Abstract
Ginseng, the root of Panax ginseng C.A. Meyer, is used as a general tonic. Recently, we isolated a novel ginseng-derived lysophosphatidic acid (LPA) receptor ligand, gintonin. Gintonin activates G protein-coupled LPA receptors with high affinity in cells endogenously expressing LPA receptors, e.g., Xenopus oocytes. P2X receptors are ligand-gated ion channels activated by extracellular ATP, and 7 receptor subtypes (P2X1–P2X7) have been identified. Most of the P2X1 receptors are expressed in the smooth muscles of genitourinary organs involved in reproduction. A main characteristic of the P2X1 receptor is rapid desensitization after repeated ATP treatment of cells or tissues expressing P2X1 receptors. In the present study, we examined the effect of gintonin on P2X1 receptor channel activity. P2X1 receptors were heterologously expressed in Xenopus oocytes. ATP treatment of oocytes expressing P2X1 receptors induced large inward currents (IATP), but repetitive ATP treatments induced a rapid desensitization of IATP. Gintonin treatment after P2X1 receptor desensitization potentiated IATP in a concentration-dependent manner. We further examined the signaling transduction pathways involved in gintonin-mediated potentiation of IATP. Gintonin-mediated IATP potentiation was blocked by Ki16425, an LPA1/3 receptor antagonist, a PKC inhibitor, a PLC inhibitor, and a PI4-Kinase inhibitor but not by a calcium chelator. In addition, mutations of the phosphoinositide binding site of the P2X1 receptor greatly attenuated the gintonin-mediated IATP potentiation. These results indicate that G protein-coupled LPA receptor activation by gintonin is coupled to the potentiation of the desensitized P2X1 receptor through a phosphoinositide-dependent pathway.
Keywords: gintonin, LPA receptor, P2X1 receptor, P2X1 receptor potentiation
INTRODUCTION
Ginseng, the root of Panax ginseng C.A. Meyer, is a general tonic consumed throughout the world. Ginseng shows multiple physiological and pharmacological effects (Nah et al., 1997). For example, ginseng affects male reproductive systems, including spermatogenesis, sperm motility, and sperm survival (Hwang et al., 2004; Jang et al., 2011; Park et al., 2006). However, the active ingredients and the underlying molecular mechanism by which ginseng exerts its diverse effects are not fully understood. Recently, we isolated a novel ligand of the ginseng-derived G protein-coupled lysophosphatidic acid (LPA) receptor, gintonin (Hwang et al., 2012; Pyo et al., 2011). We demonstrated that gintonin activates LPA receptors with high affinities in cells expressing LPA receptors endogenously or heterologously (Hwang et al., 2012). Activation of LPA receptors by gintonin affects cell survival, proliferation, migration, and morphological changes in neuronal and non-neuronal cells; LPA receptors are linked to multiple G proteins such as Gαi/o, Gα12/13, and Gαq/11 (Hwang et al., 2012). LPA receptor activation by gintonin is also coupled to diverse downstream events, including stimulation of phospholipase C, mitogen-activated protein kinases, and phosphoinositide 4-kinase (PI4-kinase) (Hwang et al., 2012). However, it is unknown whether the activation of G protein-coupled LPA receptors by gintonin regulates P2X1 receptor channel activity.
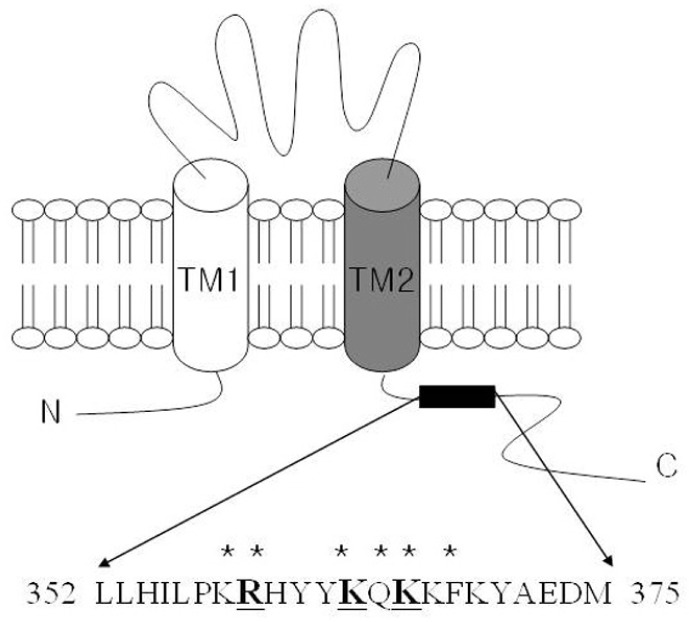
P2X receptors are ligand-gated cation channels activated by extracellular ATP (Burnstock, 1997; Valera et al., 1994). Seven P2X receptor subtypes, P2X1 to P2X7, have been identified (North et al., 2002). The P2X subtypes assemble to form either homo- or hetero-trimeric channels, leading to a great variety of phenotypes. Each P2X subunit has 2 putative membrane-spanning segments, TM1 and TM2 (Fig. 1A)-hydrophobic regions that cross the plasma membrane-and a large extracellular loop as well as intracellular N- and C-termini (Javis et al., 2009; North et al., 1996; Surprenant et al., 1995). P2X receptors are associated with diverse physiology and pathophysiology such as immune response, neuropathic pain by sensory transduction of the central nervous system, and control of smooth muscle contraction in genitourinary systems (Burnstock et al., 2007; Khakh and North, 2006). P2X1 receptors are expressed in organs containing smooth muscle cells, such as the urinary bladder, vas deferens, and other genitourinary systems, where the receptors are involved in the regulation of smooth muscle contraction (Mulryan et al., 2000; North et al., 2002). In particular, mice lacking P2X1 receptors exhibit a reduced vas deferens smooth muscle contraction and subsequent infertility, indicating that the P2X1 receptor plays an important role in male fertility (Mulryan et al., 2000). Another characteristic of the P2X1 receptor channel activity is fast desensitization, resulting in a decreased response to repeated agonist application (North et al., 2002).
Fig. 1.
Schematic of the transmembrane regions and the basic residues of the C-terminal domain of the P2X1 receptor. P2X receptors likely possess 2 transmembrane segments (TM1 and TM2) and a long extracellular loop. The N- and C-termini comprise a cytosolic domain. The proximal C-terminal region after the TM2 segment of the P2X1 receptor includes the putative phosphoinositide binding site. Amino acid residues mutated in the present study are underlined.
