Abstract
Sirtuins (SIRTs), a family of nicotinamide adenine dinucleotide (NAD)-dependent deacetylases, are emerging as key molecules that regulate aging and age-related diseases including cancers, metabolic disorders, and neurodegenerative diseases. Seven isoforms of SIRT (SIRT1–7) have been identified in mammals. SIRT1 and 6, mainly localized in the nucleus, regulate transcription of genes and DNA repair. SIRT3 in the mitochondria regulates mitochondrial bioenergetics. Initial studies in yeasts, nematodes, and flies indicated a strong connection of SIRT with the life-prolonging effects of calorie restriction (CR), a robust experimental intervention for longevity in a range of organisms. However, subsequent studies reported controversial findings regarding SIRT roles in the effect of CR. This review describes the functional roles of mammalian SIRTs and discusses their relevance to mechanisms underlying the longevity effect of CR.
Keywords: calorie restriction, longevity, metabolism, sirtuins
INTRODUCTION
Silencing information regulator 2 (Sir2) was originally identified as a protein essential for transcriptional silencing at the silent mating loci and telomeres (Gottschling et al., 1990; Rine and Herskowitz, 1987). Sir2 was later established as an important regulator for many cellular processes, including cell cycle regulation (Dryden et al., 2003), fatty acid metabolism (Starai et al., 2002), gene silencing, and lifespan extension (Kaeberlein et al., 1999; Sinclair and Guarente, 1997). In the budding yeast Saccharomyces cerevisiae, the finite replicative life span, defined as the number of times an individual cell divides before it undergoes senescence, has been established as a model for the genetic regulation of longevity (Mortimer and Johnston, 1959). Replicative aging of yeast can be regulated by ribosomal DNA (rDNA) stability resulting from recombination between rDNA repeats and the accumulation of extrachromosomal rDNA circles (Fritze et al., 1997; Kaeberlein et al., 1999; Sincclair and Guarente, 1997). Studies in S. cerevisiae demonstrated that an extra copy of the Sir2 gene increased lifespan, while deletions of this gene shortened lifespan (Kaeberlein et al. 1999). Lin et al. (2000) showed that reducing the glucose concentration in media, as a calorie restriction (CR) model in yeast, increased the replicative lifespan in wild-type yeast, whereas reducing the glucose concentration failed to extend lifespan in the Sirt2-deleted yeast model. Furthermore, in Drosophila, an increase of Sir2 (dSir2) extended lifespan, whereas a decrease in dSir2 blocked the effects of CR on extending lifespan (Rogina and Helfand, 2004). These findings indicate that Sir2 has a critical role in the effects of CR on lifespan extension. However, another study demonstrated that Sir2 was not necessary in CR-mediated lifespan extension (Lamming et al., 2005). Moreover, in another report, Sir2 overexpression coupled with CR resulted in an additive longevity effect (Kaeberlein et al., 2004), suggesting that lifespan extension by CR is unlikely to be mediated by Sir2.
Despite these controversial results, Sirt2 is currently being extensively investigated in the study of metabolism, aging and age-associated diseases, including cancer, diabetes, and neurodegenerative disorders. Understanding the function of Sir2 in the control of energy metabolism and lifespan is important, both for identifying new longevity pathways and for identifying new drug candidates for the improvement of human health. Here, we review the function of SIRTs, in particular in the areas of mammalian energy metabolism and longevity. In addition, we discuss the function of sirtuins and their relation to CR.
OVERVIEW OF SIRTUINS
Sirtuins are a family of NAD+-dependent protein deacetylases that are evolutionally conserved from yeast to human. Mammals contain seven Sir2 homologs (SIRT1-7, SIRTs, sirtuins) that share a common 275 amino acid catalytic domain. Mammalian sirtuins are localized in different subcellular compartments: SIRT1, −6, and −7 are predominately in the nucleus, SIRT2 is cytoplasmically localized, and SIRT3, −4, and −5 are located in the mitochondria (Frye, 2000; North and Verdin, 2004). SIRT1 targets a number of substrates that regulate DNA damage, stress response, mitochondrial biogenesis, and glucose and lipid metabolism (Houtkooper et al., 2012). SIRT2 controls cell cycle and glucose and lipid metabolism (Chalkiadaki and Guarente, 2012; Houtkooper et al., 2012). SIRT3, 4, and 5 regulate ATP production, metabolism, apoptosis, and cell signaling (Haigis et al., 2006; Michishita et al., 2005; Onyango et al., 2002; Schwer et al., 2002). SIRT6 is involved in genomic DNA stability and repair, and crucial in metabolism and aging (Mostoslavsky et al., 2006; Zhong et al., 2010). No robust activity has been found for SIRT7 as of yet (Table 1).
Table 1.
Localization and activity of mammalian sirtuins
| Sirtuin | Activity | Localization | Interactions | Biological connections |
|---|---|---|---|---|
| SIRT1 | Deacetylation | Nucleus | FOXO1, FOXO3, PGC1α, p53, eNOS | Cell survival & Metabolism |
| SIRT2 | Deacetylation | Cytosol | FOXO1, NF-κB | Cell cylcle & Inflammation |
| SIRT3 | Deacetylation | Mitochondria | SOD2, IDH2 | Redox regulation & Metabolism |
| SIRT4 | ADP-ribosylation | Mitochondria | GDH | Insulin secretion & Metabolism |
| SIRT5 | Deacetylation | Mitochondria | CPS1 | Urea cycle |
| SIRT6 | Deacetylation, ADP-ribosylation | Nucleus | H3K9, H3K56 | DNA repair |
| SIRT7 | Unknown | Nucleus | Unknown | rDNA transcription |
CPS1, carbamoyl phosphate synthetase 1; eNOS, endothelial nitric oxide synthase; FOXO, forkhead box O; GDH, glutamate dehydrogenase; H3K9, histone H3 lysine 9; H3K56, histone H3 lysine 56; IDH2, isocitrate dehydrogenase 2; NF-κB, nuclear factor-κB; PGC1 α, peroxisome proliferator-activated receptor-γ co-activator 1 α; SOD2, superoxide dismutase 2
FUNCTIONS OF SIRTS IN MAMMALS
SIRT1
SIRT1 in the hypothalamic arcuate nucleus
SIRT1, the most extensively studied member of the sirtuin family, regulates energy metabolism in the hypothalamus and is induced by a negative energy balance (Chen et al., 2008; Cohen et al., 2004). SIRT1 is expressed in the orexigenic neuropeptide Y (NPY)/Agouti-related peptide (AGRP) and anorexigenic proopiomelanocortin (POMC) neurons, which are major components in regulating feeding and energy expenditure in the arcuate nucleus of the hypothalamus (Sasaki and Kitamura, 2010). Several studies in mice and rats reported that SIRT1 regulates food intake and body weight by acting on the NPY/AGRP and POMC neurons (Cakir et al., 2009; Dietrich et al., 2010).
