal of pediatric gastroenterology and nutrition. 2004;39 (Suppl 2):S662–9. doi: 10.1097/00005176-200406002-00013. [DOI] [PubMed] [Google Scholar]
- 8.Soares-Weiser K, MacLehose H, Bergman H, et al. The Cochrane Collaboration. 2012. Vaccines for preventing rotavirus diarrhoea vaccines in use (Review) [DOI] [PubMed] [Google Scholar]
- 9.UNICEF, WHO. Diarrhoea: Why children are still dying and what can be done. 2009. [Google Scholar]
- 10.Cortese MM, Cunliffe NA, Oliveira LHd, Gentsch JR, Glass RI, Jiang B. Global impact of rotavirus vaccines. Expert Review of Vaccines. 2010;9:395. doi: 10.1586/erv.10.17. [DOI] [PubMed] [Google Scholar]
- 11.Schaetti C. Vaccines for enteric diseases update on recent developments. Expert Review of Vaccines. 2009;8:1653–5. doi: 10.1586/erv.09.122. [DOI] [PubMed] [Google Scholar]
- 12.Shaw AR. The rotavirus saga revisited. Annual review of medicine. 2013;64:165–74. doi: 10.1146/annurev-med-121511-093810. [DOI] [PubMed] [Google Scholar]
- 13.Patel M, Shane AL, Parashar UD, Jiang B, Gentsch JR, Glass RI. Oral rotavirus vaccines: how well will they work where they are needed most? The Journal of infectious diseases. 2009;200 (Suppl 1):S39–48. doi: 10.1086/605035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cunliffe NA, Witte D, Ngwira BM, et al. Efficacy of human rotavirus vaccine against severe gastroenteritis in Malawian children in the first two years of life: a randomized, double-blind, placebo controlled trial. Vaccine. 2012;30 (Suppl 1):A36–43. doi: 10.1016/j.vaccine.2011.09.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaman K, Anh DD, Victor JC, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in Asia: a randomised, double-blind, placebo-controlled trial. The Lancet. 2010;376:615–23. doi: 10.1016/S0140-6736(10)60755-6. [DOI] [PubMed] [Google Scholar]
- 16.Armah GE, Sow SO, Breiman RF, et al. Efficacy of pentavalent rotavirus vaccine against severe rotavirus gastroenteritis in infants in developing countries in sub-Saharan Africa: a randomised, double-blind, placebo-controlled trial. The Lancet. 2010;376:606–14. doi: 10.1016/S0140-6736(10)60889-6. [DOI] [PubMed] [Google Scholar]
- 17.Pasetti MF, Simon JK, Sztein MB, Levine MM. Immunology of gut mucosal vaccines. Immunological reviews. 2011;239:125–48. doi: 10.1111/j.1600-065X.2010.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Levine MM. Immunogenicity and efficacy of oral vaccines in developing countries lessons from a live cholera vaccine. BMC Biology. 2010:8. doi: 10.1186/1741-7007-8-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Czerkinsky C, Holmgren J. Enteric vaccines for the developing world: a challenge for mucosal immunology. Mucosal immunology. 2009;2:284–7. doi: 10.1038/mi.2009.22. [DOI] [PubMed] [Google Scholar]
- 20.Lagos R, Fasano A, Wasserman SS, et al. Effect of small bowel bacterial overgrowth on the immunogenicity of single-dose live oral cholera vaccine CVD 103-HgR. Journal of Infectious Disease. 1999;180:1709–12. doi: 10.1086/315051. [DOI] [PubMed] [Google Scholar]
- 21.Bhan A, Green SK. Improving rotavirus vaccine adoption in low- and middle-income countries. Journal of Global Health. 2011;1:148–53. [PMC free article] [PubMed] [Google Scholar]
- 22.Schlaudecker EP, Steinhoff MC, Moore SR. Interactions of diarrhea, pneumonia, and malnutrition in childhood: recent evidence from developing countries. Current opinion in infectious diseases. 2011;24:496–502. doi: 10.1097/QCO.0b013e328349287d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ramakrishna BS, Venkataraman S, Mukhopadhya A. Tropical malabsorption. Postgraduate Medical Journal. 2006;82:779–87. doi: 10.1136/pgmj.2006.048579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baker S, Mathan V. Tropical enteropathy and tropical sprue. American Journal of Clinical Nutrition. 1972;25:1047–55. doi: 10.1093/ajcn/25.10.1047. [DOI] [PubMed] [Google Scholar]
- 25.Kelly P, Menzies I, Crane R, et al. Responses of small intestinal architecture and function over time to environmental factors in a tropical population. American Journal of Tropical Medicine and Hygiene. 2004;70:412–19. [PubMed] [Google Scholar]
- 26.Prendergast A, Kelly P. Enteropathies in the developing world: neglected effects on global health. American Journal of Tropical Medicine and Hygiene. 2012;86:756–63. doi: 10.4269/ajtmh.2012.11-0743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Korpe PS, Petri Wa. Environmental enteropathy: critical implications of a poorly understood condition. Trends in Molecular Medicine. 2012;18:328–36. doi: 10.1016/j.molmed.2012.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karlsson EK, Harris JB, Tabrizi S, et al. Natural selection in a bangladeshi population from the cholera-endemic ganges river delta. Science translational medicine. 2013;5:192ra86. doi: 10.1126/scitranslmed.3006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Linhares AC, Carmo KnBd, Oliveira KK, et al. Nutritional status in relation to the efficacy of the rhesus-human reassortant, tetravalent rotavirus vaccine (RRV-TV) in infants from Belém, pará state, Brazil. Revista do Instituto de Medicina Tropical de São Paulo. 2002;44:13–6. doi: 10.1590/s0036-46652002000100003. [DOI] [PubMed] [Google Scholar]
