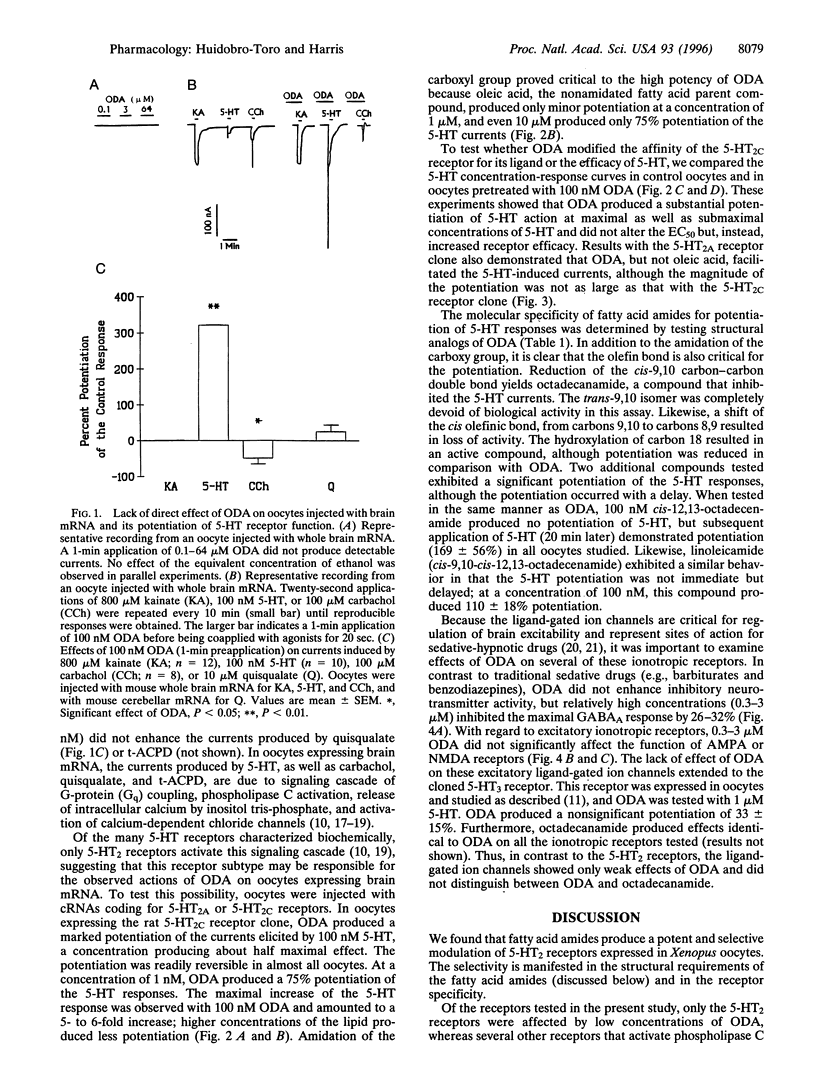
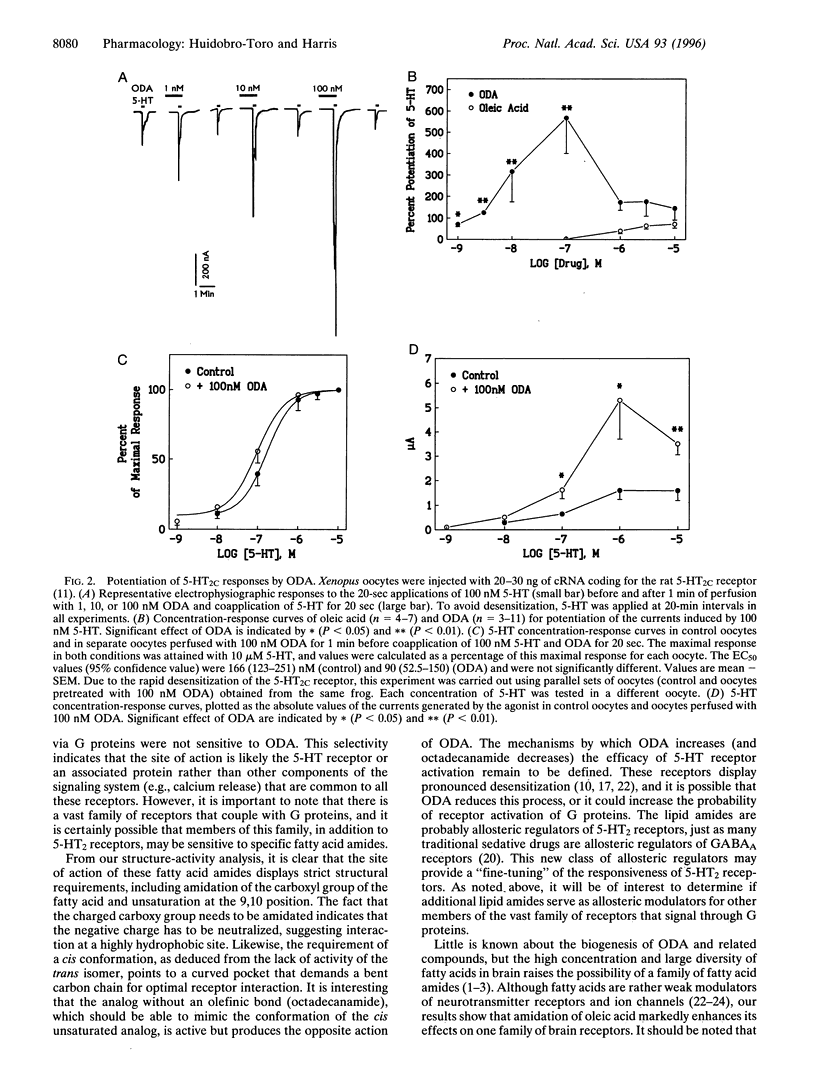
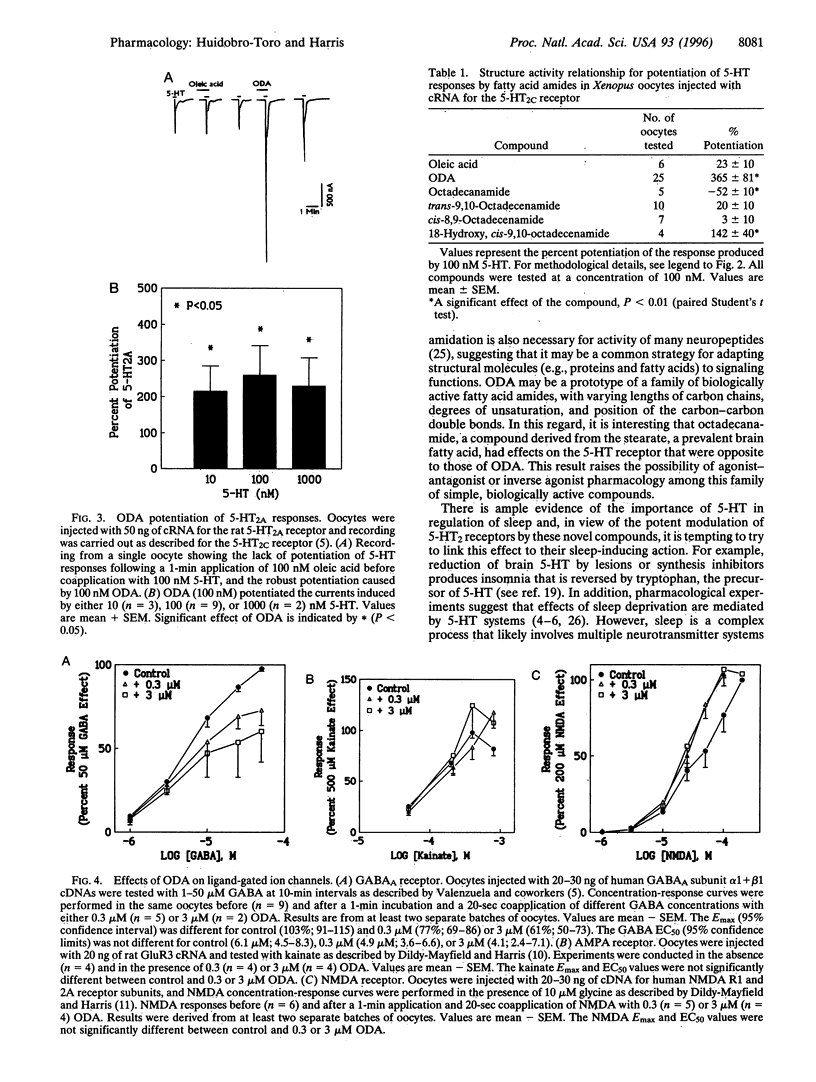
Abstract
Amide derivatives of fatty acids were recently isolated from cerebrospinal fluid of sleep-deprived animals and found to induce sleep in rats. To determine which brain receptors might be sensitive to these novel neuromodulators, we tested them on a range of receptors expressed in Xenopus oocytes. cis-9,10-Octadecenamide (ODA) markedly potentiated the action of 5-hydroxytryptamine (5-HT) on 5-HT2A and 5-HT2C receptors, but this action was not shared by related compounds such as oleic acid and trans-9,10-octacenamide. ODA was active at concentrations as low as 1 nM. The saturated analog, octadecanamide, inhibited rather than potentiated 5-HT2C responses. ODA had either no effect or only weak effects on other receptors, including muscarinic cholinergic, metabotropic glutamate, GABA(A), N-methyl-D-asparate, or alpha-amino-3-hydroxy-5-methyl-4-isoxozolepropionic acid receptors. Modulation of 5-HT2 receptors by ODA and related lipids may represent a novel mechanism for regulation of receptors that activate G proteins and thereby play a role in alertness, sleep, and mood as well as disturbances of these states.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cravatt B. F., Prospero-Garcia O., Siuzdak G., Gilula N. B., Henriksen S. J., Boger D. L., Lerner R. A. Chemical characterization of a family of brain lipids that induce sleep. Science. 1995 Jun 9;268(5216):1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- Dildy-Mayfield J. E., Harris R. A. Comparison of ethanol sensitivity of rat brain kainate, DL-alpha-amino-3-hydroxy-5-methyl-4-isoxalone proprionic acid and N-methyl-D-aspartate receptors expressed in Xenopus oocytes. J Pharmacol Exp Ther. 1992 Aug;262(2):487–494. [PubMed] [Google Scholar]
- Dildy-Mayfield J. E., Harris R. A. Ethanol inhibits kainate responses of glutamate receptors expressed in Xenopus oocytes: role of calcium and protein kinase C. J Neurosci. 1995 Apr;15(4):3162–3171. doi: 10.1523/JNEUROSCI.15-04-03162.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugovic C., Wauquier A., Leysen J. E., Marrannes R., Janssen P. A. Functional role of 5-HT2 receptors in the regulation of sleep and wakefulness in the rat. Psychopharmacology (Berl) 1989;97(4):436–442. doi: 10.1007/BF00439544. [DOI] [PubMed] [Google Scholar]