SIRT1 and cell survival
SIRT1 regulates cell survival through deacetylation and subsequent inhibition of the tumor suppressor p53, which controls cell cycle arrest and apoptosis (Luo et al., 2001; Vaziri et al., 2001). SIRT1 also deacetylates FOXO1, FOXO3a and FOXO4, modulating cell cycle and reactive oxygen species (ROS) generation by increasing p27kip1, MnSOD (manganese superoxide dismutase), and GADD45 (growth arrest and DNA damage inducible protein 45) (Brunet et al., 2004; Daitoku et al., 2004; Kobayashi et al., 2005; van der Horst et al., 2004). Studies in SIRT1-deficient and overexpressing mice identified SIRT1 as a positive regulator of telomere length, and showed that SIRT1 attenuates telomere shortening associated with aging (Palacios et al., 2010).
SIRT1 and glucose/lipid metabolism
SIRT1 has been connected with glucose and lipid metabolism (Moynihan et al., 2005; Picard et al., 2004; Rodgers and Puigserver, 2007; Rodgers et al., 2005). Glucose uptake in peripheral tissues is promoted by insulin, thus insulin-secreting pancreatic beta cells are crucial in the regulation of glucose metabolism. Studies in pancreatic beta cells demonstrated that SIRT1 positively regulates insulin secretion through inhibition of uncoupling protein-2 (UCP2) (Bernal-Mizrachi et al., 2005; Bordone et al., 2006). Glycolysis and gluconeogenesis are major pathways for maintaining glucose homeostasis, and PGC1α is key factor in regulating both pathways (Rodgers and Puigserver, 2007). SIRT1 deacetylates PGC1α and increases glucose levels (Rodgers et al., 2005). SIRT1 is also involved in lipid metabolism and enhances fat mobilization in white adipose tissue through repression of peroxisome proliferator-activated receptor gamma (PPARγ) (Picard et al., 2004). Additional studies showed that SIRT1 inhibits acetyl-CoA carboxylase and fatty acid synthase by activation of AMP-activated protein kinase (AMPK) and suppresses lipid accumulation in liver (Hou et al., 2008; Starai et al., 2002).
SIRT2
SIRT2, a predominantly cytoplasmic protein, is increased during the mitotic phase of the cell cycle, and its overexpression delays mitotic exit (Chalkiadaki and Guarente, 2012; Dryden et al., 2003; Houtkooper et al., 2012). SIRT2 also regulates NF-κB, which is involved in the immune and inflammation responses, apoptosis, cell proliferation and survival (Rothgiesser et al., 2010), suggesting potential additional functions of SIRT2. SIRT2 also controls adipogenesis, as supported by the observation that enhanced FOXO1 binding to PPARγ by SIRT2-mediated FOXO1 deacetylation suppressed PPARγ transcriptional activity, resulting in inhibition of adipocyte differentiation (Wang and Tong, 2009).
SIRT3
SIRT3, localized in the mitochondrial inner membrane, is directly linked to longevity and highly expressed in long-lived individuals (Bellizzi et al., 2005). Mitochondria are essential for cell survival and their dysfunction has been involved in metabolic diseases such as age-associated disease, neurodegenerative diseases and cancer. Functional studies showed that SIRT3 regulates mitochondrial biogenesis, and it is correlated with the expression of genes related to mitochondrial function, including PGC1α and UCP1 (Kong et al., 2010; Shi et al., 2005). Moreover, SIRT3 deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status (Yu et al., 2012). A recent study suggested that SIRT3 was regulated by nutrient excess. SIRT3 was downregulated in response to a high-fat diet leading to development of metabolic syndrome through mitochondrial protein hyperacetylation (Green and Hirschey, 2013). SIRT3 also appears to be an antioxidant protein, as SIRT3 reduced reactive oxygen species (ROS) through post-translational regulation of superoxide dismutase 2 (SOD2) via deactylation in response to oxidative stress (Chen et al., 2011). Furthermore, SIRT3 is involved in fatty acid oxidation in the liver (Giralt and Villarroya, 2012). This was supported by evidence in SIRT3-knockout mice demonstrating increased palmitoylcarnitine and triacylglycerols in the liver and various acylcarnitines in the blood (Hallows et al., 2011; Hirschey et al., 2010). SIRT3 is also expressed abundantly in the heart, and its overexpression is protective against cardiac hypertrophy, while SIRT3 depletion enhances susceptibility to hypertrophy (Sundaresan et al., 2009). Together, this suggests a link between SIRT3 and cardiac function.
Overall, the data surrounding SIRT3’s involvement in aging, cardiac homeostasis, fatty acid metabolism, mitochondrial biogenesis and adaptive thermogenesis supports its importance as a regulator of energy metabolism and a potential pharmacological target.
