Abstract
Rosiglitazone has the potential to activate peroxisome proliferator-activated receptor-γ (PPARγ), which in turn can affect bone formation and resorption. However, the mechanisms by which rosiglitazone regulates osteoclastic or osteoblastic differentiation are not fully understood. This study examines how rosiglitazone affects osteoclast formation, bone resorption and osteoblast differentiation from mouse bone marrow. Rosiglitazone treatment not only inhibited the formation of tartrate-resistant acid phosphatase-positive cells, but also prevented pit formation by bone marrow cells in a dose- and time-dependent manner. Rosiglitazone also suppressed the receptor activator of nuclear factor (NF)-κB ligand (RANKL) receptor (RANK) expression but increased PPARγ2 expression in the cells. In addition, rosiglitazone diminished RANKL-induced activation of NF-κB-DNA binding by blocking IκBα phosphorylation. Furthermore, it reduced collagen and osteocalcin levels to nearly zero and prevented mRNA expression of osteoblast-specific proteins including runt-related transcription factor-2, osteocalcin, and type I collagen. However, mRNA levels of adipocyte-specific marker, aP2, were markedly increased in the cells co-incubated with rosiglitazone. These results suggest that PPARγ activation by rosiglitazone inhibits osteoblast differentiation with increased adipogenesis in bone marrow cells and also may prevent osteoclast formation and bone resorption in the cells.
Keywords: mouse bone marrow cells, osteoblastogenesis, osteoclastogenesis, PPARγ, rosiglitazone
INTRODUCTION
Bone requires constant remodeling via a balanced activation of osteoclasts and osteoblasts (Rho et al., 2004). Osteoclasts arise from hematopoietic precursor cells of the monocyte/macrophage lineage, while osteoblasts are of mesenchymal lineage (Del Fattore et al., 2010). Abnormal activation of osteoclasts and/or reduced osteoblastogenesis disrupts bone homeostasis and eventually causes diseases such as osteoporosis, arthritis, and bone cancer (Rachner et al., 2011; Sturge et al., 2011). Increased bone marrow adipose tissue and subsequent decrease of osteoblastic differentiation from marrow progenitors may be related to bone loss caused by aging (Rahman et al., 2011).
Peroxisome proliferator-activated receptor-γ (PPARγ) is a member of the nuclear receptor superfamily of transcription factors and exists in two isoforms, PPARγ1 and PPARγ2. The PPARγ1 isoform is expressed in many cell types, including adipocytes, osteoblasts, muscle cells, and macrophages, whereas PPARγ2 expression is restricted primarily to adipose cells and is critical for fat development (Sugii and Evans, 2011). This suggests that activation of PPARγ is the most important event required for adipogenic differentiation (Kawai and Rosen, 2010). Up-regulation of PPARγ expression in myoblasts induced transdifferentiation of these cells into adipocytes (Kook et al., 2006). A prolonged and persistent activation of PPARγ leads to skewed adipogenesis activation and eventually causes bone loss due to decreased osteoblast and increased osteoclast differentiation from their progenitor cells (Lecka-Czernik, 2009; 2010).
The thiazolidinedione (TZD) class of anti-diabetic agents consists of well-known PPARγ agonists and stimulates adipogenic differentiation of cells through binding to and activation of PPARγ. Numerous studies have shown that TZDs promote adipogenesis and also inhibit osteogenic differentiation in vitro and in vivo (Okazaki et al., 1999; Schwartz, 2008). Specifically, many investigators have researched the role of rosiglitazone, a TZD, on bone metabolism because the agent is commonly used for the treatment of type 2 diabetes as a new class of oral anti-diabetic compounds. Based on previous reports, rosiglitazone is capable of reducing bone mass and strength, which is believed to be derived from the alteration of the phenotype of marrow mesenchymal stem cells (Lazarenko et al., 2007). Rosiglitazone acts as a dominant inhibitor of osteoblastogenesis by activating PPARγ2 in murine bone marrow cells (Ali et al., 2005). Oral administration of this agent also decreases the expression of osteoblastic-specific genes but increases the expression of adipocyte-specific fatty acid binding protein (aP2). These findings suggest that rosiglitazone therapy may pose a significant risk of adverse skeletal effects. Moreover, bone loss by PPARγ activation supports the theory that increased production of oxidized fatty acids with age may be an important mechanism for age-related osteoporosis in humans (Ali et al., 2005; Lazarenko et al., 2007).
Accumulated evidence suggests that the activation of osteoclastogenesis is also one of the main mechanisms by which PPARγ activation stimulates the loss of bone mass. Wan et al. (2007) reported that PPARγ activation exacerbated osteoclastic differentiation from hematopoietic stem cells. Loss of PPARγ function in mouse hematopoietic lineages caused osteopetrosis accompanied by osteoclast defects. PPARγ also appears to function as a direct regulator of c-fos expression, an essential mediator of osteoclastogenesis in mice (Wan et al., 2007). Thus, administration of TZDs might lead to bone loss by inhibiting bone formation, as well as by sustaining or increasing bone resorption. However, the roles of rosiglitazone on osteoclast formation and bone resorption are not fully understood. In addition, a different study found that rosiglitazone prevents inflammatory periodontal bone loss by inhibiting osteoclastogenesis in rat and mouse monocyte/macrophage cell line MOCP-5 cells (Hassumi et al., 2009). Furthermore, ciglitazone, another TZD class of PPARγ activators, inhibits the formation of human peripheral blood mononuclear cell (PMBC)-derived osteoclasts in a dose-dependent manner (Chan et al., 2007).
Therefore, the main purpose of this study was to explore how PPARγ activation by rosiglitazone affects osteoclastogenesis and osteoblastogenesis from bone marrow progenitors. To this end, the effects of rosiglitazone on osteoclast formation and bone resorption were examined using mouse bone marrow cells and RAW 264.7 cells. The effects of rosiglitazone on calcium and collagen accumulation and on the mRNA expression of osteoblast-specific proteins in the cells were also evaluated. This study demonstrates that PPARγ activation by rosiglitazone inhibits in vitro osteoclast formation and bone resorption as well as osteoblastic differentiation.
MATERIALS AND METHODS
Mice, chemicals, and laboratory wares
Six-week old male BALB/c mice were purchased from Orient Co. (Korea). Rosiglitazone (Cat. 5533) was a kind gift from GlaxoSmithkline (UK). Recombinant murine receptor activator of nuclear factor-kappaB (NF-κB) ligand (RANKL), tumor necrosis factor (TNF)-α, and macrophage-colony stimulating factor (M-CSF) were purchased from R & D Systems (USA). Antibodies specific for RANKL receptor (RANK), IκBα, p-IκBα, and α-tubulin were obtained from Santa Cruz Biotechnology (USA). Unless otherwise specified, additional chemicals were obtained from Sigma Chemical Co. (USA), and laboratory wares were from SPL Life Sciences (Korea).
