Abstract
Carbohydrates, mainly sucrose, that are synthesized in source organs are transported to sink organs to support growth and development. Phloem loading of sucrose is a crucial step that drives long-distance transport by elevating hydrostatic pressure in the phloem. Three phloem loading strategies have been identified, two active mechanisms, apoplastic loading via sucrose transporters and symplastic polymer trapping, and one passive mechanism. The first two active loading mechanisms require metabolic energy, carbohydrate is loaded into the phloem against a concentration gradient. The passive process, diffusion, involves equilibration of sucrose and other metabolites between cells through plasmodesmata. Many higher plant species including Arabidopsis utilize the active loading mechanisms to increase carbohydrate in the phloem to higher concentrations than that in mesophyll cells. In contrast, recent data revealed that a large number of plants, especially woody species, load sucrose passively by maintaining a high concentration in mesophyll cells. However, it still remains to be determined how the worldwide important cereal crop, rice, loads sucrose into the phloem in source organs. Based on the literature and our results, we propose a potential strategy of phloem loading in rice. Elucidation of the phloem loading mechanism should improve our understanding of rice development and facilitate its manipulation towards the increase of crop productivity.
Keywords: diffusion, phloem loading, rice, sucrose, sucrose transporter
INTRODUCTION
Sucrose (Suc) is the main carbohydrate product of photosynthesis in higher plants and is transported long distances from source to various sink organs such as flowers, seeds and roots, thus supporting plant growth and development. Long-distance transport of Suc is initiated by Suc uptake into collective phloem of minor veins of source leaves. This process is referred to as phloem loading and is the first important step of the long-distance transport of photoassimilate to sink organs. Suc is then translocated via transport phloem and unloaded into sink organs via release phloem (van Bel, 2003). The phloem contains two main cell types, sieve elements (SEs) and companion cells (CCs). This long-distance transport occurs in SEs, which lack a nucleus and have very few organelles and thus rely on associated CCs for most metabolic requirements (van Bel and Knoblauch, 2000).
High concentrations of photoassimilate loaded into source collective phloem generates the driving force, a hydrostatic pressure gradient, that enables the mass flow of Suc to sink organs via transport and release phloem (Turgeon, 2010; van Bel, 1996). That is, a high concentration of Suc inside the phloem cells in source organs creates an osmotic potential gradient that draws water into the cells. Phloem sap thus moves from source to sink by means of turgor pressure gradient.
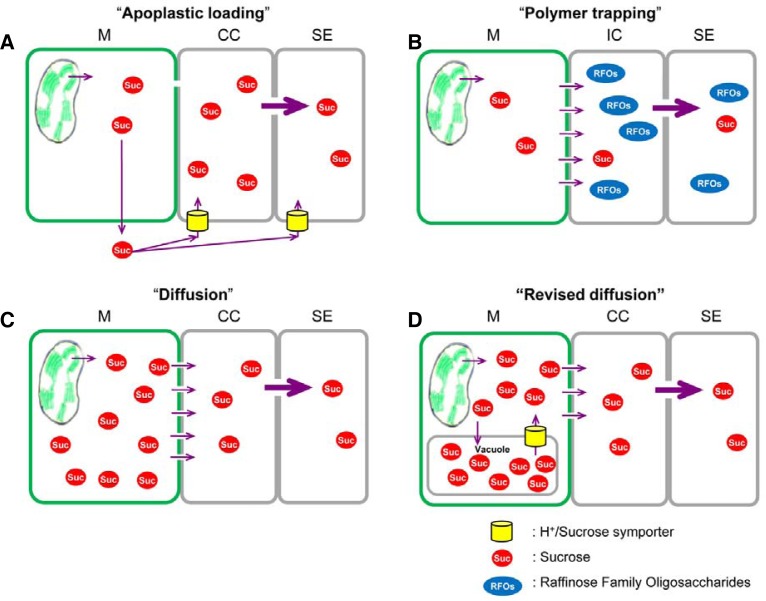
In collective phloem, the loading strategies can be divided into three major pathways: apoplastic loading, polymer trapping, and diffusion. The apoplastic loading strategy (Fig. 1A) uses the proton motive force as metabolic energy to load Suc from the apoplast (cell wall space) actively into the phloem by Suc transporters (SUTs). In the second mechanism, polymer trapping (Fig. 1B), Suc diffuses from mesophyll cells into the specialized CCs called intermediary cells, symplastically through the cell-to-cell connections called plasmodesmata. Suc then serves as a substrate to synthesize raffinose family oligosaccharides (RFOs) such as raffinose and stachyose to increase the concentration of these sugars in the phloem in this thermodynamically active process (McCaskill and Turgeon, 2007; Zhang and Turgeon, 2009). Therefore, these two strategies are metabolic energy-dependent pathways. In contrast, the diffusion strategy (Fig. 1C), a passive loading mechanism, is an energetically downhill process because Suc levels are higher in mesophyll cells than in collective phloem, and no energy is used to collect Suc in SEs.
Fig. 1.
Schematic representation of phloem loading mechanism. (A) Apoplastic phloem loading mechanism. Suc synthesized in mesophyll cells diffuses to vascular parenchyma cells, is exported to apoplast, then transported by a SUT into the phloem. (B) Symplastic polymer trapping mechanism. Suc is mobilized symplastically from mesophyll cells to the specialized CCs, intermediary cells of the collective minor veins. RFOs are then synthesized in intermediary cells. This generates high sugar concentrations in the phloem. (C) Symplastic passive loading (diffusion) mechanism. Suc is translocated passively from mesophyll cells to the phloem of minor veins. This passive loading mechanism requires high Suc concentrations in mesophyll cells to drive the diffusion via plasmodesmata into the phloem. (D) Revised symplastic passive loading mechanism mediated by vacuolar Suc trapping in rice. In this model, the tonoplast SUT is essential to regulate Suc flux to the phloem. CC, companion cell; M, mesophyll cell; SE, sieve element, IC, intermediary cell.
Rice is an important crop worldwide and is the principal food source for a large proportion of the global population. In general, the yield potential of rice crop plants is dependent upon whole plant carbohydrate partitioning mediated by Suc translocation from source to sink organs. Therefore, understanding the entire carbohydrate partitioning process whereby Suc is assimilated in source organs and translocated through the phloem to sink organs should provide new approaches to improve plant growth and crop yield (Lim et al., 2006). In this review, we summarize putative phloem loading strategies by integrating recent reports on SUTs, and propose a potential phloem loading strategy that may be especially important in rice.
