Abstract
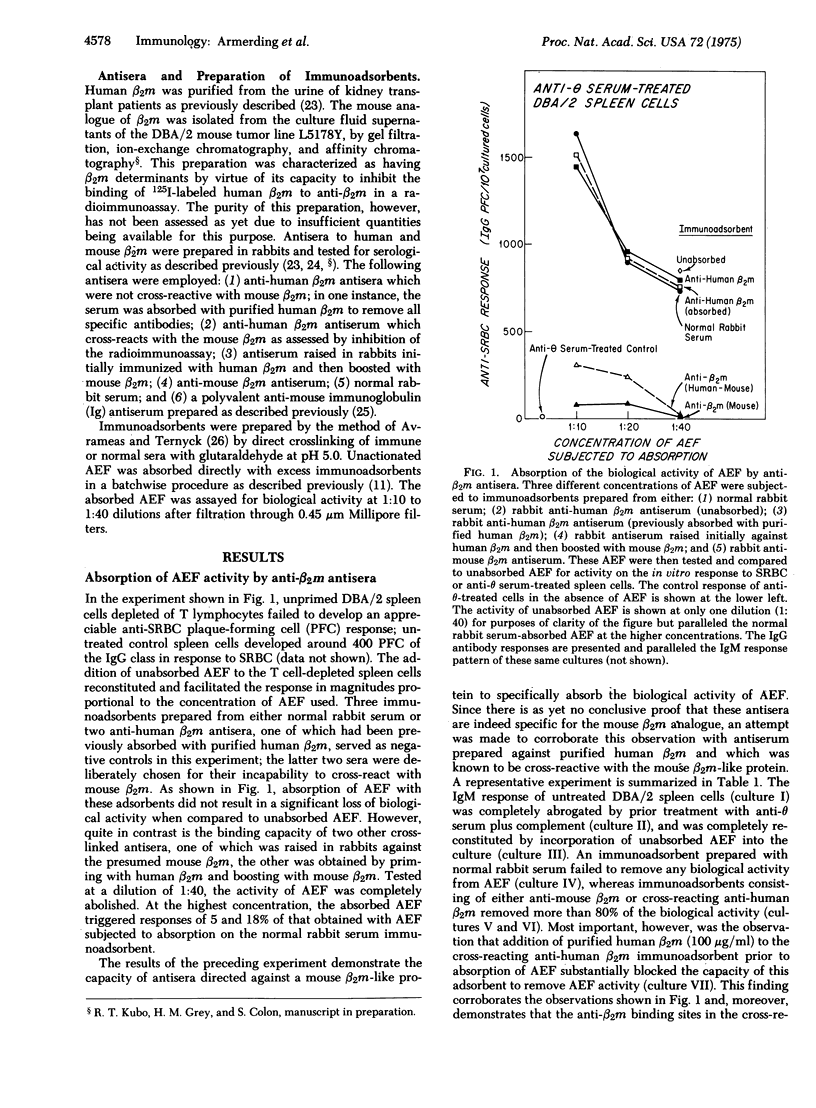
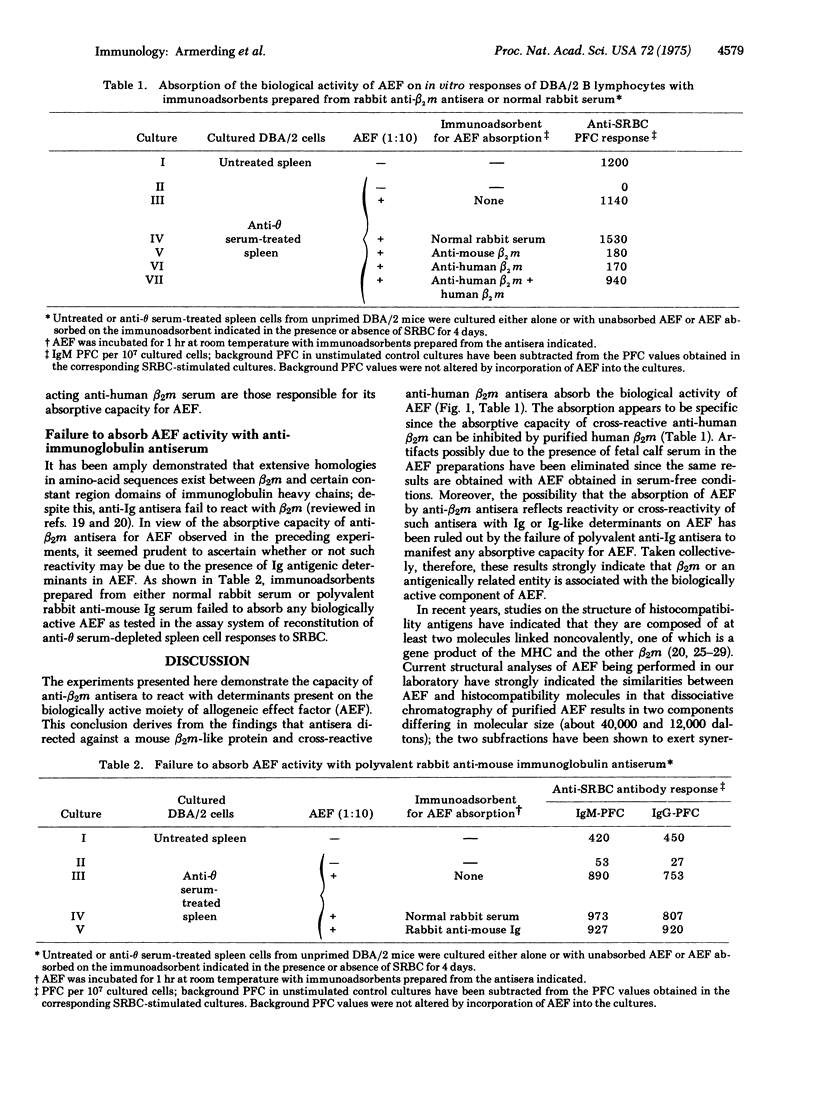
The biologically acitve entity termed allogeneic effect factor, which is produced by alloantigen-activated T cells and has been shown to regulate triggering and differentiation of B lymphocytes in vitro, has previously been demonstrated to possess antigenic determinants coded by genes in the I region of the H-2 gene complex of the mouse. The studies presented here provide evidence that allogeneic effect factor also possesses antigenic determinants identical or cross-reactive with beta2-microglobulin. These observations suggest an important role for beta2-microglobulin in the mechanism of cell-cell interactions and the consequences of such interactions on lymphocyte triggering and differentiation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson C. L., Kubo R. T., Grey H. M. Studies on the cytophilic properties of human beta2 microglobulin. J Immunol. 1975 Mar;114(3):997–1000. [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. I. Regulatory influence of bacterial lipopolysaccharide (LPS) on specific T-cell helper function. J Exp Med. 1974 Jan 1;139(1):24–43. doi: 10.1084/jem.139.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Katz D. H. Activation of T and B lymphocytes in vitro. II. Biological and biochemical properties of an allogeneic effect factor (AEF) active in triggering specific B lymphocytes. J Exp Med. 1974 Jul 1;140(1):19–37. doi: 10.1084/jem.140.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D., Sachs D. H., Katz D. H. Activation of T and B lymphocytes in vitro. III. Presence of Ia determinants on allogeneic effect factor. J Exp Med. 1974 Dec 1;140(6):1717–1722. doi: 10.1084/jem.140.6.1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armerding D. Two allotypic specifities of rat immunoglobulin. Eur J Immunol. 1971 Jan;1(1):39–45. doi: 10.1002/eji.1830010108. [DOI] [PubMed] [Google Scholar]
- Avrameas S., Ternynck T. The cross-linking of proteins with glutaraldehyde and its use for the preparation of immunoadsorbents. Immunochemistry. 1969 Jan;6(1):53–66. doi: 10.1016/0019-2791(69)90178-5. [DOI] [PubMed] [Google Scholar]
