Abstract
In field conditions, the zebra2 (z2) mutant in rice (Oryza sativa) produces leaves with transverse pale-green/yellow stripes. It was recently reported that ZEBRA2 encodes carotenoid isomerase (CRTISO) and that low levels of lutein, an essential carotenoid for non-photochemical quenching, cause leaf variegation in z2 mutants. However, we found that the z2 mutant phenotype was completely suppressed by growth under continuous light (CL; permissive) conditions, with concentrations of chlorophyll, carotenoids and chloroplast proteins at normal levels in z2 mutants under CL. In addition, three types of reactive oxygen species (ROS; superoxide [O2−], hydrogen peroxide [H2O2], and singlet oxygen [1O2]) accumulated to high levels in z2 mutants grown under short-day conditions (SD; alternate 10-h light/14-h dark; restrictive), but do not accumulate under CL conditions. However, the levels of lutein and zeaxanthin in z2 leaves were much lower than normal in both permissive CL and restrictive SD growth conditions, indicating that deficiency of these two carotenoids is not responsible for the leaf variegation phenotype. We found that the CRTISO substrate tetra-cis-lycopene accumulated during the dark periods under SD, but not under CL conditions. Its accumulation was also positively correlated with 1O2 levels generated during the light period, which consequently altered the expression of 1O2-responsive and cell death-related genes in the variegated z2 leaves. Taking these results together, we propose that the z2 leaf variegation can be largely attributed to photoperiodic accumulation of tetra-cis-lycopene and generation of excessive 1O2 under natural day-night conditions.
Keywords: carotenoid isomerase, rice, singlet oxygen, tetra-cis-lycopene, zebra2
INTRODUCTION
Carotenoids are among the most abundant phytochemicals in nature, and contain a C40 isoprenoid backbone with polyene side chains. Carotenoids contribute to the yellow, orange, and red colors of fruits, vegetables, flowers, and autumn leaves. In all photosynthetic organisms, carotenoids play important roles in harvesting light energy and also in protecting cells from the harmful effects of high light intensity. In addition, carotenoids are also important for human beings and animals because they are involved in vitamin A activity (Rock, 1997), antioxidant activity (Hadley et al., 2002), and preservation of eye health (Fraser and Bramley, 2004).
Carotenoids are essential in plants, both to increase the efficiency of photosynthesis and to protect the photosynthetic apparatus from the effects of excess light. Some carotenoids are incorporated into the light-harvesting complexes (LHCs) to transfer excitation energy to chlorophyll with a high efficiency (Grossman et al., 1995). Three kinds of xanthophylls (antheraxanthin, violaxanthin, and zeaxanthin) are involved in the xanthophyll cycle, which dissipates excess energy by non-photochemical quenching (NPQ) in LHCs to avoid photo-oxidative damage (Jahns and Holzwarth, 2011; Li et al., 2009; Muller et al., 2001). Lutein also can quench the excitation energy generated from excited chlorophyll. Mutant analysis revealed that lutein can compensate for the absence of zeaxanthin and allow NPQ function (Li et al., 2009). In the reaction center of Photosystem II, β-carotene is essential for deactivation of excited singlet oxygen (1O2) generated by excess light irradiance (Telfer, 2002).
During last two decades, the mechanisms of carotenoid metabolism have been elucidated by identification of enzymes in this pathway (Lu and Li, 2008). In higher plants, carotenoid biosynthesis begins with the conversion of geranylgeranyl pyrophosphate into 15-cis-phyotene by phyotene synthase (Giuliano et al., 1993). To synthesize lycopene, phytoene is dehydrogenated by two desaturases, phytoene desaturase and ζ-carotene desaturase (Dong et al., 2007; Norris et al., 1995), which introduce a series of four double bonds in a cis-configuration. The cis-bonds are isomerized into all-trans-conformations by carotenoid isomerase (CRTISO) (Isaacson et al., 2002; Park et al., 2002) and Z-ISO (Chen et al., 2010; Li et al., 2007). All-trans-lycopene is a branch point in the carotenoid biosynthesis pathway. It is cycled by ε-cyclase (LCYE) or β-cyclase (LCYB) to form cyclic carotenes. LCYE plays a crucial role in determining the ratio of α-carotene/β-carotene (Harjes et al., 2008). Both α- and β-carotene are then subjected to a series of oxygenation reactions to produce lutein and xanthophylls, respectively. Further modifications of xanthophylls lead to production of abscisic acid (Cornish and Zeevaart, 1988; DellaPenna and Pogson, 2006). In higher plants, the carotenoid species derived from all-trans-lycopene are associated with photosystem proteins (Grossman et al., 1995), and all-trans-lycopene by itself is the most abundant carotenoid in tomato fruits (Isaacson et al., 2002). Although the physiological and biochemical functions of most carotenoids have been intensively investigated, few studies have examined the possible effects of carotenoid intermediates on plant cells.
In recent years, it has been revealed that some chlorophyll intermediates have unique functions in plant development or physiological processes. For instance, the chlorophyll intermediate Mg-protoporphyrin IX (Mg-proto IX) acts as a retrograde signaling molecule between the chloroplast and the nucleus. In Arabidopsis, Mg-proto IX down-regulates the expression of many nuclear genes encoding chloroplast proteins, including photosynthetic proteins (Isaacson et al., 2002) and a heat shock protein HSP70 (Kropat et al., 1995). However, the particulars of this retrograde signaling are still being debated (Mochizuki et al., 2008; Moulin et al., 2008). Some chlorophyll intermediates, including protochlorophyllide a (Pchlide a), pheophorbide a (Pheide a), and red chlorophyll catabolite (RCC), also can function as strong photosensitizers.
Some of these photosensitizers act through the production of reactive oxygen species (ROS). For example, studies of the Arabidopsis flu mutant allowed the elucidation of the singlet oxygen (1O2) signaling pathway between chloroplast and nucleus (Danon et al., 2004; op den Camp et al., 2003; Wagner et al., 2004). In flu mutants, Pchlide a accumulates under diurnal light-dark conditions, leading to the generation of singlet oxygen (1O2) in plastids (Meskauskiene et al., 2001). Two Arabidopsis mutants affecting chlorophyll catabolism, acd1 encoding Pheide a oxygenase (PAO) and acd2 encoding RCC reductase (RCCR), also exhibit a cell-death phenotype similar to that of the flu mutant. In diurnal light-dark growth conditions, these mutants show cell death and leaf necrosis because the chlorophyll catabolic intermediates, Pheide a and RCC, accumulate in these mutants, respectively (Hirashima et al., 2009; Pruzinska et al., 2007). Thus, some chlorophyll intermediates have drastic effects on plant development and physiological events. Given these properties of chlorophyll intermediates, it can be thought that some of carotenoid intermediates may also have specific effects on plant cells. Because all carotenoid species are incorporated into photosystem proteins in the chloroplasts, it is possible that they may have particular characteristics similar to chlorophyll intermediates. In Arabidopsis, mutants that are defective in the carotenoid biosynthetic pathway cause accumulation of substrates for their respective enzymes as well as significant down-regulation of photosynthesis-related genes (Dong et al., 2007; Qin et al., 2007). These indicate that accumulation of carotenoid intermediates is closely associated with the decreased expression of photosynthesis-related genes, although carotenoid pigments are already incorporated in photosystem proteins (Albuquerque et al., 2009; Dall’Osto et al., 2007; Reinsberg et al., 2001).
