Abstract
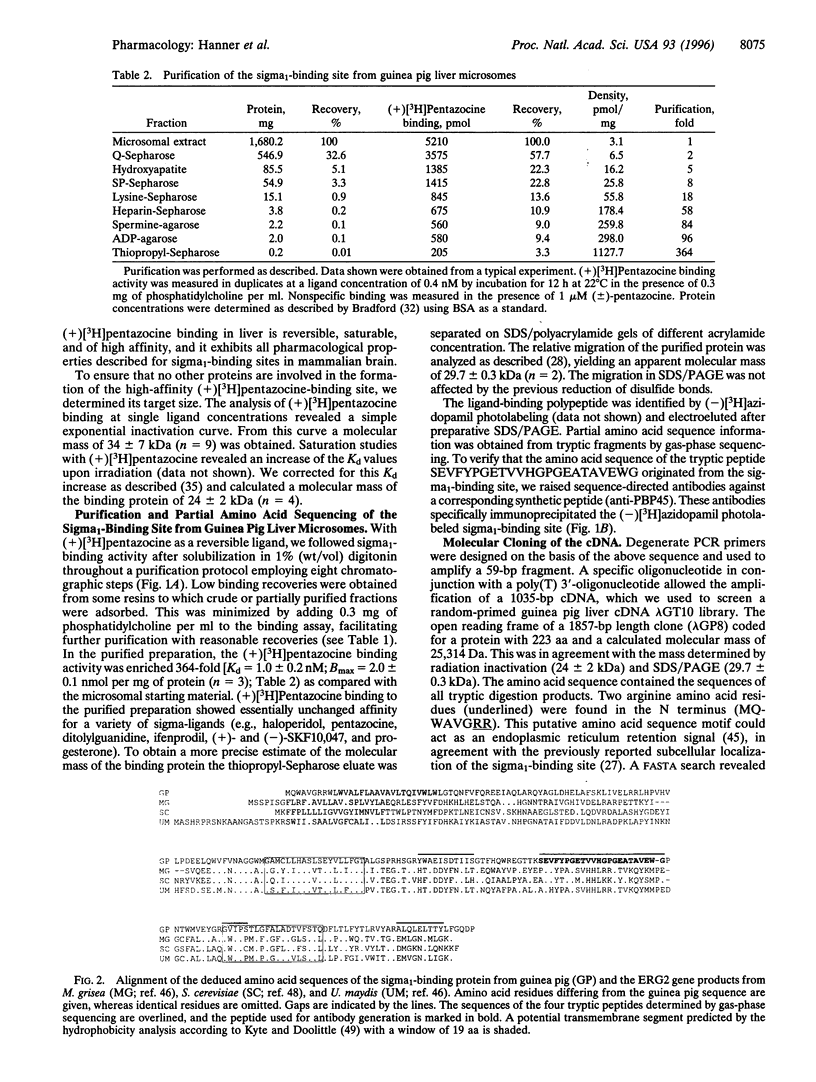
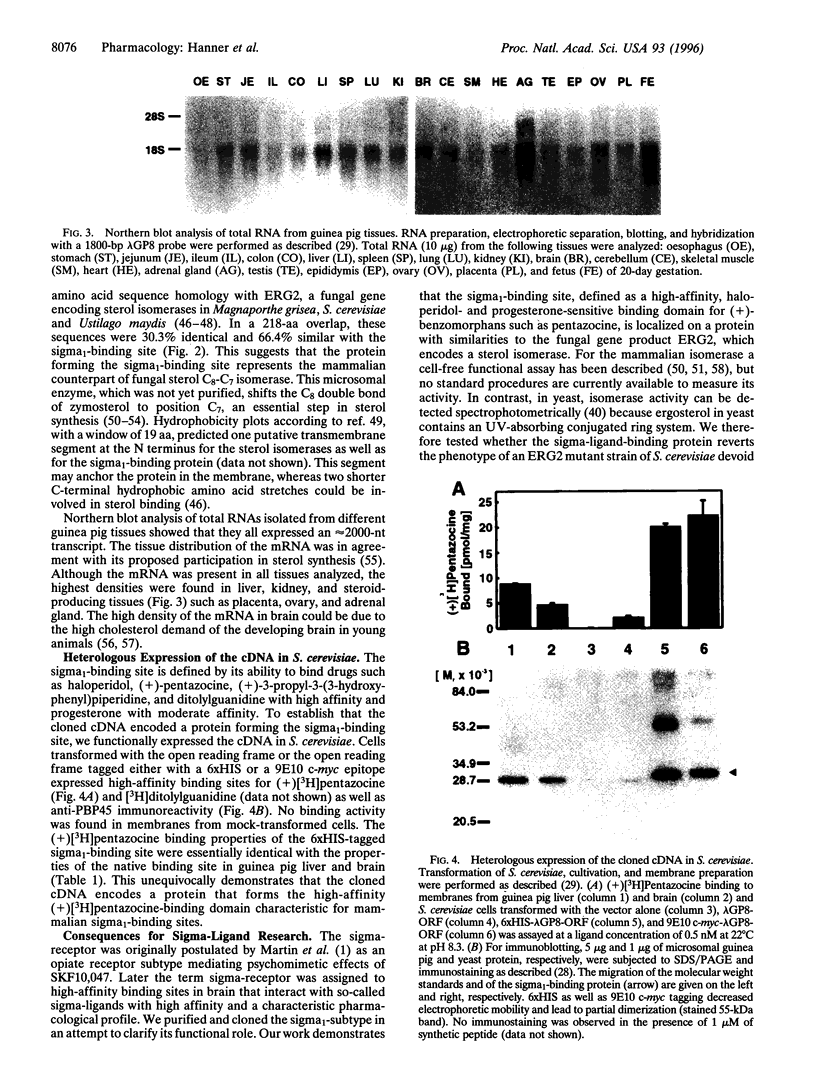
Sigma-ligands comprise several chemically unrelated drugs such as haloperidol, pentazocine, and ditolylguanidine, which bind to a family of low molecular mass proteins in the endoplasmic reticulum. These so-called sigma-receptors are believed to mediate various pharmacological effects of sigma-ligands by as yet unknown mechanisms. Based on their opposite enantioselectivity for benzomorphans and different molecular masses, two subtypes are differentiated. We purified the sigma1-binding site as a single 30-kDa protein from guinea pig liver employing the benzomorphan(+)[3H]pentazocine and the arylazide (-)[3H]azidopamil as specific probes. The purified (+)[3H]pentazocine-binding protein retained its high affinity for haloperidol, pentazocine, and ditolylguanidine. Partial amino acid sequence obtained after trypsinolysis revealed no homology to known proteins. Radiation inactivation of the pentazocine-labeled sigma1-binding site yielded a molecular mass of 24 +/- 2 kDa. The corresponding cDNA was cloned using degenerate oligonucleotides and cDNA library screening. Its open reading frame encoded a 25.3-kDa protein with at least one putative transmembrane segment. The protein expressed in yeast cells transformed with the cDNA showed the pharmacological characteristics of the brain and liver sigma1-binding site. The deduced amino acid sequence was structurally unrelated to known mammalian proteins but it shared homology with fungal proteins involved in sterol synthesis. Northern blots showed high densities of the sigma1-binding site mRNA in sterol-producing tissues. This is also in agreement with the known ability of sigma1-binding sites to interact with steroids, such as progesterone.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arthington B. A., Hoskins J., Skatrud P. L., Bard M. Nucleotide sequence of the gene encoding yeast C-8 sterol isomerase. Gene. 1991 Oct 30;107(1):173–174. doi: 10.1016/0378-1119(91)90314-2. [DOI] [PubMed] [Google Scholar]
- Ashman W. H., Barbuch R. J., Ulbright C. E., Jarrett H. W., Bard M. Cloning and disruption of the yeast C-8 sterol isomerase gene. Lipids. 1991 Aug;26(8):628–632. doi: 10.1007/BF02536427. [DOI] [PubMed] [Google Scholar]
- Bolger G. T., Skolnick P., Kempner E. S. Radiation inactivation reveals discrete cation binding sites that modulate dihydropyridine binding sites. Mol Pharmacol. 1989 Aug;36(2):327–332. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cagnotto A., Bastone A., Mennini T. [3H](+)-pentazocine binding to rat brain sigma 1 receptors. Eur J Pharmacol. 1994 Jan 15;266(2):131–138. doi: 10.1016/0922-4106(94)90102-3. [DOI] [PubMed] [Google Scholar]
