Abstract
Obesity is associated with chronic low-grade inflammation, which contributes to systemic metabolic irregularities and obesity-linked metabolic disorders. Orosomucoid (ORM), an acute phase reactant protein, was shown to be produced in response to metabolic and inflammatory signals in the adipose tissue of obese mice, which protects them from severe inflammation and subsequent metabolic dysfunction. In this study, we examined whether there are site-specific differences between visceral and subcutaneous adipose tissue (VAT and SAT, respectively) ORM gene and protein expression from individuals with a wide range of obesity and the relationship between expressed and circulating ORM levels and measures of adiposity, insulin resistance, and pro- and anti-inflammatory markers and adipokines. The level of circulating ORM correlated positively with BMI, body fat mass, and serum leptin. It also correlated with fasting insulin, HOMA-IR values and C-reactive protein in men. There were no site-specific differences in ORM mRNA and protein expression between VAT and SAT, nor did we find a relationship between circulating ORM levels and its mRNA expression in either fat depot. We found that ORM mRNA expression correlated with mRNA expression of TNF-α, IL-6, and adiponectin in VAT, and with TNF-α and adiponectin in SAT. These observations are the first description linking adipose tissue ORM and pro- and anti-inflammatory molecules in humans. The close links of ORM and measures of adiposity, insulin resistance, and adipose tissue inflammation in humans reinforce previous experimental data and warrant further studies to explore a possible role of ORM in the pathogenesis of obesity-associated metabolic derangements.
Keywords: adipokines, obesity, orosomucoid, subcutaneous fat, visceral fat
INTRODUCTION
Obesity is accompanied by a state of low grade systemic inflammation, which may contribute to the development of obesity-associated metabolic dysfunction. Chronic increase of systemic and local inflammation in peripheral tissues has been shown to play causative roles in the development of metabolic disorders such as atherosclerosis and type 2 diabetes mellitus (T2DM), which are often observed in morbidly obese individuals (Hotamisligil, 2006; Lusis, 2000). Adiposity is a major component of metabolic syndrome and a risk factor for developing T2DM and cardiovascular disease (CVD) (Alberti et al., 2006; Grundy et al., 2005). It has been suggested that dysfunctional adipocytes and adipose tissue resident macrophages release elevated amounts of proinflammatory proteins that may contribute to higher systemic levels of inflammation (Berg and Scherer, 2005; Stern et al., 2007).
The term, adipokine, refers collectively to both the proinflammatory and protective factors expressed and secreted from adipose tissue in relation to inflammatory burden (Ahima and Flier, 2000). These signaling molecules have numerous functions, which include regulation of metabolism, the inflammatory process, and body mass. There is a well-supported notion that an imbalance in pro- and anti-inflammatory adipokines contributes to metabolic dysfunction. In obese subjects, chronic inflammation in adipose tissue interferes with its normal functions, including buffering lipid metabolites and secreting adipokines; these functions are involved in regulating insulin sensitivity and the development of metabolic abnormalities (Ouchi et al., 2011).
Orosomucoid (ORM), also named α-1 acid glycoprotein, is one of the most abundant plasma proteins, accounting for approximately 1% of all plasma proteins (Fournier et al., 2000; Hochepied et al., 2003). It is a member of the acute phase protein family and is secreted primarily from the liver. Extrahepatic synthesis and secretion has also been reported from adipose tissue (Fournier et al., 2000). As an immunomodulator, ORM inhibits mitogen-induced proliferation of lymphocytes and aggregation of platelets as well as chemotaxis, superoxide generation, and aggregation of neutrophils via unknown mechanisms (Fournier et al., 2000; Hochepied et al., 2003). Injection of exogenous ORM was shown to consistently protect mice from TNF-α induced lethality (Libert et al., 1994). We recently reported that ORM expression is induced in response to both metabolic and inflammatory signals in the adipose tissue of obese mice, protecting them from severe inflammation. This occurs unless there is a serious disturbance in glucose and lipid homeostasis, which can eventually lead to systemic metabolic complications. ORM expression was elevated selectively in the adipose tissue of obese mice with increasing plasma ORM levels. Adipose ORM expression was augmented rapidly (within one week) upon receiving a high fat diet regimen (HFD), but not by acute inflammation with lipopolysaccharide (LPS) injection. In contrast, expression of liver ORM was induced by LPS, but not by HFD. These results suggest that adipose ORM, in contrast to liver ORM, is modulated by distinct mechanisms upon exposure to pathophysiological conditions (Lee et al., 2010).