- Hadingham K. L., Wingrove P., Le Bourdelles B., Palmer K. J., Ragan C. I., Whiting P. J. Cloning of cDNA sequences encoding human alpha 2 and alpha 3 gamma-aminobutyric acidA receptor subunits and characterization of the benzodiazepine pharmacology of recombinant alpha 1-, alpha 2-, alpha 3-, and alpha 5-containing human gamma-aminobutyric acidA receptors. Mol Pharmacol. 1993 Jun;43(6):970–975. [PubMed] [Google Scholar]
- Harris R. A., Mihic S. J., Dildy-Mayfield J. E., Machu T. K. Actions of anesthetics on ligand-gated ion channels: role of receptor subunit composition. FASEB J. 1995 Nov;9(14):1454–1462. doi: 10.1096/fasebj.9.14.7589987. [DOI] [PubMed] [Google Scholar]
- Julius D., Huang K. N., Livelli T. J., Axel R., Jessell T. M. The 5HT2 receptor defines a family of structurally distinct but functionally conserved serotonin receptors. Proc Natl Acad Sci U S A. 1990 Feb;87(3):928–932. doi: 10.1073/pnas.87.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizer J. S., Busby W. H., Jr, Cottle C., Youngblood W. W. Glycine-directed peptide amidation: presence in rat brain of two enzymes that convert p-Glu-His-Pro-Gly-OH into p-Glu-His-Pro-NH2 (thyrotropin-releasing hormone). Proc Natl Acad Sci U S A. 1984 May;81(10):3228–3232. doi: 10.1073/pnas.81.10.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Bourdellès B., Wafford K. A., Kemp J. A., Marshall G., Bain C., Wilcox A. S., Sikela J. M., Whiting P. J. Cloning, functional coexpression, and pharmacological characterisation of human cDNAs encoding NMDA receptor NR1 and NR2A subunits. J Neurochem. 1994 Jun;62(6):2091–2098. doi: 10.1046/j.1471-4159.1994.62062091.x. [DOI] [PubMed] [Google Scholar]
- LeMarquand D., Pihl R. O., Benkelfat C. Serotonin and alcohol intake, abuse, and dependence: clinical evidence. Biol Psychiatry. 1994 Sep 1;36(5):326–337. doi: 10.1016/0006-3223(94)90630-0. [DOI] [PubMed] [Google Scholar]