- Cuzner M. L., Davison A. N. The lipid composition of rat brain myelin and subcellular fractions during development. Biochem J. 1968 Jan;106(1):29–34. doi: 10.1042/bj1060029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeHaven-Hudkins D. L., Fleissner L. C., Ford-Rice F. Y. Characterization of the binding of [3H](+)-pentazocine to sigma recognition sites in guinea pig brain. Eur J Pharmacol. 1992 Dec 1;227(4):371–378. doi: 10.1016/0922-4106(92)90153-m. [DOI] [PubMed] [Google Scholar]
- DeLean A., Munson P. J., Rodbard D. Simultaneous analysis of families of sigmoidal curves: application to bioassay, radioligand assay, and physiological dose-response curves. Am J Physiol. 1978 Aug;235(2):E97–102. doi: 10.1152/ajpendo.1978.235.2.E97. [DOI] [PubMed] [Google Scholar]
- Dempsey M. E. Pathways of enzymic synthesis and conversion to cholesterol of delta-5,7,24-cholestatrien-3 beta-ol and other naturally occurring sterols. J Biol Chem. 1965 Nov;240(11):4176–4188. [PubMed] [Google Scholar]
- Ferris C. D., Hirsch D. J., Brooks B. P., Snowman A. M., Snyder S. H. [3H]opipramol labels a novel binding site and sigma receptors in rat brain membranes. Mol Pharmacol. 1991 Feb;39(2):199–204. [PubMed] [Google Scholar]
- Ferris R. M., Tang F. L., Chang K. J., Russell A. Evidence that the potential antipsychotic agent rimcazole (BW 234U) is a specific, competitive antagonist of sigma sites in brain. Life Sci. 1986 Jun 23;38(25):2329–2337. doi: 10.1016/0024-3205(86)90640-5. [DOI] [PubMed] [Google Scholar]
- Fletcher E. J., Church J., Abdel-Hamid K., MacDonald J. F. Selective reduction of N-methyl-D-aspartate-evoked responses by 1,3-di(2-tolyl)guanidine in mouse and rat cultured hippocampal pyramidal neurones. Br J Pharmacol. 1993 Aug;109(4):1196–1205. doi: 10.1111/j.1476-5381.1993.tb13749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gewirtz G. R., Gorman J. M., Volavka J., Macaluso J., Gribkoff G., Taylor D. P., Borison R. BMY 14802, a sigma receptor ligand for the treatment of schizophrenia. Neuropsychopharmacology. 1994 Feb;10(1):37–40. doi: 10.1038/npp.1994.5. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Alvear G. M., Werling L. L. Sigma receptor regulation of norepinephrine release from rat hippocampal slices. Brain Res. 1995 Feb 27;673(1):61–69. doi: 10.1016/0006-8993(94)01394-w. [DOI] [PubMed] [Google Scholar]
- Hanner M., Moebius F. F., Weber F., Grabner M., Striessnig J., Glossmann H. Phenylalkylamine Ca2+ antagonist binding protein. Molecular cloning, tissue distribution, and heterologous expression. J Biol Chem. 1995 Mar 31;270(13):7551–7557. doi: 10.1074/jbc.270.13.7551. [DOI] [PubMed] [Google Scholar]