- Cullen S. E., David C. S., Shreffler D. C., Nathenson S. G. Membrane molecules determined by the H-2 associated immune response region: isolation and some properties. Proc Natl Acad Sci U S A. 1974 Mar;71(3):648–652. doi: 10.1073/pnas.71.3.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham B. A., Berggård I. Structure, evolution and significance of beta2-microglobulin. Transplant Rev. 1974;21(0):3–14. doi: 10.1111/j.1600-065x.1974.tb01543.x. [DOI] [PubMed] [Google Scholar]
- Erb P., Feldmann M. Role of macrophages in in vitro induction of T-helper cells. Nature. 1975 Mar 27;254(5498):352–354. doi: 10.1038/254352a0. [DOI] [PubMed] [Google Scholar]
- Grey H. M., Kubo R. T., Colon S. M., Poulik M. D., Cresswell P., Springer T., Turner M., Strominger J. L. The small subunit of HL-A antigens is beta 2-microglobulin. J Exp Med. 1973 Dec 1;138(6):1608–1612. doi: 10.1084/jem.138.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. II. Failure of physiologic cooperative interactions between T and B lymphocytes from allogeneic donor strains in humoral response to hapten-protein conjugates. J Exp Med. 1973 Jun 1;137(6):1405–1418. doi: 10.1084/jem.137.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Hamaoka T., Dorf M. E., Benacerraf B. Cell interactions between histoincompatible T and B lymphocytes. The H-2 gene complex determines successful physiologic lymphocyte interactions. Proc Natl Acad Sci U S A. 1973 Sep;70(9):2624–2628. doi: 10.1073/pnas.70.9.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Osborne D. P., Jr The allogeneic effect in inbred mice. II. Establishment of the cellular interactions required for enhancement of antibody production by the graft-versus-host reaction. J Exp Med. 1972 Sep 1;136(3):455–465. doi: 10.1084/jem.136.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindred B., Shreffler D. C. H-2 dependence of co-operation between T and B cells in vivo. J Immunol. 1972 Nov;109(5):940–943. [PubMed] [Google Scholar]
- Lightbody J. J., Urbani L., Poulik M. D. Effect of beta 2 microglobulin antibody on effector function of T-cell mediated cytotoxicity. Nature. 1974 Jul 19;250(463):227–228. doi: 10.1038/250227a0. [DOI] [PubMed] [Google Scholar]
- McCalmon R. T., Kubo R. T., Grey H. M. Effect of anti-beta2-microglobulin on antigen and allogeneic lymphocyte-induced proliferation of human lymphocytes. J Immunol. 1975 Jun;114(6):1766–1770. [PubMed] [Google Scholar]
