Abstract
It has been suggested that activation of receptor PTKs is important for leukemogenesis and leukemia cell response to targeted therapy in hematological malignancies including leukemia. PTKs induce activation of the PI3K/Akt/mTOR pathway, which can result in prevention of apoptosis. Here, we describe an important role of the TrkC-associated molecular network in the process of leukemogenesis. TrkC was found to be frequently overexpressed in human leukemia cells and leukemia subtypes. In U937 human leukemia cells, blockade of TrkC using small hairpin RNA (shRNA) specific to TrkC or K562a, a specific inhibitor of TrkC, resulted in a significant decrease in growth and survival of the cells, which was closely associated with reduced mTOR level and Akt activity. In addition, TrkC enhances the survival and proliferation of leukemia, which is correlated with activation of the PI3K/Akt pathway. Moreover, TrkC significantly inhibits apoptosis via induction of the expression of PLK-1 and Twist-1 through activation of AKT/mTor pathway; therefore, it plays a key role in leukemogenesis. These findings reveal an unexpected physiological role for TrkC in the pathogenesis of leukemia and have important implications for understanding various hematological malignancies.
Keywords: apoptosis, hematological malignancies, PI3K/Akt/Mtor, survival and growth, TrkC
INTRODUCTION
There is growing evidence of involvement of multiple protein-tyrosine kinase (PTK) oncogenes, such as PDGFR, c-kit, and FLT3, their immediate downstream targets, and/or of proteins regulating their function in hematologic malignancies. Mutations of upstream receptors such as FLT3 (20–30%), KIT (7–17%), and FMS (12%), have been documented in acute myleloid leukermia (AML) and in myelodysplasic syndrome (MDS). Furthermore, these mutations may cause activation of the PI3K/Akt/mTOR pathway, which can result in prevention of apoptosis (Christiansen et al., 2005; Dong et al., 1995; Kiyoi et al., 1998; Padua et al., 1998; Shimada et al., 2006). Moreover, it has been suggested that coactivation of receptor PTKs is important to leukemia development and may affect the leukemia cell response to targeted therapy (Stommel et al., 2007).
TrkC, a member of the tropomyosin-related kinase (Trk) family of neurotrophin receptors, and its neurotrophin ligand (NT-3) primarily regulate growth, differentiation, and survival of neurons (Huang and Reichardt, 2003; Segal, 2003). TrkC is also highly expressed in good-prognosis neuroblastomas as well as medulloblastomas, and has been shown to be integrally involved in neuroblastoma biology (Grotzer et al., 2000; Segal et al., 1994; Yamashiro et al., 1997). Furthermore, although classical activation of TrkC occurs via receptor multimerization in response to ligand binding, TrkC activation in human tumors seems to be largely attributable to over-expression of the full-length protein. TrkC have also been found in several nonneural cell types (medullary thyroid carcinoma, breast cancer, colon cancer, lung cancer, liver cancer, prostate cancer) and may also play a crucial role in the initiation, progression, and metastasis of many tumors in humans (Chen-Tsai et al., 2004; Grotzer et al., 2000; Hisaoka et al., 2002; Jin et al., 2007; 2010; 2011; McGregor et al., 1999; Satoh et al., 2001). Importantly, there is increasing evidence of the involvement of TrkC in leukemogenesis. The breakpoints of t(12;15)(p13;q25), which result in ETV6-NTRK3, have also been identified in a case report of adult AML and breast cancer (Eguchi et al., 1999; Tognon et al., 2002). This rearrangement generates a gene fusion encoding the sterile α motif domain of the ETV6 (TEL) transcription factor linked to the PTK domain of the neurotrophin-3 receptor NTRK3 (TrkC) (Knezevich et al., 1998). The resulting ETV6-NTRK3 (EN) protein functions as a chimeric PTK with a potent transforming activity (Liu et al., 2000; Wai et al., 2000). Moreover, TrkC activation in AML patients attributable to overexpression of TrkC and shown a clear correlation of TrkC expression pattern and the FAB classification (Li et al., 2009). These results indicate that TrkC activation/overexpression may also play a crucial role in leukemogenesis. However, relatively little is known about the mechanisms of oncogenesis mediated by TrkC activation/overexpression in leukemogenesis. In this manuscript, we identified a molecular and functional network present in leukemogenesis that regulates and coordinates with TrkC. Surprisingly, we find that TrkC is mainly present in the leukemia and acted as a key regulator of PLK-1/Twist-1 through activation of PI3K/Akt/mTOR pathway. Our studies provide molecular insights into leukemogenesis and have important implications to hematological malignancies including leukemia.
