Abstract
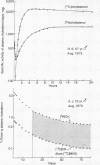
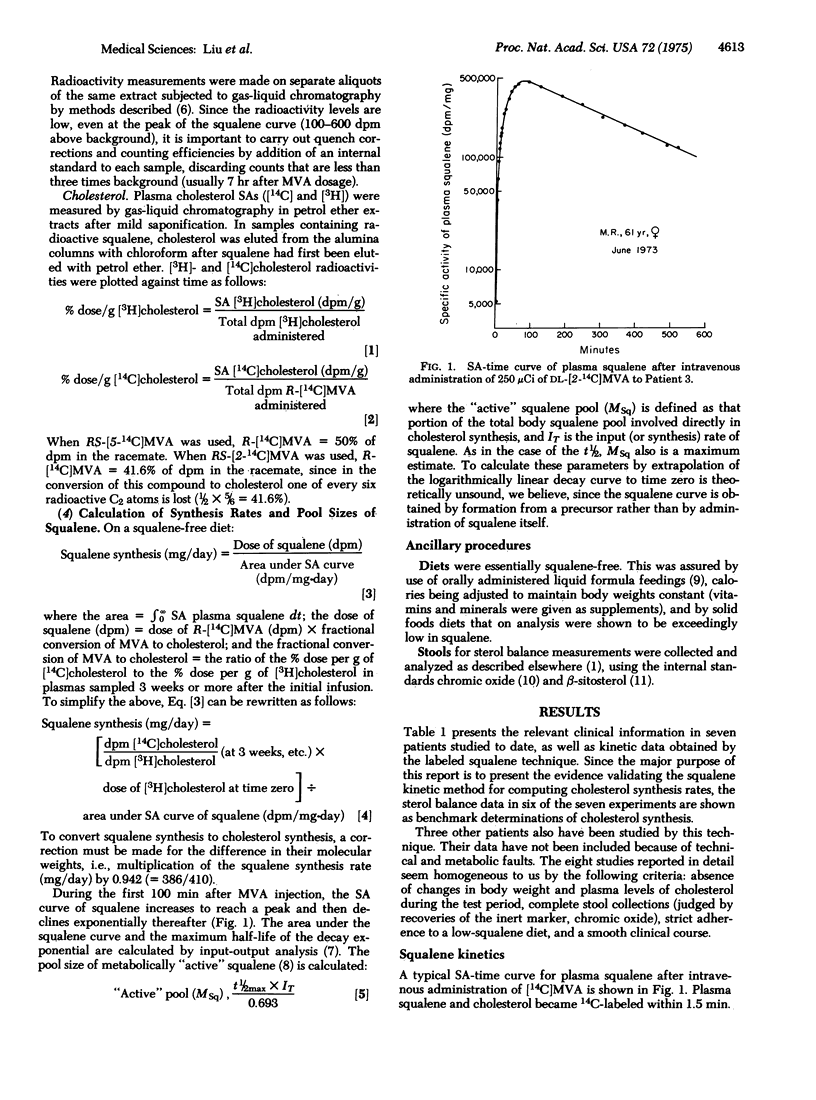
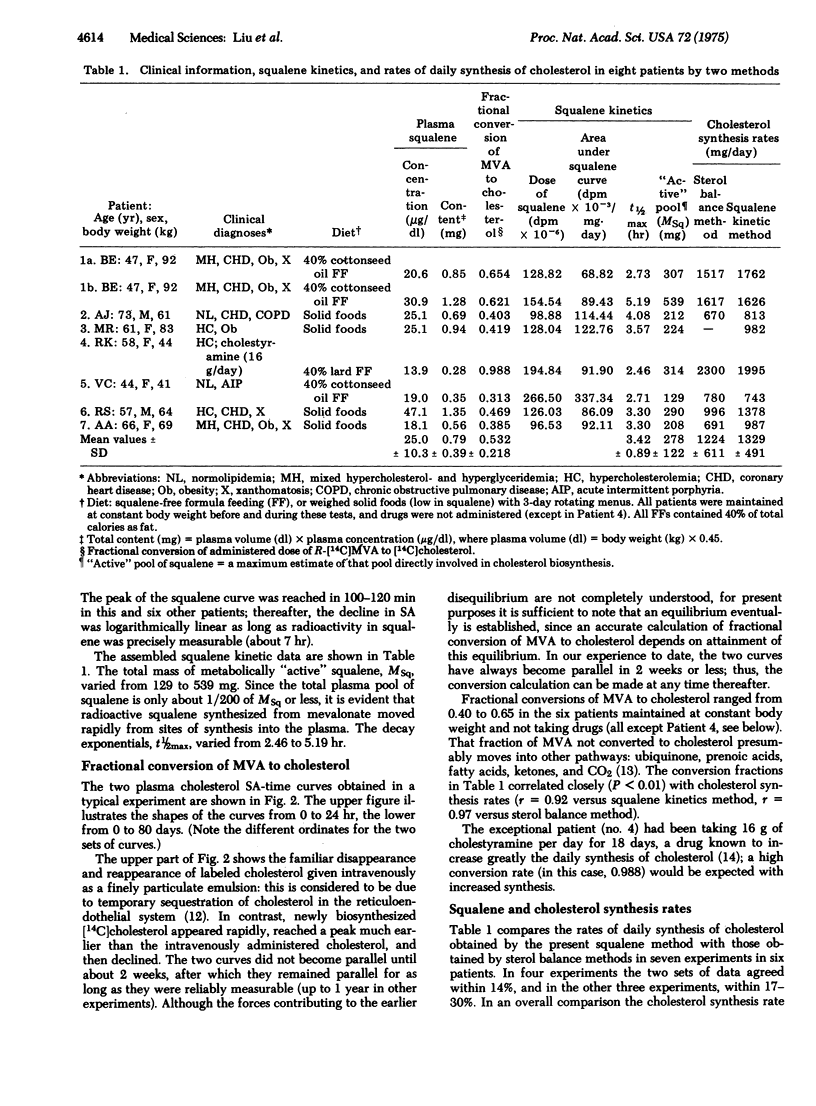
A method for measuring the rate of total daily cholesterol synthesis in man has been developed through isotope kinetic studies of squalene biosynthesis after intravenous administration of [14C]mevalonic acid. Plasma squalene becomes rapidly labeled, reaching maximal specific activity approximately 100 min after mevalonate administration and then decays exponentially to reach undetectable levels in 12 hr. The rate of daily squalene synthesis equals the percent dose of mevalonate converted to cholesterol divided by the area under the specific activity curve of squalene; the fraction of the dose of mevalonate converted to cholesterol is calculated by the simultaneous injection of [3H]- and [14C] cholesterol in plasma. The premise that squalene and cholesterol synthetic rates are equivalent was tested. In seven patients it was found that the mean daily cholesterol synthesis rates estimated simultaneously by sterol balance and by sqyalene kinetic methods agreed within 8%. In addition, fractional conversions of mevalonic acid to cholesterol were highly correlated with cholesterol synthesis rates. Maximum estimates of the pool sizes and half-lives of metabolically "active" squalene also were obtained. This measurement of daily cholesterol synthesis by squalene kinetics minimizes patient inconvenience, is suitable for outpatient studies, and yields results in 4 weeks or less. Because of the rapidity of the rate of squalene synthesis, the results obtained reflect cholesterol synthesis over a period of less than 10 hr and are therefore uniquely applicable to unsteady state situations.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Back P., Hamprecht B., Lynen F. Regulation of cholesterol biosynthesis in rat liver: diurnal changes of activity and influence of bile acids. Arch Biochem Biophys. 1969 Aug;133(1):11–21. doi: 10.1016/0003-9861(69)90482-2. [DOI] [PubMed] [Google Scholar]