- Lerner R. A., Siuzdak G., Prospero-Garcia O., Henriksen S. J., Boger D. L., Cravatt B. F. Cerebrodiene: a brain lipid isolated from sleep-deprived cats. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9505–9508. doi: 10.1073/pnas.91.20.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzoni O., Fagni L., Pin J. P., Rassendren F., Poulat F., Sladeczek F., Bockaert J. (trans)-1-amino-cyclopentyl-1,3-dicarboxylate stimulates quisqualate phosphoinositide-coupled receptors but not ionotropic glutamate receptors in striatal neurons and Xenopus oocytes. Mol Pharmacol. 1990 Jul;38(1):1–6. [PubMed] [Google Scholar]
- Ordway R. W., Singer J. J., Walsh J. V., Jr Direct regulation of ion channels by fatty acids. Trends Neurosci. 1991 Mar;14(3):96–100. doi: 10.1016/0166-2236(91)90069-7. [DOI] [PubMed] [Google Scholar]
- Pandey S. C., Davis J. M., Pandey G. N. Phosphoinositide system-linked serotonin receptor subtypes and their pharmacological properties and clinical correlates. J Psychiatry Neurosci. 1995 May;20(3):215–225. [PMC free article] [PubMed] [Google Scholar]
- Pascoe P. A. Drugs and the sleep-wakefulness continuum. Pharmacol Ther. 1994;61(1-2):227–236. doi: 10.1016/0163-7258(94)90064-7. [DOI] [PubMed] [Google Scholar]
- Roth B. L. Multiple serotonin receptors: clinical and experimental aspects. Ann Clin Psychiatry. 1994 Jun;6(2):67–78. doi: 10.3109/10401239409148985. [DOI] [PubMed] [Google Scholar]
- Sallanon M., Janin M., Buda C., Jouvet M. Serotoninergic mechanisms and sleep rebound. Brain Res. 1983 May 23;268(1):95–104. doi: 10.1016/0006-8993(83)90393-1. [DOI] [PubMed] [Google Scholar]
- Sanna E., Dildy-Mayfield J. E., Harris R. A. Ethanol inhibits the function of 5-hydroxytryptamine type 1c and muscarinic M1 G protein-linked receptors in Xenopus oocytes expressing brain mRNA: role of protein kinase C. Mol Pharmacol. 1994 May;45(5):1004–1012. [PubMed] [Google Scholar]
- Saxena P. R. Serotonin receptors: subtypes, functional responses and therapeutic relevance. Pharmacol Ther. 1995 May;66(2):339–368. doi: 10.1016/0163-7258(94)00005-n. [DOI] [PubMed] [Google Scholar]
- Sharpley A. L., Cowen P. J. Effect of pharmacologic treatments on the sleep of depressed patients. Biol Psychiatry. 1995 Jan 15;37(2):85–98. doi: 10.1016/0006-3223(94)00135-P. [DOI] [PubMed] [Google Scholar]