Cell cultures
Bone marrow cells were obtained from 6–8 week-old BALB/c mice. Animal care and use practices were approved by the Chonbuk National University Committee on Ethics in the Care and Use of Laboratory Animals (CBU 2010-0007). Mice were sacrificed by cervical dislocation, and the tibiae and femora were removed and washed several times with PBS with antibiotics. The ends of the bones were cut, and the marrow cavity was flushed with modified essential medium (MEM) supplemented with 10% fetal calf serum (FCS; Hyclone Laboratories, USA) and antibiotics. The bone marrow suspension was incubated in a 100-mm culture dish in the presence of 50 ng/ml M-CSF. After three days, adherent cells were collected using a scraper and then used as bone marrow macrophages to induce osteoclastic differentiation. A small amount of bone marrow suspension was also incubated in α-MEM containing 10% fetal bovine serum and antibiotics. After 48 h of incubation, non-adherent cells were removed and then adherent cells were subsequently cultured in the same media. When the cells reached 90% confluence in a 100-mm culture dish, they were dissociated using Trypsin/EDTA and speared onto various culture plates according to the experimental design. To induce osteoblastic differentiation, the cells were incubated in α-MEM supplemented with DAG (10 nM dexamethasone, 50 μM ascorbic acid, and 20 mM β-glycerophosphate). Media were changed every 3 days during the culture periods. In addition, the effect of rosiglitazone on osteoclast formation was explored using RAW 264.7 macrophage cells.
Osteoclastic differentiation and TRAP staining
The bone marrow cells were treated with various concentrations (0–10 μM) of rosiglitazone in the presence of 50 ng/ml M-CSF, 100 ng/ml RANKL and/or 10 ng/ml TNF-α to examine the effect of rosiglitazone on osteoclast formation in bone marrow cells. Culture media was replaced with fresh media on days 2 and 5 of co-incubation. After seven days of co-incubation, the control and experimental cultures were fixed in 4% PBS-buffered para-formaldehyde and stained with tartrate-resistant acid phosphatase (TRAP) using a Sigma Aldrich kit according to the manufacturer’s instructions. TRAP-positive cells were counted using optic microscopy, and cells containing three or more nuclei were considered to be multinucleated osteoclastic cells (MNCs). In some experiments, RAW 264.7 cells were incubated in the presence of RANKL and/or rosiglitazone, and after seven days of incubation, the cells were processed for TRAP staining.
Bone resorption assay
Bone marrow cells (1 × 105 cells/ml) were suspended in α-MEM containing M-CSF, TNF-α, and/or RANKL and then divided across a 24-well plate coated with calcium-phosphate nano crystals (OAAS-24; Osteoclast Activity Assay Substrate, Oscotec Inc., Korea) at a density of 2 × 104 cells/cm2. Seven days after incubation, the cells were removed from the plates by treatment with 5% sodium hypochlorite, and pit formation was observed under an optic microscope. The resorbed area was also measured by image analyzer and expressed as percentage (%) of the control value.
Measurement of cell viability
Cell viability was determined using water-soluble tetrazolium salt (WST)-8 reagent. In brief, bone marrow suspension was incubated with various concentrations (0–50 μM) of rosiglitazone, and WST-8 reagent was added into the cultures after 48 h of incubation. After incubation for an additional 4 h, the WST-8-specific absorbance was measured at 450 nm using a microplate reader (Packard Instrument Co., USA).
RNA preparation and polymerase chain reaction (PCR)
Total RNA was extracted from the control and experimental cells at various times according to the manufacturer’s instructions (SV Total RNA Isolation System, Promega, USA). Reverse transcription and PCR amplification were performed using an Access RT-PCR System (Promega) according to the manufacturer’s protocol. The primer sequences were as follows: 5′-ggttatgta atgagcggcagca-3′ (forward) and 5′-ttctcacg gcactgtagatctgg-3′ (reverse) for RANK and 5′-gggtcagctctt gtgaatgg-3′ (forward) and 5′-ctgatgcactgcctatgagc-3′ (reverse) for PPARγ2. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a positive control and was amplified with the primers 5′-aacctgccaaatatgatgac-3′ (forward) and 5′-ataccaggaaa tgagcttga-3′ (reverse). PCR was performed for 30–35 cycles of 94°C for 30 s, 50–60°C for 30 s, and 72°C for 1 min in a DNA thermal cycler (model PTC-100, USA). PCR products were analyzed on 1.5–2% agarose gels and visualized using ethidium bromide staining. Band intensity was calculated using a gel imaging system (model F1–F2 Fuses type T2A, BIO-RAD, Italy).
Quantitative real-time PCR amplification was also performed with osteoblastic or adipogenic gene-specific primers using an ABI Prism 7900HT Sequence Detection System (Applied Bio-systems). The primer sequences used for runt-related transcription factor 2 (Runx2), osteocalcin, type I collagen, and aP2 are described elsewhere (Rzonca et al., 2004). Relative expressions of these genes were obtained from relative standard curves run in triplicate after dividing each value by the value of β-actin.
Preparation of cell fractions
Whole lysates were prepared in a lysis buffer as described in Son et al. (2009). Nuclear proteins were prepared as described in Maulik et al. (1998), and protein concentrations were determined using the Bradford method (1976). To prepare cytosolic proteins, cells were incubated in 200 μl lysis buffer (250 mM sucrose, 20 mM Hepes, pH 7.5, 10 mM KCl, 1.5 mM MgCl2, 1 mM EGTA, 1 mM EDTA, 1 mM DTT, 1 mM PMSF, and 10 μg/ml each of leupeptin, aprotinin, and pepstatin A) on ice for 30 min. Cell lysates were gently centrifuged at 750 × g for 10 min at 4°C, and the supernatants were further centrifuged at 10,000 × g for 25 min at 4°C. The final supernatants were used as cytosolic fractions.
Western blot analysis
Equal amounts of protein extracts were separated by 12–15% SDS-PAGE and blotted onto poly vinyl difluoride membranes. The blots were probed with primary antibodies overnight at 4°C prior to incubation with secondary antibody in a blocking buffer for 1 h. The blots were developed with enhanced chemiluminescence (Amersham Pharmacia Biotech Inc., UK) and exposed on X-ray film (Eastman-Kodak Co., USA).
Electrophoretic mobility shift assay (EMSA)
DNA-protein binding reactions were performed for 30 min at room temperature, with 10–15 μg protein in 20 μl buffer containing 1 μg/ml BSA, 0.5 μg/μl poly (dI-dC), 5% glycerol, 1 mM DTT, 1 mM PMSF, 10 mM Tris-Cl (pH 7.5), 50 mM NaCl, 30,000 cpm of [α-32P] dCTP-labeled oligonucleotides, and the Klenow fragment of DNA polymerase. The samples were separated on 6% polyacrylamide gels before the dried gels were exposed to X-ray film (Eastman Kodak Co.) for 12–24 h at −70°C. The oligonucleotide primer sequences specific for NF-κB have been described elsewhere (Lee et al., 2003).
Measurement of collagen and osteocalcin
Collagen contents in the cells were determined by Sirius Red-based colorimetric assay. In brief, bone marrow cells were treated with various concentrations of rosiglitazone in the presence of DAG, and the medium was changed every two days. At ten days after treatment, cells were fixed with Bouin’s fluid for 1 h and washed several times with distilled water. The culture plates were stored at room temperature for drying and then stained with Sirius Red dye reagent for 1 h. After washing twice with 10 mM HCl, the cells were treated with 100 mM NaOH, and absorbance was measured at 550 nm. In addition, osteocalcin contents in the cells were measured ten days after the same treatment using a sandwich ELISA assay kit (Biomedical Technologies Inc., USA). All the experiments were performed according to the manufacturer’s instructions. The contents of collagen and osteocalcin were expressed as μg or ng per 106 cells. In addition, cells were stained with 40 mM alizarin red S (pH 4.2) after fixation with 70% ice-cold ethanol at 14 days after osteoblastic differentiation.