Recently, characterization and molecular identification of the rice zebra2 (z2-1) mutant has been reported (Chai et al., 2011). In this z2 mutants, the activity of CRTISO, one of the carotenoid synthetic enzymes, is absent because of a 24-bp deletion mutation by an alteration in the splicing site. In the etiolated seedling of z2 mutants, a lack of CRTISO activity caused accumulation of a carotenoid intermediate, tetra-cis-lycopene. Furthermore, under natural day-night conditions, z2 mutants contained low levels of lutein, which are essential for quenching excited energy generated from triplet chlorophylls. However, the detailed mechanism of the formation of leaf variegation during early z2 leaf development remains poorly understood.
In this study, we characterized different allele of zebra2 mutant (designated z2-2). We found that transverse green/yellow leaf sectors developed in z2 mutants under diurnal light/dark conditions (restrictive conditions), but not under continuous light (CL) (permissive conditions), although under both conditions the levels of lutein and zeaxanthin were significantly lower than wild-type (WT) plants. Here we show that tetra-cis-lycopene accumulated during the dark period, and 1O2 was generated excessively during the light period under restrictive conditions, but not under permissive conditions. The levels of tetra-cis-lycopene were positively correlated with 1O2 production. Furthermore, drastic changes in gene expression of 1O2-responsive, cell death- and photosynthesis-related genes occurred in the variegated z2 leaves. A detailed mechanism of how the zebra phenotype develops in z2 mutants and a possible function of carotenoid intermediates are discussed.
MATERIALS AND METHODS
Plant materials and growth conditions
The zebra2 (z2-2) mutant was previously isolated from a mutant pool produced by chronic gamma irradiation of the indica rice cultivar IR36 as described (Iwata and Omura, 1977). The wild-type IR36 and z2-2 mutant plants were cultivated in paddy fields (Korea; 37°N latitude) or in growth chambers. In the growth chambers, rice plants were grown in short-day (SD; 10-h light/14-h dark) or continuous light (CL) conditions under cool-white light (300 μmol m−2 s−1) at 25–28°C.
Measurement of photosynthetic pigments
For the measurement of total chlorophyll and carotenoid concentrations, pigments were extracted from the second or third leaf tissues of 2-week-old plants with 80% ice-cold acetone. The concentrations of chlorophyll and carotenoids were determined from absorbance values measured with a spectrophotometer (Lichtenthaler, 1987). The extraction and analysis of carotenoids by reverse-phase HPLC analysis were performed as previously described (Pogson et al., 1996). Briefly, a reverse-phase C18, 5 μm column (250 × 4.6 mm; RS Tech, Korea) with an ethyl acetate-based mobile phase was used. Throughout chromatography, the elution was monitored at 440 nm. The extraction and analysis of lycopene were performed as previously described (Fraser et al., 2000). Briefly, a reversephase C30, 3 μm column (250 × 4.6 mm; Prontosil, Germany) with a methanol/tert-methyl butyl ether-based mobile phase was used. The elution was monitored continuously at 290 nm. The extraction and analysis of chlorophyll intermediates were performed as previously described (Zapata et al., 2000). Briefly, the symmetry C8, 5 μm column (250 × 4.6 mm; VYDAC, USA) was used. Ultimate 3000 (Dionex, USA) HPLC system was used, and each elution was monitored continuously by photodiode detector system. Each pigment was identified by its characteristic absorption spectrum.
SDS-PAGE and immunoblot analysis
Leaf tissue (1.0 mg) was homogenized with 10 μl of SDS-PAGE sample buffer [50 mM Tris, pH 6.8, 2 mM EDTA, 10% (w/v) glycerol, 2% SDS, and 6% 2-mercaptoethanol], and denatured at 75°C for 3 min, then samples were subjected to SDS-PAGE. For immunoblot analysis, 10 μl of each protein sample was used. The resolved proteins were electroblotted onto an Immobilon-P Transfer Membrane (Millipore). Antibodies against photosystem proteins (Lhcb1, Lhcb2, Lhcb4, Lhcb5, Lhca1, Lhca2, D1, PsbO, ATPase, and Tic100) were obtained from Agrisera (Sweden). The horseradish peroxidase activity of secondary antibodies (Sigma) was detected using an ECL detection kit, WEST SAVE (AbFRONTIER, Korea), as per the manufacturer’s instructions. RbcL protein on the membrane was visualized by staining with Coomassie Brilliant Blue reagent (Sigma) after immunoblot analysis.
Detection of reactive oxygen species
Detection of hydrogen peroxide (H2O2) and superoxide (O2−) was carried out as previously described (Li et al., 2010; Wi et al., 2010) with minor modifications. Hydrogen peroxide (H2O2) and superoxide (O2−) were detected by 3,3-diaminobendizine (DAB) and nitroblue tetrazolium chloride (NBT), respectively. Leaves of 2-week-old plants grown under CL or SD conditions in the growth chambers were sampled and incubated in 0.1% DAB (Sigma) or 0.05% NBT (Duchefa) in 50 mM sodium phosphate buffer (pH 7.5) at room temperature overnight with gentle shaking. Chlorophyll was completely removed by incubating in 90% ethanol at 80°C. For singlet oxygen (1O2) detection, Singlet Oxygen Sensor Green (SOSG; Invitrogen) reagent was used. Leaves of 2-week-old plants were treated with 50 mM SOSG in 10 mM sodium phosphate buffer (pH 7.5). After 30-min incubation, fluorescence emission following excitation at 480 nm was imaged using a laser scanning confocal microscope (LSM510, Carl Zeiss-LSM510). The red autofluorescence emission from chlorophyll was also detected following excitation at 543 nm.
Quantitative real-time PCR (qRT-PCR) analysis
Total RNA was isolated from the leaves of 2-week-old plants grown under CL and SD conditions with the Total RNA Extraction Kit (iNtRON Biotechnology, Korea). Then, first-strand cDNA was prepared with 2 μg of total RNA in a 50 μl reaction volume using M-MLV reverse transcriptase and oligo(dT)15 primer (Promega). The transcript levels of photosynthesis-related genes (Lhcb1, Lhcb4, and Lhca1), cell death-associated genes (JAmyb, OsNAC4, and MT2b) and singlet oxygen-responsive genes (OsACS6, Os02g49880, and Os11g03370) were determined by qRT-PCR analysis with gene-specific primers (Supplementary Table S1). The 20 μl of qRT-PCR mixture contained 2 μl of the first-strand cDNA mixture (50 μl), 10 μl of 2X QuantiTect LightCycler 480 SYBR Green I Master (Roche) and 0.25 μM of the forward and reverse primers for each gene. PCR was performed on the Light Cycler 2.0 instrument (Roche Diagnostics, Germany). The qRT-PCR conditions used were: 95°C for 2 min, followed by 45 cycles at 95°C for 5 s, 59°C for 15 s, and 72°C for 10 s. Data were obtained from three replicates per cDNA sample, and the mRNA levels of each gene were normalized to those of glyceraldehyde phosphate dehydrogenase (GADPH) (GenBank accession number: AK064960) as previously described (Jain et al., 2006).
Genetic and physical mapping of the z2 locus
A mapping population of 1080 F2 individuals was obtained by a cross of the indica-type z2-2 mutant and a japonica cultivar ‘Sinseonchalbyeo’. To confirm the chromosomal localization of the z2 locus, we performed genetic mapping using 284 z2-type F2 plants and eight simple sequence repeat (SSR) markers distributed on chromosome 11 (marker information is available in GRAMENE; http://www.gramene.org). One SSR and nine sequence-tagged site (STS1-STS9) markers were used for physical mapping (Supplementary Table S1). To examine nucleotide sequence divergence between japonica and indica on chromosome 11, we used BLAST searches of the National Center for Biotechnology Information database (NCBI; http://www.ncbi.nlm.nih.gov/BLAST/).
