Abstract
In response to infection, insects produce a variety of antimicrobial peptides (AMPs) to kill the invading pathogens. To study their physicochemical properties and bioactivities for clinical and commercial use in the porcine industry, we chemically synthesized the mature peptides Bombyx mori moricin and Hyalophora cecropia cecropin B. In this paper, we described the antimicrobial activity of the two AMPs. Moricin exhibited antimicrobial activity on eight strains tested with minimal inhibitory concentration values (MICs) ranging between 8 and 128 μg/ml, while cecropin B mainly showed antimicrobial activity against the Gramnegative strains with MICs ranging from 0.5 to 16 μg/ml. Compared to the potent antimicrobial activity these two AMPs displayed against most of the bacterial pathogens tested, they exhibited limited hemolytic activity against porcine red blood cells. The activities of moricin and cecropin B against Haemophilus parasuis SH 0165 were studied in further detail. Transmission electron microscopy (TEM) of moricin and cecropin B treated H. parasuis SH 0165 indicated extensive damage to the membranes of the bacteria. Insights into the probable mechanism utilized by moricin and cecropin B to eliminate pathogens are also presented. The observations from this study are important for the future application of AMPs in the porcine industry.
Keywords: antimicrobial peptide, cecropin B, Haemophilus parasuis SH 0165, moricin, transmission electron microscopy
INTRODUCTION
Over the past decades, bacterial pathogens have resulted in severe economic losses to the global swine industry. Antibiotics have been in constant use among pig herds. However, to date, this method has been threatened by the emergence of antimicrobial resistance (AMR) in bacterial pathogens (Kim et al., 2011; Vanni et al., 2011). The development of multi-drug resistance in zoonotic bacteria and commensal bacterial pathogens (Frye and Fedorka-Cray, 2007) lead to fears that AMR could be transferred from livestock to humans (Cavaco et al., 2011) and lead to the complete ban on the use of antimicrobial growth promoters by the European Union in 2006 (Veldhuizen et al., 2008). Thus, alternative chemotherapeutic compounds need to be developed to control bacterial infections among pig herds.
AMPs with potent antibacterial, antiviral, and antifungal properties have been identified from a wide range of organisms including vertebrates, invertebrates, plants, protozoans, and microbes. Typically, AMPs have low molecular weight, high water solubility, and broad-spectrum antimicrobial activity with low levels of cytotoxicity. These characteristics are very important for the development of AMPs as antimicrobial agents for clinical use. More importantly, these molecules are believed to have an alternative mechanism of bactericidal action that is distinct from the mechanisms utilized by clinically used antibiotics. It has been generally accepted that AMP-mediated elimination of microorganisms occurs mainly via a physical interaction with the phospholipid membrane and the subsequent disruption of vital membrane functions (Jenssen et al., 2006). This mode of action indicates that these new types of antimicrobial agents could evade the multi-drug resistance mechanisms developed by bacteria.
Cecropins and moricin are linear amphipathic α-helical, cationic peptides and comprise one of the major sub-classes of the AMPs family. Cecropins are basic peptides of approximately 4 kDa in size and contain 35–37 amino acids. Cecropins and cecropin-like peptides have been purified from various insects, including Lepidopteran, Dipteran and Coleopteran insects (Andersson et al., 2003; Flyg et al., 1987; Kim et al., 2010). Since the discovery of the first cecropins in the immune hemolymph of the giant silk moth, Hyalophora cecropia (Hultmark et al., 1980), more than 200 AMPs have been identified in arthropods. In 1995, moricin was initially isolated from the hemolymph of the silkworm Bombyx mori (Hara and Yamakawa, 1995a). Moricin, a 42-amino-acid peptide, is highly basic, and its amino acid sequence has no significant similarity to the sequences of other AMPs (Hara and Yamakawa, 1995a). To date, moricin-like AMPs have been exclusively found in lepidopteran insects (Oizumi et al., 2005), which implies that cecropins may be distributed more widely than moricin. It has also been reported that both cecropin B and moricin have antibacterial activity against Gram-negative and Gram-positive bacteria (Hara and Yamakawa, 1995a; Sato and Feix, 2006). Among the cecropin family, cecropin B is the most active (Hultmark et al., 1982), while moricin displays a higher activity against Gram-positive bacteria than cecropins (Hara and Yamakawa, 1995a; Hemmi et al., 2002).
Numerous studies have been conducted regarding moricin and cecropin B. Both AMPs form random structures in water, and these peptides form α-helices only when they bind to a membrane or are present in other hydrophobic environments (Christensen et al., 1988; Hemmi et al., 2002; Steiner, 1982). Both moricin and cecropin B are believed to kill bacteria by affecting the integrity of the bacterial membranes (Chen et al., 2003; Hara and Yamakawa, 1995a). Furthermore, the activity of cecropin B against bacterial pathogens of fish (Sarmasik and Chen, 2003) and plants (Jan et al., 2010) has also been tested. However, to the best of our knowledge, the activities of moricin and cecropin B towards porcine bacterial pathogens have not been identified.
In this report, we described the antimicrobial activity of B. mori moricin and H. cecropia cecropin B against a broad range of porcine bacterial pathogens. In addition, the morphological changes of moricin and cecropin B treated Haemophilus parasuis SH 0165 were studied using TEM. We also discussed the possible mechanisms utilized by these two AMPs for the elimination of bacteria. Further understanding of the mechanism of interaction between AMPs and bacterial pathogens is important for the development of more effective peptide antibiotics and for any future applications in the swine industry.
MATERIALS AND METHODS
Antimicrobial agents
The peptides (Table 1) were synthesized using an automated solid-phase peptide synthesizer at the peptide synthesis facility (Merrifield, 1963) of Neweast Biosciences lnc. (China). The synthetic peptides were purified via a reverse-phase high-pressure liquid chromatography (RP-HPLC) using a C18 column (Waters Xbridge). Elution was conducted using a water-acetonitrile linear gradient (0–80% of acetonitrile) containing 0.1% (V/V) trifluoroacetic acid (TFA). Finally, the purity and accurate masses of the product peptides were determined using HPLC and mass spectrometry, respectively. Penicillin and streptomycin sulfate powders were purchased from New Century Pharmaceutical Company (China).
Table 1.