SIRT4
SIRT4, another mitochondrial protein, has ADP-ribosyl-transferase activity, but unlike SIRT1-3, lacks NAD-dependent deacetylase activity (Haigis et al., 2006). The only identified substrate found thus far for SIRT4 is glutamate dehydrogenase (GDH), an enzyme that converts glutamate to α-ketoglutarate in mitochondria. SIRT4 inhibits GDH activity through ADP-ribosylation and promotes insulin secretion through increase of ATP (Haigis et al., 2006). SIRT4 is also associated with the regulation of fatty acid oxidation in liver and muscle, although the mechanisms underlying these regulations are still unclear (Nasrin et al., 2010).
SIRT6
SIRT6 is predominantly nuclear, and reportedly resides in the nucleolus (Ford et al., 2006; Mostoslavsky et al., 2006). SIRT6 is a unique member among the SIRT proteins, in that it has both deacetylation and ADP-ribosylation activities. SIRT6 is likely linked to cellular metabolism and aging. A study of mice with a targeted deletion of SIRT6 showed that SIRT6 is essential for survival and controls glucose homeostasis through HIF-1α (Mostosalvsky et al., 2006; Zhong et al., 2010). SIRT6 interacts with NF-κB and deacetylates histone H3 lysine 9 (H3K9) at NF-κB target gene promoters leading to inactivation of many of the same transcriptional programs observed in aged tissues, including genes controlled by NF-κB (Kawahara et al., 2009). In addition, SIRT6 participates in the regulation of DNA stabilization and repair through its specific histone H3 lysine 56 (H3K56) deacetylase activity (Michishita et al., 2008; 2009; Yang et al., 2009). These data suggest that SIRT6, along with SIRT1, is a key member of the SIRT family that exerts a wide range of actions in the regulation of cellular physiology and aging.
The other SIRT proteins
The other SIRT proteins, SIRT5 and SIRT7, are not well investigated. SIRT5 was characterized as a mitochondrial protein with weak deacetylase activity (Michishita et al., 2005; North et al., 2003). SIRT5 was reported to deacetylate carbamoyl phosphate synthetase 1 (CPS1) during fasting and activate ammonia detoxification through the urea cycle (Nakagawa et al., 2009). SIRT7 is associated with activation of RNA polymerase I transcription (Ford et al., 2006), although no substrate has been found for SIRT7 as yet.
Relevance of SIRTs to the longevity effect of CR
SIRT1, 3, and 6 are the most extensively studied members of the SIRT family in regard to the longevity effects of CR. These SIRTs were likely first examined because protein levels of all three isoforms are elevated in CR animals, although this increase seems to be tissue specific (Cohen et al., 2004; 2008; Kanfi et al., 2008; Palacios et al., 2009). CR induces physiological and behavioral changes, such as reduced growth factor, glucose, and triglycerides, and increased locomotor and foraging activity (McCarter et al., 1997; Weed et al., 1997; Weindruch et al., 1988). However, the increased physiological activity in response to CR was diminished in SIRT-deficient (SIRT1-KO) mice (Chen et al., 2005), suggesting that one of the parameter of CR, increase of physiological activity requires SIRT1. CR mice live longer (Koubova and Guarente, 2003), whereas the SIRT1-KO mice had shortened lifespan in adlibitum or CR conditions compared with wild-type mice (Li et al., 2008). This data suggests that SIRT1 is a key factor in the regulation of physiological changes and lifespan extension induced by CR. Moreover, SIRT1 overexpressing mice exhibit several phenotypes resembling CR mice: they are leaner, metabolically more active, and show low insulin and fasted blood glucose levels (Bordone et al., 2007). However, the requirement for SIRT1 in CRmediated lifespan extension is under debate, as the mechanism of SIRT1 activation in response to CR remains unclear and because CR increases SIRT1 levels in some tissues (Chen et al., 2008).
Resveratrol, a polyphenolic compound found in grapes, extends lifespan in yeast, worms, flies in a Sir2-dependent manner (Howitz et al., 2003; Wood et al., 2004). However, neither resveratrol nor overexpression of SIRT1 was found to extend lifespan in mice fed standard diets (Herranz et al., 2010; Pearson et al., 2008). Resveratrol improved mitochondrial function and protected against high fat-induced obesity through SIRT1 and PGC1α (Lagouge et al., 2006). However, whether resveratrol and SRT1720 directly activate SIRT1 is still under debate, as SIRT1 activation by these compounds was induced only when a fluorophore tag was covalently attached to the peptide substrate, as in the initial compound screens (Borra et al., 2005; Kaeberlein et al., 2005; Pacholec et al., 2010). CR and resveratrol increase endothelial-derived nitric oxide (NO) (Csiszar et al., 2009; Nisoli et al., 2005), an important regulator of vascular relaxation and endothelial senescence (Oemar et al., 1998). Mattagajasingh et al. (2007) showed that SIRT1 increases NO through deacetylation of eNOS. Further studies are required to determine whether CR-mediated beneficial functions in vascular senescence result from increased SIRT1.
AMPK, a nutrient and energy sensor, is closely related to SIRT1 as a fuel-sensing molecule, as both are regulated by cellular energy balance. SIRT1 activity is regulated by AMPK-mediated increase of cellular NAD+ levels and the [NAD+]/[NADH] ratio (Cantó et al., 2009; Imai et al., 2000; Landry et al., 2000). The observation that AMPK modulates the activity of SIRT1 targets, including PGC1a, FOXO1 and FOXO3a transcription factors, raises the possibility that AMPK and SIRT1 are implicated in beneficial functions of CR on metabolic adaptation (Cantó et al., 2009). The mechanism by which CR activates SIRT1 is still under debate. In a study in yeast, CR did not affect NAD+ levels, but lowered NADH levels (Lin et al., 2004), while in mammals NADH levels were unchanged in response to CR (Cohen et al., 2004; Nemoto et al., 2004; Rodgers et al., 2005). In a subsequent study, Wang et al. (2012) demonstrated that CR enhanced phosphorylation of AMPK in skeletal muscle, indicating that activation of SIRT1 by AMPK can occur in CR, at least in skeletal muscle.