RESULTS
Phenotypic characterization of the rice zebra2 mutant
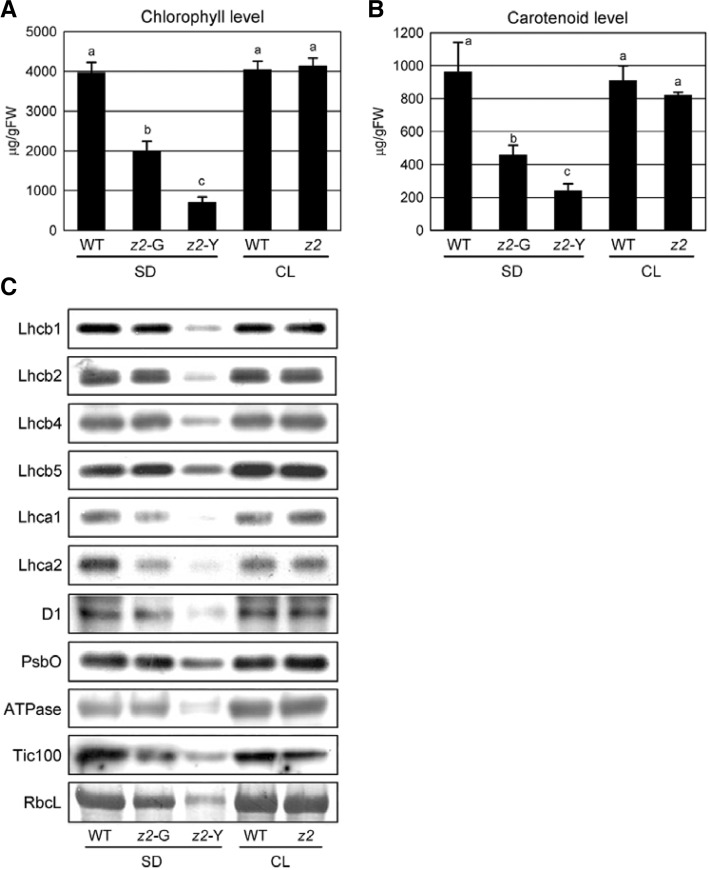
The zebra2 (z2) mutant was isolated from an M2 population of indica rice IR36 irradiated with gamma rays (Iwata and Omura, 1977). In the field, z2 mutants produce variegated leaf blades with transverse sectors of pale-green/yellow (zebra phenotype) throughout development (Fig. 1A). The zebra phenotype appears most strongly at the early seedling stage, and the expressivity gradually declines thereafter. In this study, we focused on determining the mechanisms that produce the z2 phenotype. Previously, Kusumi et al. (2000) reported a zebra mutant TCM248. The zebra phenotype of TCM248 developed under diurnal light-dark cycles (restrictive), and it was almost suppressed under continuous light (CL; permissive) conditions. Thus, to examine whether the z2 phenotype showed a similar dependence on diurnal light-dark conditions, the parental wild-type (IR36) and z2 plants were grown under cool-white light (300 μmol m−2 s−1) under short-day (SD; 10-h light/14-h dark; restrictive) or CL conditions. Like TCM248, z2 mutants exhibited the mutant phenotype under SD, but not under CL conditions (Figs. 1B and 1C). Chlorophyll and carotenoid contents of z2 leaves under SD were considerably lower in the yellow sectors, approximately 18% and 22% of WT levels, respectively, and those in the pale-green sectors of z2 leaves were approximately 50% of WT levels (Figs. 2A and 2B). Next, we examined the relative levels of chloroplast proteins including nine thylakoid proteins (Lhcb1, Lhcb2, Lhcb4, Lhcb5, Lhca1, Lhca2, D1, PsbO, and ATPase), a stroma protein (RbcL), and an envelope protein (Tic100). Under SD, all the tested proteins were considerably lower in the yellow sectors (z2-Y) of z2 mutant leaves (Fig. 2C) and the green sectors (z2-G) also contained reduced levels of LHCI. Under CL, however, the z2 mutant leaves developed no lesions or stripes, and appeared completely wild type (Figs. 1B and 1C). Moreover, under CL, the levels of chlorophyll (Fig. 2A) and chloroplast proteins (Fig. 2C) in z2 leaves were almost the same as those in WT, though total carotenoid levels were slightly lower (Fig. 2B). Under extended long-day (18-h light/ 6-h dark) conditions, the mutant phenotype became much weaker; in these conditions, z2 mutants produced leaves with transverse green/pale-green stripes or almost all pale-green color, but not a clear transverse pale-green/yellow phenotype (data not shown). Taken together, these results suggest that the phenotypic severity of the z2 mutant is closely associated with the dark period under restrictive conditions.
Fig. 1.
Phenotypic characterization of z2 mutants. (A) 80-day-old WT and z2 plants grown under natural day-night conditions in the paddy field. (B, C) 2-week-old WT and z2 plants (B) and their leaf blades (C) grown under SD (10 h-light/14 h-dark) and CL conditions at 25–28°C in the growth chambers. Cool-white light intensity was 300 μmol m−2 s−1. WT, wild type; z2, zebra2; SD, short day; CL, continuous light.
Fig. 2.
Characterization of photosynthetic parameters. (A, B) Concentrations of photosynthetic pigments. Chlorophyll (A) and carotenoids (B) were extracted from the leaves of 2-week-old plants grown under SD or CL conditions in the growth chambers (see “Materials and Methods”). The mean and SD values were obtained from more than five biological replicates. The same letter above each bar indicates that means are not significantly different at the 0.05 level as determined by ANOVA. (C) Immunoblot analysis of chloroplast proteins. Total protein was extracted from the leaves of 2-week-old plants of equal fresh weight, and extracts were subjected to SDS-PAGE. The levels of chloroplast proteins were analyzed by immunoblot analysis using specific antibodies. z2-G, pale-green sectors; z2-Y, yellow sectors of z2 mutant.
Recently, map-based cloning of the Z2 gene using the z2-1 mutant was reported by Chai et al. (2011). We had also identified the z2 locus by map-based cloning using different SSR and STS markers in 2010. It revealed that the z2 mutant in this study has a different allele (designated z2-2)(Supplementary Fig. S1 and Table S1). ZEBRA2 encodes a carotenoid isomerase (CRTISO) which is one of the carotenoid biosynthetic enzymes (Supplementary Fig. S2). CRTISO is a single-copy gene in the rice genome, and its amino acid sequence is highly conserved in higher plants (Supplementary Fig. S2). The defective phenotypes of z2-1 and zebra-leaf1 (zel1; another mutant allele of CRTISO) in rice were rescued by constitutive expression of CRTISO (Chai et al., 2011; Wei et al., 2010), indicating that the zebra phenotypes of z2/zel1 mutations result from a lack of carotenoid isomerase.