- Munro A. J., Taussig M. J., Campbell R., Williams H., Lawson Y. Antigen-specific T-cell factor in cell cooperation: physical properties and mapping in the left-hand (K) half of H-2. J Exp Med. 1974 Dec 1;140(6):1579–1587. doi: 10.1084/jem.140.6.1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller E., Persson U. Mitogenic properties of rabbit anti-human beta 2-microglobulin for murine B cells. Scand J Immunol. 1974;3(4):445–454. doi: 10.1111/j.1365-3083.1974.tb01277.x. [DOI] [PubMed] [Google Scholar]
- Rask L., Ostberg L., Lindblom B., Fernstedt Y., Peterson P. A. The subunit structure of transplantation antigens. Transplant Rev. 1974;21(0):85–105. doi: 10.1111/j.1600-065x.1974.tb01547.x. [DOI] [PubMed] [Google Scholar]
- Rosenthal A. S., Shevach E. M. Function of macrophages in antigen recognition by guinea pig T lymphocytes. I. Requirement for histocompatible macrophages and lymphocytes. J Exp Med. 1973 Nov 1;138(5):1194–1212. doi: 10.1084/jem.138.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver J., Hood L. Detergent-solubilised H-2 alloantigen is associated with a small molecular weight polypeptide. Nature. 1974 Jun 21;249(459):764–765. doi: 10.1038/249764a0. [DOI] [PubMed] [Google Scholar]
- Solheim B. G. Association between the beta-2-m-HL-A molecule and membrane structures responsible for lymphocyte activation. Transplant Rev. 1974;21(0):35–52. doi: 10.1111/j.1600-065x.1974.tb01545.x. [DOI] [PubMed] [Google Scholar]
- Strominger J. L., Cresswell P., Grey H., Humphreys R. E., Mann D., McCuneJ, Parham P., Robb R., Sanderson A. R., Springer T. A. The immunoglobulin-like structure of human histocompatibility antigens. Transplant Rev. 1974;21(0):126–143. doi: 10.1111/j.1600-065x.1974.tb01549.x. [DOI] [PubMed] [Google Scholar]
- Tanigaki N., Pressman D. The basic structure and the antigenic characteristics of HL-A antigens. Transplant Rev. 1974;21(0):15–34. doi: 10.1111/j.1600-065x.1974.tb01544.x. [DOI] [PubMed] [Google Scholar]
- Toivanen P., Toivanen A., Vainio O. Complete restoration of bursa-dependent immune system after transplantation of semiallogeneic stem cells into immunodeficient chicks. J Exp Med. 1974 May 1;139(5):1344–1349. doi: 10.1084/jem.139.5.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]