- Harmon J. T., Nielsen T. B., Kempner E. S. Molecular weight determinations from radiation inactivation. Methods Enzymol. 1985;117:65–94. doi: 10.1016/s0076-6879(85)17008-4. [DOI] [PubMed] [Google Scholar]
- Hellewell S. B., Bruce A., Feinstein G., Orringer J., Williams W., Bowen W. D. Rat liver and kidney contain high densities of sigma 1 and sigma 2 receptors: characterization by ligand binding and photoaffinity labeling. Eur J Pharmacol. 1994 Jun 15;268(1):9–18. doi: 10.1016/0922-4106(94)90115-5. [DOI] [PubMed] [Google Scholar]
- Keon J. P., James C. S., Court S., Baden-Daintree C., Bailey A. M., Burden R. S., Bard M., Hargreaves J. A. Isolation of the ERG2 gene, encoding sterol delta 8-->delta 7 isomerase, from the rice blast fungus Magnaporthe grisea and its expression in the maize smut pathogen Ustilago maydis. Curr Genet. 1994 Jun;25(6):531–537. doi: 10.1007/BF00351674. [DOI] [PubMed] [Google Scholar]
- Kolodziej P. A., Young R. A. Epitope tagging and protein surveillance. Methods Enzymol. 1991;194:508–519. doi: 10.1016/0076-6879(91)94038-e. [DOI] [PubMed] [Google Scholar]
- Kreeger J. S., Yukhananov RYu, Larson A. A. Altered N-methyl-D-aspartate (NMDA) activity in the mouse spinal cord following morphine is mediated by sigma activity. Brain Res. 1995 Feb 20;672(1-2):83–88. doi: 10.1016/0006-8993(94)01383-s. [DOI] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Largent B. L., Wikström H., Gundlach A. L., Snyder S. H. Structural determinants of sigma receptor affinity. Mol Pharmacol. 1987 Dec;32(6):772–784. [PubMed] [Google Scholar]
- Larson A. A., Sun X. Regulation of sigma activity by the amino-terminus of substance P in the mouse spinal cord: involvement of phencyclidine (PCP) sites not linked to N-methyl-D-aspartate (NMDA) activity. Neuropharmacology. 1993 Sep;32(9):909–917. doi: 10.1016/0028-3908(93)90147-u. [DOI] [PubMed] [Google Scholar]
- Lee C., Levin A., Branton D. Copper staining: a five-minute protein stain for sodium dodecyl sulfate-polyacrylamide gels. Anal Biochem. 1987 Nov 1;166(2):308–312. doi: 10.1016/0003-2697(87)90579-3. [DOI] [PubMed] [Google Scholar]
- Lee W. H., Kammereck R., Lutsky B. N., McCloskey J. A., Schroepfer G. J. Studies on the mechanism of the enzymatic conversion of delta 8-cholesten-3 beta-ol to delta 7-cholesten-3 beta-ol. J Biol Chem. 1969 Apr 25;244(8):2033–2040. [PubMed] [Google Scholar]
- Linden J. Calculating the dissociation constant of an unlabeled compound from the concentration required to displace radiolabel binding by 50%. J Cyclic Nucleotide Res. 1982;8(3):163–172. [PubMed] [Google Scholar]