Xenopus oocytes express endogenous LPA1 receptors (Kimura et al., 2001). In the present study, we examined whether activation of the endogenous G protein-coupled LPA receptor by gintonin in Xenopus oocytes affects P2X1 receptor channel activity. P2X1 receptors were heterologously expressed in Xenopus oocytes. We observed that when P2X1 receptors were stimulated by repeated treatments with ATP, the ATP-mediated inward current (IATP) dramatically decreased compared with the initial IATP. Interestingly, gintonin treatment, after induction of desensitization by repetitive application of ATP, greatly potentiated IATP. The potentiating effect of gintonin on IATP observed after desensitization was blocked by the protein-kinase C, PI4-kinase, and phospholipase C signaling pathway but not the Ca2+ signaling pathway. Site-directed mutagenesis of the phosphoinositide (PIP2)-binding site of the P2X1 receptor also attenuated the gintonin-mediated IATP potentiation. We discuss the signaling transduction pathways and the role of LPA receptors in the genitourinary systems involved in the gintonin-mediated potentiation of IATP after P2X1 receptor desensitization. LPA receptor activation by gintonin is coupled to the regulation of P2X1 receptor channel activity, and gintonin-mediated IATP potentiation might be the molecular basis of the beneficial effects of ginseng in the genitourinary systems.
MATERIALS AND METHODS
Materials
Gintonin devoid of ginseng saponins was prepared from Panax ginseng according to method of Pyo et al. (2011). Gintonin used in this study was dissolved in dimethyl sulfoxide (DMSO) and then diluted with bath medium before use. The final DMSO concentration was less than 0.01%. The cDNA of the human P2X1 receptor (GenBank accession no. NM_002558.2) was purchased from Missouri S&T cDNA Resource Center (USA). The phospholipid diC8-PI(4,5)P2 was purchased from Echelon Biosciences Inc., (USA). All other agents were purchased from Sigma-Aldrich (USA).
Preparation of Xenopus oocytes
Xenopus laevis frogs were purchased from Xenopus I (USA). The care and handling of the frogs were in accordance with institutional guidelines. The frogs underwent surgery twice, and the 2 surgeries were separated by at least 3 weeks. For isolation of oocytes, frogs were anesthetized with an aerated solution of 3-amino benzoic acid ethyl ester, and the ovarian follicles were removed. Oocytes were separated by treatment with collagenase by gentle shaking for 2 h in CaCl2-free medium containing 82.5 mM NaCl, 2 mM KCl, 1 mM MgCl2, 5 mM N-(2-hydroxyethyl)piperazine-N′-2 ethanesulfonic acid (HEPES), 2.5 mM sodium pyruvate, 100 units/ml penicillin, and 100 μg/ml streptomycin. Stage V–VI oocytes were collected and stored in ND96 medium (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, 1.8 mM CaCl2, and 5 mM HEPES, pH 7.5) supplemented with 0.5 mM theophylline and 50 μg/ml gentamycin. The oocyte-containing solution was maintained at 18°C with gentle continuous shaking, and the supplemented MD96 medium was replaced daily. All electrophysiological experiments were performed within 5 to 6 days following isolation of the oocytes, with chemicals applied to the bath.
cRNA preparation of P2X1 receptor and microinjection
A recombinant plasmid (from the Missouri S&T cDNA Resource Center), containing a human P2X1 receptor cDNA insert, was linearized by digestion with the appropriate restriction enzymes. The cRNAs were transcribed from linearized templates with an in vitro transcription kit (mMessage mMachine, Ambion, USA) using T7 polymerase. The cRNA was dissolved in RNase-free water at a final concentration of approximately 1 μg/μl, aliquoted, and stored at −70°C until used. P2X1 receptor cRNA (40 nl) was injected into the animal or vegetal pole of each oocyte using a 10 μl VWR microdispenser (VWR Scientific, USA). The injection pipette was pulled from glass capillary tubing and used for recording electrodes; the tip had a diameter of 15–20 μm.
Site-directed mutagenesis of the P2X1 receptor, and in vitro transcription of P2X1 receptor cDNA
Single or double amino acid substitutions were prepared using the QuikChange XL Site-Directed Mutagenesis Kit (Stratagene, USA) along with Pyrococcus furiosus DNA polymerase and sense and antisense primers encoding the desired mutations. Overlap extension of the target domain by sequential polymerase chain reaction (PCR) was conducted according to the manufacturer’s protocol. The final PCR products were transformed into Escherichia coil strain DH5α, screened by PCR, and confirmed by sequencing of the target regions. The mutant DNA constructs were linearized at the 3′ ends by digestion with Sma I, and run-off transcripts were prepared using the methylated cap analog, m7 G(5′)ppp(5′)G. The cRNAs were prepared using the mMessage mMachine transcription kit (Ambion) with T7 RNA polymerase. The absence of degraded RNA was confirmed by denaturing agarose gel electrophoresis followed by ethidium bromide staining. Similarly, recombinant plasmids containing P2X1 receptor cDNA inserts were linearized by digestion with the appropriate restriction enzymes, and cRNAs were obtained using the mMessage mMachine in vitro transcription kit with T7 polymerase. The final cRNA products were resuspended at a concentration of 1 μg/μl in RNase-free water and stored at −80°C.
Data recording
A custom-made Plexiglas net chamber was used for the 2-electrode voltage-clamp recordings. The chamber was constructed by milling of 2 concentric wells to the chamber bottom (diameter/height: upper well: 8/3 mm, lower well: 6/5 mm) and by gluing plastic meshes (ca. 0.4 mm grid diameter) to the bottom of the upper well. A perfusion inlet (ca. 1 mm in diameter) was added in the wall of the lower well, and a suction tube was placed on the edge of the upper well. The oocyte was then placed on the net separating the upper and lower wells; the grids of the net served as dimples to keep the oocyte in place during the electrophysiological recording. The oocytes were impaled with 2 microelectrodes filled with 3 M KCl (0.2–0.7 MΩ). The recordings were performed at a flow rate of 2 ml/min with Ca2+-free ND96 solution (96 mM NaCl, 2 mM KCl, 1 mM MgCl2, and 5 mM HEPES, pH 7.5). ND96 solution without CaCl2 was used to exclude any contributions of Ca2+-activated Cl− currents by gintonin. The electrophysiological experiments were performed at room temperature with an Oocyte Clamp (OC-725C, Warner Instrument, USA), and the stimulation and data acquisition were controlled with a pClamp 8 (Molecular Devices, USA). For most of the electrophysiological ex-periments, the oocytes were clamped at a holding potential of −70 mV, and 500 ms voltage steps were applied from −100 to +50 mV to assess the current-voltage relationship. In the indicated experiments, stimulation with 1 μM ATP (Sigma-Aldrich), dissolved in Ca2+-free ND96 solution, was performed 5 times with 5 min intervals. Because the ATP-induced P2X1 responses were stable after the second application of ATP, 3 μg/ml gintonin was applied for 30 s between the third and fourth ATP applications. Normalization of potentiation of the P2X1 receptor was defined as the ratio of the fourth over the third IATP performed by calculating the ratio of the third and fourth IATP. The phospholipid diC8-PI(4,5)P2 was injected (20 nl; 10 mM) in the cytoplasm of oocytes 30 min before recording. For all the calculations of final diC8-PI(4,5)P2 concentration, estimated the oocyte cell volume at 1 μl (Bernier et al., 2008).