MATERIALS AND METHODS
Cell culture and reagents
Human leukemia cell lines (KG-1, HL-60, Meg01, KU812, CCRF-HSB2, NB4, K252, CCRF-SB, Jurkat, ML-1, and U937) were maintained in RPMI 1640 (Gibco) supplemented with 10% heat-inactivated fetal bovine serum at 37°C in a humidified 5% CO2 incubator. The protein kinase inhibitor K252a was purchased from Calbiochem (USA).
Plasmids
Each of the two shRNA-encoding oligonucleotides against human TrkC were designed and verified to be specific for TrkC by BLAST searches against the human genome (Supplementary Table 1). The human TrkC-shRNA insert was subcloned into the pLKO lentiviral vector. A control shRNA that did not match any known human coding cDNA was used as a control.
Antibodies and Western blotting
We conducted western blotting as previously described (Jin et al., 2010). Antibodies were obtained from the following companies: anti-TrkC, anti-PLK-1, anti-Twist-1, anti-mTOR and anti-phospho-mTOR from Abcam (USA); anti-PARP, anti-caspase-3 and anti-phospho-Akt from Cell Signaling Technology (USA); anti-β-actin from Sigma-Aldrich (USA).
Reverse transcription-PCR reactions
Total RNA was extracted using Qiazol reagent (Qiagen, Inc., USA). Reverse transcription was conducted using a one-step RT-PCR kit according to the manufacturer’s instructions (Qiagen, Inc). The forward and reverse primers were GCACA TTGACTTTGGGGACT and ACTGGCCAGCAGAGTAGGAA for human mTOR, CTCTCCCAAATGCTCCACAT and TCCGG TACATGATGCTTTCA for human TrkC, and GACCCCTTC ATTGACCTCAAC and CTTCTCCATGGTGGTGAAGA for human GAPDH. PCR products were separated on 1% agarose gels and visualized by ethidium bromide.
Quantitative RT-PCR
The primer sequences are listed in the supplemental experimental procedures (Supplementary Table 2). Total RNA was isolated using RNeasy Mini Kits (Qiagen) according to the manufacturer’s instructions and reverse transcribed with hexa-nucleotide mix (Roche). The resulting cDNA was used for PCR using the SYBR-Green Master PCR mix and the Taqman master PCR mix (Applied Biosystems) and performed in triplicate. PCR and data collection were conducted using a 7900HT Fast Real-Time PCR System (Applied Biosystems). All quantifications were normalized to the endogenous control 18S RNA. The relative quantitation value for each target gene was compared to the calibrator for that target and expressed as 2−(Ct-Cc).
Cell growth and cell viability assay
U937 control-shRNA, U937 TrkC-shRNAs cells, or U937 cells treated with/without K252a were plated in six-well plates (1 × 105 cells/well), cultured, and counted. Each data point represents the mean of cells counted in three dishes. The cell viability assays were performed using MTT, which determines the mitochondrial activity in living cells. Briefly, cells were plated in 96-multiwell plates at a density of 1 × 104 cells/well and 100 μl DMEM containing MTT (0.5 mg/ml) was then added to each well and incubated in CO2 at 37°C for 3 h. After the incubation period, the media were removed and the cells were washed twice with phosphate buffered saline (PBS). Living cells can transform the tetrazolium ring into dark blue insoluble formazan crystals solubilized with dimethyl sulfoxide (DMSO), which can be quantified at 570 nm using an enzyme-linked immunosorbent assay (ELISA) reader.
Analysis of apoptosis by annexin staining
U937 control-shRNA, U937 TrkC-shRNA cells, and U937 cells treated with K252a were seeded at a density of 1 × 106 cells/ml in 60 mm culture dishes and incubated for 24 h in a CO2 incubator. Apoptosis was determined using an Annexin-V apoptosis detection kit (BD Pharmingen™) according to the manufacturer’s protocol. Flow cytometric data of 10,000 cells/sample were acquired using a FACS Calibur system (Becton Dickinson, USA). Data were analyzed using the computer program Win MDI 2.8.