Statistical analysis
Unless otherwise indicated, all data are expressed as the mean ± standard deviation (S.D.) of three or more independent experiments. A one-way ANOVA was used for multiple comparisons using SPSS version 18.0 software. A p value < 0.05 was considered statistically significant.
RESULTS
Rosiglitazone inhibits osteoclast formation and bone resorption by bone marrow cells in a dose-dependent manner
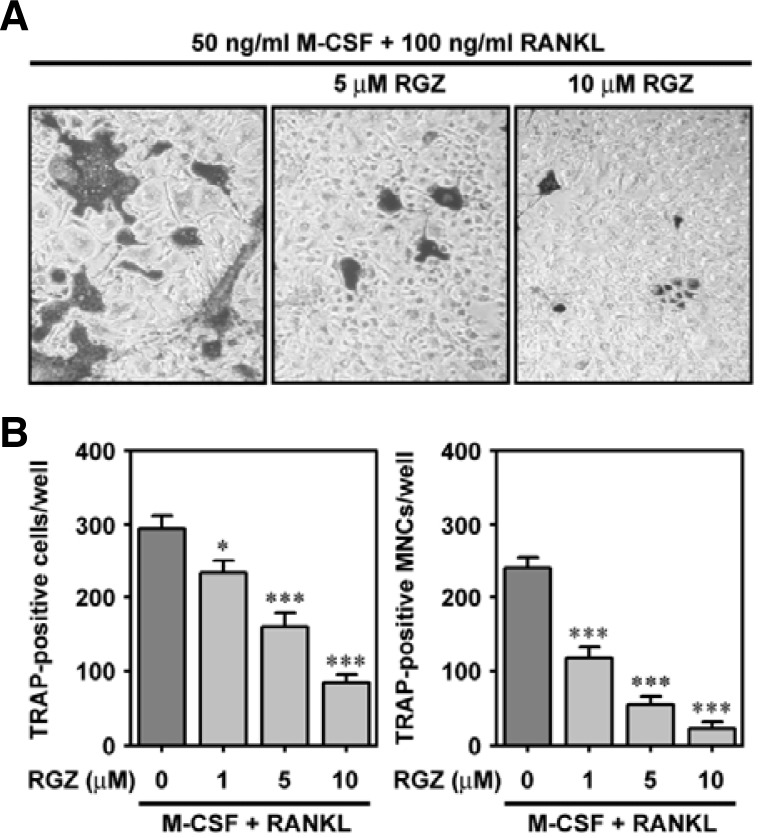
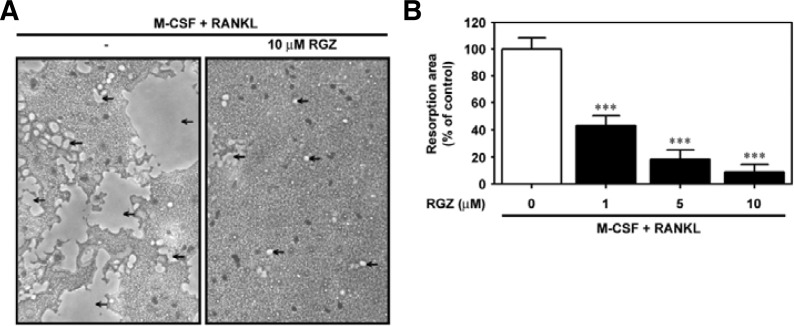
To verify the nature of rosiglitazone on osteoclast differentiation from bone marrow cells, the cells were cultured with various concentrations (0–10 μM) of rosiglitazone for seven days in the presence of 50 ng/ml M-CSF and 100 ng/ml RANKL. Figure 1A shows the RANKL-mediated osteoclast differentiation and its suppression due to combined treatment with rosiglitazone. The number of TRAP-positive cells (Fig. 1B) and TRAP-positive MNCs (Fig. 1C) were reduced in a dose-dependent manner by rosiglitazone. When the cells were co-treated with 1 μM and 10 μM rosiglitazone, the number of TRAP-positive MNCs was diminished by 48.9% and 9.5%, respectively, compared to cells supplemented with M-CSF and RANKL. Rosiglitazone also prevented RANKL-mediated bone resorption, as measured by an in vitro model system (Fig. 2A). A significant inhibition of bone resorption occurred even when the cells were incubated in combination with 1 μM rosiglitazone (Fig. 2B). The combined treatment with 10 μM rosiglitazone almost completely attenuated the pit formation by bone marrow cells.
Fig. 1.
Rosiglitazone reduces the number of TRAP-positive cells in a dose-dependent manner. Bone marrow cells were exposed to the indicated concentrations of rosiglitazone in the presence of 50 ng/ml M-CSF and 100 ng/ml RANKL. The control and experimental bone marrow cells were subjected to TRAP staining (A), and the numbers of TRAP-positive cells (B) and TRAP-positive MNCs (C) were counted seven days after exposure. *P < 0.05 and ***P < 0.001 vs. the cells containing M-CSF and RANKL.
Fig. 2.
Rosiglitazone prevents the RANKL-induced pit formation in M-CSF-treated bone marrow cells. (A) Bone marrow cells were treated with 10 μM rosiglitazone in bone-coated 24-well plates and cultured for seven days in the presence of M-CSF and RANKL. Pit formation on the plate was observed under optic microscopy. (B) Bone marrow cells were also exposed to various concentrations (0–10 μM) of rosiglitazone in the presence of M-CSF and RANKL, and seven days later, the resorbed area was quantified from three independent experiments and expressed as % of the control value. ***P < 0.001 vs. the control values without rosiglitazone.
Rosiglitazone prevents osteoclastogenesis in TNF-α-stimulated bone marrow cells and in RANKL-exposed RAW 264.7 cells
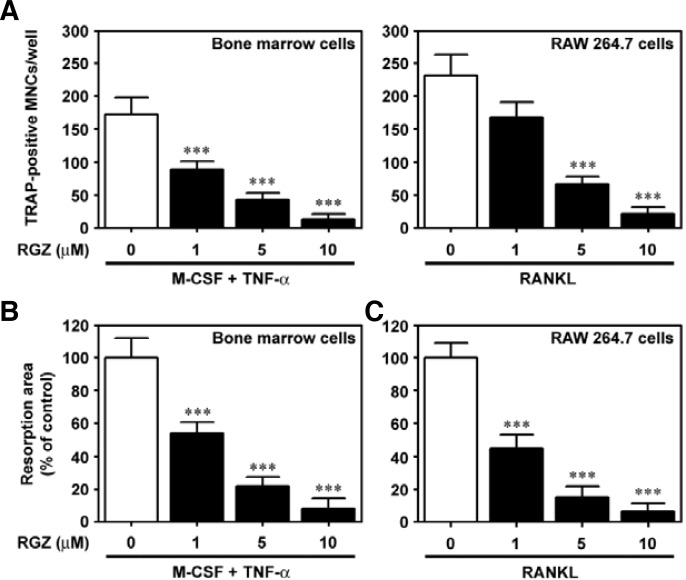
Next, it was determined whether or not rosiglitazone suppresses osteoclastic differentiation induced by TNF-α-treated bone marrow cells. Of the 96 multiwell plates, there were 173 ± 26/well TRAP-positive MNCs at seven days after treatment with 10 ng/ml TNF-α. However, combined treatment with rosiglitazone reduced TNF-α-mediated MNC formation, and addition of 10 μM rosiglitazone resulted in almost complete inhibition (Fig. 3A). Similarly, rosiglitazone treatment decreased bone resorption stimulated by TNF-α in bone marrow cells (Fig. 3B). Rosiglitazone also significantly diminished osteoclast formation (Fig. 3A) as well as bone resorption (Fig. 3B) induced by RANKL-stimulated RAW 264.7 cells.