Data analysis
To obtain concentration-response curves of the effects of gintonin on the ATP-mediated inward currents, the peak amplitudes at different concentrations of gintonin were plotted, and the Origin software (OriginLab, USA) was used to fit the data to the Hill equation: I/Imax = [A]nH/([A]nH + [EC50]nH), where I is the peak current at a given concentration of ATP, Imax is the maximal peak current, EC50 is the concentration of gintonin producing a half-maximal effect, [A] is the concentration of gintonin, and nH is the Hill coefficient. All values are presented as the mean ± S.E.M. The significance of differences between control and treatment values was determined using Student’s t-test. Values of p < 0.05 were considered statistically significant.
RESULTS
Human P2X1 receptors desensitize rapidly, and gintonin potentiates P2X1 receptor channel activity
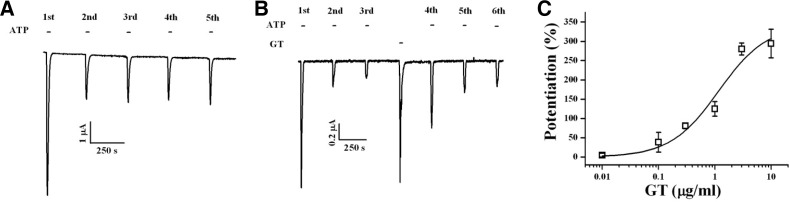
We examined the effects of gintonin on P2X1 receptor channel activity. Thus, we first expressed the human P2X1 receptor subunits in Xenopus oocytes (North et al., 2002). As shown in Fig. 2A, the addition of ATP (100 μM) to the bathing solution induced a large inward current (IATP) in oocytes injected with human P2X1 receptor subunit cRNAs at a holding potential of −70 mV, as reported previously (North et al., 2002; Rettinger and Schmalzing, 2003). When ATP was re-applied after 5 min, IATP was reduced by approximately 50–80%, corresponding to a rapid desensitization of the P2X1 receptor. Subsequent applications of ATP at 5 min intervals produced reproducible but desensitized responses (Fig. 2A). In H2O-injected control oocytes, the application of ATP did not induce inward currents (data not shown). Next, we examined whether activation of endogenous LPA receptors by gintonin in Xenopus oocytes affected P2X1 receptor-mediated channel activity. Gintonin was applied between the third and fourth ATP applications. Gintonin elicited an endogenous Ca2+-activated Cl− channel (CaCC) current (Pyo et al., 2011). Gintonin also significantly potentiated the amplitude of subsequent IATP responses by 2–3-fold, as compared with the third IATP (Fig. 2B). The potentiating effect of gintonin on IATP was concentration dependent, with saturation observed at 3 μg/ml, and the EC50 value was 0.37 ± 0.19 μg/ml (Fig. 2C).
Fig. 2.
Concentration effect of gintonin (GT) on P2X1 receptor channel activity. (A) Representative ATP-evoked IATP in Xenopus oocytes expressing human P2X1 receptors (1 μM ATP application at 5 min intervals). (B) Representative trace demonstrating that gintonin (3 μg/ml) activates endogenous CaCC and potentiates P2X1 receptor channel activity by ATP. (C) The stimulatory effects of gintonin on IATP are concentration dependent. Gintonin was added between the third and fourth ATP applications. The percentage potentiation of IATP by gintonin is defined as the ratio of the fourth IATP to the third IATP amplitude measured after gintonin stimulation. Each point represents the mean ± SEM (n = 5–7).
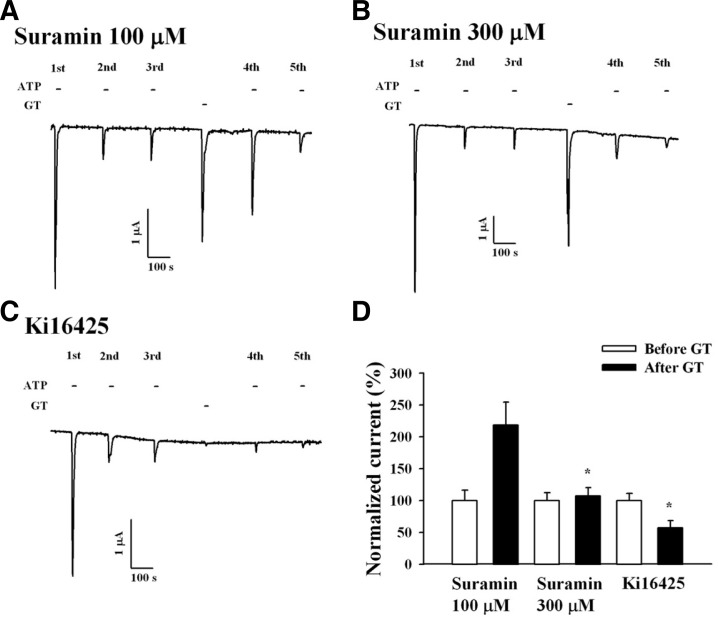
Suramin is a broad-spectrum antagonist of the P2X receptor, and human P2X1 receptors are effectively blocked by suramin (Sim et al., 2008). We examined the effect of suramin to determine whether gintonin-mediated IATP potentiation is achieved through P2X receptor activation. Co-application of gintonin (3 μg/ml) and suramin had no effect on the gintonin-induced CaCC current at a suramin concentration of 100 and 300 μM. Thus, although no effect was observed on the gintonin-induced CaCC current, suramin (300 μM) abolished the gintonin-mediated potentiation of IATP (Figs. 3A, 3B, and 3D). These results indicate that the gintonin-mediated potentiation of IATP was achieved through P2X1 receptor activation.