Microarray data analysis
To compare the expression levels of TrkC in the various hematological malignancies, the correlations between the gene expression profiles of TrkC and the gene expression profiles of tumors from patients with leukemia in the Queen’s University database (GSE13204) were determined (Haferlach et al., 2010). The TrkC gene levels in the Queen’s University dataset were then extracted and averaged in patients with leukemia. ANOVA was performed, and boxplot graphs of gene expression were plotted using GraphPad Prism v 5.0 (GraphPad Software, Inc.). The one-way ANOVA significance for each plot was P < 0.0001.
RESULTS
Leukemia cells aberrantly express TrkC
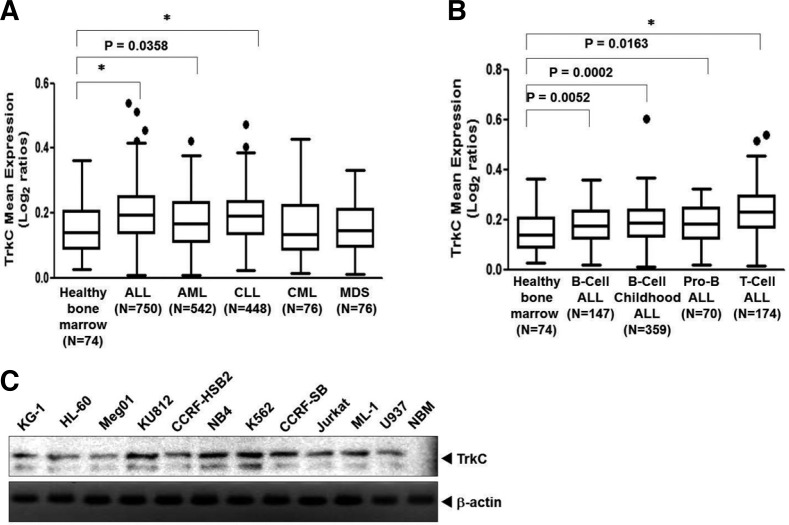
Given the lack of effect of TrkC on leukemogenesis, we attempted to use existing gene expression signatures (GESs) of various types of leukemia to uncover possible connections between TrkC expression and leukemia pathogenesis. We sought to understand the relatedness of TrkC and leukemia pathogenesis in a large cohort of leukemia samples (2,143 patients) (Haferlach et al., 2010). The TrkC gene expression profiles derived from many tumors displayed a high correlation to leukemia and hematological malignancies. TrkC expression was more significantly upregulated in Acute Lymphoblastic Leukemia (ALL), Acute Myeloid Leukemia (AML), and Chronic Lymphocytic Leukemia (CLL) than in healthy bone marrow specimens. However, the expression of TrkC in MDS, and CML did not change when compared to healthy bone marrow specimens and the correlation between TrkC expression and CML/MDS was not significant (Fig. 1A). Interestingly, TrkC expression showed greater upregulation in ALL subtypes than in healthy bone marrow specimens (Fig. 1B). Next, we evaluated TrkC expression in a panel of leukemia cell lines by immunoblotting analysis. TrkC was highly expressed in human leukemia cell lines. In contrast, normal bone marrow samples (NBM) had low to undetectable TrkC levels (Fig. 1C). Our data support the role of TrkC as a leukemogenesis enforcer and further indicate that it may function to induce leukemia progression.
Fig. 1.
TrkC is overexpressed in human leukemia cells. (A) TrkC gene expression is correlated with leukemia subtypes. The gene expression data were plotted as box plots of the mean expression of the TrkC gene. The TrkC gene level was extracted from the dataset and averaged in each tumor. Values represent the log2 ratio over control. The one-way ANOVA significance for each plot was P < 0.0001. *P < 0.0001 as determined by a Student’s t-test. (B) TrkC gene expression is correlated with human Acute Lymphoblastic Leukemia (ALL) subtypes. The gene expression data were plotted as box plots of the mean expression of the TrkC gene. The TrkC gene level was extracted from the dataset and averaged in each tumor. Values represent the log2 ratio over control. The one-way ANOVA significance for each plot was P < 0.0001. *P < 0.0001 as determined by a Student’s t-test. (C) The expression of TrkC protein in a panel of human normal bone marrow (NBM) and leukemia cell lines was examined by immunoblotting analysis. The endogenous β-actin level was measured as an internal control. NBM, Normal bone marrow.