ROS production is proportional to dark period length under diurnal light-dark conditions
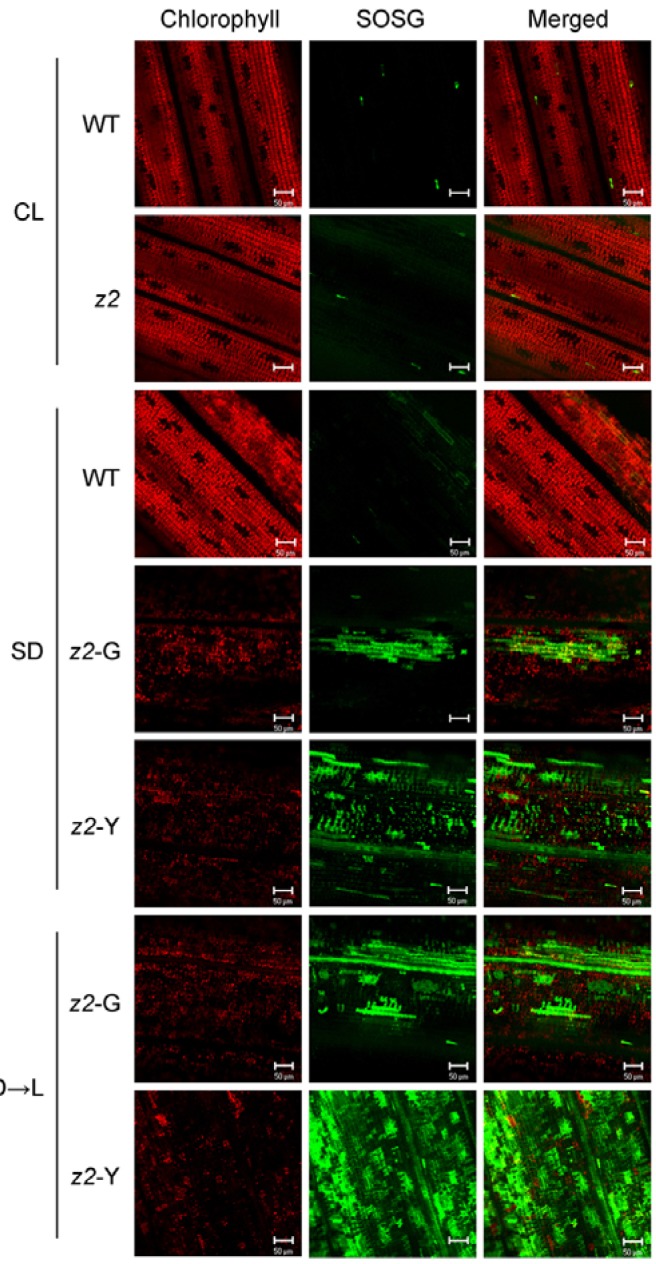
It has been reported that leaf variegation or necrosis in some rice mutants, such as preharvesting sprouting3 (phs3), zebranecrosis (zn) and Stay-green (SGR)-overexpressiong mutants, can be caused by excess accumulation of ROS during early leaf development (Fang et al., 2008; Jiang et al., 2011; Li et al., 2010). In this respect, it is possible that the z2 mutant phenotype may also be caused by ROS accumulation under restrictive conditions. We thus examined the levels of three types of ROS using different staining methods: NBT for O2− (blue precipitate), DAB for H2O2 (brown precipitate), and SOSG for 1O2 (green fluorescence). Under SD, all three types of ROS accumulated in z2 leaves, but not in WT leaves (Figs. 3 and 4). Under CL, ROS accumulation was nearly negligible in the normal green leaves of z2 mutants. Noticeably, we detected a larger amount of 1O2 in the z2 leaves when the SD-grown plants were dark-incubated for 4 days and then exposed to cool-white light (300 μmol m−2 s−1) for 3 hours (Fig. 4, bottom). The 1O2 accumulation was quite similar to the Arabidopsis acd1 mutant, which is deficient in PAO activity (Supplementary Fig. S3) (Hirashima et al., 2009). These results strongly suggest that ROS generation, especially 1O2 accumulation, in z2 mutant leaves is closely related to diurnal light-dark growth conditions.
Fig. 3.
H2O2 and O2− accumulation in z2 leaves. (A, B) Accumulation of superoxide anion radicals (O2−) and hydrogen peroxide (H2O2) in the WT and z2 leaves under SD and CL conditions visualized by NBT (A) and DAB staining (B), respectively. Photographs of the leaves before (left) and after (right) NBT or DAB staining are shown.
Fig. 4.
1O2 accumulation in z2 mutants under different growth conditions. Singlet oxygen (1O2) was detected by SOSG fluorescence (see “Materials and Methods”). For D → L conditions, the SD-grown WT and z2 plants were transferred into darkness at ZT-0, incubated for 4 days in the dark and then exposed to white light for 3 h. Red chlorophyll autofluorescence (left), green SOSG fluorescence (middle), and merged images (right) are shown. Each fluorescence signal was observed by laser scanning confocal microscopy. SOSG fluorescence was collected at 520 nm and chlorophyll autofluorescence was collected at 680 nm. Scale bar = 50 μm.
Tetra-cis-lycopene accumulates in z2 mutant only under restrictive conditions
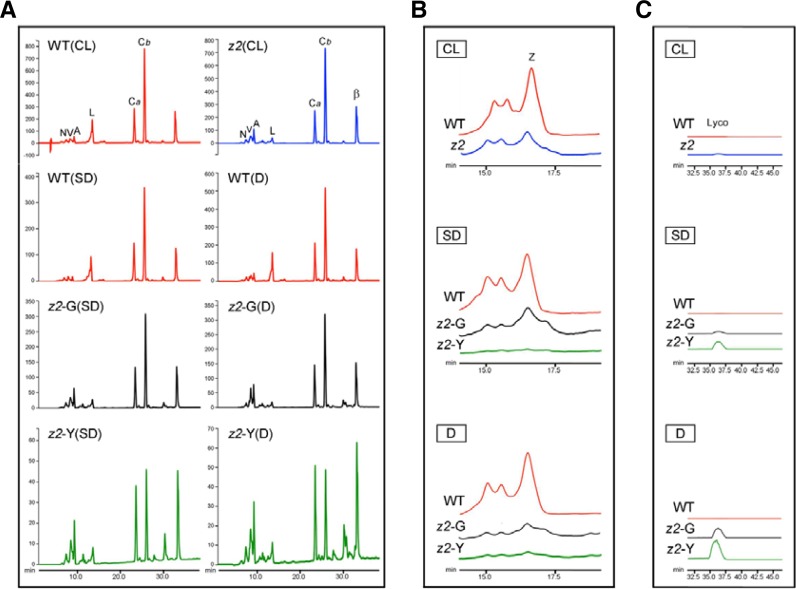
The isomerization from tetra-cis-lycopene to all-trans-lycopene by CRTISO occurs only in darkness (Fang et al., 2008; Isaacson et al., 2002; Park et al., 2002). In other words, accumulation of tetra-cis-lycopene (substrate of CRTISO) in z2 mutants is dependent on the dark period because CRTISO works only in darkness. Based on this, we postulated that accumulation of tetra-cis-lycopene during the dark period is associated with 1O2 production during the light period (Figs. 3 and 4), which leads to the development of transverse leaf variegation under natural day-night conditions (Fig. 1). To verify, levels of tetra-cis-lycopene and other carotenoids were measured using reverse-phase HPLC analysis. For plant materials under SD, we sampled just before the beginning of the light period (zeitgeber time; ZT-0). For dark treatment, the SD-grown plants were transferred into darkness at ZT-0 and incubated for 3 days (3 days dark incubation; 3 DDI). All the peaks of carotenoid pigments were normalized to the peak of chlorophyll a (Fig. 5). The amounts of lutein, zeaxanthin, and β-carotene were also quantified using standard methods (Table 1).
Fig. 5.
HPLC analysis of photosynthetic pigments in WT and z2 leaves. Comparison of (A) carotenoids and chlorophylls, (B) zeaxanthins, (C) lycopenes in 2-week-old WT and z2 leaves grown under 3 different conditions. CL, continuous light; SD, short day; D, the SD-grown plants were transferred into darkness at ZT-0 and then incubated for 3 days (D). Leaves grown under each condition were sampled just before the onset of light irradiance (ZT-0). Each peak of pigments was normalized to chlorophyll a. Peaks of carotenoids and chlorophylls were monitored at 290 nm. N, neoxanthin; V, violaxanthin; A, antheraxanthin; L, lutein; Ca, chlorophyll a; Cb, chlorophyll b; β, β-carotene; Z, zeaxanthin; Lyco, tetra-cis-lycopene.
Table 1.