Fig. 3.
Rosiglitazone inhibits osteoclast differentiation derived from TNF-α-treated bone marrow cells and from RANKL-stimulated RAW 264.7 cells. Bone marrow cells were incubated with the indicated doses of rosiglitazone in the presence of 50 ng/ml M-CSF and 10 ng/ml TNF-α. RAW 264.7 cells were also cultured in combination with 100 ng/ml RANKL and various concentrations of rosiglitazone. After seven days of coincubation, these cells were processed for TRAP staining (A) and pit formation assays (B). The results represent the mean ± S.D. from three independent experiments. ***P < 0.001 vs. the control cells without rosiglitazone.
Inhibition of osteoclastogenesis by rosiglitazone depends on when the agent is added to the cultures after RANKL stimulation
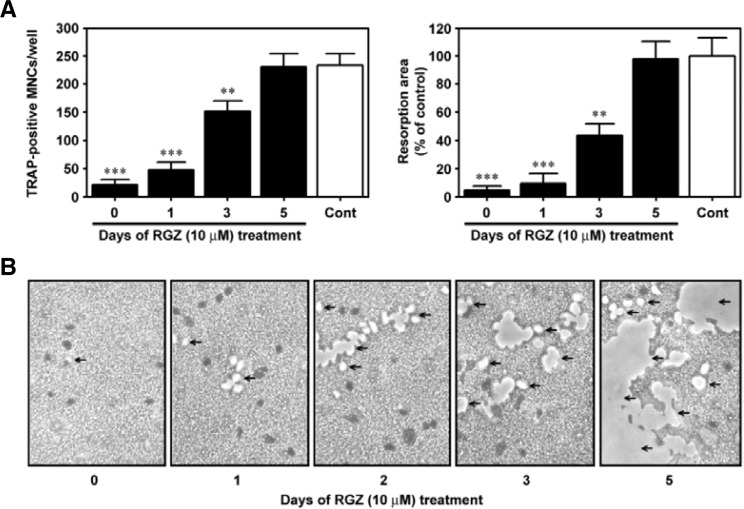
When rosiglitazone (10 μM) was added to the bone marrow cultures at time 0 and one day after the treatment with RANKL, a marked inhibition of the TRAP-positive MNC formation was observed compared to the control values (Fig. 4A). However, when rosiglitazone was added three days after RANKL stimulation, the formation of TRAP-positive MNCs was apparent, although not as prominent as in the case of RANKL treatment alone. There was no inhibitory effect of rosiglitazone on osteoclast formation when rosiglitazone was added to the cells five days after RANKL stimulation. Figure 4B shows the time-dependent suppression of bone resorption by rosiglitazone, where the inhibition of RANKL-stimulated pit formation by rosiglitazone is inversely related to the time at which rosiglitazone was added into the cultures. In particular, the addition of 10 μM rosiglitazone at time 0 or one day after stimulation by RANKL led to almost complete abrogation of pit formation (Fig. 4C).
Fig. 4.
Rosiglitazone prevents osteoclast formation and bone resorption by bone marrow cells in a time-dependent manner. Rosiglitazone (10 μM) was added to M-CSF-treated bone marrow cultures on days 0, 1, 3, and 5 after stimulation with 100 ng/ml RANKL. Seven days after the stimulation, the cells were processed for analyses of TRAP-positive MNC formation (A) and pit formation (B). The resorbed area was also quantified from three independent experiments and expressed as % of the control value (C). **P < 0.01 and ***P < 0.001 vs. the control cells incubated with M-CSF and RANKL for seven days.
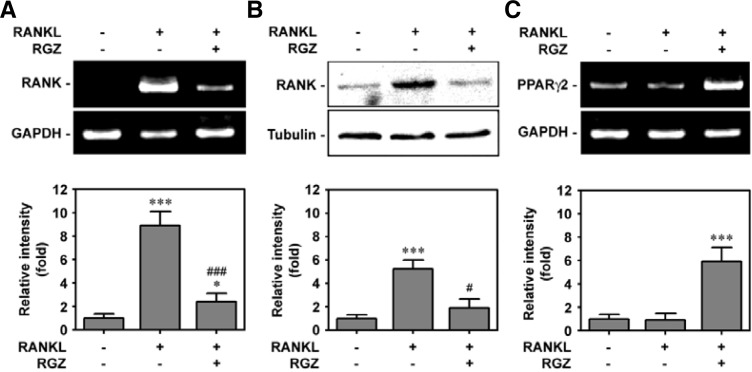
Rosiglitazone inhibits RANKL-induced RANK expression but increases PPARγ2 expression in bone marrow cells
Subsequently, the effect of rosiglitazone on the expression of RANK at the mRNA and protein levels was investigated using bone marrow cells. As shown in Fig. 5A, the mRNA level of RANK was apparently increased by RANKL-stimulated cells, whereas the addition of 10 μM rosiglitazone appeared to attenuate RANK mRNA expression. Western blot data also revealed a RANKL-mediated RANK increase at the protein level and inhibition of RANKL-mediated RANK by co-treatment with rosiglitazone (Fig. 5B). As expected, RANKL treatment alone did not change the PPARγ2 mRNA level in M-SCF-treated bone marrow cells, while incubating the cells with the combined media containing both RANKL and rosiglitazone markedly increased its mRNA level (Fig. 5C).
Fig. 5.
Combined treatment with rosiglitazone significantly diminishes the expression of RANK but increases PPARγ2 expression in RANKL-stimulated bone marrow cells. Bone marrow cells were incubated in α-MEM containing 50 ng/ml M-CSF, 100 ng/ml RANKL, and/or 10 μM rosiglitazone for 48 h, and then the levels of RANK (A) and PPARγ2 at mRNA (C) and protein (B) levels were determined by RT-PCR and Western blot analyses. *P < 0.05 and ***P < 0.001 vs. M-CSF treatment alone. #P < 0.05 and ###P < 0.001 vs. M-CSF and RANKL treatment.