PUTATIVE PHLOEM LOADING MECHANISMS
Apoplastic loading
While a portion of fixed carbon is stored transiently as starch in the chloroplasts and Suc in the vacuole, a large amount of photoassimilate fixed during the day is exported directly through the phloem. Suc is synthesized in mesophyll cells, diffuses symplastically through plasmodesmata into bundle sheath cells and subsequently vascular parenchyma cells. Suc is then exported into the apoplast. In the apoplastic loading strategy, the phloem is symplastically isolated or has rare plasmodesmata connecting to surrounding cells. Subsequently, Suc must be transported across the plasma membrane of CCs and/or SEs from the apoplast (Gottwald et al., 2000). In this apoplastic loading mechanism, the function of SUTs localized in the plasma membrane is essential for pumping Suc into the phloem. The proton motive force generated by H+-ATPases is the form of energy used to drive Suc into the phloem by SUTs against a concentration gradient (Braun and Slewinski, 2009; Lalonde et al, 2004; Sauer, 2007; Turgeon, 2010). It is also noteworthy that active phloem loading is assumed to allow plants to maintain low photoassimilate concentrations in source leaves and thus to avoid feedback inhibition of photosynthesis, as well as to elevate sufficient pressure in the phloem to enable long-distance transport (Fig. 1A; Turgeon, 2010).
A number of plant species including the model plant Arabidopsis utilize the apoplastic loading pathway. SUTs are the best characterized components involved in apoplastic phloem loading. The first SUT genes, SoSUT1 and StSUT1, were isolated from spinach (Spinacia oleracea) and potato (Solanum tuberosum), respectively (Riesmeier et al., 1992; 1993). Antisense suppression of StSUT1 in potato causes local breaching and curling of leaves and dramatic reduction in root development and tuber yield. The plant leaves contain over 20-fold higher soluble sugars and 5-fold higher starch compared with wild type plants. This work revealed an essential role of SUTs, localized in the phloem plasma membrane, in the primary step in the apoplastic loading pathway (Riesmeier et al., 1994). Subsequently, SUTs have been identified throughout the plant kingdom (Reinders et al., 2012).
In Arabidopsis, AtSUC2 is a potential apoplastic phloem loader, which acts as Suc/H+ symporter to uptake Suc into the phloem. atsuc2 mutants display a severe growth retardation phenotype that is considered typical for an apoplastic phloem loader mutant (Gottwald et al., 2000). In the monocot species maize (Zea mays), zmsut1 mutant plants develop chlorotic leaves that accumulate high concentrations of sugars and prematurely senesce. The feeding of [14C]Suc demonstrated that Suc export is diminished in the mutants compared with wild type. In addition, the zmsut1 plants display reduced plant height, fewer leaves, delayed flowering, and stunted tassel development (Slewinski et al., 2009). Therefore, in maize, the activity of ZmSUT1 is essential for phloem loading of Suc.
In the apoplastic loading strategy, the mechanism for Suc export from the vascular parenchyma cells to the apoplast as a prerequisite for phloem loading by SUTs has been elusive. However, a transporter responsible for this Suc efflux process was recently discovered to be SWEET proteins (Chen et al., 2012) that were previously known as sugar, glucose and fructose, efflux transporters in Arabidopsis and rice (Chen et al., 2010). Expression analysis of Arabidopsis AtSWEET11 and AtSWEET12 promoter::β-glucuronidase (GUS) transgenic plants indicated that these genes are preferentially expressed in vascular parenchyma cells. In addition, atsweet11/atsweet12 double mutants were defective in phloem loading (Chen et al., 2012), thus revealing an important function of SWEET-mediated uniport of Suc from the parenchyma cells feeding H+-coupled import into the phloem SE-CC complex.
It is noteworthy that while mutation of the Suc carriers such as AtSUC2 and ZmSUT1, caused a typical phenotype of apoplastic phloem loading mutants, these plants can complete their life cycle and make viable seeds (Slewinski et al., 2009; Srivastava et al., 2009). Therefore, the apoplastic loading plant species largely follow apoplastic loading pathway, but are capable to use an alternative loading mechanism such as the symplastic loading strategy that can partially compensate. In addition, apoplastic loading was found to be highly correlated with the herbaceous habit (Davidson et al., 2011).
Polymer trapping
In the polymer trapping mechanism, Suc is mobilized symplastically from mesophyll cells to intermediary cells of the collective minor veins. Polymer trapping is thus a specialized form of the symplastic phloem loading mechanism. Although polymer trapping does not involve active transport of Suc across the plasma membrane, it is considered a thermodynamically active process because Suc is converted to RFOs, a process that requires metabolic energy (Turgeon, 1996; 2010; Zhang and Turgeon, 2009). RFOs are assumed to be too large to diffuse back to mesophyll through the plasmodesmata but can proceed through wider plasmodesmata into SEs. Therefore, RFOs synthesized in intermediary cells are essential to maintain high sugar concentrations in the phloem, which is similar to that in apoplastic loading species. This allows mesophyll cells to maintain low Suc levels (Fig. 1B; Slewinski and Braun, 2010; Turgeon, 2010).
The presence of intermediary cells is always correlated with the translocation of considerable RFOs. Plasmodesmata are especially numerous between bundle sheath cells and intermediary cells in the minor veins of species such as Verbascum phoeniceum, Rehmannia glutinosa, Catalpa speciosa, Buddleja davidii, Citrullus lanatus, and Coleus blumei that transport RFOs (Gamalei, 1989; Turgeon and Gowan, 1990; Rennie and Turgeon, 2009; Zhang and Turgeon, 2009).
The polymer trapping hypothesis is supported by recent molecular and genetic evidence. In V. phoeniceum, RNAi suppression of a plasma membrane-localized SUT did not produce a typical phenotype of apoplastic phloem loader mutants (Zhang and Turgeon, 2009). Galactinol synthase (GAS) catalyzes the first committed step of RFO synthesis, which produces galactinol from myo-inositol and UDP-galactose. Galactinol then serves as the galactosyl donor in the RFO synthesis. RNAi suppression of the two VpGAS genes resulted in pronounced inhibition of RFO synthesis. As a result, transgenic plants showed carbohydrate accumulation, reduced sugar export, leaf chlorosis and severe growth retardation (McCaskill and Turgeon, 2007). This indicates that the mechanism of polymer trapping is most likely dependent on RFO synthesis in intermediary cells but does not require active Suc transport from the apoplast (Zhang and Turgeon, 2009). Notably, a floristic analysis indicated that plants with intermediary cells are over-represented in the tropics and subtropics (Davidson et al., 2011).