Fig. 3.
P2X and LPA receptor antagonists block gintonin-mediated IATP potentiation of human P2X1 receptor channel activity. (A, B) Representative trace showing 2 different concentrations of suramin, a P2X receptor antagonist, on the gintonin-mediated IATP potentiation. (C) Representative trace showing the gintonin-mediated IATP potentiation in the absence or presence of Ki16425, an LPA1/3 receptor antagonist, in Xenopus oocytes expressing human P2X1 receptors. (D) Histograms summarizing the effects of suramin and Ki16425 on the gintonin-mediated IATP potentiation (*p < 0.001, compared to the absence of suramin). Data represent the mean ± SEM (n = 4–5/group).
Potentiation of P2X1 receptor channel activity by gintonin involves the LPA receptor-PLC-PKC pathway but not the Ca2+ pathway
Xenopus oocytes express endogenous LPA1 receptors (Kimura et al., 2001). We demonstrated that gintonin induces [Ca2+]i transients and enhances CaCC currents via LPA receptor activation (Hwang et al., 2012). We examined the effect of the LPA1/3 receptor antagonist, Ki16425, on the gintonin-mediated IATP potentiation. In the absence of Ki16425, gintonin treatment enhanced IATP. However, treatment of Ki16425 (10 μM) not only abolished gintonin-mediated CaCC activation, but also attenuated gintonin-mediated IATP potentiation. This result indicates that the gintonin-mediated potentiation of IATP was achieved through the activation of endogenously expressed LPA1 receptors in Xenopus oocytes (Figs. 3C and 3D).
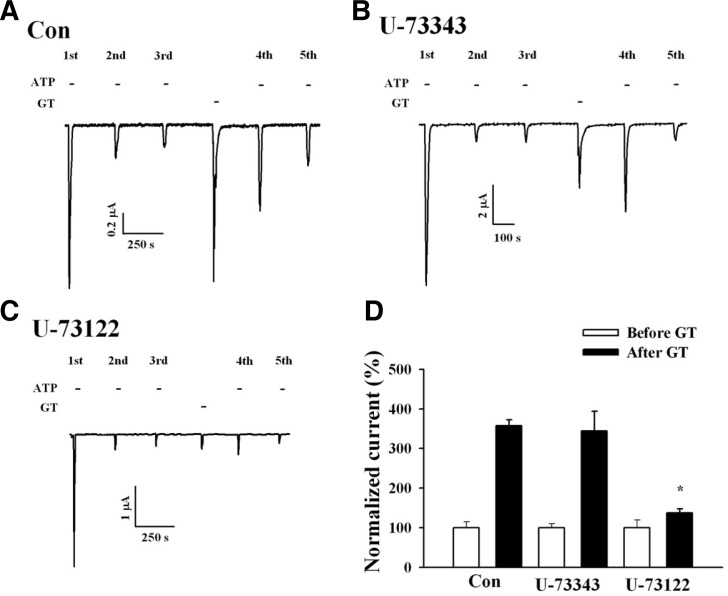
We next examined the signaling pathways involved in the gintonin-mediated IATP potentiation. Application of an inactive PLC inhibitor, U-73343, had no effect on gintonin action (Fig. 4B), whereas application of an active PLC inhibitor, U-73122, nearly abolished the gintonin-mediated P2X1 receptor potentiation (Figs. 4C and 4D). These results indicate that gintonin-mediated potentiation of IATP involves PLC activation.
Fig. 4.
Active PLC inhibitor U-73122 blocking the gintonin action on P2X1 receptor desensitization. (A) Representative trace showing the gintonin-mediated IATP potentiation in Xenopus oocytes expressing human P2X1 receptors. (B, C) Representative trace showing the effects of active (U73122) and inactive (U73343) PLC inhibitors (each 1 μM) on the gintonin-mediated IATP potentiation. The active PLC inhibitor (1 μM) blocks both gintonin-evoked CaCC and IATP potentiation. (D) Histograms summarizing the effects of the active or inactive PLC inhibitors on gintoninmediated IATP potentiation (*p < 0.001, compared to control). Data represent the mean ± SEM (n = 5/group).
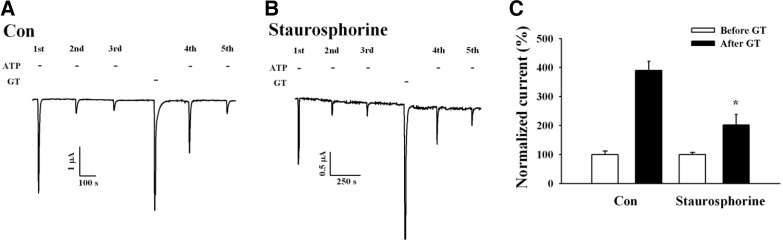
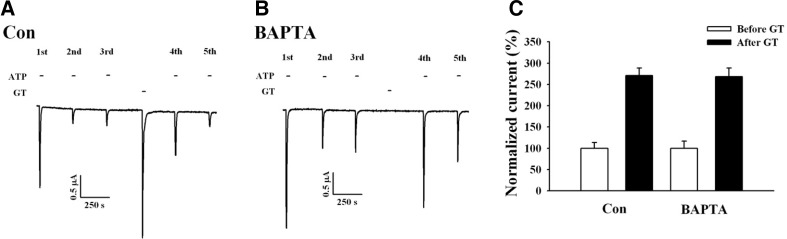
Because the above-described results suggest that gintonin-mediated IATP potentiation may involve PKC activation, we next examined the effects of the PKC inhibitor, staurosporine, on gintonin-mediated potentiation of IATP. Pre-incubation with 1 μM staurosporine for 1 h attenuated the gintonin-mediated IATP potentiation by 75% (Fig. 5). These results indicate that gintonin-mediated IATP potentiation after P2X1 receptor desensitization is achieved through PKC activation. Because gintonin-mediated IATP potentiation after P2X1 receptor desensitization was observed after the CaCC current enhancements (induced after the mobilization of intracellular Ca2+), we examined whether gintonin-mediated IATP potentiation also requires transient [Ca2+]i mobilization (Fig. 2), using the membrane-permeable calcium chelator, BAPTA-AM [1,2-bis(O-aminophenoxy)ethane-N,N,N’, N’-tetraacetic acid]. Oocytes expressing human P2X1 receptors were first pre-incubated with 100 μM BAPTA-AM for 3 h, and the gintonin-mediated IATP potentiation was then examined. Interestingly, although BAPTA-AM treatment abolished the gintonin-mediated CaCC activation (Fig. 6B), BAPTA-AM treatment had no effect on the gintonin-mediated IATP potentiation Fig. 6C). These results indicate that the gintonin-mediated IATP potentiation is independent of the intracellular calcium level.