The NAD+ salvage pathway is important in regulating SIRT1 deacetylase activity. NAD+ is hydrolyzed to NAM, which inhibits SIRT1 deacetylase activities (Bitterman et al., 2002; Fulco et al., 2008; Sauve et al., 2005) and O-acetyl-ADP-ribose (Borra et al., 2002; Tanner et al., 2000). Intracellular NAD+ levels and SIRT1 function are regulated by nicotinamide phosphoribosyltransferase (NAMPT), which functions to resynthesize NAD+ from NAM (Revollo et al., 2004). In skeletal muscle, NAMPT is activated by AMPK under glucose-deprived conditions, resulting in an increased [NAD+]/[NADH] ratio (Fulco et al., 2008). Therefore, in skeletal muscle, it is likely that activation of NAMPT by AMPK leads to increased NAD+ levels, which enhance SIRT1 deacetylase activity.
Although SIRT1 is the best-characterized mammalian sirtuin in the effects of CR, SIRT3 also seems to have an important role in CR. Someya et al. (2010) showed that SIRT3 is required for the CR-mediated prevention of age-related hearing loss and oxidative damage. Furthermore, SIRT3-KO mice showed a diminished protective effect of CR on oxidative stress (Qiu et al., 2010), indicating that SIRT3 is involved in defense programs against oxidative stress.
CR-linked metabolic reprogramming for efficient energy utilization and reduction in oxidative stress is regulated by mitochondrial protein function (Sohal and Weindruch, 1996). A recent paper showed that SIRT3 modulates mitochondrial protein acetylation in response to CR (Hebert et al., 2013). SIRT3 is also involved in human longevity. A variable number of tandem repeat (VNTR) enhancer found in the SIRT3 gene has an allele-specific enhancer activity, and the allele completely lacking enhancer activity is virtually absent in long-living individuals (Bellizzi et al., 2005).
SIRT6 shares similar functions with SIRT1, such as protection against high fat diet (HFD)-induced metabolic damage (Pfluger et al., 2008) and negative regulation of NF-κB (Kawahara et al., 2009; Yeung et al., 2004). Although only observed in male mice, SIRT6 is the only protein in the SIRT family that extends mammalian lifespan (Kanfi et al., 2012).
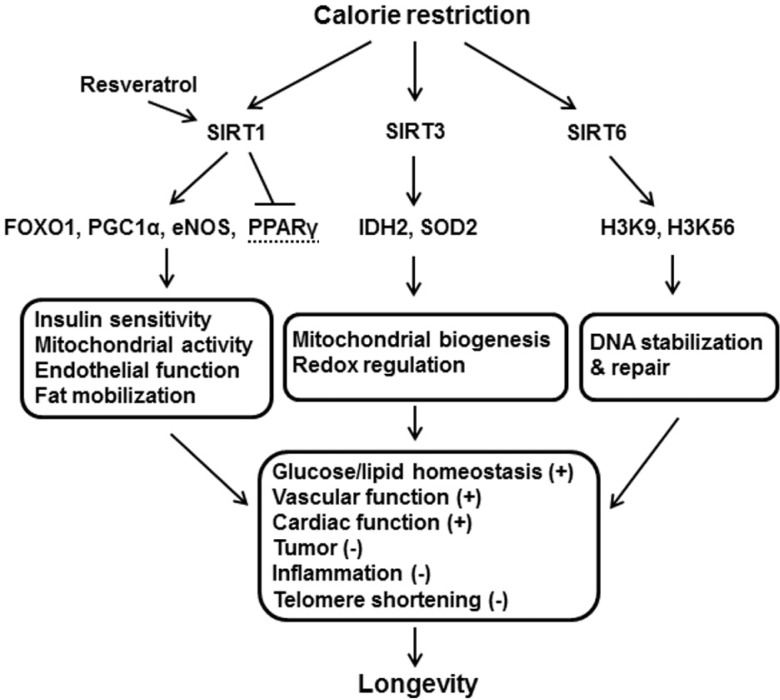
Given these functions of SIRTs in the longevity effect of CR, SIRTs could be important regulators of beneficial CR-mediated functions in mammals (see Fig. 1).
Fig. 1.
SIRTs-mediated pathways that induce anti-aging effect of CR in mammals. (+), enhancement; (−), inhibition (See Table 1 for abbreviation).
CONCLUSION
The multifunctional SIRTs regulate cellular and whole-body metabolism processes, such as cell survival, glucose/lipid metabolism, mitochondrial biogenesis, adaptive thermogenesis, and DNA stabilization and repair. Resveratrol, a well-known SIRT1 activator, helps prevent metabolic disorder by increasing glucose utilization and insulin sensitivity, and improves survival of mice fed a high-calorie diet. The numerous studies discussed here clearly support a close involvement of Sir2/SIRT1 in CRmediated lifespan extension. The results also raise the possibility that SIRT1 may not be the only factor, but rather one of several components that regulate CR-mediated lifespan extension, as neither overexpression of SIRT1 nor resveratrol extended lifespan in mammals. Notably, Chen et al. (2008) showed that CR does not increase SIRT1 in whole tissue. It is likely that the use of tissue-specific SIRT1 transgenic mice could be more effective in clarifying the role of SIRT1 in CR-mediated lifespan extension. Although there are some functional overlaps among the SIRT family members, each SIRT isoform generally exhibits a unique function in CR, indicating that SIRTs may be essential for the longevity effect of CR. Studies of overexpressed SIRT1, 3, and 6, which are increased by CR, could be a reasonable focus for future research to identify longevity mechanisms involved in CR. Moreover, further studies should be performed to identify other factors for CR-mediated lifespan extension, in particular the connections between SIRTs and other longevity factors, such as AMPK, insulin/IGF1 signaling and FOXO proteins.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (no. 22390042) and the JSPS Asian CORE Program.