Diffusion, a passive symplastic loading
A third proposed mechanism for phloem loading is a passive symplastic pathway called diffusion (Reidel et al., 2009; Slewinski and Braun, 2010; Turgeon, 2010; Turgeon and Medville, 1998). This is a passive flux of Suc from mesophyll cells to the phloem of minor veins. Plant species that utilize this passive loading mechanism maintain high levels of Suc in mesophyll cells to drive diffusion via plasmodesmata into the phloem, which is an energetically downhill process (Fig. 1C; Reidel et al., 2009; Rennie and Turgeon, 2009; Turgeon and Medville, 1998).
Plasmodesmatal frequencies in the phloem of leaf minor veins vary considerably (Davidson et al., 2011; Gamalei, 1989; van Bel and Gamalei, 1992). Plants with high, intermediate, and low plasmodesmatal frequencies in the phloem of minor veins are classified to type 1, 1–2a, and 2, respectively (Gamalei, 1989). Analysis of minor vein ultrastructure and plasmodesmatal frequencies of CCs indicates that most of the symplastic loaders are type 1, having numerous plasmodesmata to maintain the passive flux (Rennie and Turgeon, 2009; Schulz, 2005; Turgeon and Ayre, 2005). In contrast, Arabidopsis and maize belong to type 1–2a and type 2, respectively, by Gamalei’s definition (Evert et al., 1978; Gamalei, 1989; Haritatos et al., 2000). In the analysis of 45 herbaceous and woody species, 14 exhibited the characteristics of passive loading (Rennie and Turgeon, 2009). This number included 11 of 19 woody plants analyzed. This indicates strong association between passive symplastic loading and the tree growth form (Davidson et al., 2011).
Since mesophyll cells and the phloem are symplastically linked, these plants do not accumulate radiolabeled Suc in the minor veins when their leaf discs are incubated in [14C]Suc. In symplastic loaders, Suc taken up into the symplast diffuses through plasmodesmata into mesophyll cells as well as the phloem. In contrast, apoplastic loaders and polymer trapping species showed clear vein images (Rennie and Turgeon, 2009; Turgeon, 2010).
PHLOEM LOADING STRATEGY IN RICE
From observation of the ultrastructure of vascular bundles, it was suggested that both apoplastic and symplastic phloem loading pathways are possible in rice (Chonan et al., 1984; Kaneko et al., 1980), but the exact phloem loading mechanism in rice has not been clearly demonstrated.
Functions of rice SUTs
In apoplastic loaders, function of the plasma membrane-localized SUTs is essential for translocating Suc from apoplast into the phloem against concentration gradient (Braun and Slewinski, 2009; Lalonde et al., 2004; Sauer, 2007; Turgeon, 2010). Based on comparison of their deduced amino acid sequences, SUTs are categorized into three major groups: type I (specific to eudicots, plasma membrane localized), type II (present in all plants, plasma membrane localized), and type III (present in all plants, vacuolar membrane localized). Members of type I are responsible for phloem loading or Suc import into sink organs (Gottwald et al., 2000; Riesmeier et al., 1994). Monocot species utilize type II SUTs for phloem loading (Aoki et al., 2003; Kühn, 2003; Lim et al., 2006; Slewinski et al., 2009). Type III SUTs are localized at the vacuolar membrane (tonoplast) and function in Suc transport into the cytosol from the vacuole lumen (Endler et al., 2006; Eom et al., 2011; Reinders et al., 2008). Of five OsSUTs in rice, four, OsSUT1, OsSUT3, OsSUT4, and OsSUT5, are categorized into type II, and OsSUT2 belongs to the type III family of tonoplast SUT members. Of type II OsSUTs, OsSUT4 is closely related to dicot type SUTs of type II family, sharing a common feature of N-terminal extension and long central loop, whose function has not been clearly demonstrated (Aoki et al., 2003; Hirose et al., 1997). In addition, OsSUT5 was further classified to a subgroup of monocot SUTs (Kühn and Grof, 2010), and its transport activity is less sensitive to pH, than other SUTs in the type II family (Sun et al., 2010). Therefore, it is hypothesized that the remaining members of type II, OsSUT1 and/or OsSUT3, are candidates to function in apoplastic phloem loading.
OsSUT1 is most likely an ortholog of the phloem loader, ZmSUT1, of maize (Slewinski et al., 2009). In gene expression pattern analysis, OsSUT1 mRNA was highly expressed in leaves as well as in stems and filling grains (Aoki et al., 2003; Hirose et al., 1997). A detailed expression pattern of OsSUT1 was conducted by immunolocalization using an OsSUT1 peptide-specific antibody (Scofield et al., 2007a; 2007b). The results showed that during germination and early seedling growth OsSUT1 is confined to the phloem of coleoptiles and first and second leaf blades (Scofield et al., 2007a). Also, in mature rice plants, OsSUT1 is present in the mature phloem of all the vegetative tissues, from flag leaf blade to the base of the filling grain, involved in long-distance transport pathway during grain filling (Scofield et al., 2007b). The results obtained from expression and localization experiments suggest that OsSUT1 may function in apoplastic phloem loading in leaves.