Amino acid sequences of moricin and cecropin B
| Peptides | Amino acid sequences | Molecular weight | Charges |
|---|---|---|---|
| Cecropin B | KWKVFKKIEKMGRNIRN | 3834.9 | +7 |
| GIVKAGPAIAVLGEAKAL* | |||
| Moricin | AKIPIKAIKTVGKAVGKGLRA | 4543.4 | +11 |
| INIASTANDVFNFLKPKKRKH |
The C-terminus of cecropin B was amidated
Antimicrobial activity
The antimicrobial activity of moricin and cecropin B was tested against eight different bacterial strains: Escherichia coli ATCC 25922 (E. coli), Staphylococcus aureus ATCC 25923 (S. aureus), Streptococcus suis NCTC 10234 (S. suis), Salmonella chloleraesuis C 500 (S. chloleraesuis), and clinical isolates of Actinobacillus pleuropneumoniae (A. pleuropneumoniae), Haemophilus parasuis SH 0165 (H. parasuis), Bordetella bronchiseptica HH 0809 (B. brochiseptica) and Pasteurella multocida 03507 (P. multocida). The minimum inhibitory concentration values (MICs) of the peptides against the bacterial strains mentioned above were determined using a standard broth micro-dilution method with some modifications (Steinberg et al., 1997). In brief, the bacteria were grown in Tryptic Soy Broth (TSB, Difco) overnight at 37°C and shaken at 200 rpm for 3–8 h until the bacteria reached the mid-log phase. The cultures were washed twice with autoclaved 10 mM sodium phosphate buffer (pH 7.4) and were re-suspended in fresh TSB to a final concentration of 1 × 105 CFU/ml. The stock peptide solution was prepared at a concentration of 1,280 μg/ml in 0.01% acetic acid and 0.2% BSA and then serial twofold dilutions were made to a final concentration of 2.5 μg/ml. Equal aliquots (90 μl) of a bacterial suspension (1 × 105 CFU/ml) in TSB were distributed to each well of 96-well polypropylene microtiter plates (Corning, USA), and then each well was inoculated with 10 μl of the diluted peptides. The MICs were defined as the lowest concentrations that completely inhibited bacterial growth. The tests were performed in triplicate, and MICs that were reproduced twice or three times in three independent measurements were recorded. The Minimum Bactericidal concentration (MBC) was evaluated by plating the contents of the first three wells showing no visible growth of bacteria onto TSA plates (Difco, America) and incubating at 37°C for 24 h. The lowest concentration that prevented any residual colony formation is considered the MBC (Yoshiro Nakajima et al., 2003).
Because a high density of approximately 108 CFU/ml H. parasuis is needed to observe TEM images, the supra-MICs were determined under high initial inoculation dose (108 CFU/ml of H. parasuis) in parallel with the regularly used inoculum dose of 105 CFU/ml. The following steps were the same as previously described.
Kill-curve studies
One hundred and twenty-five microliters of 4 μg/ml cecropin B and 512 μg/ml moricin (final concentrations, 2 and 256 μg/ml, respectively) were mixed with 125 μl of 2 × 106 CUF/ml H. parasuis SH 0165 at the mid-logarithmic phase and were incubated anaerobically at 37°C. At various time points, 25 μl aliquots were taken from the mixture, serially diluted in TSB, and plated out on TSA plates. Plates were incubated at 37°C after which bacterial colonies were counted. As a negative control, the bacterial suspension was incubated with 100 μl of solvent (0.01% acetic acid, 0.2% BSA).
Hemolytic activity
The hemolytic assay was performed using porcine erythrocytes as previously described with slight modifications (Klüver et al., 2005). Briefly, 8 ml of porcine blood was centrifuged at 1,800 × g for 10 min at RT to collect the erythrocytes. The pellets were washed three times in assay buffer A (3 g/l TSB, 287 mM glucose) and centrifuged at 1,800 × g for 10 min. An 100 μl cell suspension was diluted with buffer A (final concentration around 2%). Subsequently, aliquots of 15 μl peptide (final concentration 0.25–256 μg/ml) solutions were added to the 135 μl of the diluted blood cells in polypropylene 96-well microtiter plates (Corning, USA), and the mixture was incubated for 1 h at 37°C. After incubation, each plate was centrifuged for 5 min at 1,800 × g, and the 100 μl supernatant of each well was transferred to a new 96-well plate. The OD was determined at 450 nm. The values for 0% and 100% hemolysis were determined using erythrocyte suspensions incubated in buffer A or 1% Tween-20. Additionally, another similar experiment was performed in buffer B (5 mM phosphate, pH 7.4, 5 mM glucose, 154 mM NaCl, final concentration), which is a better reflection of the physiological environment of erythrocytes. Hemolysis was calculated relative to the total hemolysis caused by Tween-20 according to the following formula: hemolysis (%) = (Epeptide − Eneg) / (Epos − Eneg) × 100.
Transmission electron microscopy
Mid-logarithmic phase H. parasuis SH 0165 cells (∼2 × 108 CFU/ml) were treated with moricin (either 128 μg/ml or 512 μg/ml) and cecropin B (either 2 μg/ml or 512 μg/ml) as described above. The mixtures containing each concentration of peptides were kept for either 20 min or 120 min at 37°C under aerobic conditions. The negative control was run in the absence of the peptide. After treatment, the cells were centrifuged at 5,000 × g for 10 min, fixed in 3% glutaraldehyde solution and washed 6 times in PBS buffer for 20 min each wash; this was followed by another fixation with 1% osmic acid solution for 2 h and 2 consecutive 5 min washes in PBS buffer. Then the samples were dehydrated at 4°C in a series of 10 min incubations in graded ethanol solutions: 50% (once), 70% (once), 80% (twice), 90% (twice), and 100% (twice). Then, the samples were infiltrated with a solution of 100% acetone and epoxide resin Epon812 (Beijing XXBR Technology co., LTD, China) (1:1) overnight. Samples were then embedded with the epoxide resin Epon812 at 60°C for 48 h, and sectioned with a diamond knife under an anatomical microscope. Microtome sections of 1 μm were placed on copper grids (Beijing XXBR Technology co., LTD, China). The sections were stained separately for 3 min with a saturated uranylacetate in 70% ethanol solution and a lead dye, followed by an examination under a transmission electron microscope (FEI Tecnai G2 20, Oregon, America) operated at 200 kV.