TrkC enhances growth of leukemia cells by induction of PI3K/Akt/mTOR pathways
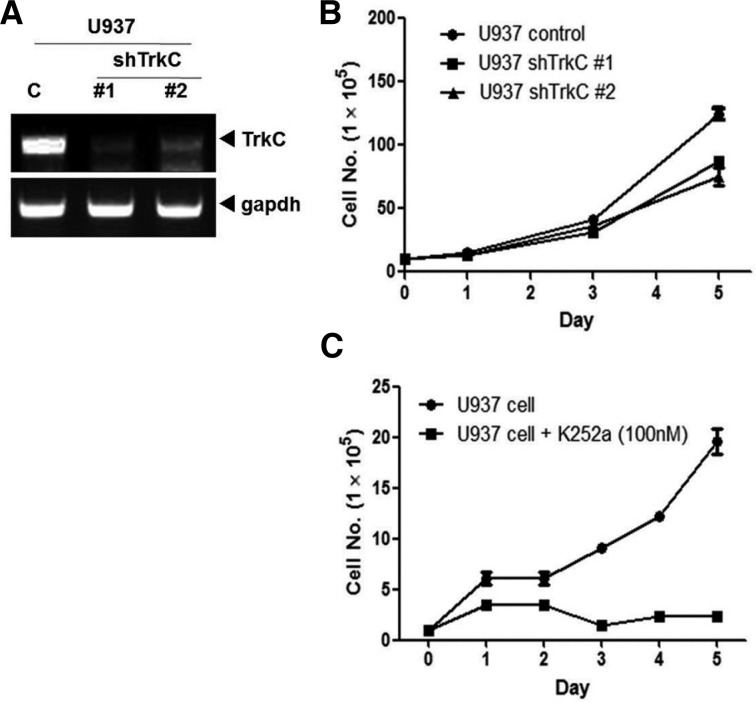
Leukemia cells, but not normal bone marrow samples, expressed high levels of TrkC, suggesting that this protein may be a promising molecular therapeutic target for the treatment of leukemia. To determine the necessity of TrkC in the regulation of leukemogenesis, we used a lentivirus to stably express shRNA against TrkC in leukemia cells. As shown in Fig. 2A, TrkC shRNAs partially suppressed the expression of endogenous TrkC (i.e., an 70% reduction). Moreover, we tested whether inhibition of TrkC expression in U937 cells affected their proliferation in vitro. Stable knockdown of TrkC by shRNA reduced proliferation of U937 cells when compared with control shRNA transfected cells (Fig. 2B), demonstrating the important role of endogenous TrkC in the regulation of leukemia proliferation. In addition, to determine whether TrkC is an important mediator of the tumorigenic potential of U937 cells, we examined whether the pharmacological inhibition of TrkC by K252a, an inhibitor of Trk (NTRK) tyrosine kinases, would influence the ability of U937 cells to proliferate. U937 cells that were not treated with K252a proliferated well, whereas U937 cells treated with K252a had significantly lower growth. These findings suggest that the tyrosine kinase activity of TrkC affects U937 cell survival (Fig. 2C).
Fig. 2.
Suppression of TrkC expression with stable TrkC-shRNA reduces cell proliferation. (A) The expression of TrkC was examined by RT-PCR in U937 cells stably expressing control-shRNA, TrkC-shRNA #1, and TrkC-shRNA #2. GAPDH was used as a loading control. (B) The population doubling of U937 control-shRNA and U937 TrkC-shRNAs cells. Each data point represents the mean of cells counted in three dishes. (C) The population doubling of U937 cells with or without treatment of K252a. U937 cells were grown in 6-well plates and treated with K252a (100 nM) or vehicle control [dimethyl sulfoxide (DMSO)] for five days. Each data point represents the mean of cells counted in three dishes.