Carotenoid concentrations in WT and z2 leaves under SD and CL conditions
| SD | CL | ||||
|---|---|---|---|---|---|
|
| |||||
| WT | z2-G | z2-Y | WT | z2 | |
| Lutein | 164.3 (18.8) a | 37.9 (7.7) b | 17.9 (5.6) c | 174.8 (14.2) a | 44.9 (10.5) b |
| Zeaxanthin | 14.5 (2.9) a | 2.8 (0.9) b | 0.8 (0.3) c | 15.1 (3.0) a | 3.2 (1.0) b |
| β-Carotene | 82.3 (21.3) b | 41.3 (11.3) c | 23.3 (5.1) d | 80.3 (12.4) b | 119.3 (13.5) a |
Units for carotenoid content are μg/g fresh weight. Numbers in parentheses indicate SD value. Carotenoid contents were quantified by reverse-phase
HPLC analysis (see “Materials and Methods”).
SD, short-day; CL, continuous light; WT, wild type; z2, zebra2; G, green sectors; Y, yellow sectors. Means with the same letter within each column are not significantly different at the 0.05 level as determined by ANOVA.
Compared with WT, the levels of lutein and zeaxanthin were significantly lower in z2 mutants under CL (Table 1; Figs. 5A and 5B). However, total carotenoids were slightly lower (Fig. 2B) because β-carotene and other xanthophylls (neoxanthin, violaxanthin, and antheraxanthin) were significantly higher. This may explain why z2 mutants can produce normal green leaves under CL; i.e. higher levels of β-carotene and other xanthophylls nearly compensate for lower levels of lutein and zeaxanthin. In addition, the levels of tetra-cis-lycopene were so low as to be nearly absent (Fig. 5C).
Under SD, however, the levels of lutein, zeaxanthin, and β-carotene were significantly lower in z2 mutants (Table 1; Figs. 5A and 5B). As expected, the peaks of tetra-cis-lycopene both in the green (z2-G) and yellow (z2-Y) sectors were detected but no peak was detected in WT (Fig. 5C). Noticeably, tetra-cis-lycopene accumulated to higher levels in the 3 DDI-treated leaves than in the SD-grown leaves of z2 mutant (Fig. 5C). These results suggest that accumulation of tetra-cis-lycopene depends on the dark period, similar to the patterns of ROS production (Figs. 3 and 4). We further examined chlorophyll intermediates Pheide a and Pchlide a, which are strong photosensitizers (op den Camp et al., 2003; Pruzinska et al., 2007). No detectable levels of Pheide a and Pchilde a were found in the SD-grown z2 leaves (Supplementary Fig. S4).
Taken together, the levels of lutein and zeaxanthin in z2 leaves were consistently lower regardless of permissive and restrictive conditions, indicating that low levels of these carotenoid pigments are not a critical factor for leaf variegation and 1O2 production in z2 mutants. Instead, accumulation of tetra-cis-lycopene (substrate of CRTISO) during the dark period might be critical for leaf variegation under restrictive conditions, although tetra-cis-lycopene levels detected in this series of experiment (using green tissue) were considerably lower than that in etiolated seedlings of z2 mutant (Chai et al., 2011).
Altered expression of 1O2-responsive, photosynthesis- and cell death-related genes in z2 mutants under restrictive conditions
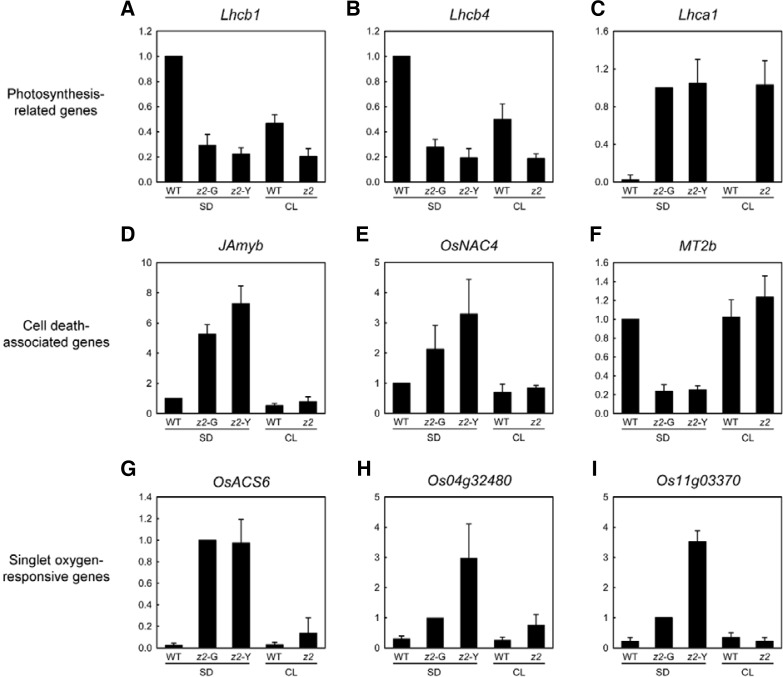
In Fig. 4, we showed that light-induced 1O2 production in z2 leaves is a crucial factor for transverse variegation. It was previously reported that 1O2 is not only a cytotoxic molecule, but is also involved in the control of nuclear gene expression in plants (Kim et al., 2008). In addition to stress-related genes, 1O2 triggers ectopic expression of programmed cell death-related genes, leading to the formation of necrotic lesions in plant tissues (Danon et al., 2004; op den Camp et al., 2003). Moreover, 1O2 down-regulates photosystem-related genes (Khandal et al., 2009). Thus, we examined the correlation between alteration of nuclear gene expression and the severity of leaf variegation. By quantitative real-time RT-PCR (qRT-PCR) analysis, we measured the expression levels of three photosynthesis-related genes (Lhcb1, Lhcb4, and Lhca1), three cell death-related genes (JAmyb, OsNAC4, and MT2b), and three singlet oxygen-responsive genes [OsACS6 (Supplementary Fig. S5), Os04g32480, and Os11g03370] (Jiang et al., 2011).
The two LHCII genes, Lhcb1 and Lhcb4, were down-regulated in both pale-green (z2-G) and yellow (z2-Y) sectors of z2 mutants grown under SD (Figs. 6A and 6B) as previously reported (Chai et al., 2011). Unexpectedly, they were also down-regulated in the normal green leaves of z2 mutants under CL, inconsistent with their protein levels (Fig. 2C). Furthermore, a LHCI gene, Lhca1, was highly up-regulated in z2 leaves regardless of photoperiod conditions (Fig. 6C), which could be related to lower protein levels of Lhca1 subunits under SD (Fig. 2C). This result suggests that alteration of photosynthesis-related genes in z2 mutants is not largely associated with the development of leaf variegation.
Fig. 6.
Expression analysis of WT and z2 leaves by qRT-PCR. (A–C) Expression levels of photosynthesis-related genes, Lhcb1 (Os01g41740), Lhcb4 (Os07g37240), and Lhca1 (Os06g21590). (D–F) Expression levels of cell death-related genes, JAmyb (Os11g45740), OsNAC4 (Os01g60020), and MT2b (Os05g02070). (G-I) Expression levels of 1O2-responsive genes, OsACS6 (Os04g48850; Fig. S5), Os04g32480, and Os11g03370. Relative mRNA levels were normalized to the transcript levels of glyceraldehyde phosphate dehydrogenase (GAPDH; GenBank accession number AK064960). The mean and SD values were obtained from more than nine biological replicates.