- Davignon J., Simmonds W. J., Ahrens E. H. Usefulness of chromic oxide as an internal standard for balance studies in formula-fed patients and for assessment of colonic function. J Clin Invest. 1968 Jan;47(1):127–138. doi: 10.1172/JCI105703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Edmond J., Popjåk G. Metabolism of mevalonate in rats and man not leading to sterols. J Biol Chem. 1975 Mar 10;250(5):1771–1775. [PubMed] [Google Scholar]
- Goodman D. S., Noble R. P., Dell R. B. Three-pool model of the long-term turnover of plasma cholesterol in man. J Lipid Res. 1973 Mar;14(2):178–188. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Davignon J. The interaction of cholesterol absorption and cholesterol synthesis in man. J Lipid Res. 1969 May;10(3):304–315. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr Measurements of cholesterol turnover, synthesis, and absorption in man, carried out by isotope kinetic and sterol balance methods. J Lipid Res. 1969 Jan;10(1):91–107. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Dietary beta-sitosterol as an internal standard to correct for cholesterol losses in sterol balance studies. J Lipid Res. 1968 May;9(3):374–387. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G. Interruption of the enterohepatic circulation of bile acids in man: comparative effects of cholestyramine and ileal exclusion on cholesterol metabolism. J Lab Clin Med. 1971 Jul;78(1):94–121. [PubMed] [Google Scholar]
- Grundy S. M., Ahrens E. H., Jr, Salen G., Schreibman P. H., Nestel P. J. Mechanisms of action of clofibrate on cholesterol metabolism in patients with hyperlipidemia. J Lipid Res. 1972 Jul;13(4):531–551. [PubMed] [Google Scholar]