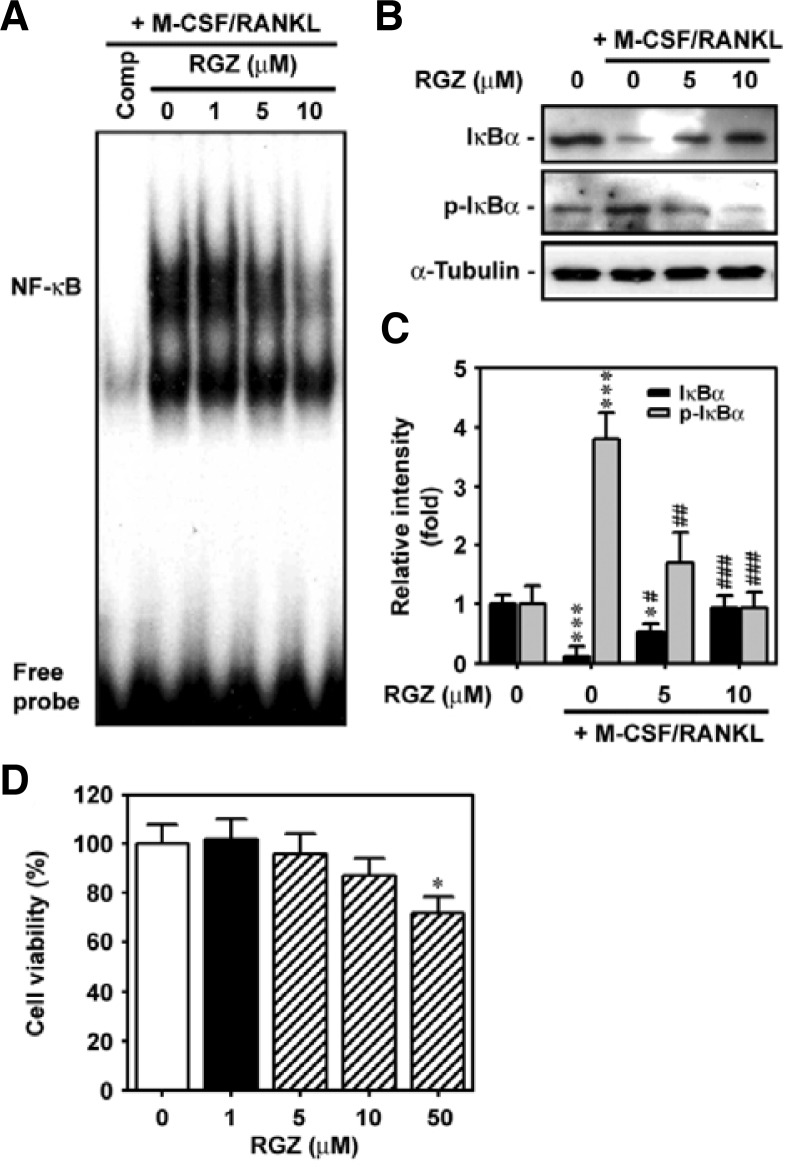
Rosiglitazone inhibits NF-κB-DNA binding activation by blocking IκBα phosphorylation in RANKL-stimulated bone marrow cells
The effects of rosiglitazone on NF-κB binding activity and IκB degradation were determined in RANKL-stimulated bone marrow cells. As expected, RANKL treatment increased the DNA-binding of NF-κB (Fig. 6A). Combined treatment of cells with RANKL and rosiglitazone decreased RANKL-induced activation of NF-κB binding in a dose-dependent manner. Rosiglitazone also inhibited RANKL-stimulated phosphorylation of IκBα and its subsequent degradation in bone marrow cells (Fig. 6B). The addition of 10 μM rosiglitazone completely inhibited both the degradation and activation of IκBα (Fig. 6C). This inhibition by rosiglitazone was not related to reduction in cell viability because similar concentrations of the agent did not have a significantly toxic effect on the cells (Fig. 6D).
Fig. 6.
Rosiglitazone suppresses NF-κB-DNA binding and IκBα phosphorylation in RANKL-stimulated bone marrow cells. Bone marrow cells suspended in α-MEM containing 50 ng/ml M-CSF were spread onto 6-well plates, then exposed to increasing concentrations (0–10 μM) of rosiglitazone in the presence of 100 ng/ml RANKL for 48 h. Bone marrow cells were also incubated with increasing concentrations (0–50 μM) of rosiglitazone for 48 h and then processed for WST-8 assay. *P < 0.05 and ***P < 0.001 vs. the control cells without M-CSF and RANKL. #P < 0.05, ##P < 0.01 and ###P < 0.001 vs. the cultures containing both M-CSF and RANKL.
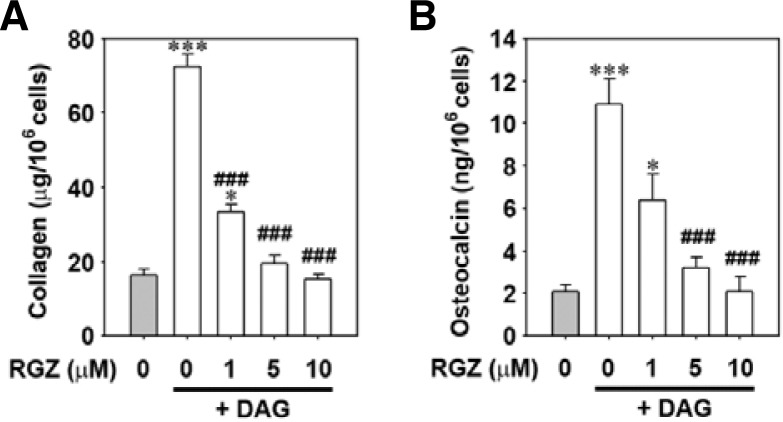
Rosiglitazone inhibits osteoblastogenesis in bone marrow cells
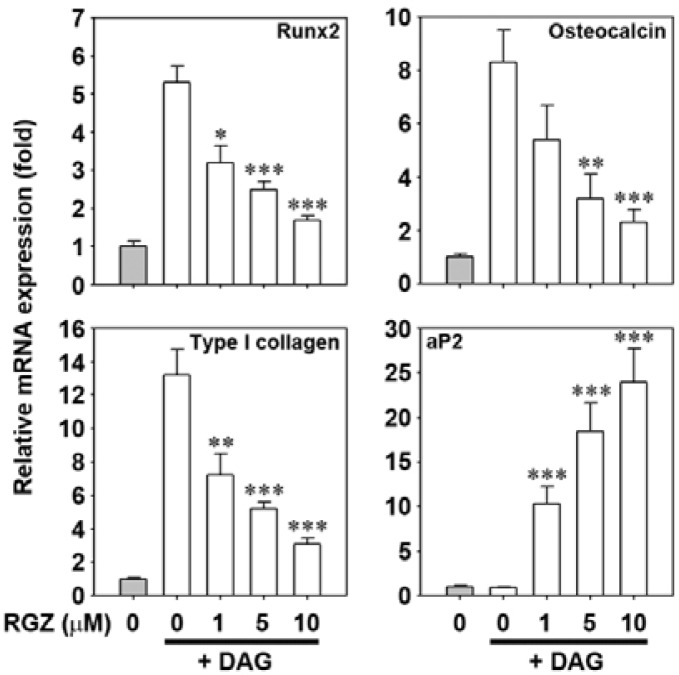
The role of rosiglitazone on osteoblastic differentiation in bone marrow cells was further investigated. As shown in Fig. 7A, DAG treatment alone increased the collagen contents in the cells, whereas co-incubation with 10 μM rosiglitazone reduced collagen to basal level (Fig. 7A). Similarly, osteocalcin levels increased in DAG-treated bone marrow cells but decreased dramatically in cells subjected to a combination of DAG treatment and rosiglitazone (Fig. 7B). These results were supported by the results obtained from alizarin red S staining where the DAG-mediated mineralization was suppressed by rosiglitazone in a dose-dependent manner (Supplementary Fig. 1). When the mRNA levels of several osteoblast-specific proteins were determined by real-time RT-PCR, rosiglitazone almost completely prevented the DAG-mediated increases in the levels of Runx2, osteocalcin, and type I collagen mRNAs (Fig. 8). However, mRNA levels of the adipocyte-specific marker aP2 were markedly increased in a dose-dependent manner in cells co-incubated with rosiglitazone.