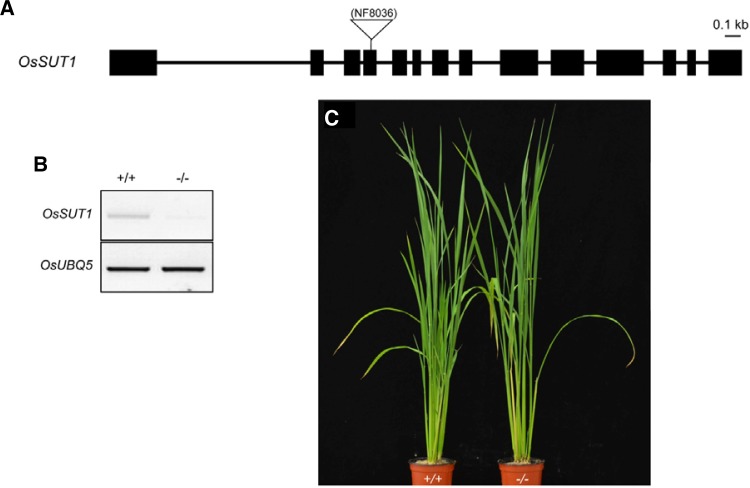
However, transgenic rice plants with antisense suppression of OsSUT1 do not accumulate Suc or show significant effects on photosynthetic rates in source leaves. Also, their vegetative growth does not show any visible symptom (Ishimaru et al., 2001; Scofield et al., 2002), raising the possibility that apoplastic phloem loading is a minor pathway or not largely contributed by OsSUT1 in rice vegetative tissues. Although OsSUT1 antisense rice plants under the control of CaMV35S (Ishimaru et al., 2001) or maize ubiquitin (Scofield et al., 2002) promoter were assessed, it would be valuable to examine null mutants because the antisense lines have still low expression of OsSUT1 that can be sufficient to maintain phloem loading (Ishimaru et al., 2001; Schofield et al., 2002). Recent analysis of Tos17 insertional mutant of OsSUT1 revealed that germination of mutant pollen is impaired (Hirose et al., 2010). Therefore, homozygous mutant plants of OsSUT1 have not been produced by normal self-pollination. In our present study, we generated homozygous ossut1 mutants from the Tos17 insertional heterozygous line of OsSUT1 by using anther culture (Fig. 2A). RT-PCR analysis indicated that the ossut1 mutant plants are null lacking any expression of OsSUT1 (Fig. 2B). During their vegetative growth stage, the ossut1 mutants are indistinguishable from wild type plants (Fig. 2C), which is therefore consistent with those of OsSUT1 antisense transgenic rice plants (Ishimaru et al., 2001; Scofield et al., 2002). This indicates that OsSUT1-mediated pathway may not be a primary route for phloem loading in source leaves of rice. It is therefore now assumed that OsSUT1 may function in retrieval by phloem reloading of Suc leaked from the phloem (Scofield et al., 2007b).
Fig. 2.
Isolation and characterization of the ossut1 mutant. (A) Schematic diagram of the rice OsSUT1 gene and the Tos17 insertion position of line NF8036. The 14 exons are indicated by boxes. (B) RT-PCR analysis of wild type (+/+) and homozygous (−/−) mutant. OsSUT1 transcripts are not detectable in the homozygous mutant. (C) Growth phenotype of wild type (left) and homozygous ossut1 mutant (right) at heading stage.
It is noteworthy that, by in situ localization, OsSUT1 is expressed in the maternal nucellar projection and nucellar epidermis, and the filial aleurone tissues but not in the starchy endosperm of mid-developing seeds (Furbank et al., 2001). This is consistent with the severe defects in grain filling in OsSUT1 antisense plants as well as retarded development at the early stage of vegetative growth (Scofield et al., 2002). In addition, in tissue slices of filling grains, treatment of p-chloromercuriben-zene sulfonate, a SUT inhibitor, interferes with Suc transport (Furbank et al., 2001). These results suggest that OsSUT1 may play a role in Suc transport from maternal to aleurone tissues via an apoplastic route.
OsSUT3 is also a type II SUT. In situ localization was used to show that OsSUT3 is highly expressed in developing pollen (Ngampanya et al., 2002; Takeda et al., 2001), suggesting a role in Suc translocation into pollen. Our preliminary analysis of OsSUT3 promoter::GUS transgenic rice plants confirms that it is preferentially expressed in pollen. This expression suggests that OsSUT3 may function in developing pollen rather than in the phloem loading of source organs. In summary, apoplastic loading mechanism may not be predominant for phloem loading in source leaves of rice.
Although phylogenetic analysis of SUTs above indicated that OsSUT4 and OsSUT5 are classified within different subgroups than OsSUT1 (Aoki et al., 2003; Kühn and Grof, 2010; Lim et al., 2006), we cannot completely rule out the possibility that OsSUT4 and/or OsSUT5 may have redundant function in apoplastic loading. Cellular localization assay in detail on OsSUT4 and OsSUT5 may aid understanding of the in planta roles of these rice SUTs. In addition, at present the mutant lines of OsSUT3, OsSUT4, and OsSUT5 are available from our rice T-DNA mutant population and their single, double, and multiple mutants are being analyzed in our laboratory.
Involvement of tonoplast SUT in phloem loading
In photoautotrophic cells, vacuoles store excess Suc during the day and export it at night (Ayre, 2011; Linka and Weber, 2010; Martinoia et al., 2007; Neuhaus, 2007). Recently, through proteomic and/or GFP fusion analyses, tonoplast-localized SUTs have been identified (Endler et al., 2006; Eom et al., 2011; Okhubo-Kurihara et al., 2011; Payyavula et al., 2011; Reinders et al., 2008; Schneider et al., 2012; Schulz et al., 2011). Among them, expression of PtaSUT4 was highest in mature source leaves. By in situ localization, PtaSUT4 expression was found in epidermal cells, spongy mesophyll, the lower layer of the palisade mesophyll and minor phloem traces (Payyavula et al., 2011). In rice, OsSUT2 expression was mainly found in mesophyll and bundle sheath cells of leaf blades and sheaths, rarely in CCs and SEs of the phloem (Eom et al., 2011). Thus, these have been suggested to function in the transport and vacuolar storage of photosynthetically derived Suc.
Functional characterization of tonoplast SUTs have been performed in Populus, rice and Arabidopsis (Eom et al., 2011; Payyavula et al., 2011; Schneider et al., 2012). In poplar, PtaSUT4 RNAi transgenic plants exhibit 1.5 to 2-fold higher Suc content in source leaves. An increased ratio of leaf-to-stem biomass indicates a link between vacuolar transport of Suc and biomass partitioning (Payyavula et al., 2011). In rice, the T-DNA insertional mutant, ossut2 has increased Suc content both at the end of day (2-fold) and at the end of night (4-fold). The ossut2 mutants also have diminished plant growth, tiller number, plant height, root dry weight and 1,000 grain weight. Decreased translocation of Suc from source leaves to sink parts of the ossut2 mutant was also demonstrated (Eom et al., 2011). These data suggest that the tonoplast SUTs influence Suc export from source to sink organs.