RESULTS
Characterization of moricin and cecropin B
The complete open reading frame of the B. mori moricin encodes a 66-residue sequence (MNILKLFFVFIVAMSLVACSTAAPAKIPIKAIKTVGKAVGKGLRAINIASTANDVFNFLKPKKRKH) with the underlined part representing a predicted signal peptide that is rich in hydrophobic residues (Hemmi et al., 2002). The mature sequence of moricin from B. mori showed a high degree of homology to the moricin of M. sexta (67%) and S. litura (75%) (Fig. 1A). The mature peptide of H. cecropia cecropin B is processed from a 62-amino acid precursor molecule (MNFSRIFFFVFALVLALSTVSAAPEPKWKVFKKIEKMGRNIRNGIVKAGPAIAVLGEAKALG) with an underlined 26-residue leader peptide (Van Hofsten et al., 1985). We synthesized mature cecropin B starting from Lys27 and ending at Leu61 with an amidated C-terminus. The amidated C-terminus may contribute to the broad spectrum property of cecropin B. The amino acid sequence of cecropin B from H. cecropia showed a high degree of homology to that of B. mori (85%) and D. melanogaster (71%) (Fig. 1B). Most of the amino acid changes of the two peptides involve substitutions of residues with similar chemical properties (Fig. 1). Moricin has a special structural domain that was composed of a cluster of five basic amino acid residues at the C-terminus (Fig. 1B). This region has been thought to interact with the surface of the bacterial membrane.
Fig. 1.

Comparison of the moricin amino acid sequence from B. mori with those from M. sexta and S. litura (A), and Comparison of the cecropin B amino acid sequence from H. cecropia with those from B. mori and D. melanogaster (B). The multiple sequence alignment was conducted using the CLUSTAL W program. Gaps that have been inserted to optimize the alignment are indicated as dashes. The symbol “ * ” indicated that the residues are identical in all columns; “ : ” indicated the conserved substitutions; “ . ” indicated the semi-conserved substitutions, respectively. In (A), B. m. moricin from B. mori (NP_001036829.2); M. s. moricin from M. sexta (AA074637.1); S. l. moricin from S. litura (BAC79440.1). In (B), H. c. cecropin B from H. cecropia (AAA29184.1); B. m. cecropin B from B. mori (BAA01889.1); D. m. cecropin B from D. melanogaster (AAF57027.1).
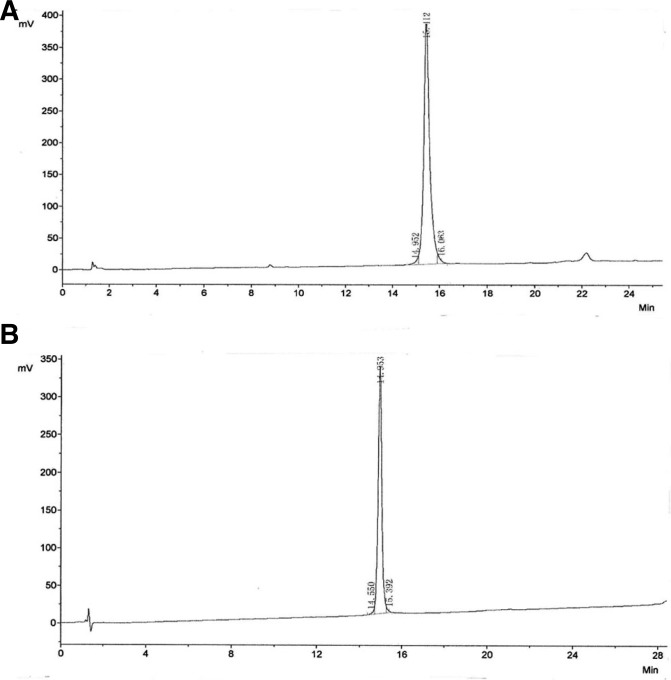
Reversed-phase HPLC (Fig. 2A) of the chemically synthesized moricin indicated a > 95% purity, and mass spectrometry showed a molecular mass of 4543.4, which is in close agreement with the calculated mass of 4543.5. The calculated isoelectric point is 11.36 (according to the online tool http://web.expasy.org/compute_pi/). The reversed-phase HPLC (Fig. 2B) of cecropin B indicated a > 95% purity, and mass spectrometry showed a molecular mass of 3834.9, which is in close agreement with the calculated mass of 3835.7. The calculated isoelectric point is 10.73.
Fig. 2.
Reversed-phase HPLC chromatogram of moricin (A), cecropin B (B). Both of the peptides eluted at 15 min using a 0–100% acetonitrile gradient (1.5%/min) in water.
Antimicrobial activity
The antimicrobial activity of moricin, cecropin B, penicillin, and streptomycin sulfate were analyzed against eight Gram-positive and Gram-negative bacteria related to porcine bacterial diseases. The MICs are shown in Table 2. Briefly, moricin exhibited antimicrobial activity on all the strains tested with MICs ranging between 8 and 128 μg/ml. While cecropin B showed activity mainly against the Gram-negative strains with MIC values between 0.5 and 16 μg/ml. Overall, the MICs of cecropin B against the Gram-negative strains were lower and were approximately 1 to 64 times more active compared to the MICs of moricin. The bacteria that were most susceptible to moricin were B. bronchiseptica and A. pleuropneumoniae, both with MICs of 8 μg/ml. The bacteria that were most susceptible to cecropin B were B. bronchiseptica and H. parasuis, with MICs of 0.5 μg/ml and 2 μg/ml, respectively.
Table 2.
Antimicrobial activity of AMPs and antibiotics towards Gram-negative and Gram-positive bacteria
| Microorganism | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Cecropin B | Moricin | Penicillin | Streptomycion sulfate | |
| Gram-negative bacteria | ||||
| E. coli | 16 | 32 | 32 | 4 |
| S. chloleraesuis | 8 | 32 | 0.5 | > 32 |
| A. pleuropneumoniae | 8 | 8 | 0.5 | 16 |
| H. parasuis | 2 | 128 | 0.5 | 16 |
| B. bronchiseptica | 0.5 | 8 | > 32 | > 32 |
| P. multocida | 8 | 128 | 2 | 2 |
| Gram-positive bacteria | ||||
| S. aureus | > 128 | 16 | < 0.0625 | 4 |
| S. suis | > 128 | 16 | < 0.0625 | 16 |
MIC values at various initial bacterial densities and MBC values against H. parasuis SH 0165
The bacteria density must be high for high-magnification TEM imaging. To identify the appropriate sub- and supra-MICs for the following TEM preparation, it was necessary to compare the MICs tested under an approximately 100-fold-higher concentration using the standard inoculation cell density. The results are shown in Table 3, indicating that MICs increased 4-fold for moricin and 256-fold for cecropin B. The corresponding suband supra-MICs for samples prepared with moricin and cecropin B were also presented in Table 3. The MBC value was the same as the MIC value for cecropin B, while it increased 2 fold for moricin (Table 3).