Fig. 7.
Rosiglitazone treatment decreases the contents of collagen and osteocalcin in DAG-treated bone marrow cells. Bone marrow suspension was incubated in the presence of DAG with and without the indicated concentrations (0–10 μM) of rosiglitazone, and cellular levels of collagen (A) and osteocalcin (B) were determined after ten days of incubation. *P < 0.05 and ***P < 0.001 vs. the control cells without rosiglitazone and DAG. ###P < 0.001 vs. DAG treatment only.
Fig. 8.
Combined treatment with rosiglitazone dramatically attenuates the expression of osteoblast-specific proteins but increases aP2 mRNA levels in DAG-treated bone marrow cells. Bone marrow suspension was incubated in DAG-contained medium with and without the increasing rosiglitazone concentrations (0–10 μM), and the mRNA levels of Runx2, osteocalcin, type I collagen, and aP2 were determined by real-time RT-PCR after ten days of incubation. *P < 0.05, **P < 0.01, and ***P < 0.001 vs. DAG treatment only.
DISCUSSION
Rosiglitazone, a PPARγ agonist and anti-diabetic agent, is known to affect bone metabolism. However, there are conflicting findings regarding how this agent affects bone loss, and it appears to both inhibit and stimulate osteoclastogenesis depending on experimental conditions. Rosiglitazone has great potential as a clinical treatment for type 2 diabetic patients, so understanding its exact role in bone metabolism is important. The present study demonstrated that rosiglitazone not only inhibits osteoclast formation and bone resorption, but also prevents osteoblastogenesis in mouse bone marrow cells.
Accumulated evidence suggests that rosiglitazone accelerates osteoclast formation and bone resorption, indicating its in vivo association with bone loss (Gruntmanis et al., 2010; Harsløf et al., 2011). These findings are not consistent with results from the present study showing that rosiglitazone suppressed osteoclastogenesis in a dose-dependent manner. Wan et al. (2007) examined TieCre/flox mutant mice in which PPARγ was deleted in osteoclasts but not in osteoblasts and observed impaired osteoclast differentiation and bone resorption, resulting in osteopetrosis and extramedullary hematopoiesis in the spleen. Function was restored by ligand activation of PPARγ-facilitated osteoclast formation and bone resorption in a receptor-dependent manner. Thus, it is possible that prolonged usage of rosiglitazone may result in decreased bone formation and increased bone resorption in patients with type 2 diabetes (Wan, 2010).
Other studies have demonstrated the inhibitory effects of TZDs such as rosiglitazone, troglitazone, and ciglitazone, on osteoclastogenesis. Okazaki et al. (1999) revealed that troglitazone inhibits osteoclast-like cell formation and bone resorption in mouse bone marrow cells. Ciglitazone also inhibited the formation of osteoclasts from PBMCs stimulated with RANKL and M-CSF (Chan et al., 2007). Similar to the results of this study, rosiglitazone not only inhibited the formation of TRAP-positive cells, but also attenuated the levels of TRAP mRNA and RANK protein that were increased after RANKL stimulation in mouse monocyte/macrophage cell line (Hassumi et al., 2009). Koufany et al. (2008) also reported that TZDs such as rosiglitazone and pioglitazone prevent bone resorption rather than cartilage changes in experimental polyarthritis by acting as anti-inflammatory mediators. Although the mechanisms by which rosiglitazone inhibits osteoclastogenesis are still unclear, one possibility is that the inhibition of osteoclast formation and bone resorption by rosiglitazone are associated in part with its potential to inhibit cytokine production. This is because PPARγ agonists are capable of preventing the production of osteoclastogenic-stimulating cytokines including interleukin-1 and TNF-α (Jiang et al., 1998). The present study also showed that treatment of rosiglitazone inhibits osteoclast induction and pit formation induced by TNF-α in bone marrow cells. Another possibility is that rosiglitazone inhibits the differentiation of hematopoietic precursors into macrophage lineages, resulting in increased adipogenic differentiation (Lazarenko et al., 2007). This is partly supported by previous findings showing that PPARγ activation by TZDs leads to the facilitation of adipogenesis with the attendant reduction of osteoblastic differentiation from marrow progenitors. It is also important to note that osteoblasts play critical roles in the activation of osteoclast differentiation and bone resorption (Neve et al., 2011). However, more detailed experiments are required to explore these possibilities and to understand the roles of rosiglitazone in the process of early differentiation of marrow progenitors.
This study also shows the inhibitory effects of rosiglitazone on osteoblastogenesis in bone marrow cells, as evidenced by the decreases in collagen and osteocalcin contents after treatment with rosiglitazone. In parallel with this result, rosiglitazone treatment decreased osteoblastogenesis in human mesenchymal stem cells (Benvenuti et al., 2007). Administration of rosiglitazone also causes bone loss in mice by suppressing osteoblast differentiation and bone formation (Ali et al., 2005). These effects of rosiglitazone are believed to be closely related to the increase in marrow adipocytes with the attendant induction of adipogenesis in bone marrows (Nishimura et al., 2007). In other words, these results suggest that the inhibition of bone formation by rosiglitazone is due, at least in part, to the decreased differentiation of osteoblasts from bone marrow progenitors. Rosiglitazone does not affect osteoblast life span, whereas it diverts bipotential mesenchymal progenitors from the osteoblast to the adipocyte lineage and also suppresses the differentiation of monopotential osteoblast progenitors (Ali et al., 2005; Lecka-Czernik et al., 2002). There is also a report showing that PPARγ haploinsufficiency in mice caused an increase in bone mass with increased osteoblastogenesis and decreased adipogenesis from marrow-derived mesenchymal stem cells (Akune et al., 2004). In addition to ciglitazone, a PPARγ activator, 15-deoxy-Δ 12,14-prostaglandin-J2, inhibited the expression of osteocalcin in primary osteoblasts, which was significantly suppressed by treating the cells with a PPARγ antagonist, GW9662 (Lin et al., 2007). These findings strongly suggest that PPARγ suppresses osteoblast differentiation and induces adipogenic differentiation under normal physiological circumstances. Of course, the common origin of adipocytes and osteoblasts contributes to reciprocal alterations in a number of these cells, but rosiglitazone may suppress the differentiation of monopotential osteoblast progenitors.