Suc transport into Xenopus oocytes by LjSUT4, a Lotus japonicus tonoplast SUT, was found to be inhibited by the protonophore carbonyl cyanide m-chlorophenylhydrazone (CCCP) and the extracellular application of Suc induced membrane depolarization in LjSUT4-expressing Xenopus oocytes (Reinders et al., 2008). Suc transport by OsSUT2 expressed in yeast was inhibited by CCCP (Eom et al., 2011). These findings indicate that tonoplast SUTs function as Suc/H+ symporters. Considering the normal pH gradient across the tonoplast, they function in Suc transport across the tonoplast from the vacuole lumen to the cytosol. It is probable that the Suc transport activity of OsSUT2 in yeast is due to mis-targeting of the overexpressed protein to the plasma membrane in the heterologous system. In summary, tonoplast SUTs are involved in Suc transport from the vacuole lumen to the cytosol, playing an essential role in Suc export from source leaves to sink organs. It is noteworthy that poplar utilizes passive symplastic export (Russin and Evert, 1985; Turgeon and Medville, 1998). In contrast, in the apolastic phloem loading species, Arabidopsis, mutation of the tonoplast AtSUC4 showed no visible phenotype in normal growth conditions (Schneider et al., 2012), suggesting a relatively a minor role for the tonoplast SUT during Suc translocation.
Proposed phloem loading mechanism in rice
Based on the concentration and form of transport sugars, function of SUTs, and vein structures in source leaves of various plant species, a potential phloem loading mechanism is considered (Turgeon, 2010). Although the phloem loading mechanism in rice is still uncertain and need more consideration, previous and our present data provide some evidence that rice may utilize a passive loading strategy in source leaves as a primary phloem loading pathway. It has been shown that rice stores relatively high ratios of Suc to transitory starch in leaves, which differs from other plant species including Arabidopsis that primarily stores starch (Lee et al., 2008; Murchie et al., 2002; Nakano et al., 1995; Trevanion, 2002; Winder et al., 1998). It is noteworthy that concentrations of transport sugars in leaves correlate with a qualitative assessment of plasmodesmatal frequencies. Most of the type 1 and type 1–2a species with high and intermediate numbers of plasmodesmata have high concentrations of transport sugars, while many of the type 2 species have low concentrations of transport sugars (Rennie and Turgeon, 2009). It is known that the former group utilizes passive loading while the latter adopted an apoplastic loading strategy. In poplar the osmolalities between the phloem and mesophyll cells are approximately the same, as they are in the passive loading species willow (Salix babylonica) (Russin and Evert, 1985; Turgeon and Medville, 1998). High concentration of Suc in leaves is a diagnostic feature of plants that load passively (Rennie and Turgeon, 2009).
Numerous plasmodesmatal connections between parenchyma cells and CCs were observed in rice leaves in ultrastructural studies of small and large vascular bundles, suggesting that Suc loading could occur via symplastic pathway (Botha et al., 2008; Chonan et al., 1981; Kaneko et al., 1980). In a dye-feeding experiment, the low molecular weight dye, 5,6-carbo-xyfluorescein diacetate was observed to move freely out of the phloem into the surrounding tissues including the mesophyll cells in the flag leaf blade (Scofield et al., 2007b), further supporting the possibility of a symplastic pathway in source leaves.
Antisense suppression of OsSUT1 (Ishimaru et al., 2001; Schofield et al., 2002) and Tos17 transposon insertional homozygous plant (Fig. 2) did not show a visible abnormal phenotype of apoplastic phloem loader mutants during vegetative growth. Therefore, OsSUT1 might not function in the main phloem loading pathway in source leaves. There is no current evidence that another Suc transport activity compensates for the loss of OsSUT1. A closely related type II SUT in rice, OsSUT3, appeared to be preferentially expressed in pollen (Ngampanya et al., 2002; Takeda et al., 2001). Unless the expression pattern of OsSUT3 is altered in the OsSUT1 mutant and antisense lines, the possibility that OsSUT3 functions in apoplastic phloem loading in source leaves can be excluded. OsSUT4 and OsSUT5 are also type II SUTs but are less related to OsSUT1 and the phloem loader ZmSUT1. Although the functions of OsSUT4 and OsSUT5 in plants have not been determined (Aoki et al., 2003; Kühn and Grof, 2010; Lim et al., 2006), it is not likely that they have redundant function in apoplastic loading in source organs. Therefore, we are now proposing that rice does not utilize SUT-mediated apoplastic phloem loading pathway as a primary route.
Considering that Suc is temporarily stored in the vacuoles of photosynthetic assimilatory tissues (Linka and Weber, 2010; Martinoia et al., 2007; Neuhaus, 2007; Riens et al., 1991; Winter et al., 1993), this raises the question of the involvement and function of tonoplast-localized SUT in plant growth and development in rice. In this regard, it is noteworthy that the ossut2 phenotype resembles those of mutants of apoplastic loaders (Eom et al., 2011; Gottwald et al., 2000; Slewinski et al., 2009). Rice plants lacking OsSUT2 display severe growth defects compared with wild type and OsSUT2-complemented lines (Eom et al., 2011). In the ossut2 mutant, Suc was accumulated in source leaves. Thus, it is probable that the accumulation of sugar in ossut2, due to the decreased transport of Suc from the vacuole lumen to the cytosol, interferes with Suc translocation to sink organs, and thus affects plant growth.
In summary, in rice, Suc most likely diffuses through plasmodesmata into the phloem of the minor vein via passive process. Suc concentrations of mesophyll cells and thus in the entire leaves are relatively higher than those in the minor veins. In this diffusion process, the tonoplast SUT, OsSUT2 controls mesophyll cytosolic Suc concentration and therefore functions as a valve to regulate Suc flux into the phloem (Fig. 1D).
FUTURE PROSPECTS
Elucidating the phloem loading mechanism in source leaves is essential for our understanding of whole plant carbon partitioning via long-distance phloem translocation of photoassimilate. Here, we summarized current putative phloem loading mechanism of Suc in plant species and hypothesized the potential primary phloem loading pathway in rice. Several lines of evidence now support our proposal that rice may utilize a passive symplastic pathway to translocate Suc through plasmodesmata into the phloem of the minor vein. In particular, we hypothesized that “vacuolar trapping” regulated by the tonoplast OsSUT2 is an essential process for Suc translocation into the phloem. In poplar and rice, tonoplast SUTs were found to strongly influence plant growth and development. Now both species are believed to utilize passive phloem loading pathway as primary Suc translocation in source leaves. Therefore, it would be interesting to determine whether in most of passive loading species tonoplast SUTs have the similar function that have been found in poplar and rice.