Table 3.
MBC values toward H. parasuis and MIC values at different initial bacteria densities
| Values | Concentrations (μg/ml)
|
|
|---|---|---|
| Moricin | Cecropin B | |
| MBC | 256 | 2 |
| MIC under standard cell density | 128 | 2 |
| MIC under high cell density suitable for TEM | 512 | 512 |
| Sub-MIC for TEM | 128 | 2 |
| Supra-MIC for TEM | 512 | 512 |
Killing kinetics toward H. parasuis
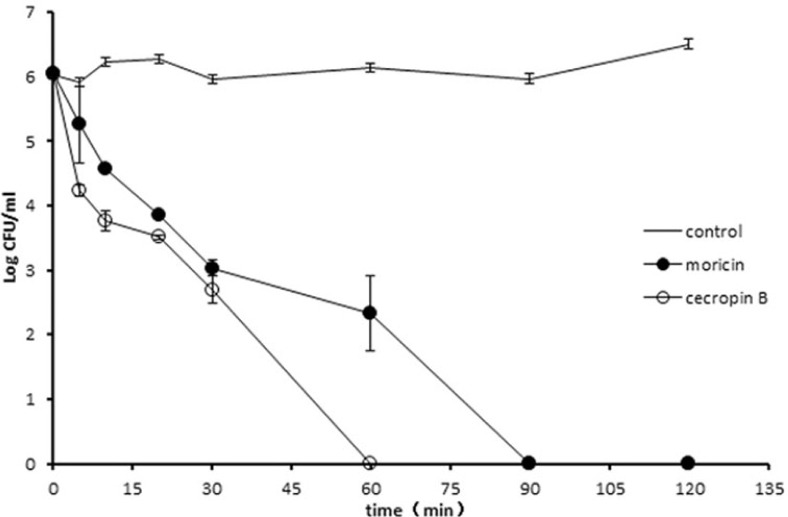
Kill-curve studies were performed with moricin and cecropin B on a logarithmic-phase H. parasuis culture. An approximately 3-log unit decrease in viable H. parasuis cells was detected after a 30 min treatment with 256 μg/ml moricin (Fig. 3). After 90 min, a decrease below the detection limit was accomplished. Sixty minutes were sufficient for cecropin B to kill all the bacteria (Fig. 3). The cecropin B killing effect toward H. parasuis appeared to be stronger than that of moricin.
Fig. 3.
Killing kinetics of peptides against H. parasuis. Bacteria were treated with 2 μg/ml cecropin B (open circles) or 256 μg/ml moricin (closed circles). Samples were taken after various incubation times and viable bacteria were determined using colony count assays. Data represent mean ± SD.
Hemolyic activity
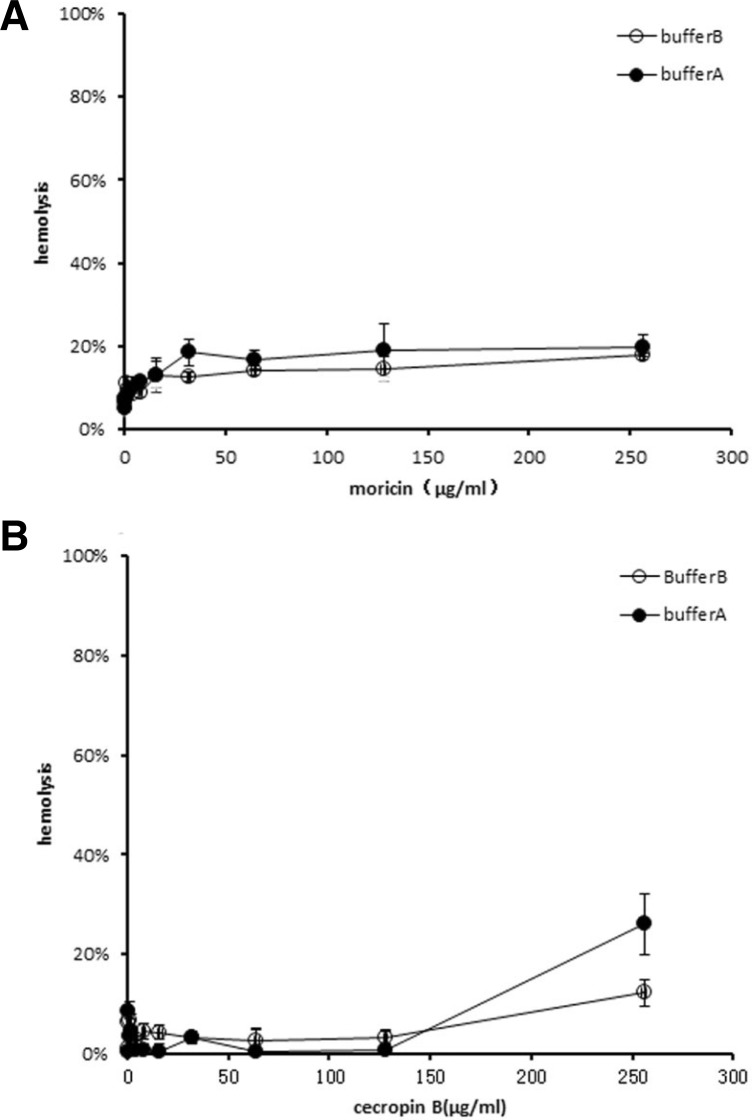
To assess the cytotoxicity of the peptides against mammalian cells, erythrocytes were collected from fresh porcine blood and incubated with moricin and cecropin B. The release of hemoglobin was measured as an indicator of the hemolytic activity of moricin and cecropin B. The test was performed in two different test media with a similar outcome (Fig. 4). In the two types of medium, hemolysis was observed to be less than 20% even at the highest concentration of moricin at 256 μg/ml, while the values of hemolysis for cecropin B were even less than 10% under the tested concentrations and up to 128 μg/ml. At a concentration of 256 μg/ml, cecropin B hemolysis increased to 26% in the medium containing 287 mM glucose and 1/10 TSB (Fig. 4).
Fig. 4.