TrkC inhibits apoptosis of leukemia
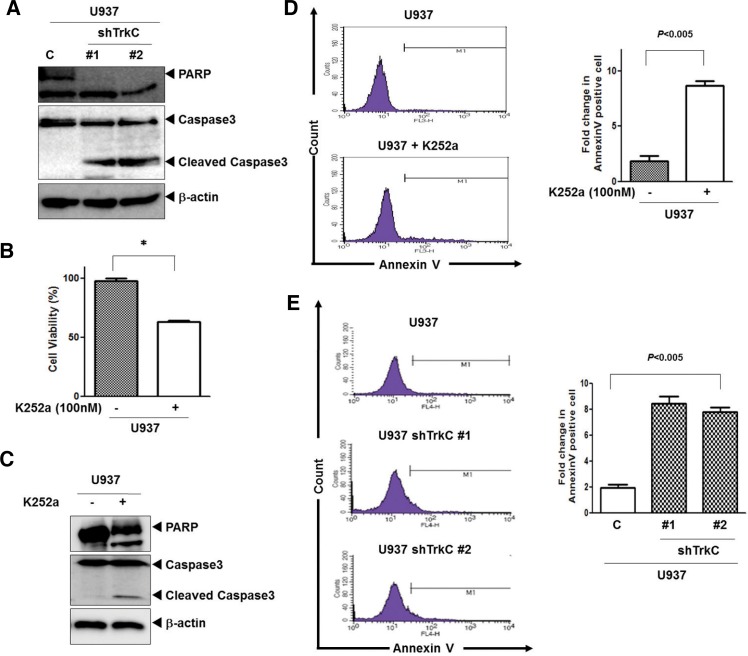
To further identify the inhibition mechanism of apoptosis of TrkC, we investigated the activation of caspase-3 and cleavage of PARP. As shown in Fig. 3A, U937 TrkC-shRNA cells significantly increased the activation of caspase-3 and the cleavage of PARP (an endogenous substrate of caspase-3) when compared to U937 control-shRNA cells. Moreover, we examined whether pharmacological inhibition of TrkC with K252a, an inhibitor of Trk (NTRK) tyrosine kinases, would influence the ability of U937 cells to survive and proliferate by MTT assay. Indeed, U937 cells treated with K252a grew more slowly than the parental cells (Fig. 3B). Moreover, U937 cells treated with K252a significantly increased the activation of caspase-3 and the cleavage of PARP when compared to untreated K252a (Fig. 3C). To further characterize the inhibition of apoptosis by TrkC, we assessed the translocation of phosphatidylserine using annexin V. As shown in Figs. 3D and 3E, the numbers of early apoptosis cells (annexin V-positive) increased by up to 5-fold in U937 cells treated with K252a and U937 cells expressing TrkC-shRNA, but they were markedly decreased in untreated U937 cells or U937 control-shRNA cells. These findings suggest that Inhibition of caspase-3 activation and the cleavage of PARP by TrkC inhibits apoptosis.
Fig. 3.
Loss of TrkC leads to loss of cell viability. (A) Cleavage of procaspase-3 and PARP of U937 control-shRNA or U937 TrkC-shRNA cells was analyzed by Western blotting. (B) Cell viability of U937 cells after treatment with K252a (100 nM) or vehicle control [dimethyl sulfoxide (DMSO)] was determined by MTT assay. Experiments were performed and the results shown reflect the mean and standard mean error (SEM) of each group. The experiments were repeated three times with similar results. *P < 0.0001 as determined by a Student’s t-test. (C) Cleavage of procaspase-3 and PARP of U937 cells with or without treatment with K252a was analyzed by Western blotting. (D) Representative FACS histograms indicating the percentage of apoptotic U937 cells after treatment with K252a as determined by binding of FITC-conjugated Annexin V. FACS analyses were conducted 24 h after culture. Right columns, mean number of Annexin V-positive cells expressed as a percentage of total cells. (E) Representative FACS histograms indicating the percentage of apoptotic U937 control-shRNA or U937 TrkC-shRNA cells as determined by binding of FITC-conjugated Annexin V. FACS analyses were conducted 24 h after culture. Right columns, mean number of Annexin V-positive cells expressed as a percentage of total cells.
TrkC inhibits apoptosis of leukemia through activation of Akt/mTOR pathway
Akt or protein kinase B (Akt/PKB), a serine/threonine protein kinase, has emerged as a crucial regulator of widely divergent cellular processes involved in leukemic cell biology, including apoptosis, proliferation, differentiation, and metabolism (Min et al., 2003). Moreover, mTOR serine/threonine kinase belongs to two multi-protein complexes, referred to as mTORC1 and mTORC2. mTOR-generated signals play critical roles in leukemic cell biology by controlling mRNA translation of genes that promote proliferation and survival (Evangelisti et al., 2011).