Expression and phylogenetic analysis suggested that OsSUT3, OsSUT4, and OsSUT5 have distinct functions from apoplastic loaders. Nevertheless, future characterization of the single and multiple mutants of OsSUT3, OsSUT4, and OsSUT5 should help us understand their function in rice. In preliminary experiments, we did not see visible abnormal plant growth of any of single, double, and triple homozygous mutant plants of OsSUT3, OsSUT4, and OsSUT5. Detailed analysis of these resources is further needed to determine their function.
Recently, SWEET proteins in Arabidopsis were found to export Suc from vascular parenchyma cells to apoplast for phloem loading by SUTs (Chen et al., 2012). Rice also has the SWEET homologs including OsSWEET11/Xa13 and OsWEET14. Interestingly, OsSWEET11 functions as a rice susceptibility gene for specific pathovars of Xanthomonas oryzae pv. oryzae (Chen et al., 2012). Rice SWEETs may have evolved to regulate the efflux from parenchyma cells and highly localized transfer of Suc into the phloem, and thus reduce Suc release to the apoplast in order to prevent pathogen infections. There is the possibility that OsSWEETs may function in apoplastic loading in concert with OsSUT1 under particular circumstances that require more Suc translocation into the phloem. In this regard, in rice that appears to be mainly dependent on passive symplastic loading, it would be interesting to see whether double or multiple mutations of these functionally redundant OsSWEETs may cause growth a defect phenotype like atsweet11/atsweet12.
Acknowledgments
This work was supported by grants from the Next-Generation BioGreen 21 Program (PJ008114022011 to J.-S.J.), Rural Development Administration of the Korean Ministry of Food, Agriculture, Forestry, and Fisheries, and from the World Class University program (R33-2008-000-10168-0 to J.-S.J.) and the Mid-Career Researcher Program (2010-0026679 to J.-S.J.) from the Korean Ministry of Education, Science and Technology. The Division of Chemical Sciences, Geosciences, and Biosciences, Office of Basic Energy Sciences of the U.S. Department of Energy (grant DE-FG02-10ER15886) is gratefully acknowledged for support to J.M.W.
REFERENCES
- Aoki N., Hirose T., Scofield G.N., Whitfeld P.R., Furbank R.T. The sucrose transporter gene family in rice. Plant Cell Physiol. 2003;44:223–232. doi: 10.1093/pcp/pcg030. [DOI] [PubMed] [Google Scholar]
- Ayre B.G. Membrane-transport systems for sucrose in relation to whole-plant carbon partitioning. Mol. Plant. 2011;4:377–394. doi: 10.1093/mp/ssr014. [DOI] [PubMed] [Google Scholar]
- Botha C.E., Aoki N., Scofield G.N., Liu L., Furbank R.T., White R.G. A xylem sap retrieval pathway in rice leaf blades: evidence of a role for endocytosis? J. Exp. Bot. 2008;59:2945–2954. doi: 10.1093/jxb/ern150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun D.M., Slewinski T.L. Genetic control of carbon partitioning in grasses: roles of sucrose transporters and tiedyed loci in phloem loading. Plant Physiol. 2009;149:71–81. doi: 10.1104/pp.108.129049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chonan N., Kaneko M., Kawahara H., Matsuda T. Ultrastructure of the large vascular bundles in the leaves of rice plants. Jpn. J. Crop Sci. 1981;50:323–331. [Google Scholar]
- Chonan N., Kawahara H., Matsuda T. Ultrastructure of vascular bundles and fundamental parenchyma in relation to movement of photosynthate in leaf sheath of rice. Jpn. J. Crop Sci. 1984;53:435–444. [Google Scholar]
- Chen L.Q., Hou B.H., Lalonde S., Takanaga H., Hartung M.L., Qu X.Q., Guo W.J., Kim J.G., Underwood W., Chaudhuri B., et al. Sugar transporters for intercellular exchange and nutrition of pathogens. Nature. 2010;468:527–532. doi: 10.1038/nature09606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L.Q., Qu X.Q., Hou B.H., Sosso D., Osorio S., Fernie A.R., Frommer W.B. Sucrose efflux mediated by SWEET proteins as a key step for phloem transport. Science. 2012;335:207–211. doi: 10.1126/science.1213351. [DOI] [PubMed] [Google Scholar]
- Davidson A., Keller F., Turgeon R. Phloem loading, plant growth form, and climate. Protoplasma. 2011;248:153–163. doi: 10.1007/s00709-010-0240-7. [DOI] [PubMed] [Google Scholar]
- Endler A., Meyer S., Schelbert S., Schneider T., Weschke W., Peters S.W., Keller F., Baginsky S., Martinoia E., Schmidt U.G. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006;141:196–207. doi: 10.1104/pp.106.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eom J.S., Cho J.I., Reinders A., Lee S.W., Yoo Y., Tuan P.Q., Choi S.B., Bang G., Park Y.I., Cho M.H., et al. Impaired function of the tonoplast-localized sucrose transporter in rice, OsSUT2, limits the transport of vacuolar reserve sucrose and affects plant growth. Plant Physiol. 2011;157:109–119. doi: 10.1104/pp.111.176982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evert R.F., Eschrich W., Heyse W. Leaf structure in relation to solute transport and phloem loading in Zea mays L. Planta. 1978;138:279–294. doi: 10.1007/BF00386823. [DOI] [PubMed] [Google Scholar]
- Furbank R.T., Scofield G.N., Hirose T., Wang X-D., Patrick J.W., Offler C.E. Cellular localization and function of a sucrose transporter OsSUT1 in developing rice grains. Aust. J. Plant Physiol. 2001;28:1187–1196. [Google Scholar]
- Gamalei Y. Structure and function of leaf minor veins in trees and herbs. Trees. 1989;3:96–110. [Google Scholar]
- Gottwald J.R., Krysan P.J., Young J.C., Evert R.F., Sussman M.R. Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc. Natl. Acad. Sci. USA. 2000;97:13979–13984. doi: 10.1073/pnas.250473797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haritatos E., Medville R., Turgeon R. Minor vein structure and sugar transport in Arabidopsis thaliana. Planta. 2000;211:105–111. doi: 10.1007/s004250000268. [DOI] [PubMed] [Google Scholar]