- 30.Perez-Schael I, Salinas B, Tomat M, et al. Efficacy of the human rotavirus vaccine RIX4414 in malnourished children. The Journal of infectious diseases. 2007;196:537–40. doi: 10.1086/519687. [DOI] [PubMed] [Google Scholar]
- 31.Ueno PM, Oriá RB, Maier Ea, et al. Alanyl-glutamine promotes intestinal epithelial cell homeostasis in vitro and in a murine model of weanling undernutrition. American journal of physiology, gastrointestinal and liver physiology. 2011;301:G612–22. doi: 10.1152/ajpgi.00531.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNeal M, Broome R, Ward R. Active immunity against rotavirus infection in mice is correlated with viral replication and titers of serum rotavirus IgA following vaccination. Virology. 1994;204:642–50. doi: 10.1006/viro.1994.1579. [DOI] [PubMed] [Google Scholar]
- 33.Teodosio N, Lago E, Romani S, Guedes R. A regional basic diet from northeast Brazil as a dietary model of experimental malnutrition. Arch Latinoam Nutr. 1990;40:533–47. [PubMed] [Google Scholar]
- 34.Knowlton DR, Spector DM, Ward RL. Development of an improved method for measuring neutralizing antibody to rotavirus. Journal of virological methods. 1991;33:127–34. doi: 10.1016/0166-0934(91)90013-p. [DOI] [PubMed] [Google Scholar]
- 35.Ward RL, Bernstein DI, Young EC, Sherwood JR, Knowlton DR, Schiff GM. Human rotavirus studies in volunteers: determination of infectious dose and serological response to infection. Journal of Infectious Disease. 1986;154:871–80. doi: 10.1093/infdis/154.5.871. [DOI] [PubMed] [Google Scholar]
- 36.EO, MR-T, PLO Effect of malnutrition on rotavirus infection in suckling mice: kinetics of early infection. Proc Soc Exp Biol Med. 1985;178:85–90. doi: 10.3181/00379727-178-41987. [DOI] [PubMed] [Google Scholar]
- 37.Costa LB, JohnBull Ea, Reeves JT, et al. Cryptosporidium-malnutrition interactions: mucosal disruption, cytokines, and TLR signaling in a weaned murine model. Journal of Parasitology. 2011;97:1113–20. doi: 10.1645/GE-2848.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bartelt LA, Roche J, Kolling G, et al. Persistent G lamblia impairs growth in a murine malnutrition model. Journal of Clinical Investigation. 2013;123:2672–84. doi: 10.1172/JCI67294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bolick DT, Roche JK, Hontecillas R, Bassaganya-Riera J, Nataro JP, Guerrant RL. Enteroaggregative Escherichia coli strain in a novel weaned mouse model: exacerbation by malnutrition, biofilm as a virulence factor and treatment by nitazoxanide. Journal of Medical Microbiology. 2013;62:896–905. doi: 10.1099/jmm.0.046300-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Unicomb L, Kilgore P, Faruque S, et al. Anticipating rotavirus vaccines: hospital-based surveillance for rotavirus diarrhea and estimates of disease burden in Bangladesh. Pediatric Infectious Disease Journal. 1997;16:947–51. doi: 10.1097/00006454-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 41.Preidis GA, Saulnier DM, Blutt SE, et al. Host response to probiotics determined by nutritional status of rotavirus-infected neonatal mice. Journal of pediatric gastroenterology and nutrition. 2012;55:299–307. doi: 10.1097/MPG.0b013e31824d2548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Green F, Heyworth B. Immunoglobulin-containing cells in jejunal mucosa of children with protein-energy malnutrition and gastroenteritis. Arch Dis Child. 1980;55:380–3. doi: 10.1136/adc.55.5.380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Neumann C, Lawlor G, Stiehm E, et al. Immunologic responses in malnourished children. The American Journal of Clinical Nutrition. 1975;28:89–104. doi: 10.1093/ajcn/28.2.89. [DOI] [PubMed] [Google Scholar]
- 44.Cripps AW, Otczyk DC, Barker J, Lehmann D, Alpers MP. The relationship between undernutrition and humoral immune status in children with pneumonia in Papua New Guinea. Papua and New Guinea medical journal. 2008;51:120–30. [PubMed] [Google Scholar]
- 45.Cooper PJ, Chico ME, Losonsky G, et al. Albendazole treatment of children with ascariasis enhances the vibriocidal antibody response to the live attenuated oral cholera vaccine CVD 103-HgR. The Journal of infectious diseases. 2000;182:1199–206. doi: 10.1086/315837. [DOI] [PubMed] [Google Scholar]
- 46.Ahmed T, Arifuzzaman M, Lebens M, Qadri F, Lundgren A. CD4+ T-cell responses to an oral inactivated cholera vaccine in young children in a cholera endemic country and the enhancing effect of zinc supplementation. Vaccine. 2009;28:422–9. doi: 10.1016/j.vaccine.2009.10.032. [DOI] [PubMed] [Google Scholar]
- 47.Chan J, Nirwati H, Triasih R, et al. Maternal antibodies to rotavirus: could they interfere with live rotavirus vaccines in developing countries? Vaccine. 2011;29:1242–7. doi: 10.1016/j.vaccine.2010.11.087. [DOI] [PubMed] [Google Scholar]
- 48.Lopman BA, Pitzer VE, Sarkar R, et al. Understanding reduced rotavirus vaccine efficacy in low socioeconomic settings. PloS one. 2012;7:e41720. doi: 10.1371/journal.pone.0041720. [DOI] [PMC free article] [PubMed] [Google Scholar]