Studies have further established that adipose tissue distribution has a significant impact on disease risk. Compared to gluteo-femoral fat, higher levels of central abdominal fat increase the risk of both CVD and T2DM. Such differences in risk may be attributable to the depot-specific differences in the expression and secretion of adipokines (Fisher et al., 2002). Differences in gene expression of adipocyte-secreted molecules suggest that there are intrinsic fat depot-specific differences in the endocrine function of adipose tissue. Differentially expressed adipokines include leptin (Montague et al., 1998), plasminogen activator inhibitor-1 (Alessi et al., 1997), IL-6 (Fried et al., 1998) and visfatin (Fukuhara et al., 2005).
In order to further understand the role of ORM in human obesity, we examined circulating levels and adipose tissue expression of ORM in human subjects with different levels of adiposity. We aimed to determine the following: 1) if circulating ORM levels correlate with measures of obesity, insulin resistance and circulating levels of leptin, adiponectin and C-reactive protein (CRP), 2) if there are site-specific differences between visceral and subcutaneous fat depots in ORM expression and 3) if ORM expression in the visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT) correlate with expression of adiponectin and two proinflammatory markers, TNF-α and IL-6.
MATERIALS AND METHODS
Study population
The subjects were individuals undergoing elective abdominal surgery (n = 64), for cholecystectomy and weight reduction surgery. The subjects’ ages ranged from 17 to 71 years, and all were free of acute inflammation, infection, or malignant conditions. Nine subjects had T2DM, all of which were treated with diet alone. All subjects had a stable weight with no fluctuations > 2% of their body weight for at least 2 months prior to surgery. This study was performed at the Obesity Research Center, King Saud University, Riyadh, Saudi Arabia. The Institutional Review Board approved this study and all participants gave informed consent.
Prior to surgery, all patients gave their medical history and underwent a physical examination. Weight (in kilograms) was measured in light clothing, without shoes, to the nearest 0.1 kg; height was measured using a stadiometer to the nearest centimeter; and body mass index (BMI) was calculated (weight/height squared; in kilograms per square meter). BMI ranged from 17.4 to 66.6 kg/m2. Percentage body fat was measured by a bioelectrical impedance analyzer system (BT-905 Body Composition Analyzer, Skylark Co. Ltd., Taiwan).
After overnight fasting, blood samples were obtained and sera were stored at −80°C until analytical measurements could be performed. Paired VAT and SAT samples were obtained from 20 subjects during the surgical procedure. All tissues were immediately frozen in liquid nitrogen and stored at −80°C.
Analytical methods
Levels of serum glucose, triglycerides, total cholesterol, and high-density lipoprotein (HDL) cholesterol were determined using a Dimension Xpand Plus integrated clinical chemistry autoanalyzer (Siemens Healthcare Diagnostics, USA). Serum levels of low-density lipoprotein (LDL) cholesterol were calculated using Friedewald’s equation (Friedewald et al., 1972). Plasma insulin quantity was determined by electrochemiluminescence using a Cobas e411 immunoanalyzer (Roche Diagnostics, USA). Insulin resistance was represented using the “homeostasis model assessment of insulin resistance” (HOMAIR), which was determined according to the following equation: HOMA-IR = fasting plasma glucose (mmol/L) × fasting plasma insulin (mU/ml)/22.5 (Matthews et al., 1985). Glycosylated hemoglobin (HbA1c) was measured using a turbidimetric inhibition immunoassay with the Dimension Xpand Plus autoanalyzer (Siemens Healthcare Diagnostics).
Serum orosomucoid concentrations were measured by ELISA (AssayMax Human α-1-Acid Glycoprotein, AssayPro, USA). The intra- and inter-assay coefficients of variance (CVs) were 4.4% and 7.2%, respectively. Commercially available ELISA kits were used to measure serum concentrations of leptin and adiponectin (Millipore Corporation, USA), and CRP (Immunodiagnostik AG, Germany) according to the manufacturers’ recommended protocols (leptin intra-assay CV = 4.9% inter-assay CV = 8.6%, adiponectin intra-assay CV = 7.4% and inter-assay CV = 8.4%, and CRP intra-assay CV = 6.0% and inter-assay CV = 11.6%).
RNA extraction and real-time PCR
RNA was extracted from adipose tissue samples using an RNeasy Lipid Tissue kit (Qiagen GmbH, Germany). Extraction was followed by a DNase digestion step to remove any contaminating genomic DNA. RNA was quantitated using a Nanodrop ND-1000 spectrophotometer (Labtech International Ltd.). The quality of the RNA was evaluated by agarose gel electrophoresis and visual inspection of the 28S and 18S ribosomal bands. From each sample, 300 ng of RNA was reverse transcribed using a high capacity RNA to cDNA Kit (Applied Biosystems, USA) according to the manufacturer’s instructions.