Under SD, JAmyb and OsNAC4, positive regulators of cell death in rice, were significantly up-regulated (Figs. 6D and 6E), and a negative regulator, MT2b, was down-regulated (Fig. 6F). This result indicates that 1O2 signaling induces the expression of cell death-related genes, leading to programmed cell death (PCD) or cytotoxicity-induced cell death (CICD) in z2 leaves, especially in the yellow (z2-Y) sectors. The mRNA levels of three 1O2-responsive genes were significantly up-regulated in the variegated leaves of z2 mutants grown under SD, especially in the yellow (z2-Y) sectors, but not significantly in the normal green z2 leaves under CL (Figs. 6G–6I), consistent with the levels of 1O2 produced in z2 leaves (Fig. 4). It indicates that the 1O2 signaling pathway is being activated under restrictive conditions, leading to leaf variegation in z2 mutants.
DISCUSSION
Low levels of lutein and zeaxanthin are not responsible for leaf variegation in z2 mutants
Previously, Chai et al. (2011) suggested that transverse leaf variegation in z2 mutants is caused by low levels of lutein. In this study, we showed that z2 mutants did not exhibit leaf variegation under CL (Fig. 1), even though lutein and zeaxanthin levels were considerably lower in z2 mutants compared with those in WT (Fig. 5). This finding strongly suggests that leaf variegation in z2 mutant under restrictive conditions cannot be attributed to low levels of lutein and zeaxanthin.
Arabidopsis mutants with low levels of lutein or zeaxanthin have been reported previously (Li et al., 2009; Pogson et al., 1996; 1998). Despite low levels of these carotenoids, phenotypes and chlorophyll accumulation of these mutants were almost the same as WT. Instead, other carotenoid species accumulate to higher levels in these mutants (Li et al., 2009; Pogson et al., 1996), which may compensate for reduced lutein and zeaxanthin functions including photoprotection, energy transfer, and accumulation of photosystem proteins. A similar phenomenon was observed in z2 mutants. Under CL (permissive) conditions, total carotenoid levels in z2 mutants are only slightly lower than that of WT (Fig. 2B), because higher amounts of β-carotene, antheraxanthin, neoxanthin, and violaxanthin accumulated instead of lower amounts of lutein and zeaxanthin (Figs. 5A and 5B). Furthermore, levels of three ROS (1O2, H2O2, and O2−) in the normal green leaves of CL-grown z2 mutants were not substantially different from those of WT (Figs. 3 and 4), indicating that photoprotective and energy-transfer functions of carotenoids are almost normal due to compensation by other carotenoid species. These results further support our conclusion that low levels of lutein and zeaxanthin in z2 mutants are not responsible for leaf variegation under restrictive conditions.
Tetra-cis-lycopene accumulation in the dark is correlated with light-induced singlet oxygen (1O2) production under restrictive conditions
By SOSG analysis, we found that 1O2 levels in the 3 DDI-treated leaves of SD-grown z2 mutants were much higher than those in the untreated SD-grown plants (Fig. 4), and that 1O2 levels are closely associated with tetra-cis-lycopene levels in each condition (Fig. 5C). These results suggest that, at least in part, tetra-cis-lycopene accumulation during the dark period might induce 1O2 generation during the light period, leading to transverse variegation only under diurnal light-dark conditions.
There are two possibilities for the involvement of tetra-cis-lycopene in leaf variegation of z2 mutants under restrictive conditions. One possibility is that tetra-cis-lycopene may act as an antioxidant. Generally, carotenoids at low concentrations serve as antioxidants, and inhibit 1O2 generation (Stahl and Sies, 2003; Stahl et al., 1998). Studies have revealed that lycopene (all-trans-lycopene) is one of the most potent antioxidants among major carotenoids (Di Mascio et al., 1989; Stahl et al., 1998), and tomato lycopene extracts have been found to inhibit proliferation of several types of cancer cells (Amir et al., 1999). However, at high concentrations or in the presence of chronic oxidative stress, they can function as pro-oxidants by promoting free radical-induced reactions, and stimulate cell-death machinery (Jakus and Farkas, 2005; Melnikova et al., 1999). In this scenario, it is highly possible that a trace amount of tetra-cis-lycopene also functions as an antioxidant in plant cells. However, high concentrations of free tetra-cis-lycopene may promote light-induced 1O2 generation indirectly, which leads to leaf variegation under restrictive conditions. Another possibility is that tetra-cis-lycopene by itself functions as a photosensitizer. Tetra-cis-lycopene is a precursor and tetra-cis-isomer of all-trans-lycopene. This structure enables it to achieve a high-energy transition state upon light exposure after dark, which may cause it to act as a photosensitizer. In chlorophyll metabolism, seven chlorophyll intermediates have been reported to act as photosensitizers in plants (Hörtensteiner and Kräutler, 2011; Tanaka and Tanaka, 2007). These photosensitizers are considered to control gene expression in the nucleus by generating 1O2 when exposed to light. Hence, it is reasonable that carotenoid intermediates including tetra-cis-lycopene might also act as photosensitizers. In this respect, our characterization of the causes of the z2 phenotype provides new insight into the physiological functions of a carotenoid intermediate, tetra-cis-lycopene, as a possible photosensitizer in developing chloroplasts in rice.
Altered expression of 1O2-responsive and cell death-related genes is a critical factor for leaf variegation in z2 mutants
By qRT-PCR analysis, we found that some 1O2-responsive genes were significantly up-regulated in z2 mutants under SD (Figs. 6G, 6H, and 6I), whereas their mRNA levels were almost the same as those in WT plant under CL, indicating that the 1O2-mediated plastid-to-nucleus signaling pathway is highly activated in z2 mutants only under restrictive conditions. 1O2 causes severe damage to plant tissues by two mechanisms. One is direct peroxidation of lipids and oxidative damage to another macromolecules, and the other is induction of PCD (Kim et al., 2008). These responses depend on the levels of 1O2 in plant cells. We showed that mRNA levels of cell death-related genes changed significantly in z2 leaves only under SD, and these changes were larger in the yellow (z2-Y) sectors than the pale-green (z2-G) sectors (Figs. 6D, 6D, and 6F). Considering the photoperiodic cycle under restrictive conditions, 1O2 production in z2 leaves would not stay constant because 1O2 is not generated in the dark. It is probable that 1O2 accumulates excessively during the day period, in the presence of tetra-cis-lycopene, which may act as a photosensitizer. Taken altogether, we suggest that the photoperiodic accumulation of tetra-cis-lycopene and singlet oxygen causes severe damage to developing chloroplasts during early leaf development, and that this is a critical factor for leaf variegation of z2 mutants.
Supplementary Material
Acknowledgments
This work was supported by a grant from the Next-Generation BioGreen 21 Program (Plant Molecular Breeding Center No. PJ008128), Rural Development Administration, Republic of Korea.