REFERENCES
- Bellizzi D, Rose G, Cavalcante P, Covello G, Dato S, De Rango F, Greco V, Maggiolini M, Feraco E, Mari V, et al. (2005). A novel VNTR enhancer within the SIRT3 gene, a human homologue of SIR2, is associated with survival at oldest ages. Genomics. 85, 258–263 [DOI] [PubMed] [Google Scholar]
- Bernal-Mizrachi C, Gates AC, Weng S, Imamura T, Knutsen RH, DeSantis P, Coleman T, Townsend RR, Muglia LJ, Semenkovich CF. (2005). Vascular respiratory uncoupling increases blood pressure and atherosclerosis. Nature. 435, 502–506 [DOI] [PubMed] [Google Scholar]
- Bitterman KJ, Anderson RM, Cohen HY, Latorre-Esteves M, Sinclair DA. (2002). Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem. 277, 45099–45107 [DOI] [PubMed] [Google Scholar]
- Bordone L, Motta MC, Picard F, Robinson A, Jhala US, Apfeld J, McDonagh T, Lemieux M, McBurney M, Szilvasi A, et al. (2006). Sirt1 regulates insulin secretion by repressing UCP2 in pancreatic beta cells. PLoS Biol. 4, e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordone L, Cohen D, Robinson A, Motta MC, van Veen E, Czopik A, Steele AD, Crowe H, Marmor S, Luo J, et al. (2007). SIRT1 transgenic mice show phenotypes resembling calorie restriction. Aging Cell. 6, 759–767 [DOI] [PubMed] [Google Scholar]
- Borra MT, O’Neill FJ, Jackson MD, Marshall B, Verdin E, Foltz KR, Denu JM. (2002). Conserved enzymatic production and biological effect of O-acetyl-ADP-ribose by silent information regulator 2-like NAD+-dependent deacetylases. J Biol Chem. 277, 12632–12641 [DOI] [PubMed] [Google Scholar]
- Borra MT, Smith BC, Denu JM. (2005). Mechanism of human SIRT1 activation by resveratrol. J Biol Chem. 280, 17187–17195 [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, et al. (2004). Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science. 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- Cakir I, Perello M, Lansari O, Messier NJ, Vaslet CA, Nillni EA. (2009). Hypothalamic Sirt1 regulates food intake in a rodent model system. PLoS One. 4, e8322.Cell136, 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cantó C, Gerhart-Hines Z, Feige JN, Lagouge M, Noriega L, Milne JC, Elliott PJ, Puigserver P, Auwerx J. (2009). AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 458, 1056–1060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalkiadaki A, Guarente L. (2012). Sirtuins mediate mammalian metabolic responses to nutrient availability. Nat Rev Endocrinol. 8, 287–296 [DOI] [PubMed] [Google Scholar]
- Chen D, Steele AD, Lindquist S, Guarente L. (2005). Increase in activity during calorie restriction requires Sirt1. Science. 310, 1641. [DOI] [PubMed] [Google Scholar]
- Chen D, Bruno J, Easlon E, Lin SJ, Cheng HL, Alt FW, Guarente L. (2008). Tissue-specific regulation of SIRT1 by calorie restriction. Genes Dev. 22, 1753–1757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Zhang J, Lin Y, Lei Q, Guan KL, Zhao S, Xiong Y. (2011). Tumour suppressor SIRT3 deacetylates and activates manganese superoxide dismutase to scavenge ROS. EMBO Rep. 12, 534–41 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA. (2004). Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science. 305, 390–392 [DOI] [PubMed] [Google Scholar]
- Csiszar A, Labinskyy N, Pinto JT, Ballabh P, Zhang H, Losonczy G, Pearson K, de Cabo R, Pacher P, Zhang C, et al. (2009). Resveratrol induces mitochondrial biogenesis in endothelial cells. Am J Physiol Heart Circ Physiol. 297, 13–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daitoku H, Hatta M, Matsuzaki H, Aratani S, Ohshima T, Miyagishi M, Nakajima T, Fukamizu A. (2004). Silent information regulator 2 potentiates Foxo1-mediated transcription through its deacetylase activity. Proc Natl Acad Sci USA. 101, 10042–10047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dietrich MO, Antunes C, Geliang G, Liu ZW, Borok E, Nie Y, Xu AW, Souza DO, Gao Q, Diano S, et al. (2010). Agrp neurons mediate Sirt1’s action on the melanocortin system and energy balance: roles for Sirt1 in neuronal firing and synaptic plasticity. J Neurosci. 30, 11815–11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. (2003). Role for human SIRT2 NAD-dependent deace- tylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 23, 3173–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ford E, Voit R, Liszt G, Magin C, Grummt I, Guarente L. (2006). Mammalian Sir2 homolog SIRT7 is an activator of RNA polymerase I transcription. Genes Dev. 20, 1075–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritze CE, Verschueren K, Strich R, Easton Esposito R. (1997). Direct evidence for SIR2 modulation of chromatin structure in yeast rDNA. EMBO J. 16, 6495–6509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frye RA. (2000). Phylogenetic classification of prokaryotic and eukaryotic Sir2-like proteins. Biochem Biophys Res Commun. 273, 793–798 [DOI] [PubMed] [Google Scholar]
- Fulco M, Cen Y, Zhao P, Hoffman EP, McBurney MW, Sauve AA, Sartorelli V. (2008). Glucose restriction inhibits skeletal myoblast differentiation by activating SIRT1 through AMPK-mediated regulation of Nampt. Dev. Cell. 14, 661–673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giralt A, Villarroya F. (2012). SIRT3, a pivotal actor in mitochondrial functions: metabolism, cell death and aging. Biochem J. 444, 1–10 [DOI] [PubMed] [Google Scholar]
- Gottschling DE, Aparicio OM, Billington BL, Zakian VA. (1990). Position effect at S. cerevisiae telomeres: Reversible repression of Pol II transcription. Cell. 63, 751–762 [DOI] [PubMed] [Google Scholar]