Hemolytic activity of moricin (A) and cecropin B (B). Freshly isolated porcine red blood cells were incubated with various concentrations of moricin and cecropin B (0–256 μg/ml) in 5 mM phosphate, pH 7.4, 5 mM glucose, 154 mM NaCl (open circles), or 3 g/l TSB, 287 mM glucose (closed circles), separately. Release of hemoglobin, as a measure of hemolysis, was measured at 450 nm. Release of hemoglobin upon addition of Tween-20 was set at 100 %. Data represent mean ± SD.
Effects of moricin and cecropin B treatment on the ultrastructure of H. parasuis SH 0165
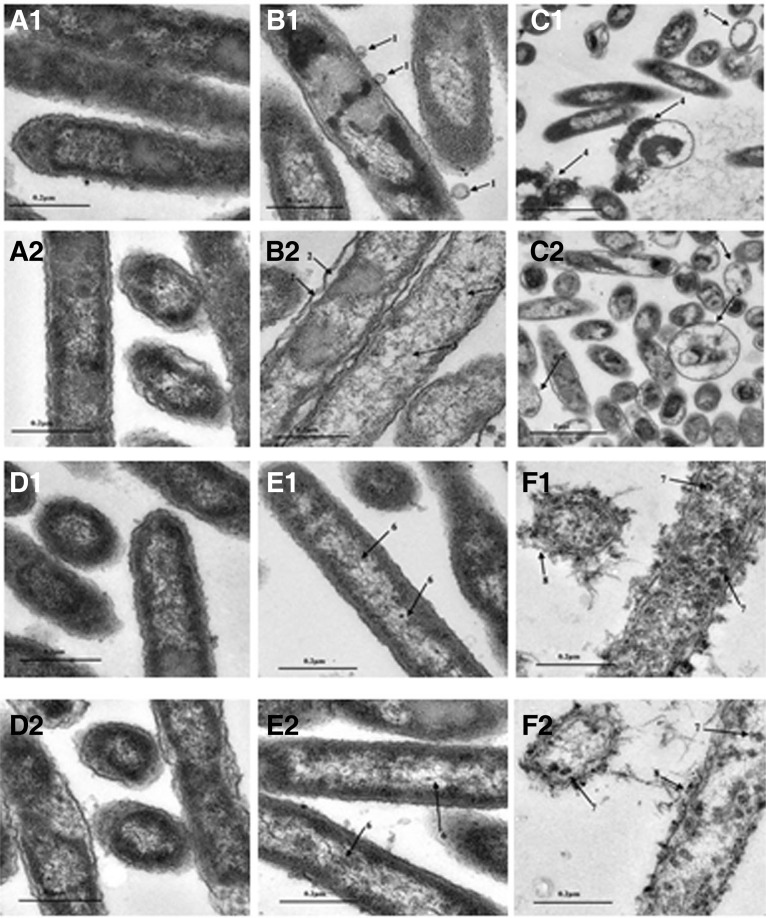
To further elucidate the killing mechanisms employed by moricin and cecropin B, H. parasuis SH 0165 was treated separately with sub- and supra-MIC of moricin and cecropin B (for either 20 min or 120 min). The morphological changes in the bacteria were analyzed using TEM. Untreated cells of H. parasuis SH 0165 in medium showed a normal cell shape with an undamaged structure of the inner membrane and a complete, slightly waved outer membrane (Figs. 5A1 and 5A2). The periplasmic space was thin and had a uniform appearance (Figs. 5A1 and 5A2). After incubation with a sub-MIC of moricin for 20 min, numerous blebs protruded from the outer bacterial membrane (Fig. 5B1). At a sub-MIC for 120 min, we observed similar membrane disturbances with the cytosol being filled with electron-dense fibrous structures (Fig. 5B2). After incubation with a supra-MIC of moricin for 20 min, H. parasuis SH 0165 was characterized by a ghost-like appearance, and a large amount of the cytoplasmic contents leaked out from the inside of the bacteria (Fig. 5C1). Increased numbers of ghost-like cells were detected after treatment with a supra-MIC of moricin for 120 min (Fig. 5C2). In all cases (Fig. 5), the most significant changes were a retraction of the cytoplasm from the membrane, membrane blebs and the leakage of the cytoplasmic contents. Other morphological changes were electron fibrous structures and the damage of the cell membrane. Finally, ghost-like appearances or completely lysed bacteria were observed at higher concentrations and a longer incubation time.
Fig. 5.
TEM micrographs of untreated H. parasuis SH 0165 (A1, A2, D1, and D2), treated with sub-MIC moricin for 20 min (B1) or for 120 min (B2), with supra-MIC moricin for 20 min (C1) or for 120 min (C2), treated with sub-MIC cecropin B for 20 min (E1) or for 120 min (E2), with supra-MIC cecropin B for 20 min (F1) or for 120 min (F2). Arrows: (1) membrane bubbles; (2) cytoplasm detachment; (3) electron-dense fibrous; (4) leaked out contens; (5) “ghost-like” cells; (6) heterogeneous electron density; (7) electron dots; (8) membrane lysed.
TEM micrographs of H. parasuis SH 0165 in medium without peptide showed round, proliferating cells with well-defined membranes (Figs. 5D1 and 5D2). At the sub-MIC of cecropin B, numerous vacuoles along with a heterogeneous cytosolic electron density could be observed in the cytoplasm (Figs. 5E1 and 5E2). For a supra-MIC of cecropin B (Figs. 5F1 and 5F2), numerous completely lysed cells were detected, and many electron dots appeared compared with controls. However, unlike moricin, the morphological appearances of the cells treated with the same dose of cecropin B did not change substantially with increasing incubation times.
DISCUSSION
In this report, we described the antibacterial and hemolytic activity of synthetic moricin and cecropin B in vitro. The two AMPs inhibited the growth of most of the porcine bacterial pathogens tested. Moreover, they displayed limited hemolytic activity under the two different incubation conditions of the tested concentrations.