Synthesized cDNAs served as templates with specific primers in the presence of dNTPs and TaqDNA polymerase. Real time reverse transcription-PCR amplification reactions were brought to a final volume of 20 μl and contained 1.875 ng of total RNA, 10 pmol of the forward and reverse primers, and SYBR Green (Invitrogen, USA). The iQ5 multicolor real time PCR detection system (Bio-Rad, USA) was used for PCR amplification in 96-well plates. All reactions were performed in duplicate. Protocol conditions consisted of denaturation at 95°C for 120 s, followed by 40 cycles at 95°C for 20 s, 60°C for 30 s, and 72°C for 30 s. After cycling, the melting curve was analyzed. The relative amounts of each mRNA were calculated by using the comparative threshold cycle (CT) method. For an invariant control, we used 18S rRNA. PCR products were stained with ethidium bromide and visualized by ultraviolet fluorescence of the electrophoresed product in 1.2% agarose gels. The sequences of the primers used were as follow: ORM 5′-GGGAATCCTAGCAGGACACA-3′ (sense) and ORM 5′-GCAAGTGAGGGAAAAAGCTG-3′ (antisense); adiponectin 5′-GGCAGGAAAGGAGAACCTGG-3′ (sense) and adiponectin 5′-AGCCTTGTCCTTCTTGAAGAG-3′ (antisense); TNF-α 5′-AGCCCATGTTGTAGCAAACC-3′ (sense) and TNF-α 5′-GGAAGACCCCTCCCAGATAG-3′ (antisense); and IL-6 5′-TACCCCCAGGAGAAGATTCC-3′ (sense) and IL-6 5′-TTTTCTGCCAGTGCCTCTTT-3′ (antisense).
Identification of ORM by Western blotting
Tissue samples were homogenized in RIPA buffer (50 mM Tris-HCL pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM sodium chloride, 1 mM EDTA and protease inhibitors). The total protein present in the samples was measured using a DC protein assay kit (Bio-Rad). A total of 5–10 μg of protein from each sample was loaded onto each well in a 10% polyacrylamide gel and electrophoresis was performed at 120 V for 1 h. After polyacrylamide gel electrophoresis (PAGE), proteins were transferred to a PVDF membrane to which was applied a 15V electrical field overnight. The blots were then blocked with 2% bovine serum albumin (Invitrogen) in phosphate buffered saline with 1% Tween-20 (PBS-T) for 1 h. This was followed by 2 h of incubation with anti-ORM1/2 mouse IgG, ascites antibody (Santa Cruz Biotechnology, USA) diluted to 1:1000 in PBS-T. The blots were then washed three times with PBS-T for 10 min each. The membrane was incubated for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-mouse IgG, diluted to 1:2500 in PBS-T, and followed by three washes as before. The blots were developed using Pierce ECL Western blot substrate according to the manufacturer’s instructions. Exposed X-ray films (BioMax Light Chemiluminescence film, Eastman Kodak, USA) were developed in a Kodak X-OMAT film processor (Eastman Kodak). The blots were stripped and re-probed with antibodies for the anti-β-actin mouse monoclonal as a loading control. The blots were then quantified using GeneTools analysis software (Syngene, UK) and the values for ORM were normalized to loading control values. The results are expressed as a percent of the loading control.
Statistical analysis
Data are shown as the mean ± SD unless stated otherwise. Before statistical analysis, logarithmic transformation of the non-normally distributed parameters was performed to approximate a normal distribution. P values < 0.05 were considered statistically significant. Associations between variables were evaluated by Pearson correlation analysis, and the results are presented as correlation coefficients (r).