- Green MF, Hirschey MD. (2013). SIRT3 weighs heavily in the metabolic balance: a new role for SIRT3 in metabolic syndrome. J Gerontol A Biol Sci Med Sci. 68, 105–107 [DOI] [PubMed] [Google Scholar]
- Haigis MC, Mostoslavsky R, Haigis KM, Fahie K, Christodoulou DC, Murphy AJ, Valenzuela DM, Yancopoulos GD, Karow M, Blander G, et al. (2006). SIRT4 inhibits glutamate dehydrogenase and opposes the effects of calorie restriction in pancreatic β cells. Cell. 126, 941–954 [DOI] [PubMed] [Google Scholar]
- Hallows WC, Yu W, Smith BC, Devries MK, Ellinger JJ, Someya S, Shortreed MR, Prolla T, Markley JL, Smith LM, et al. (2011). Sirt3 promotes the urea cycle and fatty acid oxidation during dietary restriction. Mol Cell. 41, 139–149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hebert AS, Dittenhafer-Reed KE, Yu W, Bailey DJ, Selen ES, Boersma MD, Carson JJ, Tonelli M, Balloon AJ, Higbee AJ, et al. (2013). Calorie restriction and SIRT3 trigger global reprogramming of the mitochondrial protein acetylome. Mol Cell. 49, 186–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herranz D, Muñoz-Martin M, Cañamero M, Mulero F, Martinez-Pastor B, Fernandez-Capetillo O, Serrano M. (2010). Sirt1 improves healthy ageing and protects from metabolic syndrome-associated cancer. Nat Commun. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschey MD, Shimazu T, Goetzman E, Jing E, Schwer B, Lombard DB, Grueter CA, Harris C, Biddinger S, Ilkayeva OR, et al. (2010). SIRT3 regulates mitochondrial fatty-acid oxidation by reversible enzyme deacetylation. Nature. 464, 121–125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X, Xu S, Maitland-Toolan KA, Sato K, Jiang B, Ido Y, Lan F, Walsh K, Wierzbicki M, Verbeuren TJ, et al. (2008). SIRT1 regulates hepatocyte lipid metabolism through activating AMP-activated protein kinase. J Biol Chem. 283, 20015–20026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper RH, Pirinen E, Auwerx J. (2012). Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 13, 225–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, et al. (2003). Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 425, 191–196 [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L. (2000). Transcriptional silencing and longevity protein Sir2 is an NAD dependent histone deacetylase. Nature. 403, 795–800 [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L. (1999). The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev. 13, 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, Kirkland KT, Fields S, Kennedy BK. (2004). Sir2-independent life span extension by calorie restriction in yeast. PLoS Biol. 2, E296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaeberlein M, McDonagh T, Heltweg B, Hixon J, Westman EA, Caldwell SD, Napper A, Curtis R, DiStefano PS, Fields S, et al. (2005). Substrate-specific activation of sirtuins by resveratrol. J Biol Chem. 280, 17038–17045 [DOI] [PubMed] [Google Scholar]
- Kanfi Y, Shalman R, Peshti V, Pilosof SN, Gozlan YM, Pearson KJ, Lerrer B, Moazed D, Marine JC, De Cabo R, et al. (2008). Regulation of SIRT6 protein levels by nutrient availability. FEBS Lett. 582, 543–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanfi Y, Naiman S, Amir G, Peshti V, Zinman G, Nahum L, Bar-Joseph Z, Cohen HY. (2012). The sirtuin SIRT6 regulates lifespan in male mice. Nature. 483, 218–221 [DOI] [PubMed] [Google Scholar]
- Kawahara TL, Michishita E, Adler AS, Damian M, Berber E, Lin M, McCord RA, Ongaigui KC, Boxer LD, Chang HY, et al. (2009). SIRT6 links histone H3 lysine 9 deacetylation to NF-kappaB-dependent gene expression and organismal life span. Cell. 136, 62–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Furukawa-Hibi Y, Chen C, Horio Y, Isobe K, Ikeda K, Motoyama N. (2005). SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int J Mol Med. 16, 237–243 [PubMed] [Google Scholar]
- Kong X, Wang R, Xue Y, Liu X, Zhang H, Chen Y, Fang F, Chang Y. (2010). Sirtuin 3, a new target of PGC-1alpha, plays an important role in the suppression of ROS and mitochondrial biogenesis. PLoS One. 5, e11707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koubova J, Guarente L. (2003). How does calorie restriction work? . Genes Dev. 17, 313–321 [DOI] [PubMed] [Google Scholar]
- Lagouge M, Argmann C, Gerhart-Hines Z, Meziane H, Lerin C, Daussin F, Messadeq N, Milne J, Lambert P, Elliott P, et al. (2006). Resveratrol improves mitochondrial function and protects against metabolic disease by activating SIRT1 and PGC-1alpha. Cell. 127, 1109–1122 [DOI] [PubMed] [Google Scholar]
- Lamming DW, Latorre-Esteves M, Medvedik O, Wong SN, Tsang FA, Wang C, Lin SJ, Sinclair DA. (2005). HST2 mediates SIR2-independent life-span extension by calorie restriction. Science. 309, 1861–1864 [DOI] [PubMed] [Google Scholar]
- Landry J, Sutton A, Tafrov ST, Heller RC, Stebbins J, Pillus L, Sternglanz R. (2000). The silencing protein SIR2 and its homologs are NAD dependent protein deacetylases. Proc Natl Acad Sci USA. 97, 5807–5811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Xu W, McBurney MW, Longo VD. (2008). SirT1 inhibition reduces IGF-I/IRS-2/Ras/ERK1/2 signaling and protects neurons. Cell Metab. 8, 38–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L. (2000). Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science. 289, 2126–2128 [DOI] [PubMed] [Google Scholar]
- Lin SJ, Ford E, Haigis M, Liszt G, Guarente L. (2004). Calorie restriction extends yeast life span by lowering the level of NADH. Genes Dev. 18, 12–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L. (2001). Negative control of p53 by Sir2α promotes cell survival under stress. Cell. 107, 137–148 [DOI] [PubMed] [Google Scholar]