- Kim D. N., Lee K. T., Reiner J. M., Thomas W. A. Restraint of cholesterol accumulation in tissue pools associated with drastic short-term lowering of serum cholesterol levels by clofibrate or cholestyramine in hypercholesterolemic swine. J Lipid Res. 1974 Jul;15(4):326–331. [PubMed] [Google Scholar]
- LOUD A. V., BUCHER N. L. The turnover of squalene in relation to the biosynthesis of cholesterol. J Biol Chem. 1958 Jul;233(1):37–41. [PubMed] [Google Scholar]
- MIETTINEN T. A., AHRENS E. H., Jr, GRUNDY S. M. QUANTITATIVE ISOLATION AND GAS--LIQUID CHROMATOGRAPHIC ANALYSIS OF TOTAL DIETARY AND FECAL NEUTRAL STEROIDS. J Lipid Res. 1965 Jul;6:411–424. [PubMed] [Google Scholar]
- Nikkari T., Schreibman P. H., Ahrens E. H., Jr In vivo studies of sterol and squalene secretion by human skin. J Lipid Res. 1974 Nov;15(6):563–573. [PubMed] [Google Scholar]
- Perl W., Samuel P. Input-output analysis for total input rate and total traced mass of body cholesterol in man. Circ Res. 1969 Aug;25(2):191–199. doi: 10.1161/01.res.25.2.191. [DOI] [PubMed] [Google Scholar]
- Samuel P., Lieberman S. Improved estimation of body masses and turnover of cholesterol by computerized input--output analysis. J Lipid Res. 1973 Mar;14(2):189–196. [PubMed] [Google Scholar]
- Sedaghat A., Samuel P., Crouse J. R., Ahrens E. H., Jr Effects of neomycin on absorption, synthesis, and/or flux of cholesterol in man. J Clin Invest. 1975 Jan;55(1):12–21. doi: 10.1172/JCI107902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro D. J., Imblum R. L., Rodwell V. W. Thin-layer chromatographic assay for HMG-CoA reductase and mevalonic acid. Anal Biochem. 1969 Oct 1;31(1):383–390. doi: 10.1016/0003-2697(69)90279-6. [DOI] [PubMed] [Google Scholar]
- Zilversmit D. B., Hughes L. B. Validation of a dual-isotope plasma ratio method for measurement of cholesterol absorption in rats. J Lipid Res. 1974 Sep;15(5):465–473. [PubMed] [Google Scholar]