The current study supports the idea that inhibitory effects of rosiglitazone on osteoblastogenesis are related to increased expressions of PPARγ2 and aP2. This study also emphasizes the involvement of reduced mRNA expression of osteoblasts-pecific proteins, including Runx2, osteocalcin, and type I collagen. Runx2 is the main transcription factor required for osteoblastogenesis (Baek and Kim, 2011; Marie, 2008). Data from this study together with data from previous studies (Ali et al., 2005; Benvenuti et al., 2007; Lecka-Czernik et al., 1999) indicate that inhibition of Runx2 by rosiglitazone may be critical to understanding how rosiglitazone strongly prevents osteobla stogenesis in bone marrow cultures. Additional research is needed to determine whether or not there is a direct relationship between Runx2 induction and adipogenesis.
As such, our present findings demonstrate that rosiglitazone treatment in the presence of RANKL or TNF-α prevents osteoclast differentiation and bone resorption derived from M-CSF-stimulated mouse bone marrow cells, which suggests that rosiglitazone plays a role in bone loss. Interestingly however, rosiglitazone also prevents osteoblast differentiation, as demonstrated by the dose-dependent reductions of osteoblast-specific factors at the mRNA and protein levels after treatment with the agent. Considering the dual effects of rosiglitazone, it is still unclear exactly how the agent affects bone growth. Interestingly, rosiglitazone has been shown to strongly inhibit osteoblastic differentiation of bone marrow progenitors but not calvaria-derived osteoblasts (Ali et al., 2005). This indicates that rosiglitazone might affect the early, rather than late, stages of differentiation from marrow progenitor cells. There are also many reports showing that TZDs exert anti-inflammatory effects through inhibition of NF-αB activation (Hisada et al., 2005; Ohga et al., 2007; Sung et al., 2006; Wan et al., 2008). The present study also indicates that rosiglitazone is able to suppress NF-κB-DNA binding activation. Many types of proinflammatory cytokines are involved in the stimulation of osteoclastic differentiation, and PPARγ agonists attenuate cytokine production (Ohga et al., 2007; Sung et al., 2006). In addition, it is likely that the in vitro effects of rosiglitazone on osteoclastogenesis are not consistent with those in in vivo conditions. Therefore, it appears that rosiglitazone-mediated inhibition of osteoclast differentiation in vitro is at least in part associated with its anti-inflammatory potential.
In summary, osteoblasts are more critical for regulating bone metabolism and maintaining bone mass than osteoclasts. This is because osteoblasts are capable of differentiating into bone cells as well as affecting the differentiation of progenitor cells into osteoclasts or adipocytes, although osteoclasts also participate in the induction of osteoblasts. Moreover, the blockage of osteoblast-specific transcription factors can have a major impact on the loss of bone mass prior to an influence by osteoclasts. Consequently, long-term administration of rosiglitazone might result in a skewed differentiation of marrow progenitor cells into adipocytes via PPARγ activation, which leads to the in vivo suppression of osteoblastic differentiation with subsequent bone loss. These processes are unlikely to be affected by a direct impact of rosiglitazone on osteoclastic differentiation.
Supplementary Material
Acknowledgments
This work was supported by the Mid-career Researcher Program through a National Research Foundation grant by the Ministry of Education, Science and Technology (MEST), Republic of Korea (2011-0027469). A part of this work was supported by the Exchange Professor Program of Chonbuk National University for Dr. J.-C. Lee at the University of Kentucky, KY, USA.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Akune T., Ohba S., Kamekura S., Yamaguchi M., Chung U.I., Kubota N., Terauchi Y., Harada Y., Azuma Y., Nakamura K., et al. PPARgamma insufficiency enhances osteogenesis through osteoblast formation from bone marrow progenitors. J. Clin. Invest. 2004;113:846–855. doi: 10.1172/JCI19900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ali A.A., Weinstein R.S., Stewart S.A., Parfitt A.M., Manolagas S.C., Jilka R.L. Rosiglitazone causes bone loss in mice by suppressing osteoblast differentiation and bone formation. Endocrinology. 2005;146:1226–1235. doi: 10.1210/en.2004-0735. [DOI] [PubMed] [Google Scholar]
- Baek W.Y., Kim J.E. Transcriptional regulation of bone formation. Front. Biosci. (Schol Ed.) 2011;3:126–135. doi: 10.2741/s138. [DOI] [PubMed] [Google Scholar]
- Benvenuti S., Cellai I., Luciani P., Deledda C., Baglioni S., Giuliani C., Saccardi R., Mazzanti B., Dal Pozzo. S., Mannucci E., et al. Rosiglitazone stimulates adipogenesis and decreases osteoblastogenesis in human mesenchymal stem cells. J. Endocrinol. Invest. 2007;30:RC26–30. doi: 10.1007/BF03350807. [DOI] [PubMed] [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Chan B.Y., Gartland A., Wilson P.J., Buckley K.A., Dillon J.P., Fraser W.D., Gallagher J.A. PPAR agonists modulate human osteoclast formation and activity in vitro. Bone. 2007;40:149–159. doi: 10.1016/j.bone.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Del Fattore A., Capannolo M., Rucci N. Bone and bone marrow: the same organ. Arch. Biochem. Biophys. 2010;503:28–34. doi: 10.1016/j.abb.2010.07.020. [DOI] [PubMed] [Google Scholar]
- Gruntmanis U., Fordan S., Ghayee H.K., Abdullah S.M., See R., Ayers C.R., McGuire D.K. The peroxisome proliferator-activated receptor-gamma agonist rosiglitazone increases bone resorption in women with type 2 diabetes: a randomized, controlled trial. Calcif. Tissue Int. 2010;86:343–349. doi: 10.1007/s00223-010-9352-5. [DOI] [PubMed] [Google Scholar]
- Harsløf T., Wamberg L., Møller L., Stødkilde-Jørgensen H., Ringgaard S., Pedersen S.B., Langdahl B.L. Rosiglitazone decreases bone mass and bone marrow fat. J. Clin. Endocrinol. Metab. 2011;96:1541–1548. doi: 10.1210/jc.2010-2077. [DOI] [PubMed] [Google Scholar]
- Hassumi M.Y., Silva-Filho V.J., Campos-Júnior J.C., Vieira S.M., Cunha F.Q., Alves P.M., Alves J.B., Kawai T., Gonçalves R.B., Napimoga M.H. PPAR-gamma agonist rosiglitazone prevents inflammatory periodontal bone loss by inhibiting osteoclastogenesis. Int. Immunopharmacol. 2009;9:1150–1158. doi: 10.1016/j.intimp.2009.06.002. [DOI] [PubMed] [Google Scholar]
- Hisada S., Shimizu K., Shiratori K., Kobayashi M. Peroxisome proliferator-activated receptor gamma ligand prevents the development of chronic pancreatitis through modulating NF-kappaB-dependent proinflammatory cytokine production and pancreatic stellate cell activation. Rocz. Akad. Med. Bialymst. 2005;50:142–147. [PubMed] [Google Scholar]