RESULTS
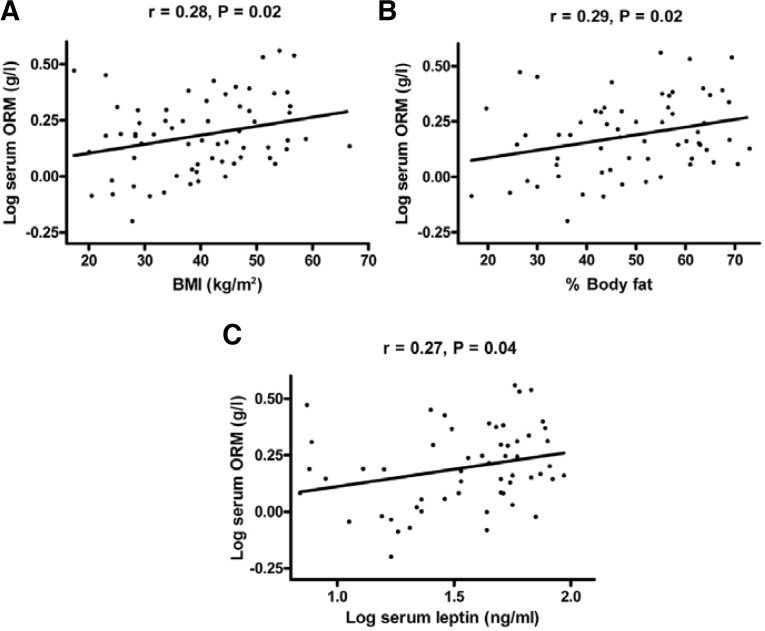
Most of our studied population was either obese or overweight, with significantly higher body fat in women as compared to men. Serum ORM concentrations were not significantly different between men (1.56 ± 0.8 g/L) and women (1.7 ± 0.6 g/L). There were, however, significant differences between the genders in circulating levels of leptin and CRP, with higher levels of both leptin and CRP detected in the women (Table 1). There was a significantly positive correlation of serum ORM concentrations with BMI (Fig. 1A), body fat content (Fig. 1B), and serum leptin (Fig. 1C). There were no correlations of serum ORM concentrations with fasting plasma glucose, or adiponectin levels in the entire study population. There were significant correlations between serum ORM concentrations and fasting insulin (r = 0.42, P = 0.05), HOMA-IR values (r = 0.47, P = 0.03), serum triglycerides (r = −0.59, P = 0.00), and CRP (r = 0.58, P = 0.01) in men, but not in women (Table 2).
Table 1.
Clinical and biochemical characteristics of the study subjects
| Variable | All | Male | Female | P value |
|---|---|---|---|---|
| N | 64 | 23 | 41 | |
| Age (years) | 37.66 ± 14.77 | 35.65 ± 13.68 | 38.78 ± 15.29 | 0.42 |
| BMI (kg/m2) | 40.24 ± 11.43 | 38.7 ± 12.52 | 41.1 ± 10.84 | 0.42 |
| Body fat (%) | 47.54 ± 16 | 38.54 ± 17 | 52.71 ± 12.99 | 0.00 |
| Glucose (mmol/L) | 5.91 ± 1.68 | 5.91 ± 1.24 | 5.91 ± 1.4 | 0.98 |
| Hemoglobin A1c (%) | 5.77 ± 0.54 | 5.72 ± 0.42 | 5.79 ± 0.59 | 0.66 |
| Insulin (mIU/L) | 15.53 ± 9.11 | 16.22 ± 10.43 | 15.14 ± 8.4 | 0.65 |
| HOMA-IR | 4.13 ± 2.9 | 4.19 ± 2.65 | 4.1 ± 3.07 | 0.91 |
| Cholesterol (mmol/L) | 4.8 ± 0.95 | 4.74 ± 0.97 | 4.83 ± 0.95 | 0.71 |
| LDL-cholesterol (mmol/L) | 2.96 ± 0.82 | 2.9 ± 0.85 | 3 ± 0.82 | 0.61 |
| HDL-cholesterol (mmol/L) | 1.17 ± 0.24 | 1.17 ± 0.21 | 1.18 ± 0.26 | 0.87 |
| Triglycerides (mmol/L) | 1.32 ± 0.61 | 1.47 ± 0.61 | 1.24 ± 0.59 | 0.14 |
| CRP (ng/ml) | 9.04 ± 8.65 | 5.65 ± 4.3 | 9.92 ± 6.6 | 0.02 |
| Leptin (ng/ml) | 41.35 ± 23.87 | 27.24 ± 20.25 | 49.98 ± 21.91 | 0.00 |
| Adiponectin (μg/ml) | 6.75 ± 2.71 | 6.36 ± 2.73 | 7 ± 2.71 | 0.39 |
| ORM (g/L) | 1.64 ± 0.68 | 1.56 ± 0.8 | 1.7 ± 0.6 | 0.44 |
Value data are presented as the mean ± standard deviation. Abbreviations: BMI, body mass index; HOMA-IR, homeostasis model assessment of insulin resistance; CRP, C-reactive protein; LDL, low-density lipoprotein; HDL, high-density lipoprotein
Fig. 1.
Correlation of serum ORM concentration and (A) BMI (n = 64), (B) % body fat (n = 61), and (C) serum leptin concentration (n = 56). Serum ORM and leptin concentrations were log transformed to achieve normal distribution.
Table 2.