- Martin W. R., Eades C. G., Thompson J. A., Huppler R. E., Gilbert P. E. The effects of morphine- and nalorphine- like drugs in the nondependent and morphine-dependent chronic spinal dog. J Pharmacol Exp Ther. 1976 Jun;197(3):517–532. [PubMed] [Google Scholar]
- Matsuno K., Senda T., Matsunaga K., Mita S. Ameliorating effects of sigma receptor ligands on the impairment of passive avoidance tasks in mice: involvement in the central acetylcholinergic system. Eur J Pharmacol. 1994 Aug 11;261(1-2):43–51. doi: 10.1016/0014-2999(94)90298-4. [DOI] [PubMed] [Google Scholar]
- Maurice T., Hiramatsu M., Itoh J., Kameyama T., Hasegawa T., Nabeshima T. Low dose of 1,3-di(2-tolyl)guanidine (DTG) attenuates MK-801-induced spatial working memory impairment in mice. Psychopharmacology (Berl) 1994 Apr;114(3):520–522. doi: 10.1007/BF02249345. [DOI] [PubMed] [Google Scholar]
- Maurice T., Su T. P., Parish D. W., Nabeshima T., Privat A. PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice. Pharmacol Biochem Behav. 1994 Dec;49(4):859–869. doi: 10.1016/0091-3057(94)90235-6. [DOI] [PubMed] [Google Scholar]
- Mercer E. I. Sterol biosynthesis inhibitors: their current status and modes of action. Lipids. 1991 Aug;26(8):584–597. doi: 10.1007/BF02536422. [DOI] [PubMed] [Google Scholar]
- Moebius F. F., Burrows G. G., Hanner M., Schmid E., Striessnig J., Glossmann H. Identification of a 27-kDa high affinity phenylalkylamine-binding polypeptide as the sigma 1 binding site by photoaffinity labeling and ligand-directed antibodies. Mol Pharmacol. 1993 Nov;44(5):966–971. [PubMed] [Google Scholar]
- Moebius F. F., Burrows G. G., Striessnig J., Glossmann H. Biochemical characterization of a 22-kDa high affinity antiischemic drug-binding polypeptide in the endoplasmic reticulum of guinea pig liver: potential common target for antiischemic drug action. Mol Pharmacol. 1993 Feb;43(2):139–148. [PubMed] [Google Scholar]
- Moebius F. F., Hanner M., Knaus H. G., Weber F., Striessnig J., Glossmann H. Purification and amino-terminal sequencing of the high affinity phenylalkylamine Ca2+ antagonist binding protein from guinea pig liver endoplasmic reticulum. J Biol Chem. 1994 Nov 18;269(46):29314–29320. [PubMed] [Google Scholar]
- Molzahn S. W., Woods R. A. Polyene resistance and the isolation of sterol mutants in Saccharomyces cerevisiae. J Gen Microbiol. 1972 Sep;72(2):339–348. doi: 10.1099/00221287-72-2-339. [DOI] [PubMed] [Google Scholar]