- Mattagajasingh I, Kim CS, Naqvi A, Yamamori T, Hoffman TA, Jung SB, DeRicco J, Kasuno K, Irani K. (2007). SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc Natl Acad Sci USA. 104, 14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarter RJ, Shimokawa I, Ikeno Y, Higami Y, Hubbard GB, Yu BP, McMahan CA. (1997). Physical activity as a factor in the action of dietary restriction on aging: effects in Fischer 344 rats. Aging (Milano). 9, 73–79 [DOI] [PubMed] [Google Scholar]
- Michishita E, Park JY, Burneskis JM, Barrett JC, Horikawa I. (2005). Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 16, 4623–4635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Berber E, Kioi M, Padilla-Nash H, Damian M, Cheung P, Kusumoto R, Kawahara TL, Barrett JC, et al. (2008). SIRT6 is a histone H3 lysine 9 deacetylase that modulates telomeric chromatin. Nature. 452, 492–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michishita E, McCord RA, Boxer LD, Barber MF, Hong T, Gozani O, Chua KF. (2009). Cell cycle-dependent deacetylation of telomeric histone H3 lysine K56 by human SIRT6. Cell Cycle. 8, 2664–2666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer RK, Johnston JR. (1959). Life span of individual yeast cells. Nature. 183, 1751–1752 [DOI] [PubMed] [Google Scholar]
- Mostoslavsky R, Chua KF, Lombard DB, Pang WW, Fischer MR, Gellon L, Liu P, Mostoslavsky G, Franco S, Murphy MM, et al. (2006). Genomic instability and aginglike phenotype in the absence of mammalian SIRT6. Cell. 124, 315–329 [DOI] [PubMed] [Google Scholar]
- Moynihan KA, Grimm AA, Plueger MM, Bernal-Mizrachi E, Ford E, Cras-Méneur C, Permutt MA, Imai S. (2005). Increased dosage of mammalian Sir2 in pancreatic beta cells enhances glucose-stimulated insulin secretion in mice. Cell Metab. 2, 105–117 [DOI] [PubMed] [Google Scholar]
- Nakagawa T, Lomb DJ, Haigis MC, Guarente L. (2009). SIRT5 deacetylates carbamoyl phosphate synthetase 1 and regulates the urea cycle. Cell. 137, 560–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasrin N, Wu X, Fortier E, Feng Y, Bare’ OC, Chen S, Ren X, Wu Z, Streeper RS, Bordone L. (2010). SIRT4 regulates fatty acid oxidation and mitochondrial gene expression in liver and muscle cells. J Biol Chem. 285, 31995–32002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T. (2004). Nutrient availability regulates SIRT1 through a forkheaddependent pathway. Science. 306, 2105–2108 [DOI] [PubMed] [Google Scholar]
- Nisoli E, Tonello C, Cardile A, Cozzi V, Bracale R, Tedesco L, Falcone S, Valerio A, Cantoni O, Clementi E, et al. (2005). Calorie restriction promotes mitochondrial biogenesis by inducing the expression of eNOS. Science. 310, 314–317 [DOI] [PubMed] [Google Scholar]
- North BJ, Verdin E. (2004). Sirtuins: Sir2-related NAD-dependent protein deacetylases of FOXO transcription factors by the SIRT1 deacetylase. Science. 303, 2011–2015 [DOI] [PubMed] [Google Scholar]
- North BJ, Marshall BL, Borra MT, Denu JM, Verdin E. (2003). The human Sir2 ortholog, SIRT2, is an NAD+-dependent tubulin deacetylase. Mol Cell. 11, 437–444 [DOI] [PubMed] [Google Scholar]
- Oemar BS, Tschudi MR, Godoy N, Brovkovich V, Malinski T, Lüscher TF. (1998). Reduced endothelial nitric oxide synthase expression and production in human atherosclerosis. Circulation. 97, 2494–2498 [DOI] [PubMed] [Google Scholar]
- Onyango P, Celic I, McCaffery JM, Boeke JD, Feinberg AP. (2002). SIRT3, a human SIR2 homologue, is an NAD-dependent deacetylase localized to mitochondria. Proc Natl Acad Sci USA. 99, 13653–13658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacholec M, Bleasdale JE, Chrunyk B, Cunningham D, Flynn D, Garofalo RS, Griffith D, Griffor M, Loulakis P, Pabst B, et al. (2010). SRT1720, SRT2183, SRT1460, and resveratrol are not direct activators of SIRT1. J Biol Chem. 285, 8340–8351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios OM, Carmona JJ, Michan S, Chen KY, Manabe Y, Ward JL, 3rd, Goodyear LJ, Tong Q. (2009). Diet and exercise signals regulate SIRT3 and activate AMPK and PGC-1alpha in skeletal muscle. Aging. 1, 771–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios JA, Herranz D, De Bonis ML, Velasco S, Serrano M, Blasco MA. (2010). SIRT1 contributes to telomere maintenance and augments global homologous recombination. J Cell Biol. 191, 1299–1313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson KJ, Baur JA, Lewis KN, Peshkin L, Price NL, Labinskyy N, Swindell WR, Kamara D, Minor RK, Perez E, et al. (2008). Resveratrol delays age-related deterioration and mimics transcriptional aspects of dietary restriction without extending life span. Cell Metab. 8, 157–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. (2008). Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci USA. 105, 9793–9798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picard F, Kurtev M, Chung N, ToparkNgarm A, Senawong T, de Machado O, Leid M, McBurney MW, Guarente L. (2004). Sirt1 promotes fat mobilization in white adipocytes by repressing PPAR-gamma. Nature. 429, 771–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. (2010). Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 12, 662–667 [DOI] [PubMed] [Google Scholar]
- Revollo JR, Grimm AA, Imai S. (2004). The NAD biosynthesis pathway mediated by nicotinamide phosphoribosyltransferase regulates Sir2 activity in mammalian cells. J Biol Chem. 279, 50754–50763 [DOI] [PubMed] [Google Scholar]