Fig. 5.
Effects of PKC inhibitor on gintonin-mediated IATP potentiation of the human P2X1 receptor. (A) Representative trace showing the prevention of gintonin-mediated P2X1 receptor desensitization in Xenopus oocytes expressed the P2X1 receptor. (B) Representative traces showing that the prevention of P2X1 receptor desensitization by gintonin is affected by the PKC inhibitor, staurosporine. (C) Histograms summarizing the effects of PKC inhibitor on gintonin-mediated IATP potentiation (*p < 0.005, compared to control). Data represent the mean ± SEM (n = 4–5/group).
Fig. 6.
Gintonin-mediated IATP potentiation does not involve intracellular calcium. (A) Representative trace showing the gintonin-mediated IATP potentiation in Xenopus oocytes expressing human P2X1 receptors. (B) Representative trace showing that although the treatment with the calcium chelator BAPTA-AM (100 μM) abolished gintonin-mediated CaCC activation, BAPTA-MA had no effect on IATP potentiation by gintonin. (C) Histograms summarizing the gintonin-mediated IATP potentiation in the absence or presence of BAPTA-AM. Data represent the mean ± SEM (n = 4–5/group).
Effects of wortmannin, an inhibitor of PI4-kinase, phosphatidylinositol 4,5-bisphosphate (PIP2), or mutations of the phosphoinositide binding sites on gintonin-mediated IATP potentiation
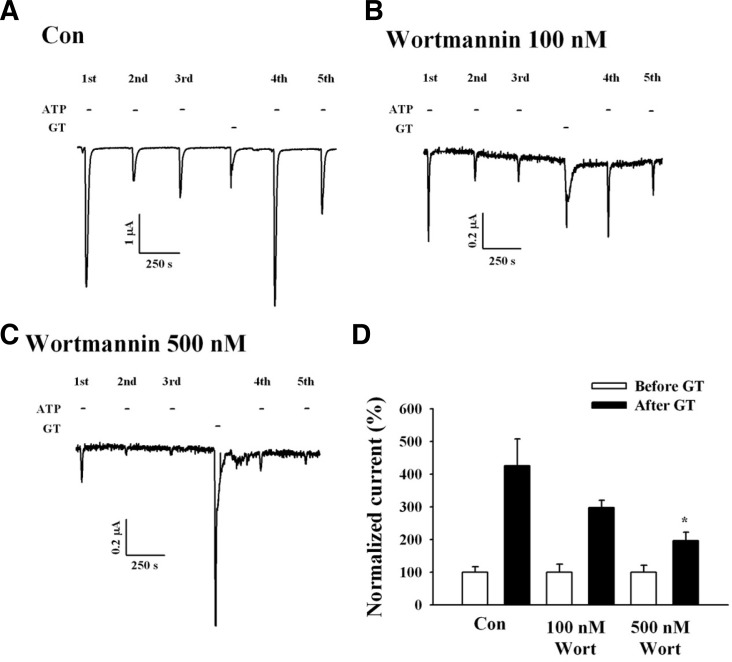
Phosphoinositides are membrane lipids involved in many cellular signaling processes. Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2 or PIP2) is abundant phosphoinositide in the cell membrane and regulates numerous ion channels and transporters (Suh et al, 2005; Zhao et al., 2007b). To study the relationship between gintonin-mediated IATP potentiation and the role of phosphoinositides, we used wortmannin. Wortmannin inhibits phosphoinositide phosphatidylinositol 4-kinase (PI4-kinase) (Balla et al., 1998). Wortmannin also attenuates PI(4,5)P2 synthesis (Zhao et al., 2007a). Oocytes expressing P2X1 receptors were pre-incubated with 100 or 500 nM wortmannin for 2 h. In oocytes pre-incubated with 100 nM wortmannin, the gintonin-mediated IATP potentiation decreased by 30% (Figs. 7B and 7D), and in oocytes pre-incubated with 500 nM wortmannin, the potentiation decreased by 55% (Figs. 7C and 7D). Interestingly, we observed that the presence of wortmannin itself diminished IATP compared with the control. These results indicate that gintonin-mediated IATP potentiation is achieved through the activation of PI4-kinase.
Fig. 7.
Wortmannin, a PI4-kinase inhibitor, attenuates gintonin-mediated IATP potentiation. (A) Representative trace showing the gintoninmediated IATP potentiation in Xenopus oocytes expressing human P2X1 receptors. (B, C) Traces showing that wortmannin (500 but not 100 nM) attenuates gintonin-mediated IATP potentiation. (D) Histograms summarizing the gintoninmediated IATP potentiation in the absence or presence of wortmannin (WT). (*p < 0.05, compared to control). Data represent the mean ± SEM (n = 6–7/group).
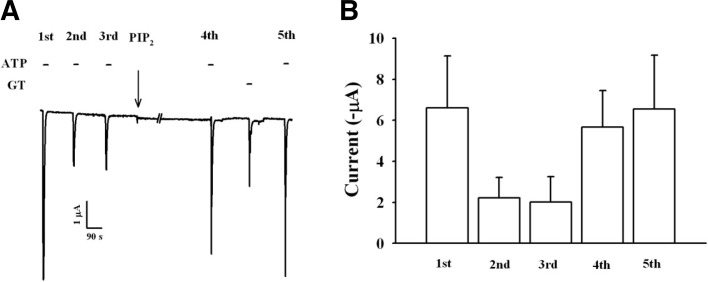
To confirm the involvement of PIP2 in gintonin-mediated IATP potentiation, we injected PIP2 into oocytes and examined gintonin-mediated IATP potentiation. As shown in Fig. 8, although PIP2 injection rescued IATP from desensitization induced by repeated treatment, treatment of gintonin did not further potentiate IATP in oocytes injected with PIP2. These results indicate that gintonin-mediated IATP potentiation might be achieved via the stimulation of PIP2 production.
Fig. 8.