- Monnet F. P., Debonnel G., Bergeron R., Gronier B., de Montigny C. The effects of sigma ligands and of neuropeptide Y on N-methyl-D-aspartate-induced neuronal activation of CA3 dorsal hippocampus neurones are differentially affected by pertussin toxin. Br J Pharmacol. 1994 Jun;112(2):709–715. doi: 10.1111/j.1476-5381.1994.tb13134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnet F. P., Mahé V., Robel P., Baulieu E. E. Neurosteroids, via sigma receptors, modulate the [3H]norepinephrine release evoked by N-methyl-D-aspartate in the rat hippocampus. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):3774–3778. doi: 10.1073/pnas.92.9.3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musacchio J. M. The psychotomimetic effects of opiates and the sigma receptor. Neuropsychopharmacology. 1990 Jun;3(3):191–200. [PubMed] [Google Scholar]
- Nakagawara M., Sato Y. [Neuropeptide Y: psychopharmacological and clinical aspects]. Nihon Shinkei Seishin Yakurigaku Zasshi. 1994 Jun;14(3):117–127. [PubMed] [Google Scholar]
- Ness G. C., Sukalski K. A., Sample C. E., Pendleton L. C., McCreery M. J., Nordlie R. C. Radiation inactivation analysis of rat liver microsomal glucose-6-phosphatase. J Biol Chem. 1989 May 5;264(13):7111–7114. [PubMed] [Google Scholar]
- Okuyama S., Ogawa S., Nakazato A., Tomizawa K. Effect of NE-100, a novel sigma receptor ligand, on phencyclidine- induced delayed cognitive dysfunction in rats. Neurosci Lett. 1995 Apr 7;189(1):60–62. doi: 10.1016/0304-3940(95)11440-8. [DOI] [PubMed] [Google Scholar]
- Ozasa S., Kempner E. S., Erickson S. K. Functional size of acyl coenzyme A:diacylglycerol acyltransferase by radiation inactivation. J Lipid Res. 1989 Nov;30(11):1759–1762. [PubMed] [Google Scholar]
- Paik Y. K., Billheimer J. T., Magolda R. L., Gaylor J. L. Microsomal enzymes of cholesterol biosynthesis from lanosterol. Solubilization and purification of steroid 8-isomerase. J Biol Chem. 1986 May 15;261(14):6470–6477. [PubMed] [Google Scholar]
- Quirion R., Bowen W. D., Itzhak Y., Junien J. L., Musacchio J. M., Rothman R. B., Su T. P., Tam S. W., Taylor D. P. A proposal for the classification of sigma binding sites. Trends Pharmacol Sci. 1992 Mar;13(3):85–86. doi: 10.1016/0165-6147(92)90030-a. [DOI] [PubMed] [Google Scholar]
- Ramsey R. B., Aexel R. T., Jones J. P., Nicholas H. J. Formation of methyl sterols in brain cholesterol biosynthesis. Sterol formation in vitro in actively myelinating rat brain. J Biol Chem. 1972 Jun 10;247(11):3471–3475. [PubMed] [Google Scholar]
- Rao T. S., Cler J. A., Emmett M. R., Mick S., Iyengar S., Wood P. L. BMY-14802 antagonizes harmaline- and D-serine-induced increases in mouse cerebellar cyclic GMP: neurochemical evidence for a sigma receptor-mediated functional modulation of responses mediated by the N-methyl-D-aspartate receptor complex in vivo. Mol Pharmacol. 1990 Jun;37(6):978–982. [PubMed] [Google Scholar]