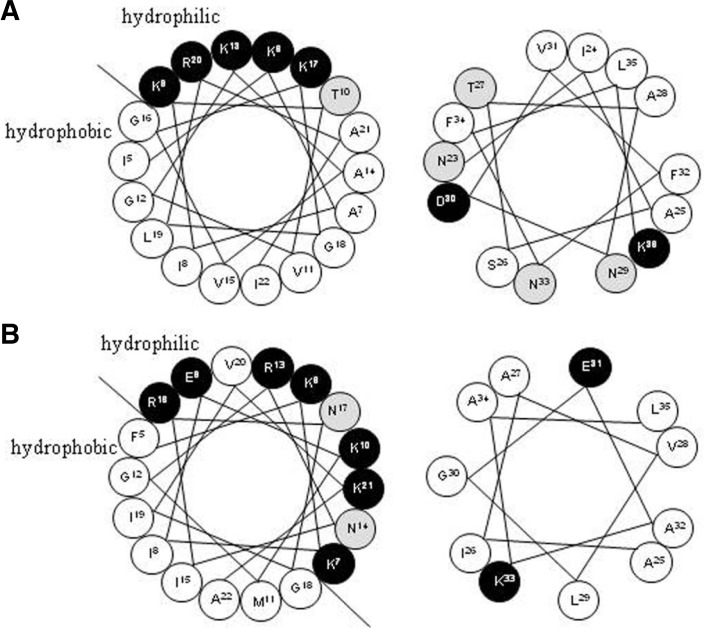
Moricin has a unique structure composed of a long bent α-helix containing eight turns over almost the entire length of the molecule except for the four N-terminal residues and the six C-terminal residues (Hemmi et al., 2002). Unlike the complete long α-helix of moricin, cecropin B has an amphipathic helix (residues 5–22) at the N-terminus, a hydrophobic α-helix at the C-terminus (residues 25–35), and a G23-P24 hinge area (Fig. 6B). The hinge area is highly conserved among cecropins (Durell et al., 1992). At the N-terminal, half of both moricin and cecropin B (residues 5–22) charged amino acids appear at intervals of several residues (Fig. 1), which indicated a unique structure in antibacterial proteins containing an amphipathic α-helix (Kreil, 1994). Furthermore, the findings reported by Hemmi et al suggest that the N-terminal fragment (residues 1–29) of moricin was responsible for the increase in membrane permeability (Hemmi et al., 2002). The N-terminal sequence of cecropin B was also found to be more important than the C-terminal domain from a functional point of view (Wu et al., 2009). These common structural properties of moricin and cecropin B strongly suggest the functional importance of the N-terminal segments of these two AMPs. Interestingly, the hydrophilic N-terminal region of cecropin B is obviously larger than that of moricin (Fig. 6). This feature could strengthen the adsorptive ability of cecropin B to the bacterial membrane. The C-terminal segment of moricin (residues 23–36) is slightly more hydrophilic than that of cecropin B (Fig. 6). Such a feature of moricin may limit this helical AMP from spanning the outer membrane of bacteria. The differences in structures could partially explain the reason why cecropin B was more potent than moricin towards Gram-negative bacteria.
Fig. 6.
Helical wheel projections of the helices in moricin and cecropin B. Residues 5–22 (left panel) and 23–36 (right panel) of moricin (A) and cecrpin B (B). Hydrophilic, hydrophobic, and charged residues are shown in gray, white and black backgrounds, respectively.
Moricin and cecropin B have been shown to have an antibacterial activity towards many Gram-positive and Gram-negative bacteria. Cecropin B has been confirmed to have higher antibacterial activity towards Gram-negative bacteria than Gram-positive bacteria (Qu et al., 1982; Van Hofsten et al., 1985). On the other hand, moricin showed higher antibacterial activity against Gram-positive bacteria than cecropins did (Hemmi et al., 2002). The MICs of the two AMPs regarding the bacteria studied in this report were in agreement with previously reported conclusions. The MICs of moricin towards the bacteria tested ranged from 8 to 128 μg/ml, while cecropin B mainly showed activity against Gram-negative strains with MICs being between 0.5 and 16 μg/ml. However, the previously reported moricin and cecropin B activity cannot be entirely compared with our own findings because of the use of different activity assignments and different experimental set-ups. It is clear that moricin and cecropin B can inhibit most of the bacterial pathogens that infect pig herds. From a pharmaceutical standpoint, a low hemolytic activity against erythrocytes is desirable. Compared with the antibacterial activity, moricin showed low levels of hemolytic activity against porcine erythrocytes (< 20% hemolysis) at all tested concentrations, whereas cecropin B displayed almost no hemolytic activity against porcine red blood cells up to a concentration of 128 μg/ml. This may be explained by the fact that the outer leaflet of mammalian membranes lacks negatively charged phospholipids and has higher cholesterol levels; both features prevent many AMPs from binding to mammalian membranes (Ishitsuka et al., 2006).
To elucidate the killing mechanism utilized by moricin and cecropin B, we used TEM to study the affect of AMPs on the ultrastructure of H. parasuis SH 0165. Because the peptide concentration and incubation time have been suggested to be important factors for the killing mechanism (Hancock and Rozek, 2002; Hartmann et al., 2010), samples of H. parasuis SH 0165 were treated with peptides at different concentrations (sub- and supra-dose) and different incubation times (20 min and 120 min). These observations are shown in Fig. 5, and several distinct signs of cell damage, such as membrane ruffling, blisters, protruding bubbles, membrane detachment and ghost-like cells, are clearly shown in the TEM micrographs.
Previous studies have demonstrated that moricin could kill bacteria by interacting with the membrane (Hara and Yamakawa, 1995b). Nevertheless, the direct morphological changes of moricin treated bacteria have not been visualized. In the TEM observations, we noticed ruffling of the outer membrane of H. parasuis SH 0165 when treated with moricin (Fig. 5B2). It was suggested that the positively charged moricin replace the Mg2+ ions in the lipopolysaccharide layer on the outer membrane of the Gram-negative bacteria and thereby result in the disturbance of the outer surface. The appearance of membrane ruffling and blisters has also been reported for Gramicidin S and NK-2 (Hammer et al., 2010). Destabilization of the outer membrane would promote the penetration of moricin and lead to a local disruption of the inner membrane causing the cytoplasmic contents to locally leak out and fill the periplasmic space. This may explain the formation of blisters without any damage to the outer membrane (Fig. 5B1). In the case of moricin, at higher concentrations or longer incubation periods, increased ghost-like and completely lysed cells were observed (Figs. 5C1 and 5C2). These results support the mechanism that moricin can kill the bacteria through the formation of ion channels in the membrane. Reports regarding the killing mechanism utilized by cecropin B are more extensive than those for moricin. Earlier studies have indicated that cecropins can form partially selective ion-permeable “channels” in planar lipid bilayer membranes (Christensen et al., 1988; Durell et al., 1992). Alternative models have also been proposed; the carpet model states that cecropins saturate the surface of the bacterial membrane before causing the membrane’s complete disruption (Gazit et al., 1995; Steiner et al., 1988). At a sub-MIC dose of cecropin B, vacuoles inside the cells were observed, while the cell membrane is kept mostly intact (Figs. 5E1 and 5E2). This indicated that the cytoplasmic contents could have leaked out while the cell membrane was left unharmed. Previously reported TEM observations of cecropin B treated bacteria showed vacuoles in cells with an intact membrane (Chen et al., 2003). In our study, we found something intriguing. At a supra-MIC of cecropin B, some of the cells were completely lysed and the membranes were completely damaged (Figs. 5F1 and 5F2). For the cells treated with a supra-dose of cecropin B, most cytoplasmic membranes were severely damaged (Figs. 5F1 and 5F2). On the other hand, the morphological changes of cells treated with a sub-dose were not very obvious (Figs. 5E1 and 5E2). Thus, it’s tempting to speculate that the dose of cecropin B can affect the mechanism of action.