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Albuquerque R.J., Hayashi T., Cho W.G., Kleinman M.E., Dridi S., Takeda A., Baffi J.Z., Yamada K., Kaneko H., Green M.G., et al. Alternatively spliced vascular endothelial growth factor receptor-2 is an essential endogenous inhibitor of lymphatic vessel growth. Nat. Med. 2009;15:1023–1030. doi: 10.1038/nm.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amir H., Karas M., Giat J., Danilenko M., Levy R., Yermiahu T., Levy J., Sharoni Y. Lycopene and 1,25-dihydroxy-vitamin D3 cooperate in the inhibition of cell cycle progression and induction of differentiation in HL-60 leukemic cells. Nutr Cancer. 1999;33:105–112. doi: 10.1080/01635589909514756. [DOI] [PubMed] [Google Scholar]
- Chai C., Fang J., Liu Y., Tong H., Gong Y., Wang Y., Liu M., Wang Y., Qian Q., Cheng Z., et al. ZEBRA2, encoding a carotenoid isomerase, is involved in photoprotection in rice. Plant Mol. Biol. 2011;75:211–221. doi: 10.1007/s11103-010-9719-z. [DOI] [PubMed] [Google Scholar]
- Chen Y., Li F., Wurtzel E.T. Isolation and characterization of the Z-ISO gene encoding a missing component of carotenoid biosynthesis in plants. Plant Physiol. 2010;153:66–79. doi: 10.1104/pp.110.153916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornish K., Zeevaart J.A. Phenotypic expression of wild-type tomato and three wilty mutants in relation to abscisic acid accumulation in roots and leaflets of reciprocal grafts. Plant Physiol. 1988;87:190–194. doi: 10.1104/pp.87.1.190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dall’Osto L., Fiore A., Cazzaniga S., Giuliano G., Bassi R. Different roles of a- and b-branch xanthophylls in photosystem assembly and photoprotection. J. Biol. Chem. 2007;282:35056–35068. doi: 10.1074/jbc.M704729200. [DOI] [PubMed] [Google Scholar]
- Danon A., Miersch O., Felix G., Camp R.G.L., Apel K. Concurrent activation of cell death-regulating signaling pathways by singlet oxygen in Arabidopsis thaliana. Plant J. 2004;41:68–80. doi: 10.1111/j.1365-313X.2004.02276.x. [DOI] [PubMed] [Google Scholar]
- DellaPenna D., Pogson B.J. Vitamin synthesis in plants: tocopherols and carotenoids. Annu. Rev. Plant Biol. 2006;57:711–738. doi: 10.1146/annurev.arplant.56.032604.144301. [DOI] [PubMed] [Google Scholar]
- Di Mascio P., Kaiser S., Sies H. Lycopene as the most efficient biological carotenoid singlet oxygen quencher. Arch. Biochem. Biophys. 1989;274:532–538. doi: 10.1016/0003-9861(89)90467-0. [DOI] [PubMed] [Google Scholar]
- Dong H., Deng Y., Mu J., Lu Q., Wang Y., Xu Y., Chu C., Chong K., Lu C., Zuo J. The Arabidopsis Spontaneous Cell Death1 gene, encoding a ζ-carotene desaturase essential for carotenoid biosynthesis, is involved in chloroplast development, photoprotection and retrograde signalling. Cell Res. 2007;17:458–470. doi: 10.1038/cr.2007.37. [DOI] [PubMed] [Google Scholar]
- Fang J., Chai C., Qian Q., Li C., Tang J., Sun L., Huang Z., Guo X., Sun C., Liu M., et al. Mutations of genes in synthesis of the carotenoid precursors of ABA lead to pre-harvest sprouting and photo-oxidation in rice. Plant J. 2008;54:177–189. doi: 10.1111/j.1365-313X.2008.03411.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser P.D., Bramley P.M. The biosynthesis and nutritional uses of carotenoids. Prog. Lipid Res. 2004;43:228–265. doi: 10.1016/j.plipres.2003.10.002. [DOI] [PubMed] [Google Scholar]
- Fraser P.D., Pinto M.E., Holloway D.E., Bramley P.M. Technical advance: application of high-performance liquid chromatography with photodiode array detection to the metabolic profiling of plant isoprenoids. Plant J. 2000;24:551–558. doi: 10.1046/j.1365-313x.2000.00896.x. [DOI] [PubMed] [Google Scholar]
- Giuliano G., Bartley G.E., Scolnik P.A. Regulation of carotenoid biosynthesis during tomato development. Plant Cell. 1993;5:379–387. doi: 10.1105/tpc.5.4.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman A.R., Bhaya D., Apt K.E., Kehoe D.M. Light-harvesting complexes in oxygenic photosynthesis: diversity, control, and evolution. Annu. Rev. Genet. 1995;29:231–288. doi: 10.1146/annurev.ge.29.120195.001311. [DOI] [PubMed] [Google Scholar]
- Hadley C.W., Miller E.C., Schwartz S.J., Clinton S.K. Tomatoes, lycopene, and prostate cancer: progress and promise. Exp. Biol. Med. 2002;227:869–880. doi: 10.1177/153537020222701006. [DOI] [PubMed] [Google Scholar]
- Harjes C.E., Rocheford T.R., Bai L., Brutnell T.P., Kandianis C.B., Sowinski S.G., Stapleton A.E., Vallabhaneni R., Williams M., Wurtzel E.T., et al. Natural genetic variation in lyco-pene epsilon cyclase tapped for maize biofortification. Science. 2008;319:330–333. doi: 10.1126/science.1150255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirashima M., Tanaka R., Tanaka A. Light-independent cell death induced by accumulation of pheophorbide a in Arabidopsis thaliana. Plant Cell Physiol. 2009;50:719–729. doi: 10.1093/pcp/pcp035. [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S., Kräutler B. Chlorophyll breakdown in higher plants. Biochim. Biophys Acta. 2011;1807:977–988. doi: 10.1016/j.bbabio.2010.12.007. [DOI] [PubMed] [Google Scholar]
- Isaacson T., Ronen G., Zamir D., Hirschberg J. Cloning of tangerine from tomato reveals a carotenoid isomerase essential for the production of b-carotene and xanthophylls in plants. Plant Cell. 2002;14:333–342. doi: 10.1105/tpc.010303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwata N., Omura T. Linkage studies in rice (Oryza sativa L.). On some mutants derived from chronic gamma irradiation. J. Fac. Agr. Kyushu Univ. 1977;21:117–127. [Google Scholar]
- Jahns P., Holzwarth A.R. The role of the xanthophyll cycle and of lutein in photoprotection of photosystem II. Biochim. Biophys. Acta. 2011 doi: 10.1016/j.bbabio.2011.04.012. [DOI] [PubMed] [Google Scholar]
- Jain M., Nijhawan A., Tyagi A.K., Khurana J.P. Validation of housekeeping genes as internal control for studying gene expression in rice by quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2006;345:646–651. doi: 10.1016/j.bbrc.2006.04.140. [DOI] [PubMed] [Google Scholar]
- Jakus J., Farkas O. Photosensitizers and antioxidants: a way to new drugs? Photochem. Photobiol. Sci. 2005;4:694–698. doi: 10.1039/b417254j. [DOI] [PubMed] [Google Scholar]
- Jiang H., Chen Y., Li M., Xu X., Wu G. Overexpression of SGR results in oxidative stress and lesion-mimic cell death in rice seedlings. J. Integr. Plant Biol. 2011;53:375–387. doi: 10.1111/j.1744-7909.2011.01037.x. [DOI] [PubMed] [Google Scholar]
- Khandal D., Samol I., Buhr F., Pollmann S., Schmidt H., Clemens S., Reinbothe S., Reinbothe C. Singlet oxygen-dependent translational control in the tigrina-d.12 mutant of barley. Proc. Natl. Acad. Sci USA. 2009;106:13112–13117. doi: 10.1073/pnas.0903522106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim C., Meskauskiene R., Apel K., Laloi C. No single way to understand singlet oxygen signalling in plants. EMBO Rep. 2008;9:435–439. doi: 10.1038/embor.2008.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kropat J., von Gromoff E.D., Muller F.W., Beck C.F. Heat shock and light activation of a Chlamydomonas HSP70 gene are mediated by independent regulatory pathways. Mol. Gen. Genet. 1995;248:727–734. doi: 10.1007/BF02191713. [DOI] [PubMed] [Google Scholar]