Effects of PIP2 injected into oocytes on gintonin-mediated IATP potentiation. (A) Representative trace showing the gintonin-mediated IATP potentiation in oocytes injected with PIP2. PIP2 was injected into oocytes 30 min before 4th IATP recording. Treatment of gintonin to ooytes injected with PIP2 did not induce potentiation of IATP, although PIP2 rescued of IATP from desensitization. (B) Histograms summarizing the gintonin-mediated IATP potentiation before or after PIP2 injection. Data represent the mean ± SEM (n = 5/group).
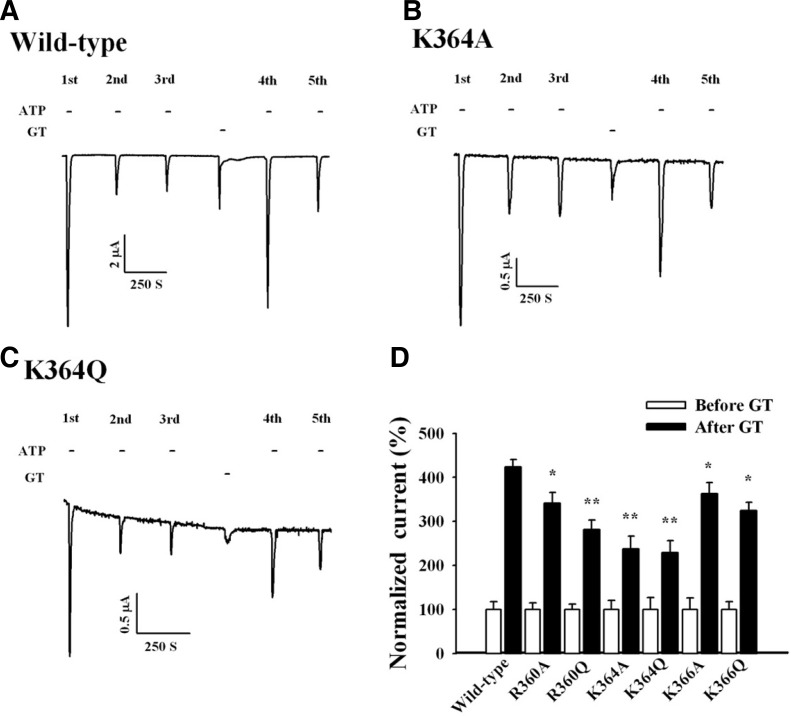
Interactions between PIP2 and ion channels are mainly electrostatic, and anionic PIP2 sites interact with a cluster of positively charged amino acid residues on the ion channels (Lopes et al., 2002). In several families of ion channels that are regulated by PIP2, the region proximal to the cytoplasmic C-terminal domain contains basic residues that interact with PIP2 (Fujiwara and Kubo, 2006). Previous reports showed that P2X1 receptors have phosphoinositide binding sites, and site-directed mutations of PIP2 binding sites attenuate IATP potentiation by Gαq/11-mediated receptor activation (Bernier et al., 2008). To further investigate the direct interaction between P2X1 receptors and phosphoinositides, we constructed several P2X1 receptor mutants that have been reported to disrupt the interaction with phosphoinositides (Bernier et al., 2008). We selected R360, K364, and K366 in the C-terminal domain of the P2X1 receptor and mutated each of these basic residues, lysine (K) and arginine (R), to glutamine (Q) or alanine (A). We then examined the effects of each mutant receptor on the gintonin-mediated IATP potentiation. The K364A and K364Q mutant receptors significantly attenuated the IATP potentiation by gintonin (Figs. 9B and 9C). In addition, the R360Q mutant receptor caused a slight attenuation of gintonin-mediated IATP potentiation (Fig. 9D). These results again indicate that gintonin induces the production of phosphoinositides via PI4-kinase activation, and the produced phosphoinositides might interact with lysine residues in the C-terminal domain of the P2X1 receptor, further potentiating IATP.
Fig. 9.
Effects of site-directed mutations of the C-terminal domain of the human P2X1 receptor on gintonin-mediated IATP. potentiation. (A) Representative trace showing the gintonin-mediated IATP potentiation in Xenopus oocytes expressing human P2X1 receptors. (B, C) Representative traces showing that mutations of the basic amino acid residue K364 in the C-terminal domain to alanine (A) or glutamine (Q) significantly attenuated the gintonin-mediated IATP. potentiation. (D) Histograms summarizing the gintonin-mediated IATP potentiation in wild-type and various mutant P2X1 receptors (*p < 0.05, **p < 0.005, compared to control). Data represent the mean ± SEM (n = 6–7/group).
DISCUSSION
P2X receptors are ligand-gated cation channels activated by extracellular ATP (Burnstock et al., 1997). P2X receptors are widely distributed in the brain, peripheral nervous system, smooth muscles, and blood cells and have physiological and pathophysiological roles (Jarvis et al., 2009; North et al., 2002). P2X1 receptors are expressed in smooth muscle cells, e.g., in the urinary bladder, vas deferens, and genitourinary systems, regulating smooth muscle contraction in rodents (Mulryan et al., 2000) and humans (Banks et al., 2006). A main characteristic of the P2X1 receptor is rapid desensitization after repeated treatment with receptor agonists (North et al., 2002).
In the present sturdy, we found that gintonin, a novel ginseng-derived LPA receptor ligand, potentiates P2X1 receptor channel activity. We further investigated how activation of G protein-coupled LPA receptor by gintonin is coupled to potentiation of ligand-gated P2X1 receptor channel activity. Three key observations indicate that gintonin regulates P2X1 receptor channel activity via phosphoinositides. First, gintonin-mediated IATP potentiation was achieved through PLC and PKC activation. Second, gintonin-mediated IATP potentiation was independent of the intracellular Ca2+ level. Third, gintonin-mediated IATP potentiation was blocked by a PI4-kinase inhibitor, and mutations of the phosphoinositide binding site of the P2X1 receptor significantly attenuated the action of gintonin on the P2X1 receptor channel activity. These results indicate that activation of the LPA receptor-signaling pathway by gintonin causes PI4-kinase activation and the production of PIP2. The produced PIP2 might interact with the P2X1 receptor C-terminal domain and hence potentiate IATP even after P2X1 receptor desensitization by ATP.