- Rine J, Herskowitz I. (1987). Four genes responsible for a position effect on expression from HML and HMR in Saccharomyces cerevisiae. Genetics. 116, 9–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Puigserver P. (2007). Fasting-dependent glucose and lipid metabolic response through hepatic sirtuin 1. Proc Natl Acad Sci USA. 104, 12861–12866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P. (2005). Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature. 434, 113–118 [DOI] [PubMed] [Google Scholar]
- Rogina B, Helfand SL. (2004). Sir2 mediates longevity in the fly through a pathway related to calorie restriction. Proc Natl Acad Sci USA. 101, 15998–16003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothgiesser KM, Erener S, Waibel S, Lüscher B, Hottiger MO. (2010). SIRT2 regulates NF-κB dependent gene expression through deacetylation of p65 Lys310. J Cell Sci. 123, 4251–4258 [DOI] [PubMed] [Google Scholar]
- Sasaki T, Kitamura T. (2010). Roles of FoxO1 and Sirt1 in the central regulation of food intake. Endocr J. 57, 939–946 [DOI] [PubMed] [Google Scholar]
- Sauve AA, Moir RD, Schramm VL, Willis IM. (2005). Chemical activation of Sir2-dependent silencing by relief of nicotinamide inhibition. Mol Cell. 17, 595–601 [DOI] [PubMed] [Google Scholar]
- Schwer B, North BJ, Frye RA, Ott M, Verdin E. (2002). The human silent information regulator (Sir)2 homologue hSIRT3 is a mitochondrial nicotinamide adenine dinucleotide-dependent deacetylase. J Cell Biol. 158, 647–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Wang F, Stieren E, Tong Q. (2005). SIRT3, a mitochondrial sirtuin deacetylase, regulates mitochondrial function and thermogenesis in brown adipocytes. J Biol Chem. 280, 13560–13567 [DOI] [PubMed] [Google Scholar]
- Sinclair DA, Guarente L. (1997). Extrachromosomal rDNA circles--a cause of aging in yeast. Cell. 91, 1033–1042 [DOI] [PubMed] [Google Scholar]
- Sohal RS, Weindruch R. (1996). Oxidative stress, caloric restriction, and aging. Science. 273, 59–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Someya S, Yu W, Hallows WC, Xu J, Vann JM, Leeuwenburgh C, Tanokura M, Denu JM, Prolla TA. (2010). Sirt3 mediates reduction of oxidative damage and prevention of age-related hearing loss under caloric restriction. Cell. 143, 802–812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starai VJ, Celic I, Cole RN, Boeke JD, Escalante-Semerena JC. (2002). Sir2-dependent activation of acetyl-CoA synthetase by deacetylation of active lysine. Science. 298, 2390–2392 [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Gupta M, Kim G, Rajamohan SB, Isbatan A, Gupta MP. (2009). Sirt3 blocks the cardiac hypertrophic response by augmenting Foxo3a-dependent antioxidant defense mechanisms in mice. J Clin Invest. 119, 2758–2771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanner KG, Landry J, Sternglanz R, Denu JM. (2000). Silent information regulator 2 family of NAD-dependent histone/ protein deacetylases generates a unique product, 1-Oacetyl-ADP-ribose. Proc Natl Acad Sci USA. 97, 14178–14182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Horst A, Tertoolen LG, de Vries-Smits LM, Frye RA, Medema RH, Burgering BM. (2004). FOXO4 is acetylated upon peroxide stress and deacetylated by the longevity protein hSir2 (SIRT1). J Biol Chem. 279, 28873–28879 [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA. (2001). hSIR2 (SIRT1) functions as an NAD-dependent p53 deacetylase. Cell. 107, 149–159 [DOI] [PubMed] [Google Scholar]
- Wang F, Tong Q. (2009). SIRT2 suppresses adipocyte differentiation by deacetylating FOXO1 and enhancing FOXO1’s repressive interaction with PPARgamma. Mol Biol Cell. 20, 801–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Zhang RY, Song J, Guan YF, Xu TY, Du H, Viollet B, Miao CY. (2012). Loss of AMP-activated protein kinase-α2 impairs the insulin-sensitizing effect of calorie restriction in skeletal muscle. Diabetes. 61, 1051–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weed JL, Lane MA, Roth GS, Speer DL, Ingram DK. (1997). Activity measures in rhesus monkeys on long-term calorie restriction. Physiol Behav. 62, 97–103 [DOI] [PubMed] [Google Scholar]
- Weindruch R, Naylor PH, Goldstein AL, Walford RL. (1988). Influences of aging and dietary restriction on serum thymosin alpha 1 levels in mice. J Gerontol. 43, B40–B42 [DOI] [PubMed] [Google Scholar]
- Wood JG, Rogina B, Lavu S, Howitz K, Helfand SL, Tatar M, Sinclair D. (2004). Sirtuin activators mimic caloricrestriction and delay ageing in metazoans. Nature. 430, 686–689 [DOI] [PubMed] [Google Scholar]
- Yang B, Zwaans BM, Eckersdorff M, Lombard DB. (2009). The sirtuin SIRT6 deacetylates H3 K56Ac in vivo to promote genomic stability. Cell Cycle. 8, 2662–2663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeung F, Hoberg JE, Ramsey CS, Keller MD, Jones DR, Frye RA, Mayo MW. (2004). Modulation of NF-kappaB-dependent transcription and cell survival by the SIRT1 deacetylase. EMBO J. 23, 2369–2380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Dittenhafer-Reed KE, Denu JM. (2012). SIRT3 protein deacetylates isocitrate dehydrogenase 2 (IDH2) and regulates mitochondrial redox status. J Biol Chem. 287, 14078–14086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L, D’Urso A, Toiber D, Sebastian C, Henry RE, Vadysirisack DD, Guimaraes A, Marinelli B, Wikstrom JD, Nir T, et al. (2010). The histone deacetylase Sirt6 regulates glucose homeostasis via Hif1α. Cell. 140, 280–293 [DOI] [PMC free article] [PubMed] [Google Scholar]