- Reinhart M. P., Billheimer J. T., Faust J. R., Gaylor J. L. Subcellular localization of the enzymes of cholesterol biosynthesis and metabolism in rat liver. J Biol Chem. 1987 Jul 15;262(20):9649–9655. [PubMed] [Google Scholar]
- Schroepfer G. J., Jr Sterol biosynthesis. Annu Rev Biochem. 1982;51:555–585. doi: 10.1146/annurev.bi.51.070182.003011. [DOI] [PubMed] [Google Scholar]
- Schutze M. P., Peterson P. A., Jackson M. R. An N-terminal double-arginine motif maintains type II membrane proteins in the endoplasmic reticulum. EMBO J. 1994 Apr 1;13(7):1696–1705. doi: 10.1002/j.1460-2075.1994.tb06434.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelly L. L., Lei K. J., Pan C. J., Sakata S. F., Ruppert S., Schutz G., Chou J. Y. Isolation of the gene for murine glucose-6-phosphatase, the enzyme deficient in glycogen storage disease type 1A. J Biol Chem. 1993 Oct 15;268(29):21482–21485. [PubMed] [Google Scholar]
- Spady D. K., Dietschy J. M. Sterol synthesis in vivo in 18 tissues of the squirrel monkey, guinea pig, rabbit, hamster, and rat. J Lipid Res. 1983 Mar;24(3):303–315. [PubMed] [Google Scholar]
- Su T. P., London E. D., Jaffe J. H. Steroid binding at sigma receptors suggests a link between endocrine, nervous, and immune systems. Science. 1988 Apr 8;240(4849):219–221. doi: 10.1126/science.2832949. [DOI] [PubMed] [Google Scholar]
- Su T. P. Sigma receptors. Putative links between nervous, endocrine and immune systems. Eur J Biochem. 1991 Sep 15;200(3):633–642. doi: 10.1111/j.1432-1033.1991.tb16226.x. [DOI] [PubMed] [Google Scholar]
- Trzaskos J. M., Bowen W. D., Fisher G. J., Billheimer J. T., Gaylor J. L. Microsomal enzymes of cholesterol biosynthesis from lanosterol: a progress report. Lipids. 1982 Mar;17(3):250–256. doi: 10.1007/BF02535112. [DOI] [PubMed] [Google Scholar]
- Walker J. M., Bowen W. D., Walker F. O., Matsumoto R. R., De Costa B., Rice K. C. Sigma receptors: biology and function. Pharmacol Rev. 1990 Dec;42(4):355–402. [PubMed] [Google Scholar]
- Wu X. Z., Bell J. A., Spivak C. E., London E. D., Su T. P. Electrophysiological and binding studies on intact NCB-20 cells suggest presence of a low affinity sigma receptor. J Pharmacol Exp Ther. 1991 Apr;257(1):351–359. [PubMed] [Google Scholar]
- Yamamoto H., Yamamoto T., Sagi N., Klenerová V., Goji K., Kawai N., Baba A., Takamori E., Moroji T. Sigma ligands indirectly modulate the NMDA receptor-ion channel complex on intact neuronal cells via sigma 1 site. J Neurosci. 1995 Jan;15(1 Pt 2):731–736. doi: 10.1523/JNEUROSCI.15-01-00731.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]