- Hirose T., Imaizumi N., Scofield G.N., Furbank R.T., Ohsugi R. cDNA cloning and tissue specific expression of a gene for sucrose transporter from rice (Oryza sativa L.) Plant Cell Physiol. 1997;38:1389–1396. doi: 10.1093/oxfordjournals.pcp.a029134. [DOI] [PubMed] [Google Scholar]
- Hirose T., Zhang Z., Miyao A., Hirochika H., Ohsugi R., Terao T. Disruption of a gene for rice sucrose transporter, OsSUT1, impairs pollen function but pollen maturation is unaffected. J. Exp. Bot. 2010;61:3639–3646. doi: 10.1093/jxb/erq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishimaru K., Hirose T., Aoki N., Takahashi S., Ono K., Yamamoto S., Wu J., Saji S., Baba T., Ugaki M., et al. Antisense expression of a rice sucrose transporter OsSUT1 in rice (Oryza sativa L.) Plant Cell Physiol. 2001;42:1181–1185. doi: 10.1093/pcp/pce148. [DOI] [PubMed] [Google Scholar]
- Kaneko M., Chonan N., Matsuda T. Ultrastructure of the small vascular bundles and transfer pathways for photosynthate in the leaves of rice plant. Jpn. J. Crop Sci. 1980;49:42–50. [Google Scholar]
- Kühn C. A comparison of the sucrose transporter systems of different plant species. Plant Biol. 2003;5:215–232. [Google Scholar]
- Kühn C., Grof C.P.L. Sucrose transporters of higher plants. Curr. Opin. Plant Biol. 2010;13:288–298. doi: 10.1016/j.pbi.2010.02.001. [DOI] [PubMed] [Google Scholar]
- Lalonde S., Wipf D., Frommer W.B. Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu. Rev. Plant Biol. 2004;55:341–372. doi: 10.1146/annurev.arplant.55.031903.141758. [DOI] [PubMed] [Google Scholar]
- Lee S.K., Jeon J.S., Börnke F., Voll L., Cho J.I., Goh C.H., Jeong S.W., Park Y.I., Kim S.J., Choi S.B., et al. Loss of cytoso-lic fructose-1,6-bisphosphatase limits photosynthetic sucrose synthesis and causes severe growth retardation in rice (Oryza sativa) Plant Cell Environ. 2008;31:1851–1863. doi: 10.1111/j.1365-3040.2008.01890.x. [DOI] [PubMed] [Google Scholar]
- Lim J.D., Cho J.I., Park Y.I., Hahn T.R., Choi S.B., Jeon J.S. Sucrose transport from source to sink seeds in rice. Physiol. Planta. 2006;126:572–584. [Google Scholar]
- Linka N., Weber A.P. Intracellular metabolite transporters in plants. Mol. Plant. 2010;3:21–53. doi: 10.1093/mp/ssp108. [DOI] [PubMed] [Google Scholar]
- Martinoia E., Maeshima M., Neuhaus H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007;58:83–102. doi: 10.1093/jxb/erl183. [DOI] [PubMed] [Google Scholar]
- McCaskill A., Turgeon R. Phloem loading in Verbascum phoeniceum L. depends on the synthesis of raffinose-family oligosaccharides. Proc. Natl. Acad. Sci. USA. 2007;104:19619–19624. doi: 10.1073/pnas.0707368104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murchie E.H., Yang J., Hubbart S., Horton P., Peng S. Are there associations between grain-filling rate and photosynthesis in the flag leaves of field-grown rice? J. Exp. Bot. 2002;53:2217–2224. doi: 10.1093/jxb/erf064. [DOI] [PubMed] [Google Scholar]
- Nakano H., Makino A., Mae T. Effects of panicle removal on the photosynthetic characteristics of the flag leaf of rice plants during the ripening stage. Plant Cell Physiol. 1995;36:653–659. [Google Scholar]
- Neuhaus H.E. Transport of primary metabolites across the plant vacuolar membrane. FEBS Lett. 2007;581:2223–2226. doi: 10.1016/j.febslet.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Ngampanya B., Takeda T., Sonoda Y., Narangajavana J., Yamaguchi J. Characterization of OsSUT2 cDNA expressed before flowering stage. Rice Genet. Newslett. 2002;19:49–51. [Google Scholar]
- Okubo-Kurihara E., Higaki T., Kurihara Y., Kutsuna N., Yamaguchi J., Hasezawa S. Sucrose transporter NtSUT4 from tobacco BY-2 involved in plant cell shape during miniprotoplast culture. J. Plant Res. 2011;124:395–403. doi: 10.1007/s10265-010-0377-7. [DOI] [PubMed] [Google Scholar]
- Payyavula R.S., Tay K.H., Tsai C.J., Harding S.A. The sucrose transporter family in Populus: the importance of a tonoplast PtaSUT4 to biomass and carbon partitioning. Plant J. 2011;65:757–770. doi: 10.1111/j.1365-313X.2010.04463.x. [DOI] [PubMed] [Google Scholar]
- Reinders A., Sivitz A.B., Starker C.G., Gantt J.S., Ward J.M. Functional analysis of LjSUT4, a vacuolar sucrose transporter from Lotus japonicas. Plant Mol. Biol. 2008;68:289–299. doi: 10.1007/s11103-008-9370-0. [DOI] [PubMed] [Google Scholar]
- Reinders A., Sivitz A.B., Ward J.M. Evolution of plant sucrose uptake transporters (SUTs) Front. Plant Sci. 2012;3:00022. doi: 10.3389/fpls.2012.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie E.A., Turgeon R. A comprehensive picture of phloem loading strategies. Proc. Natl. Acad. Sci. USA. 2009;106:14162–14167. doi: 10.1073/pnas.0902279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reidel E.J., Rennie E.A., Amiard V., Cheng L., Turgeon R. Phloem loading strategies in three plant species that transport sugar alcohols. Plant Physiol. 2009;149:1601–1608. doi: 10.1104/pp.108.134791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riens B., Lohaus G., Heineke D., Heldt H.W. Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol. 1991;97:227–233. doi: 10.1104/pp.97.1.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J.W., Willmitzer L., Frommer W.B. Isolation and characterization of a sucrose carrier cDNA from spinach by functional expression in yeast. EMBO J. 1992;11:4705–4713. doi: 10.1002/j.1460-2075.1992.tb05575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J.W., Hirner B., Frommer W.B. Potato sucrose transporter expression in minor veins indicates a role in phloem loading. Plant Cell. 1993;5:1591–1598. doi: 10.1105/tpc.5.11.1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riesmeier J.W., Willmitzer L., Frommer W.B. Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partition. EMBO J. 1994;13:1–7. doi: 10.1002/j.1460-2075.1994.tb06229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russin W.A., Evert R.F. Studies on the leaf of Populous deltoids (Salicaceae): ultrastructure, plasmodesmatal frequency, and solute concentrations. Am. J. Bot. 1985;72:1232–1247. [Google Scholar]