- Li F., Murillo C., Wurtzel E.T. Maize Y9 encodes a product essential for 15-cis-zeta-carotene isomerization. Plant Physiol. 2007;144:1181–1189. doi: 10.1104/pp.107.098996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z., Ahn T.K., Avenson T.J., Ballottari M., Cruz J.A., Kramer D.M., Bassi R., Fleming G.R., Keasling J.D., Niyogi K.K. Lutein accumulation in the absence of zeaxanthin restores nonphotochemical quenching in the Arabidopsis thaliana npq1 mutant. Plant Cell. 2009;21:1798–1812. doi: 10.1105/tpc.109.066571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Pandeya D., Nath K., Zulfugarov I.S., Yoo S.C., Zhang H., Yoo J.H., Cho S.H., Koh H.J., Kim D.S., et al. ZEBRA-NECROSIS, a thylakoid-bound protein, is critical for the photoprotection of developing chloroplasts during early leaf development. Plant J. 2010;62:713–725. doi: 10.1111/j.1365-313X.2010.04183.x. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler F.W. Karl Freudenberg, Burckhardt Helferich, Hermann O.L. Fischer: a centennial tribute. Carbohydr. Res. 1987;164:1–22. doi: 10.1016/0008-6215(87)80114-3. [DOI] [PubMed] [Google Scholar]
- Lu S., Li L. Carotenoid metabolism: Biosynthesis, regulation, and beyond. J. Integr. Plant Biol. 2008;50:778–785. doi: 10.1111/j.1744-7909.2008.00708.x. [DOI] [PubMed] [Google Scholar]
- Melnikova V., Bezdetnaya L., Belitchenko I., Potapenko A., Merlin J.L., Guillemin F. Meta-tetra(hydroxyphenyl) chlorin-sensitized photodynamic damage of cultured tumor and normal cells in the presence of high concentrations of a-tocopherol. Cancer Lett. 1999;139:89–95. doi: 10.1016/s0304-3835(99)00023-3. [DOI] [PubMed] [Google Scholar]
- Meskauskiene R., Nater M., Goslings D., Kessler F., op den Camp R., Apel K. FLU: a negative regulator of chlorophyll biosynthesis in Arabidopsis thaliana. Proc. Natl. Acad. Sci USA. 2001;98:12826–12831. doi: 10.1073/pnas.221252798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mochizuki N., Tanaka R., Tanaka A., Masuda T., Nagatani A. The steady-state level of Mg-protoporphyrin IX is not a determinant of plastid-to-nucleus signaling in Arabidopsis. Proc. Natl. Acad. Sci USA. 2008;105:15184–15189. doi: 10.1073/pnas.0803245105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moulin M., McCormac A.C., Terry M.J., Smith A.G. Tetrapyrrole profiling in Arabidopsis seedlings reveals that retrograde plastid nuclear signaling is not due to Mg-protoporphyrin IX accumulation. Proc. Natl. Acad. Sci USA. 2008;105:15178–15183. doi: 10.1073/pnas.0803054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P., Li X.P., Niyogi K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001;125:1558–1566. doi: 10.1104/pp.125.4.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris S.R., Barrette T.R., DellaPenna D. Genetic dissection of carotenoid synthesis in Arabidopsis defines plasto-quinone as an essential component of phytoene desaturation. Plant Cell. 1995;7:2139–2149. doi: 10.1105/tpc.7.12.2139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- op den Camp R.G., Przybyla D., Ochsenbein C., Laloi C., Kim C., Danon A., Wagner D., Hideg E., Gobel C., Feussner I., et al. Rapid induction of distinct stress responses after the release of singlet oxygen in Arabidopsis. Plant Cell. 2003;15:2320–2332. doi: 10.1105/tpc.014662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H., Kreunen S.S., Cuttriss A.J., DellaPenna D., Pogson B.J. Identification of the carotenoid isomerase provides insight into carotenoid biosynthesis, prolamellar body formation, and photomorphogenesis. Plant Cell. 2002;14:321–332. doi: 10.1105/tpc.010302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B., McDonald K.A., Truong M., Britton G., DellaPenna D. Arabidopsis carotenoid mutants demonstrate that lutein is not essential for photosynthesis in higher plants. Plant Cell. 1996;8:1627–1639. doi: 10.1105/tpc.8.9.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogson B.J., Niyogi K.K., Bjorkman O., DellaPenna D. Altered xanthophyll compositions adversely affect chlorophyll accumulation and nonphotochemical quenching in Arabidopsis mutants. Proc. Natl. Acad. Sci USA. 1998;95:13324–13329. doi: 10.1073/pnas.95.22.13324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzinska A., Anders I., Aubry S., Schenk N., Tapernoux-Luthi E., Muller T., Krautler B., Hortensteiner S. In vivo participation of red chlorophyll catabolite reductase in chlorophyll breakdown. Plant Cell. 2007;19:369–387. doi: 10.1105/tpc.106.044404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin G., Gu H., Ma L., Peng Y., Deng X.W., Chen Z., Qu L.-J. Disruption of phytoene desaturase gene results in albino and dwarf phenotypes in Arabidopsis by impairing chl-orophyll, carotenoid, and gibberellin biosynthesis. Cell Res. 2007;17:471–482. doi: 10.1038/cr.2007.40. [DOI] [PubMed] [Google Scholar]
- Reinsberg D., Ottmann K., Booth P.J., Paulsen H. Effects of chlorophyll a, chlorophyll b, and xanthophylls on the in vitro assembly kinetics of the major light-harvesting chlorophyll a/b complex. LHCIIb. J. Mol. Biol. 2001;308:59–67. doi: 10.1006/jmbi.2001.4573. [DOI] [PubMed] [Google Scholar]
- Rock C.L. Carotenoids: biology and treatment. Pharmacol. Ther. 1997;75:185–197. doi: 10.1016/s0163-7258(97)00054-5. [DOI] [PubMed] [Google Scholar]
- Stahl W., Sies H. Antioxidant activity of carotenoids. Mol. Aspects Med. 2003;24:345–351. doi: 10.1016/s0098-2997(03)00030-x. [DOI] [PubMed] [Google Scholar]
- Stahl W., Junghans A., de Boer B., Driomina E.S., Briviba K., Sies H. Carotenoid mixtures protect multilamellar liposomes against oxidative damage: synergistic effects of lycopene and lutein. FEBS Lett. 1998;427:305–308. doi: 10.1016/s0014-5793(98)00434-7. [DOI] [PubMed] [Google Scholar]
- Tanaka R., Tanaka A. Tetrapyrrole biosynthesis in higher plants. Annu. Rev. Plant Biol. 2007;58:321–346. doi: 10.1146/annurev.arplant.57.032905.105448. [DOI] [PubMed] [Google Scholar]
- Telfer A. What is b-carotene doing in the photosystem II reaction centre? Philos. Trans. R Soc. Lond B Biol. Sci. 2002;357:1431–1439. doi: 10.1098/rstb.2002.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D., Przybyla D., Op den Camp R., Kim C., Landgraf F., Lee K.P., Wursch M., Laloi C., Nater M., Hideg E., et al. The genetic basis of singlet oxygen-induced stress responses of Arabidopsis thaliana. Science. 2004;306:1183–1185. doi: 10.1126/science.1103178. [DOI] [PubMed] [Google Scholar]
- Wei J., Xu M., Zhang D., Mi H. The role of carotenoid isomerase in maintenance of photosynthetic oxygen evolution in rice plant. Acta Biochim. Biophys. Sin (Shanghai) 2010;42:457–463. doi: 10.1093/abbs/gmq044. [DOI] [PubMed] [Google Scholar]
- Wi S.J., Jang S.J., Park K.Y. Inhibition of biphasic ethylene production enhances tolerance to abiotic stress by reducing the accumulation of reactive oxygen species in Nicotiana tabacum. Mol Cells. 2010;30:37–49. doi: 10.1007/s10059-010-0086-z. [DOI] [PubMed] [Google Scholar]
- Zapata M., Rodriguez F., Garrido J.L. Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C-8 column and pyridine-containing mobile phases. Mar. Ecol-Prog. Ser. 2000;195:29–45. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.