We previously demonstrated that gintonin evoked a [Ca2+]i transient and activated endogenous CaCC in Xenopus oocytes via LPA receptor activation (Hwang et al., 2012; Pyo et al., 2011). Thus, the main action of gintonin is to induce a transient elevation of Ca2+ from the endoplasmic reticulum, and the released Ca2+ can subsequently influence diverse Ca2+-dependent ion channel and receptor activities (Hwang et al., 2012; Pyo et al., 2011). However, in the present study, although we observed that gintonin-mediated Ca2+ release is coupled to endogenous CaCC activation, we found that gintonin-mediated Ca2+ release did not mediate IATP potentiation because the membrane-permeable Ca2+ chelator, BAPTA-AM, had no effect on the gintonin-mediated IATP potentiation. Thus, the present study showed that LPA receptor activation by gintonin might be coupled to the production of PIP2, because wortmannin, a PI4-kinase inhibitor, attenuated the gintonin-mediated IATP potentiation. Supporting this notion is that direct injection of PIP2 into oocytes expressing P2X1 receptor also caused a rescue of IATP from desensitization but gintonin did not further potentiate IATP.
In previous reports, the activation of G protein-coupled receptors, such as the 5-HT2A and mGlu receptors coupled to the Gαq/11 signaling pathway, has been shown to potentiate IATP after P2X1 receptor desensitization (Ase et al., 2005; Bernier et al., 2008; Vial et al., 2004). These reports showed that phosphoinositides (and not calcium) are involved in 5-HT2A or mGlu receptor-mediated potentiation of IATP after P2X1 receptor desensitization and further showed that site-directed mutagenesis of the phosphoinositide binding site greatly attenuates the receptor agonist-mediated IATP potentiation of the P2X1 receptor. In the present study, we also showed that the gintonin-mediated IATP potentiation of the P2X1 receptor through LPA receptor activation (coupled to the Gαq/11 signaling pathway) was attenuated in the presence of wortmannin, a PI4-kinase inhibitor. Furthermore, mutations of the phosphoinositide binding site, such as K364A and K364Q, also attenuated the gintoninmediated IATP potentiation. However, we could observe that mutations of phosphoinositide binding sites did not completely abolish gintonin-mediated IATP potentiation. These results are well consistent with previous report that mutations of phosphoinositide binding sites did not completely abolish 5-HT2A receptor-mediated IATP potentiation (Bernier et al., 2008). These results indicate that LPA receptor activation by gintonin utilizes phosphoinositides in addition to Ca2+ as signaling mediators.
As described above, P2X1 receptors are mainly expressed in reproductive organs and are involved in smooth muscle contraction in reproductive organs such as the prostate (Banks et al., 2006; Dunn et al., 2000; Mulryan et al., 2000). The rapid desensitization of the P2X1 receptor is associated with reduced contractive activity (Banks et al., 2007; Burnstock et al., 2007; Sneddon et al., 2000). Thus, P2X1 receptors are essential for reproductive functions, and selective P2X1 receptor antagonists and agonists that potentiate the ATP-action on P2X1 receptors are under development (Dunn et al., 2000). LPA receptors, especially LPA3 receptors, are also abundantly expressed in female and male reproductive organs, e.g., the uterus, and are important during embryo implantation (Diao et al., 2010). Currently, it is unknown whether LPA receptors are co-expressed with P2X1 receptors in the same cells or organs. In the present study, we found that gintonin potentiates IATP via LPA receptor activation after P2X1 receptor desensitization; however, it is unclear whether gintonin-mediated potentiation of IATP is beneficial to smooth muscles in reproductive organs. Further studies are therefore required for clinical evaluation of gintonin for reproductive smooth muscle function in organs such as vas deferens.
In summary, we found that gintonin potentiates IATP after P2X1 receptor desensitization via the LPA receptor-PLC-PKC-PI4-kinase signaling pathways and that mutations of the phosphoinositide binding site of the P2X1 receptor attenuate the effect of gintonin on P2X1 receptor channel activity. These results suggest that gintonin, via LPA receptor activation, stimulates phosphoinositide turnover, and the gintonin-mediated production of phosphoinositides contributes to the potentiation of P2X1 receptor channel activity.
Acknowledgments
This work was supported by the SMART Research Professor Program of Konkuk University.
REFERENCES
- Ase A.R., Raouf R., Bélanger D., Hamel E., Séguéla P. Potentiation of P2X1 ATP-gated currents by 5-hydroxytryptamine 2A receptors involves diacylglycerol-dependent kinases and intracellular calcium. J. Phamacol. Exp. Ther. 2005;315:144–154. doi: 10.1124/jpet.105.089045. [DOI] [PubMed] [Google Scholar]
- Balla T. Phosphatidylionsitol 4-kinases. Biochem. Biophys. Acta. 1998;1436:69–85. doi: 10.1016/s0005-2760(98)00134-9. [DOI] [PubMed] [Google Scholar]
- Banks F.C.L., Knight G.E., Calvert R.C., Thompson C.S., Morgan R.J., Burnstock G. The purinergic component of human vas deferens contraction. Fertil. Steril. 2006;85:932–939. doi: 10.1016/j.fertnstert.2005.09.024. [DOI] [PubMed] [Google Scholar]
- Bernier A.P., Ase A.R., Tong X., Hamel E., Blais D., Zhao Q., Logothetis D.E., Séguéla P. Direct modulation of P2X1 receptor-channels by the lipid phosphatidylinositol 4,5-bisphospate. Mol. Phamacol. 2008;74:785–792. doi: 10.1124/mol.108.047019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. The past, present and future of purine nucleotides as signaling molecules. Neuropharm. Rev. 1997;36:1127–1139. doi: 10.1016/s0028-3908(97)00125-1. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Physiology and pathophysiology of purinergic neurotransmission. Physiol. Rev. 2007;87:659–797. doi: 10.1152/physrev.00043.2006. [DOI] [PubMed] [Google Scholar]