Correlation of serum ORM concentration and metabolic parameters
| Variable | All
|
Male
|
Female
|
|||
|---|---|---|---|---|---|---|
| r | P value | r | P value | r | P value | |
| Glucose (mmol/L) | −0.09 | 0.47 | 0.09 | 0.68 | −0.2 | 0.21 |
| Insulin (mIU/L) | 0.21 | 0.1 | 0.42 | 0.05 | 0.05 | 0.77 |
| HOMA-IR | 0.17 | 0.19 | 0.47 | 0.03 | −0.03 | 0.84 |
| Cholesterol (mmol/L) | −0.11 | 0.41 | −0.3 | 0.17 | 0.02 | 0.91 |
| LDL-cholesterol (mmol/L) | 0.07 | 0.6 | −0.22 | 0.3 | 0.26 | 0.09 |
| HDL-cholesterol (mmol/L) | 0.01 | 0.94 | 0.06 | 0.78 | −0.02 | 0.91 |
| Triglycerides (mmol/L) | −0.35 | 0.00 | −0.59 | 0.00 | −0.19 | 0.23 |
| CRP (ng/ml) | 0.16 | 0.3 | 0.58 | 0.01 | −0.3 | 0.14 |
| Adiponectin (μg/ml) | 0.01 | 0.95 | 0.03 | 0.89 | −0.04 | 0.8 |
r, correlation coefficient
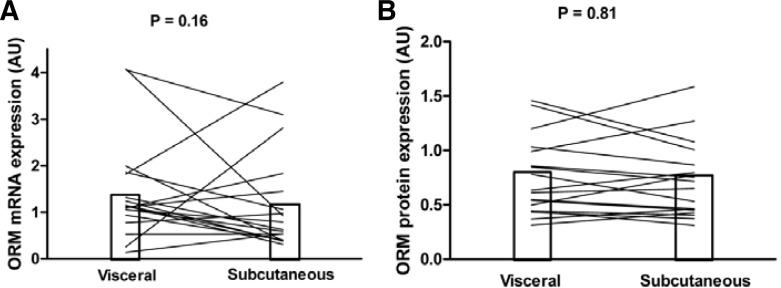
The clinical and biochemical characteristics for subjects studied for ORM mRNA and protein expression are shown in Table 3. We used real-time PCR to quantify and compare the expression of ORM in the VAT and SAT, using total RNA of paired samples for both fat depots. The mRNA expression was not significantly different between VAT and SAT (Fig. 2A). This finding was corroborated by the study of ORM expression at the protein level. Western blot revealed no significant difference in ORM protein expression between fat depots (Fig. 2B). No gender dimorphism was detected for ORM mRNA or protein expression. We did not find a relationship between circulating ORM levels and its mRNA expression in fat depots. There was no correlation of ORM mRNA expression in VAT and SAT with BMI, body fat content, glycosylated hemoglobin, fasting plasma glucose, fasting insulin, CRP, adiponectin or HOMA-IR values (data not shown).
Table 3.
Clinical and biochemical characteristics of subjects studied for ORM mRNA and protein expression
| Variable | Mean ± SD |
|---|---|
| N | 20 |
| Females | 12 |
| Age (years) | 37.75 ± 13.96 |
| BMI (kg/m2) | 42.59 ± 11.14 |
| Body fat (%) | 49.91 ± 13.62 |
| Glucose (mmol/L) | 6.03 ± 1.57 |
| Hemoglobin A1c (%) | 5.71 ± 0.34 |
| Insulin (mIU/L) | 17.29 ± 10.57 |
| HOMA-IR | 4.45 ± 2.74 |
| Cholesterol (mmol/L) | 4.82 ± 1.07 |
| LDL-cholesterol (mmol/L) | 3.11 ± 0.95 |
| HDL-cholesterol (mmol/L) | 1.12 ± 0.24 |
| Triglycerides (mmol/L) | 1.26 ± 0.73 |
| CRP (ng/ml) | 7.66 ± 5.36 |
| Leptin (ng/ml) | 44.61 ± 21.68 |
| Adiponectin (μg/ml) | 6.32 ± 1.8 |
| ORM (g/L) | 1.76 ± 0.77 |
SD, standard deviation
Fig. 2.
ORM mRNA expression and protein expression in human VAT and SAT. (A) Total RNA of paired samples from VAT and SAT was quantified using real-time PCR. Mean ORM mRNA expression was not significantly different between VAT and SAT (n = 20). (B) ORM protein expression of paired samples from VAT and SAT was analyzed by Western blot. Mean ORM protein expression was not significantly different between VAT and SAT (n = 18).