- Sieghart W. Structure and pharmacology of gamma-aminobutyric acidA receptor subtypes. Pharmacol Rev. 1995 Jun;47(2):181–234. [PubMed] [Google Scholar]
- Singer D., Boton R., Moran O., Dascal N. Short- and long-term desensitization of serotonergic response in Xenopus oocytes injected with brain RNA: roles for inositol 1,4,5-trisphosphate and protein kinase C. Pflugers Arch. 1990 Apr;416(1-2):7–16. doi: 10.1007/BF00370215. [DOI] [PubMed] [Google Scholar]
- Snutch T. P., Mandel G. Tissue RNA as source of ion channels and receptors. Methods Enzymol. 1992;207:297–309. doi: 10.1016/0076-6879(92)07019-k. [DOI] [PubMed] [Google Scholar]
- Snutch T. P. The use of Xenopus oocytes to probe synaptic communication. Trends Neurosci. 1988 Jun;11(6):250–256. doi: 10.1016/0166-2236(88)90102-6. [DOI] [PubMed] [Google Scholar]
- Tanabe Y., Masu M., Ishii T., Shigemoto R., Nakanishi S. A family of metabotropic glutamate receptors. Neuron. 1992 Jan;8(1):169–179. doi: 10.1016/0896-6273(92)90118-w. [DOI] [PubMed] [Google Scholar]
- Valenzuela C. F., Kazlauskas A., Brozowski S. J., Weiner J. L., Demali K. A., McDonald B. J., Moss S. J., Dunwiddie T. V., Harris R. A. Platelet-derived growth factor receptor is a novel modulator of type A gamma-aminobutyric acid-gated ion channels. Mol Pharmacol. 1995 Dec;48(6):1099–1107. [PubMed] [Google Scholar]
- Witt M. R., Nielsen M. Characterization of the influence of unsaturated free fatty acids on brain GABA/benzodiazepine receptor binding in vitro. J Neurochem. 1994 Apr;62(4):1432–1439. doi: 10.1046/j.1471-4159.1994.62041432.x. [DOI] [PubMed] [Google Scholar]
- Xiao Y. F., Kang J. X., Morgan J. P., Leaf A. Blocking effects of polyunsaturated fatty acids on Na+ channels of neonatal rat ventricular myocytes. Proc Natl Acad Sci U S A. 1995 Nov 21;92(24):11000–11004. doi: 10.1073/pnas.92.24.11000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates M., Leake A., Candy J. M., Fairbairn A. F., McKeith I. G., Ferrier I. N. 5HT2 receptor changes in major depression. Biol Psychiatry. 1990 Mar 1;27(5):489–496. doi: 10.1016/0006-3223(90)90440-d. [DOI] [PubMed] [Google Scholar]