- Choi S., Rho S.H., Jung S.Y., Kim S.C., Park C.S., Nah S.Y. A novel activation of Ca2+-activated Cl− channel in Xenopus oocytes by ginseng saponins: evidence for the involvement of phospholipase C and intracellular Ca2+ mobilization. Br. J. Pharmacol. 2001;132:641–648. doi: 10.1038/sj.bjp.0703856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao H., Xiao S., Zhao F., Ye X. Uterine luminal epithelium-specific proline-rich acidic protein 1 (PRAP1) as a marker for successful embryo implantation. Fertil. Steril. 2010;94:2808–2811. doi: 10.1016/j.fertnstert.2010.06.034. [DOI] [PubMed] [Google Scholar]
- Dunn P.M. Purineric receptors and the male contraceptive pill. Curr. Biol. 2000;10:R305–R307. doi: 10.1016/s0960-9822(00)00436-x. [DOI] [PubMed] [Google Scholar]
- Fujiwara Y., Kubo Y. Regulation of desensitization and ion selectivity of ATP-gated P2X2 channels by phosphoinositides. J. Physiol. 2006;576:135–149. doi: 10.1113/jphysiol.2006.115246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang S.Y., Kim W.J., Wee J.J., Choi J.S., Kim S.K. Panax ginseng improves survival and sperm quality in guinea pigs exposed to 2,3,7,8-tetrachlorodibenzo-p-dioxin. BJU Int. 2004;94:663–668. doi: 10.1111/j.1464-410X.2004.05019.x. [DOI] [PubMed] [Google Scholar]
- Hwang S.H., Shin T.J., Choi S.H., Cho H.J., Lee B.H., Pyo M.K., Lee J.H., Kang J., Kim H.J., Park C.W., et al. Gintonin, newly identified compounds from ginseng, is novel lysophosphatidic acids-protein complexes and activates G protein-coupled lysophosphatidic acid receptors with high affinity. Mol. Cells. 2012;33:151–162. doi: 10.1007/s10059-012-2216-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang M., Min J.W., In J.G., Yang D.C. Effects of red ginseng extract on the epididymal sperm motility of mice exposed to ethanol. Int. J. Toxicol. 2011;30:435–442. doi: 10.1177/1091581811405074. [DOI] [PubMed] [Google Scholar]
- Jarvis M.F., Khakh B.S. ATP-gated P2X cation-channels. Neuropharm. Rev. 2009;56:208–215. doi: 10.1016/j.neuropharm.2008.06.067. [DOI] [PubMed] [Google Scholar]
- Khakh B.S., North R.A. P2X receptors as cell surface ATP sensors in health and disease. Nature. 2006;442:527–532. doi: 10.1038/nature04886. [DOI] [PubMed] [Google Scholar]
- Kimura Y., Schmitt A., Fukushima N., Ishii I., Kimura H., Nebreda A.R., Chun J. Two novel Xenopus homologs of mammalian LP(A1)/EDG-2 function as lysophosphatidic acid receptors in Xenopus oocytes and mammalian cells. J. Biol. Chem. 2001;276:15208–15215. doi: 10.1074/jbc.M011588200. [DOI] [PubMed] [Google Scholar]
- Lopes C.M., Zhang H., Rohacs T., Jin T., Yang J., Logothetis D.E. Alteration in conserved Kir channel-PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- Mulryan K., Gitterman D.P., Lewis C.J., Vial C., Leckie B.J., Cobb A.L., Brown J.E., Conley E.C., Buell G., Pritchard C.A., et al. Reduced vas deferens contraction and male infertility in mice lacking P2X1 receptors. Nature. 2000;403:86–89. doi: 10.1038/47495. [DOI] [PubMed] [Google Scholar]
- Nah S.Y. Ginseng, recent advances and trend. Korean J. Ginseng Sci. 1997;21:1–12. [Google Scholar]
- North R.A. P2X receptors: a third major class of ligand-gated ion channels. Ciba Found. Symp. 1996;198:91–105. doi: 10.1002/9780470514900.ch5. [DOI] [PubMed] [Google Scholar]
- North R.A. Molecular physiology of P2X receptors. Physiol. Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Park J.S., Hwang S.Y., Lee W.S., Yu K.W., Paek K.Y., Hwang B.Y., Han K. The therapeutic effect of tissue cultured root of wild Panax ginseng C.A. Mayer on spermatogenetic disorder. Arch. Pharm. Res. 2006;29:800–807. doi: 10.1007/BF02974082. [DOI] [PubMed] [Google Scholar]
- Pyo M.K., Choi S.H., Hwang S.H., Shin T.H., Lee B.H., Lee S.M., Lim Y.H., Kim D.H., Nah S.Y. Novel glycoli-poproteins from ginseng. J. Ginseng Res. 2011;35:92–103. [Google Scholar]
- Rettinger J., Schmalzing G. Activation and desensitization of recombinant P2X1 receptor at nanomolar ATP concentrations. J. Gen. Physiol. 2003;121:451–461. doi: 10.1085/jgp.200208730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sim J.A., Broomhead H.E., North R.A. Ectodomain lysines and suramin block of P2X1 receptors. J. Biol. Chem. 2008;44:29841–29846. doi: 10.1074/jbc.M802523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon P. Electrophysiology of autonomic neuromuscular transmission involving ATP. J. Auton. Nerv. Syst. 2000;81:218–224. doi: 10.1016/s0165-1838(00)00141-7. [DOI] [PubMed] [Google Scholar]
- Suh B.C., Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr. Opin. Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Surprenant A., Buell G., North R.A. P2X receptors bring new structure to ligand-gated ion channels. Trends Neuroci. 1995;18:224–229. doi: 10.1016/0166-2236(95)93907-f. [DOI] [PubMed] [Google Scholar]
- Toullec D., Pianetti P., Coste H., Bellevergue P., Grand-Perret T., Ajakane M., Baudet V., Boissin P., Boursier E., Loriolle F., et al. The bisindolymaleimide GF109203X is a potent and selective inbihitor of protein kinase C. J. Biol. Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Valera S., Hussy A., Evans R.J., Adami N., North R.A., Surprenant A., Buell G. A new class of ligand-gated ion channel defined by P2X receptor for extracellular ATP. Nature. 1994;317:516–519. doi: 10.1038/371516a0. [DOI] [PubMed] [Google Scholar]
- Vial C., Tobin A.B., Evans R.J. G-protein-coupled receptor regulation of P2X1 receptors does not involve direct channel phosphorylation. Biochem. J. 2004;382:101–110. doi: 10.1042/BJ20031910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner P., Seward E.P., Buell G.N., North R.A. Domains of P2X receptors involved in desensitization. Proc. Natl. Acad. Sci. USA. 1996;93:15485–15490. doi: 10.1073/pnas.93.26.15485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Q., Yang M., Ting A.T., Logothetis D.E. PIP2 regulates the ionic current of P2X receptors and P2X7 receptor-mediated cell death. Channels. 2007a;1:46–55. [PubMed] [Google Scholar]
- Zhao Q., Logothetis D.E., Séguéla P. Regulation of ATP-gated P2X receptors by phosphoinositides. Eur. J. Physiol. 2007b;455:181–185. doi: 10.1007/s00424-007-0271-x. [DOI] [PubMed] [Google Scholar]