Along with membranes, intracellular structures have also been discussed as potential targets for AMPs (Brogden, 2005). Moricin and cecropin B also elicit bacteria-specific effects inside the cytoplasm, such as the formation of filamentous structures and electron dots (Figs. 5B2 and 5E2), suggesting that the intracellular structures, such as DNA could be the secondary targets of moricin and cecropin B, or that the impact of peptides on the bacterial membrane may result in the rearrangement of these intracellular structures. These hypotheses require further investigation.
In conclusion, we determined the antimicrobial activity of moricin and cecropin B against porcine bacterial pathogens. Both of these AMPs exhibit potent antimicrobial activity against most of the bacteria tested with a limited hemolytic activity. The main target of the two AMPs was the bacterial membrane, although they may also interact with targets inside the cells. These results indicated that moricin and cecropin B could play an important role in reducing the occurrence of bacterial infections in pig herds and could be developed as new types of drugs that can replace or be used in conjunction with antibiotics. The results shown in this study can provide useful information for the future use of custom antimicrobial peptides in pig farms.
Supplementary Material
Acknowledgments
The authors would like to thank Dr. Edwin J.A. Veldhuizen for critical comments on the manuscript and Dr. Marina Berditsch for advice on TEM sample preparation. We would also like to thank Dr. HaoBo Jiang for helping with the helical wheel projections of moricin and cecropin B. This research was supported by the China Postdoctoral Science Foundation funded project (52201-12970), National Natural Science Foundation (31201953) and the Ministry of Agriculture of China (CARS-36).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Andersson M., Boman A., Boman H. Ascaris nematodes from pig and human make three antibacterial peptides: isolation of cecropin P1 and two ASABF peptides. Cell. Mol. Life Sci. 2003;60:599–606. doi: 10.1007/s000180300051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boman H.G., Hultmark D. Cell-free immunity in insects. Trends Biochem. Sci. 1981;6:306–309. [Google Scholar]
- Brogden K.A. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Cavaco L.M., Hasman H., Aarestrup F.M. Zinc resistance of Staphylococcus aureus of animal origin is strongly associated with methicillin resistance. Vet. Microbiol. 2011;150:344–348. doi: 10.1016/j.vetmic.2011.02.014. [DOI] [PubMed] [Google Scholar]
- Chen H.M., Chan S.C., Lee J.C., Chang C.C., Murugan M., Jack R.W. Transmission electron microscopic observations of membrane effects of antibiotic cecropin B on Escherichia coli. Microsc. Res. Tech. 2003;62:423–430. doi: 10.1002/jemt.10406. [DOI] [PubMed] [Google Scholar]
- Christensen B., Fink J., Merrifield R., Mauzerall D. Channel-forming properties of cecropins and related model compounds incorporated into planar lipid membranes. Proc. Nat. Acad. Sci. USA. 1988;85:5072–5076. doi: 10.1073/pnas.85.14.5072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durell S.R., Raghunathan G., Guy H.R. Modeling the ion channel structure of cecropin. Biophys. J. 1992;63:1623–1631. doi: 10.1016/S0006-3495(92)81730-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flyg C., Dalhammar G., Rasmuson B., Boman H.G. Insect immunity: inducible antibacterial activity in Drosophila. Insect Biochem. 1987;17:153–160. [Google Scholar]
- Frye J.G., Fedorka-Cray P.J. Prevalence, distribution and characterisation of ceftiofur resistance in Salmonella enterica isolated from animals in the USA from 1999 to 2003. Int. J. Antimicrob. Agents. 2007;30:134–142. doi: 10.1016/j.ijantimicag.2007.03.013. [DOI] [PubMed] [Google Scholar]
- Gazit E., Boman A., Boman H.G., Shai Y. Interaction of the mammalian antibacterial peptide cecropin P1 with phospholipid vesicles. Biochemistry. 1995;34:11479–11488. doi: 10.1021/bi00036a021. [DOI] [PubMed] [Google Scholar]
- Hammer M.U., Brauser A., Olak C., Brezesinski G., Goldmann T., Gutsmann T., Andrä J. Lipopolysaccharide interaction is decisive for the activity of the antimicrobial peptide NK-2 against Escherichia coli and Proteus mirabilis. Biochem. J. 2010;427:477–488. doi: 10.1042/BJ20091607. [DOI] [PubMed] [Google Scholar]
- Hancock R.E.W., Rozek A. Role of membranes in the activities of antimicrobial cationic peptides. FEMS Microbiol. Lett. 2002;206:143–149. doi: 10.1111/j.1574-6968.2002.tb11000.x. [DOI] [PubMed] [Google Scholar]
- Hara S., Yamakawa M. A novel antibacterial peptide family isolated from the silkworm, Bombyx mori. Biochem. J. 1995a;310:651–656. doi: 10.1042/bj3100651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara S., Yamakawa M. Moricin, a novel type of antibacterial peptide isolated from the silkworm, Bombyx mori. J. Biol. Chem. 1995b;270:29923–29927. doi: 10.1074/jbc.270.50.29923. [DOI] [PubMed] [Google Scholar]