- Sauer N. Molecular physiology of higher plant sucrose transporters. FEBS Lett. 2007;581:2309–2317. doi: 10.1016/j.febslet.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Schneider S., Hulpke S., Schulz A., Yaron I., Höll J., Imlau A., Schmitt B., Batz S., Wolf S., Hedrich R., et al. Vacuoles release sucrose via tonoplast-localized SUC4-type transporters. Plant Biol. (Stuttg) 2012;14:325–336. doi: 10.1111/j.1438-8677.2011.00506.x. [DOI] [PubMed] [Google Scholar]
- Schulz A. Role of plasmodesmata in solute loading and unloading. In: Oparka KJ, editor. Plasmodesmata. Vol. 18. Blackwell; Oxford: 2005. pp. 135–161. Vol. Annual Plant Reviews. [Google Scholar]
- Schulz A., Beyhl D., Marten I., Wormit A., Neuhaus E., Poschet G., Buttner M., Schneider S., Sauer N., Hedrich R. Proton-driven sucrose symport and antiport are provided by the vacuolar transporters SUC4 and TMT1/2. Plant J. 2011;68:129–136. doi: 10.1111/j.1365-313X.2011.04672.x. [DOI] [PubMed] [Google Scholar]
- Scofield G.N., Hirose T., Gaudron J.A., Upadhyaya N.M., Ohsugi R., Furbank R.T. Antisense suppression of the rice sucrose transporter gene, OsSUT1, leads to impaired grain filling and germination but does not affect photosynthesis. Funct. Plant Biol. 2002;29:815–826. doi: 10.1071/PP01204. [DOI] [PubMed] [Google Scholar]
- Scofield G.N., Aoki N., Hirose T., Takano M., Jenkins C.L., Furbank R.T. The role of the sucrose transporter, OsSUT1, in germination and early seedling growth and development of rice plants. J. Exp. Bot. 2007a;58:483–495. doi: 10.1093/jxb/erl217. [DOI] [PubMed] [Google Scholar]
- Scofield G.N., Hirose T., Aoki N., Furbank R.T. Involvement of the sucrose transporter, OsSUT1, in the long-distance pathway for assimilate transport in rice. J. Exp. Bot. 2007b;58:3155–3169. doi: 10.1093/jxb/erm153. [DOI] [PubMed] [Google Scholar]
- Slewinski T.L., Braun D.M. Current perspectives on the regulation of whole-plant carbohydrate partitioning. Plant Sci. 2010;178:341–349. [Google Scholar]
- Slewinski T.L., Meeley R., Braun D.M. Sucrose transporter1 functions in phloem loading in maize leaves. J. Exp. Bot. 2009;60:881–892. doi: 10.1093/jxb/ern335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srivastava A.C., Dasgupta K., Ajieren E., Costilla G., McGarry R.C., Ayre B.G. Arabidopsis plants harboring a mutation in AtSUC2, encoding the predominant sucrose/proton symporter necessary for efficient phloem transport, are able to complete their life cycle and produce viable seed. Ann. Bot. 2009;104:1121–1128. doi: 10.1093/aob/mcp215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Reinders A., LaFleur K.R., Mori T., Ward J.M. Transport activity of rice sucrose transporters OsSUT1 and Os-SUT5. Plant Cell Physiol. 2010;51:114–122. doi: 10.1093/pcp/pcp172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda T., Toyofuku K., Matsukura C., Yamaguchi J. Sugar transporters involved in flowering and grain development of rice. J. Plant Physiol. 2001;158:465–470. [Google Scholar]
- Trevanion S.J. Regulation of sucrose and starch synthesis in wheat (Triticum aestivum L.) leaves: role of fructose 2,6-bisphosphate. Planta. 2002;215:653–665. doi: 10.1007/s00425-002-0792-7. [DOI] [PubMed] [Google Scholar]
- Turgeon R. Phloem loading and plasmodemata. Trends Plant Sci. 1996;1:415–423. [Google Scholar]
- Turgeon R. The role of phloem loading reconsidered. Plant Physiol. 2010;152:1817–1823. doi: 10.1104/pp.110.153023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Ayre B.G. Pathways and mechanisms of phloem loading. In: Holbrook N.M., Zwieniecki M.A., editors. Vascular Transport in Plants. Elsevier/Academic Press; Oxford, UK: Elsevier/Acadermic Press; 2005. pp. 45–67. [Google Scholar]
- Turgeon R., Gowan E. Phloem loading in Coleus blumei in the absence of carrier-mediated uptake of export sugar from the apoplast. Plant Physiol. 1990;94:1244–1249. doi: 10.1104/pp.94.3.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R., Medville R. The absence of phloem loading in willow leaves. Proc. Natl. Acad. Sci. USA. 1998;95:12055–12060. doi: 10.1073/pnas.95.20.12055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Bel A.J.E. Interaction between sieve element and companion cell and the consequences for photoassimilate distribution. Two structural hardware frames with associated physiological software packages in dicotyledons? J. Exp. Bot. 1996;47:1129–1140. doi: 10.1093/jxb/47.Special_Issue.1129. [DOI] [PubMed] [Google Scholar]
- van Bel A.J.E. Phloem, a miracle of ingenuity. Plant Cell Environ. 2003;26:125–149. [Google Scholar]
- van Bel A.J.E., Gamalei Y. Ecophysiology of phloem loading in source leaves. Plant Cell Environ. 1992;15:265–270. [Google Scholar]
- van Bel A.J.E., Knoblauch M. Sieve element and companion cell: the story of the comatose patient and the hyperactive nurse. Funct. Plant Biol. 2000;27:477–487. [Google Scholar]
- Winder T.L., Sun J., Okita T.W., Edwards G.E. Evidence for the occurrence of feedback inhibition of photosynthesis in rice. Plant Cell Physiol. 1998;154:665–677. [Google Scholar]
- Winter H., Robinson D.G., Heldt H.W. Subcellular volumes and metabolite concentrations in barley leaves. Planta. 1993;191:180–190. [Google Scholar]
- Zhang C., Turgeon R. Downregulating the sucrose transporter VpSUT1 in Verbascum phoeniceum does not inhibit phloem loading. Proc. Natl. Acad. Sci. USA. 2009;106:18849–18854. doi: 10.1073/pnas.0904189106. [DOI] [PMC free article] [PubMed] [Google Scholar]