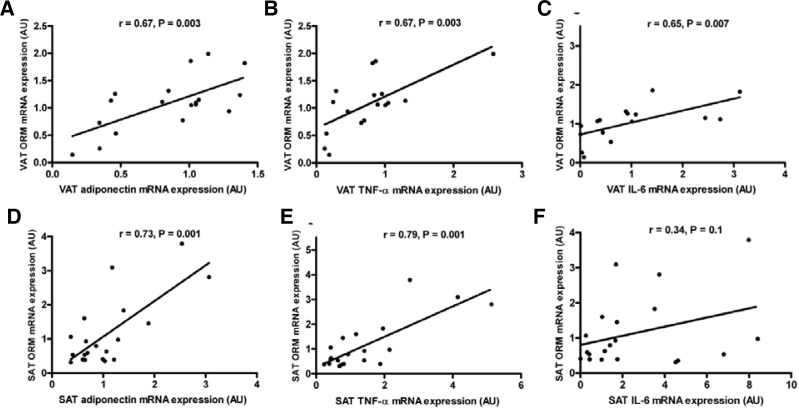
ORM mRNA expression in VAT and SAT was examined against the mRNA expression of adipose tissue products of either pro- or anti-inflammatory functions. In VAT, there were significant correlations of ORM expression with adiponectin expression (Fig. 3A), TNF-α expression (Fig. 3B), and IL-6 expression (Fig. 3C). In SAT, there were significant correlations of ORM expression with adiponectin expression (Fig. 3D) and TNF-α expression (Fig. 3E), but not with IL-6 expression (Fig. 3F).
Fig. 3.
Correlation of VAT ORM mRNA expression and (A) VAT adiponectin mRNA expression (n = 18), (B) VAT TNF-α mRNA expression (n = 17), and (C) VAT IL-6 mRNA expression (n = 16). Correlation of SAT ORM mRNA expression and (D) SAT adiponectin mRNA expression (n = 20), (E) SAT TNF-α mRNA expression (n = 20), and (F) SAT IL-6 mRNA expression (n = 20)
DISCUSSION
We had previously shown in mice that ORM is secreted from adipose tissue in response to metabolic stimuli in order to maintain metabolic homeostasis by suppressing local and systemic inflammation. ORM mRNA expression in adipose tissue was elevated in obese mice with increasing ORM plasma levels, and its expression was augmented by a high fat diet. In adipocytes, ORM suppressed proinflammatory gene expression and pathways such as IKK/NF-κB and mitogen-activated protein kinase (MAPK) signaling and reactive oxygen species (ROS) generation (Lee et al., 2010). These results suggest that ORM modulates immune responses to protect adipose tissue from the effects of excessive inflammation and metabolic dysfunction.
In humans, circulating ORM levels have been widely studied and the levels have been reported to fluctuate due to disease states (Jackson et al., 1982; Piafsky et al., 1978) and stress (Edwards et al., 1982). As an acute phase protein, ORM is synthesized mainly by the liver, but extrahepatic synthesis has also been reported (Fournier et al., 2000). Circulating ORM levels in our study group were more than twice the average levels reported in healthy people (Fournier et al., 2000). A previous study involving morbidly obese females and moderately obese males also showed that ORM protein concentrations were doubled compared to lean controls (Benedek et al., 1983; 1984). We also showed previously that serum ORM levels were significantly elevated in obese people as compared to healthy people (Lee et al., 2010). The correlation of circulating ORM levels with BMI and percentage body fat suggests that adipose tissue may be a contributing source for circulating ORM. Furthermore, the association between circulating ORM protein and measures of insulin resistance and CRP levels in males supports the usefulness of using the circulating amount of this protein as a potential marker for obesity-induced metabolic disorders and inflammation as suggested by experimental data. It is unclear, however, why the association was not found in females. This might be explained by the difference in fat distribution between genders. Previous studies showed that males have more visceral fat than females. Visceral fat is a major source of circulating fatty acids and inflammatory mediators, such as TNF-α, interleukins, and adipokines, which are directly delivered via the portal vein to the liver inducing insulin resistance (Saltevo et al., 2008).
We were unable to identify any published data examining the expression of ORM in human adipose tissue. In mice, high levels of ORM mRNA and protein expression were observed in liver, white adipose tissue and brown adipose tissue (Fournier et al., 2000; Hochepied et al., 2003; Lin et al., 2001). In this study, we reported for the first time that ORM is expressed in human VAT and SAT, and that there were no site-specific differences in ORM mRNA and protein expression between VAT and SAT. In contrast to our previous findings in mice, we did not find a relationship between circulating ORM levels and its mRNA expression in fat depots. The reason for this discrepancy cannot be explained easily. Hormonal regulation of ORM circulating levels or mRNA expression in adipose tissue has not been studied.