- Hartmann M., Berditsch M., Hawecker J., Ardakani M.F., Gerthsen D., Ulrich A.S. Damage of bacterial cell envelope by antimicrobial peptides Gramicidin S and PGLa as revealed by transmission and scanning electron microscopy. Antimicro. Agents Chemother. 2010;54:3132–3142. doi: 10.1128/AAC.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hemmi H., Ishibashi J., Hara S., Yamakawa M. Solution structure of moricin, an antibacterial peptide, isolated from the silkworm Bombyx mori. FEBS Lett. 2002;518:33–38. doi: 10.1016/s0014-5793(02)02637-6. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Steiner H., Rasmuson T., Boman H.G. Insect immunity: purification and properties of three inducible bactericidal proteins from hemolymph of immunized pupae of Hyalophora cecropia. Eur. J. Biochem. 1980;106:7–16. doi: 10.1111/j.1432-1033.1980.tb05991.x. [DOI] [PubMed] [Google Scholar]
- Hultmark D., Engström Å., Bennich H., Kapur R., Boman H.G. Insect immunity: isolation and structure of cecropin D and four minor antibacterial components from Cecropia pupae. Eur. J. Biochem. 1982;127:207–217. doi: 10.1111/j.1432-1033.1982.tb06857.x. [DOI] [PubMed] [Google Scholar]
- Ishitsuka Y., Pham D.S., Waring A.J., Lehrer R.I., Lee K.Y.C. Insertion selectivity of antimicrobial peptide protegrin-1 into lipid monolayers: effect of head group electrostatics and tail group packing. Biochim. Biophys. Acta (BBA)-Biomembranes. 2006;1758:1450–1460. doi: 10.1016/j.bbamem.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Jan P.S., Huang H.Y., Chen H.M. Expression of a synthesized gene encoding cationic peptide cecropin B in transgenic tomato plants protects against bacterial diseases. Appl. Environ. Microb. 2010;76:769–775. doi: 10.1128/AEM.00698-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenssen H., Hamill P., Hancock R.E.W. Peptide antimicrobial agents. Clin. Microbiol. Rev. 2006;19:491–511. doi: 10.1128/CMR.00056-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S.R., Hong M.Y., Park S.W., Choi K.H., Yun E.Y., Goo T.W., Kang S.W., Suh H.J., Kim I., Hwang J.S. Characterization and cDNA cloning of a cecropin-like antimicrobial peptide, papiliocin, from the swallowtail butterfly, Papilio xuthus. Mol. Cells. 2010;29:419–423. doi: 10.1007/s10059-010-0050-y. [DOI] [PubMed] [Google Scholar]
- Kim H.B., Baek H., Lee S.J., Jang Y.H., Jung S.C., Kim A., Choe N.H. Prevalence and antimicrobial resistance of Salmonella spp. and Escherichia coli isolated from pigs at slaughterhouses in Korea. Afr. J. Microbiol. Res. 2011;5:823–830. [Google Scholar]
- Klüver E., Schulz-Maronde S., Scheid S., Meyer B., Forssmann W.G., Adermann K. Structure-activity relation of human β-defensin 3: influence of disulfide bonds and cysteine substitution on antimicrobial activity and cytotoxicity. Biochemistry. 2005;44:9804–9816. doi: 10.1021/bi050272k. [DOI] [PubMed] [Google Scholar]
- Kreil G. Antimicrobial peptides from amphibian skin: an overview. Ciba Found. Symp. 1994;186:77–85. doi: 10.1002/9780470514658.ch5. [DOI] [PubMed] [Google Scholar]
- Merrifield R.B. Solid phase peptide synthesis. l. The synthesis of a tetrapeptide. J. Am. Chem. Soc. 1963;85:2149–2154. [Google Scholar]
- Oizumi Y., Hemmi H., Minami M., Asaoka A., Yamakawa M. Isolation, gene expression and solution structure of a novel moricin analogue, antibacterial peptide from a lepidop-teran insect, Spodoptera litura. Biochim. Biophys. Acta. 2005;1752:83–92. doi: 10.1016/j.bbapap.2005.07.013. [DOI] [PubMed] [Google Scholar]
- Sarmasik A., Chen T.T. Bactericidal activity of cecropin B and cecropin P 1 expressed in fish cells (CHSE-214): application in controlling fish bacterial pathogens. Aquaculture. 2003;220:183–194. [Google Scholar]
- Sato H., Feix J.B. Peptide-membrane interactions and mechanisms of membrane destruction by amphipathic α-helical antimicrobial peptides. Biochim. Biophys. Acta. 2006;1758:1245–1256. doi: 10.1016/j.bbamem.2006.02.021. [DOI] [PubMed] [Google Scholar]
- Steinberg D.A., Hurst M.A., Fujii C.A., Kung A., Ho J., Cheng F., Loury D.J., Fiddes J.C. Protegrin-1: a broad-spectrum, rapidly microbicidal peptide with in vivo activity. Antimicrob. Agents Chemother. 1997;41:1738–1742. doi: 10.1128/aac.41.8.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner H. Secondary structure of the cecropins: antibacterial peptides from the moth Hyalophora cecropia. FEBS Lett. 1982;137:283–287. doi: 10.1016/0014-5793(82)80368-2. [DOI] [PubMed] [Google Scholar]
- Steiner H., Andreu D., Merrifield R.B. Binding and action of cecropin and cecropin analogues: antibacterial peptides from insects. Biochim. Biophys. Acta. 1988;939:260–266. doi: 10.1016/0005-2736(88)90069-7. [DOI] [PubMed] [Google Scholar]
- Van Hofsten P., Faye I., Kockum K., Lee J., Xanthopoulos K., Boman I., Boman H., Engström A., Andreu D., Merrifield R. Molecular cloning, cDNA sequencing, and chemical synthesis of cecropin B from Hyalophora cecropia. Proc. Nat. Acad. Sci. USA. 1985;82:2240. doi: 10.1073/pnas.82.8.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanni M., Merenda M., Barigazzi G., Garbarino C., Luppi A., Tognetti R., Intorre L. Antimicrobial Resistance of Actinobacillus Pleuropneumoniae Isolated from Swine. Vet. Microbiol. 2011;156:172–177. doi: 10.1016/j.vetmic.2011.10.022. [DOI] [PubMed] [Google Scholar]
- Veldhuizen E.J.A., Rijnders M., Claassen E.A., Van Dijk A., Haagsman H.P. Porcine β-defensin 2 displays broad antimicrobial activity against pathogenic intestinal bacteria. Mol. Immunol. 2008;45:386–394. doi: 10.1016/j.molimm.2007.06.001. [DOI] [PubMed] [Google Scholar]
- Wu J.M., Jan P.S., Yu H.C., Haung H.Y., Fang H.J., Chang Y.I., Cheng J.W., Chen H.M. Structure and function of a custom anticancer peptide, CB1a. Peptides. 2009;30:839–848. doi: 10.1016/j.peptides.2009.02.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.