- Jiang C., Ting A.T., Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1999;391:82–86. doi: 10.1038/34184. [DOI] [PubMed] [Google Scholar]
- Kawai M., Rosen C.J. PPARγ: a circadian transcription factor in adipogenesis and osteogenesis. Nat. Rev. Endocrinol. 2010;6:629–636. doi: 10.1038/nrendo.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kook S.H., Choi C., Son Y.O., Lee K.Y., Hwang I.H., Lee H.J., Chang J.S., Choi I.H., Lee J.C. Satellite cells isolated from adult Hanwoo muscle can proliferate and differentiate into myoblasts and adipose-like cells. Mol Cells. 2006;22:239–245. [PubMed] [Google Scholar]
- Koufany M., Moulin D., Bianchi A., Muresan M., Sebillaud S., Netter P., Weryha G., Jouzeau J.Y. Anti-inflammatory effect of antidiabetic thiazolidinediones prevents bone resorption rather than cartilage changes in experimental polyarthritis. Arthritis. Res. Ther. 2008;10:R6. doi: 10.1186/ar2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarenko O.P., Rzonca S.O., Hogue W.R., Swain F.L., Suva L.J., Lecka-Czernik B. Rosiglitazone induces decreases in bone mass and strength that are reminiscent of aged bone. Endocrinology. 2007;148:2669–2680. doi: 10.1210/en.2006-1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B. Bone as a target of type 2 diabetes treatment. Curr. Opin. Investig. Drugs. 2009;10:1085–1090. [PubMed] [Google Scholar]
- Lecka-Czernik B. Bone loss in diabetes: use of antidiabetic thiazolidinediones and secondary osteoporosis. Curr. Osteoporos. Rep. 2010;8:178–184. doi: 10.1007/s11914-010-0027-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecka-Czernik B., Gubrij I., Moerman E.J., jkenova O., Lipschitz D.A., Manolagas S.C., Jilka R.L. Inhibition of Osf2/Cbfa1 expression and terminal osteoblast differentiation by PPARgamma2. J. Cell. Biochem. 1999;74:357–371. [PubMed] [Google Scholar]
- Lecka-Czernik B., Moerman E.J., Grant D.F., Lehmann J.M., Manolagas S.C., Jilka R.L. Divergent effects of selective peroxisome proliferator-activated receptor-gamma 2 ligands on adipocyte versus osteoblast differentiation. Endocrinology. 2002;143:2376–2384. doi: 10.1210/endo.143.6.8834. [DOI] [PubMed] [Google Scholar]
- Lee J.C., Kim J., Park J.K., Chung G.H., Jang Y.S. The antioxidant, rather than prooxidant, activities of quercetin on normal cells: quercetin protects mouse thymocytes from glucose oxidase-mediated apoptosis. Exp. Cell. Res. 2003;291:386–397. doi: 10.1016/s0014-4827(03)00410-5. [DOI] [PubMed] [Google Scholar]
- Lin T.H., Yang R.S., Tang C.H., Lin C.P., Fu W.M. PPARγ inhibits osteogenesis via the down-regulation of the expression of COX-2 and iNOS in rats. Bone. 2007;41:562–574. doi: 10.1016/j.bone.2007.06.017. [DOI] [PubMed] [Google Scholar]
- Marie P.J. Transcription factors controlling osteoblastogenesis. Arch. Biochem. Biophys. 2008;473:98–105. doi: 10.1016/j.abb.2008.02.030. [DOI] [PubMed] [Google Scholar]
- Maulik N., Sato M., Price B.D., Das D.K. An essential role of NF-kappaB in tyrosine kinase signaling of p38 MAP kinase regulation of myocardial adaptation to ischemia. FEBS Lett. 1998;429:365–369. doi: 10.1016/s0014-5793(98)00632-2. [DOI] [PubMed] [Google Scholar]
- Neve A., Corrado A., Cantatore F.P. Osteoblast physiology in normal and pathological conditions. Cell. Tissue Res. 2011;343:289–302. doi: 10.1007/s00441-010-1086-1. [DOI] [PubMed] [Google Scholar]
- Ohga S., Shikata K., Yozai K., Okada S., Ogawa D., Usui H., Wada J., Shikata Y., Makino H. Thiazolidinedione ameliorates renal injury in experimental diabetic rats through anti-inflammatory effects mediated by inhibition of NF-kappaB activation. Am. J. Physiol. Renal. Physiol. 2007;292:F1141–F1150. doi: 10.1152/ajprenal.00288.2005. [DOI] [PubMed] [Google Scholar]
- Okazaki R., Toriumi M., Fukumoto S., Miyamoto M., Fujita T., Tanaka K., Takeuchi Y. Thiazolidinediones inhibit osteoclast-like cell formation and bone resorption in vitro. Endocrinology. 1999;140:5060–5065. doi: 10.1210/endo.140.11.7116. [DOI] [PubMed] [Google Scholar]
- Rachner T.D., Khosla S., Hofbauer L.C. Osteoporosis: now and the future. Lancet. 2011;377:1276–1287. doi: 10.1016/S0140-6736(10)62349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman M.M., Halade G.V., Williams P.J., Fernandes G. t10c12-CLA maintains higher bone mineral density during aging by modulating osteoclastogenesis and bone marrow adiposity. J. Cell. Physiol. 2011;226:2406–2414. doi: 10.1002/jcp.22578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rho J., Takami M., Choi Y. Osteoimmunology: interactions of the immune and skeletal systems. Mol Cells. 2004;17:1–9. [PubMed] [Google Scholar]
- Rzonca S.O., Suva L.J., Gaddy D., Montague D.C., Lecka-Czernik B. Bone is a target for the antidiabetic compound rosiglitazone. Endocrinology. 2004;145:401–406. doi: 10.1210/en.2003-0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A.V. TZDs and bone: a review of the recent clinical evidence. PPAR Res. 2008;2008:297893. doi: 10.1155/2008/297893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y.O., Jang Y.S., Heo J.S., Chung W.T., Choi K.C., Lee J.C. Apoptosis-inducing factor plays a critical role in cas-pase-independent, pyknotic cell death in hydrogen peroxideexposed cells. Apoptosis. 2009;14:796–808. doi: 10.1007/s10495-009-0353-7. [DOI] [PubMed] [Google Scholar]
- Sturge J., Caley M.P., Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat. Rev. Clin. Oncol. 2011;8:357–368. doi: 10.1038/nrclinonc.2011.67. [DOI] [PubMed] [Google Scholar]
- Sugii S., Evans R.M. Epigenetic codes of PPARγ in metabolic disease. FEBS Lett. 2011;585:2121–2128. doi: 10.1016/j.febslet.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung B., Park S., Yu B.P., Chung H.Y. Amelioration of age-related inflammation and oxidative stress by PPAR gamma activator: suppression of NF-kappaB by 2,4-thiazolidinedione. Exp. Gerontol. 2006;41:590–599. doi: 10.1016/j.exger.2006.04.005. [DOI] [PubMed] [Google Scholar]
- Wan Y. PPARγ in bone homeostasis. Trends Endocrinol. Metab. 2010;21:722–728. doi: 10.1016/j.tem.2010.08.006. [DOI] [PubMed] [Google Scholar]
- Wan Y., Chong L.W., Evans R.M. PPAR-gamma regulates osteoclastogenesis in mice. Nat. Med. Dec. 2007;13:1496–1503. doi: 10.1038/nm1672. [DOI] [PubMed] [Google Scholar]
- Wan H., Yuan Y., Qian A., Sun Y., Qiao M. Pioglitazone, a PPARgamma ligand, suppresses NFkappaB activation through inhibition of IkappaB kinase activation in cerulein-treated AR42. J cells. Biomed. Pharmacother. 2008;62:466–472. doi: 10.1016/j.biopha.2007.10.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.