We found a positive correlation between ORM mRNA expression and adiponectin mRNA expression in both VAT and SAT. The mechanism for this association is unclear and led us to speculate that there exists a regulatory mechanism between both adipokines acting in a coordinated manner in obesity. Elucidation of such a mechanism in humans will clearly require more detailed studies. ORM mRNA expression was also associated with mRNA expression of TNF-α and IL-6 in VAT, and with TNF-α in SAT. TNF-α expression has been shown to increase in the adipose tissue from experimental animal models of obesity and T2DM (Hotamisligil et al., 1993). Accordingly, neutralization of TNF-α-induced signaling in obese animals leads to improved insulin sensitivity, which is associated with enhanced insulin signaling in both muscle and adipose tissue (Hotamisligil et al., 1993; 1994). We previously showed that TNF-α induces ORM mRNA levels in mature adipocytes. Furthermore, ORM suppressed TNF-α gene expression and generation of ROS in adipocytes and macrophages through the suppression of IKK/NF-κB and MAPK pathways. Induction of a high circulating level of ORM in db/db mice by continuous infusion of purified ORM protein reduced the expression of inflammatory cytokine genes, namely TNF-α and IL-6 (Lee et al., 2010). Due to the association of ORM with these inflammatory cytokines, it is possible that ORM plays a role in limiting inflammation in human adipose tissue.
In conclusion, we have shown that ORM, a newly proposed anti-inflammatory adipokine, is expressed in human adipose tissue with no differential expression between VAT and SAT. The close relationship of ORM with measures of adiposity and insulin resistance, and its association with adiponectin and proinflammatory cytokines in human adipose tissue reinforce previous experimental data and warrant further studies to explore a possible role of ORM in the pathogenesis of obesity-associated metabolic disorders.
Acknowledgments
We would like to thank Dr. Afshan Masood and Mr. Shahid Nawaz for their technical assistance. This work was supported by a grant from the National Plan for Science and Technology, King Saud University and King Abdulaziz City for Science and Technology, Riyadh, Saudi Arabia (grant no. 08-MED513-02), and the Center of Excellence in Biotechnology Research, King Saud University, Riyadh, Saudi Arabia (grant no. CEBR09).
REFERENCES
- Ahima R.S., Flier J.S. Adipose tissue as an endocrine organ. Trends Endocrinol. Metab. 2000;11:327–332. doi: 10.1016/s1043-2760(00)00301-5. [DOI] [PubMed] [Google Scholar]
- Alberti K.G., Zimmet P., Shaw J. Metabolic syndrome-a new world-wide definition. A consensus statement from the international diabetes federation. Diabet. Med. 2006;23:469–480. doi: 10.1111/j.1464-5491.2006.01858.x. [DOI] [PubMed] [Google Scholar]
- Alessi M.C., Peiretti F., Morange P., Henry M., Nalbone G., Juhan-Vague I. Production of plasminogen activator inhibitor 1 by human adipose tissue: possible link between visceral fat accumulation and vascular disease. Diabetes. 1997;46:860–867. doi: 10.2337/diab.46.5.860. [DOI] [PubMed] [Google Scholar]
- Benedek I.H., Fiske W.D., 3rd, Griffen W.O., Bell R.M., Blouin R.A., McNamara P.J. Serum alpha 1-acid glycoprotein and the binding of drugs in obesity. Br. J. Clin. Pharmacol. 1983;16:751–754. doi: 10.1111/j.1365-2125.1983.tb02258.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedek I.H., Blouin R.A., McNamara P.J. Serum protein binding and the role of increased alpha 1-acid glycoprotein in moderately obese male subjects. Br. J. Clin. Pharmacol. 1984;18:941–946. doi: 10.1111/j.1365-2125.1984.tb02567.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg A.H., Scherer P.E. Adipose tissue, inflammation and cardiovascular disease. Circ. Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- Edwards D.J., Lalka D., Cerra F., Slaughter R.L. Alpha1-acid glycoprotein concentration and protein binding in trauma. Clin. Pharmacol. Ther. 1982;31:62–67. doi: 10.1038/clpt.1982.10. [DOI] [PubMed] [Google Scholar]
- Fisher F.M., McTernan P.G., Valsamakis G., Chetty R., Harte A.L., Anwar A.J., Starcynski J., Crocker J., Barnett A.H., McTernan C.L., et al. Differences in adiponectin protein expression: effect of fat depots and type 2 diabetic status. Horm. Metab. Res. 2002;34:650–654. doi: 10.1055/s-2002-38246. [DOI] [PubMed] [Google Scholar]
- Fournier T., Medjoubi N.N., Porquet D. Alpha-1-acid glycoprotein. Biochim. Biophys. Acta. 2000;1482:157–171. doi: 10.1016/s0167-4838(00)00153-9. [DOI] [PubMed] [Google Scholar]
- Fried S.K., Bunkin D.A., Greenberg A.S. Omental and subcutaneous adipose tissues of obese subjects release interleukin-6 depot difference and regulation by glucocorticoid. J. Clin. Endocrinol. Metab. 1998;83:847–850. doi: 10.1210/jcem.83.3.4660. [DOI] [PubMed] [Google Scholar]
- Friedewald W.T., Levy R.I., Fredrickson D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma without use of the preparative ultracentrifuge. Clin. Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Fukuhara A., Matsuda M., Nishizawa M., Segawa K., Tanaka M., Kishimoto K., Matsuki Y., Murakami M., Ichisaka T., Murakami H., et al. Visfatin: a protein secreted by visceral fat that mimics the effects of insulin. Science. 2005;307:426–430. doi: 10.1126/science.1097243. [DOI] [PubMed] [Google Scholar]
- Grundy S.M., Cleeman J.I., Daniels S.R., Donato K.A., Eckel R.H., Franklin B.A., Gordon D.J., Krauss R.M., Savage P.J., Smith S.C., Jr, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. doi: 10.1161/CIRCULATIONAHA.105.169404. [DOI] [PubMed] [Google Scholar]
- Hochepied T., Berger F.G., Baumann H., Libert C. Alpha(1)-acid glycoprotein an acute phase protein with inflammatory and immunomodulating properties. Cytokine Growth Factor Rev. 2003;14:25–34. doi: 10.1016/s1359-6101(02)00054-0. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S. Inflammation and metabolic disorders. Nature. 2006;444:860–867. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Shargill N.S., Spiegelman B.M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G.S., Budavari A., Murray D., Spiegelman B.M. Reduced tyrosine kinase activity of the insulin receptor in obesity-diabetes. Central role of tumor necrosis factor-alpha. J. Clin. Invest. 1994;94:1543–1549. doi: 10.1172/JCI117495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson P.R., Tucker G.T., Woods H.F. Altered plasma drug binding in cancer: role of alpha 1-acid glycoprotein and albumin. Clin. Pharmacol. Ther. 1982;32:295–302. doi: 10.1038/clpt.1982.163. [DOI] [PubMed] [Google Scholar]
- Lee Y.S., Choi J.W., Hwang I., Lee J.W., Lee J.H., Kim A.Y., Huh J.Y., Koh Y.J., Koh G.Y., Son H.J., et al. Adipocytokine orosomucoid integrates inflammatory and metabolic signals to preserve energy homeostasis by resolving immoderate inflammation. J. Biol. Chem. 2010;285:22174–22185. doi: 10.1074/jbc.M109.085464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Libert C., Brouckaert P., Fiers W. Protection by alpha 1-acid glycoprotein against tumor necrosis factor-induced lethality. J. Exp. Med. 1994;180:1571–1575. doi: 10.1084/jem.180.4.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y., Rajala M.W., Berger J.P., Moller D.E., Barzilai N., Scherer P.E. Hyperglycemia-induced production of acute phase reactants in adipose tissue. J. Biol. Chem. 2001;276:42077–42083. doi: 10.1074/jbc.M107101200. [DOI] [PubMed] [Google Scholar]
- Lusis A.J. Atherosclerosis. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D.R., Hosker J.P., Rudenski A.S., Naylor B.A., Treacher D.F., Turner R.C. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- Montague C.T., Prins J.B., Sanders L., Zhang J., Sewter C.P., Digby J., Byrne C.D., O’Rahilly S. Depot-related gene expression in human subcutaneous and omental adipocytes. Diabetes. 1998;47:1384–1391. doi: 10.2337/diabetes.47.9.1384. [DOI] [PubMed] [Google Scholar]
- Ouchi N., Parker J.L., Lugus J.J., Walsh K. Adipokines in inflammation and metabolic disease. Nat. Rev. Immunol. 2011;11:85–97. doi: 10.1038/nri2921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piafsky K.M., Borga O., Odar-Cederlof I., Johansson C., Sjoqvist F. Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma alpha1 acid glycoprotein. N. Engl. J. Med. 1978;299:1435–1439. doi: 10.1056/NEJM197812282992604. [DOI] [PubMed] [Google Scholar]
- Saltevo J., Laakso M., Jokelainen J., Keinanen-Kiukaanniemi S., Kumpusalo E., Vanhala M. Levels of adiponectin, C-reactive protein and interleukin-1 receptor antagonist are associated with insulin sensitivity: a population-based study. Diabetes Metab. Res. Rev. 2008;24:378–383. doi: 10.1002/dmrr.831. [DOI] [PubMed] [Google Scholar]
- Stern N., Osher E., Greenman Y. Hypoadiponectinemia as a marker of adipocyte dysfunction--part II the functional significance of low adiponectin secretion. J. Cardiometab. Syndr. 2007;2:288–294. doi: 10.1111/j.1559-4564.2007.07297.x. [DOI] [PubMed] [Google Scholar]