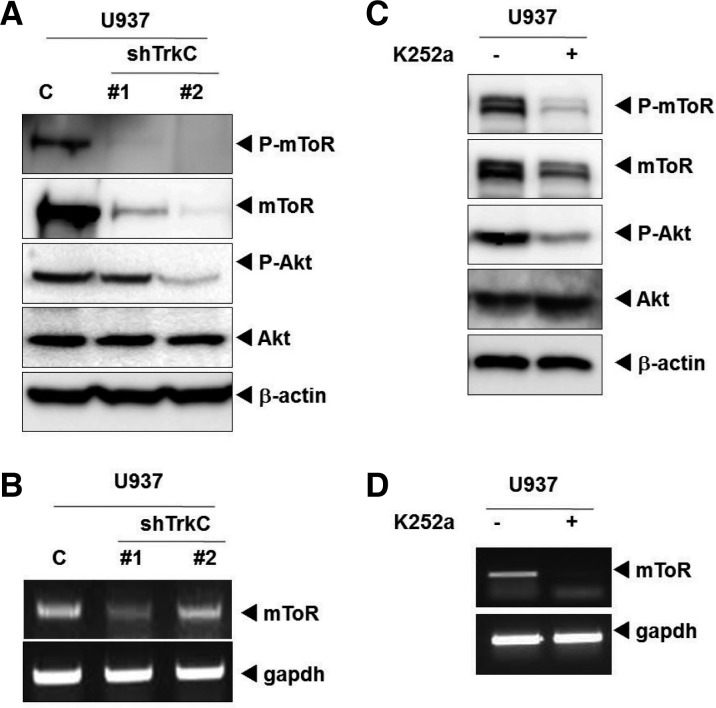
To better understand the mechanism underlying enhancement of the growth of leukemia cells by TrkC, we examined whether TrkC expression affects PI3K/Akt/mTOR pathways. During immunoblotting analysis, Akt and mTOR phosphorylation levels were markedly reduced in U937 TrkC-shRNA cells when compared to U937 control-shRNA cells. Interestingly, mTOR expression levels were also markedly reduced in U937 TrkC-shRNA cells when compared to U937 control-shRNA cells (Fig. 4A). Because TrkC knockdown reduced the total mTOR expression level, we examined transcriptionally regulated mToR expression in U937 control-shRNA or TrkC-shRNA cells. TrkC knockdown significantly reduced mTOR mRNA levels (Fig. 4B), suggesting that TrkC regulates mTOR expression at the transcriptional level. Moreover, U937 cells treated with the Trk inhibitor K252a significantly reduced P-mTOR, mTOR, and P-Akt expression levels (Fig. 4C). Finally, inhibition of tyrosine kinase activity of TrkC by treatment with K252a significantly reduced mToR mRNA levels (Fig. 4D), suggesting that tyrosine kinase activity of TrkC regulates mTOR expression at the transcriptional level, and affects U937 cell survival.
Fig. 4.
TrkC regulates Akt activity and directly regulates its downstream target, mTOR. (A) The expression of phospho-Akt, phosphor-mTOR, and mTOR was examined in U937 control-shRNA or U937 TrkC-shRNA cells by immunoblotting. β-actin was used as a loading control. (B) The relative expression level of mRNAs encoding mTOR in U937 control-shRNA or U937 TrkC-shRNA cells, as determined by real-time PCR. The endogenous GAPDH mRNA level was measured as an internal control. (C) The expression of phospho-Akt, phosphor-mTOR, Akt, and mTOR of U937 cells with or without treatment with K252a. The U937 cells were grown and treated with K252a (100 nM) or vehicle control [dimethyl sulfoxide (DMSO)]. (D) The relative expression level of mRNAs encoding mTOR of U937 cells with or without treatment by K252a, as determined by real-time PCR. The endogenous GAPDH mRNA level was measured as an internal control.
TrkC induces PLK-1 and Twist-1 expression
PLK-1 is overexpressed in AML, and its inhibition selectively reduces proliferation and induces apoptosis of leukemic cells (Ikezoe et al., 2009). In addition, PLK-1 inhibition or depletion using pharmacological and siRNA approaches decreases the phosphorylation of two mToR substrates in AML cells (Renner et al., 2010) and PLK-1 expression in leukemia cells is regulated through a PI3K- and p38-dependent pathway (Tang et al., 2008). Another novel key prognostic factor in leukenogenesis, Twist-1, is overexpressed in CML patients who later develop cytogenetic resistance to imatinib, whereas Twist-1 overexpression is involved in the resistance phenotype (Cosset et al., 2011). Also, Akt/protein kinase B (PKB)-mediated Twist1 phosphorylation promotes epithelial-mesenchymal transition (EMT) and breast cancer metastasis by modulating its transcriptional target TGF-β2, leading to enhanced TGF-β receptor signaling (Xue et al., 2012).
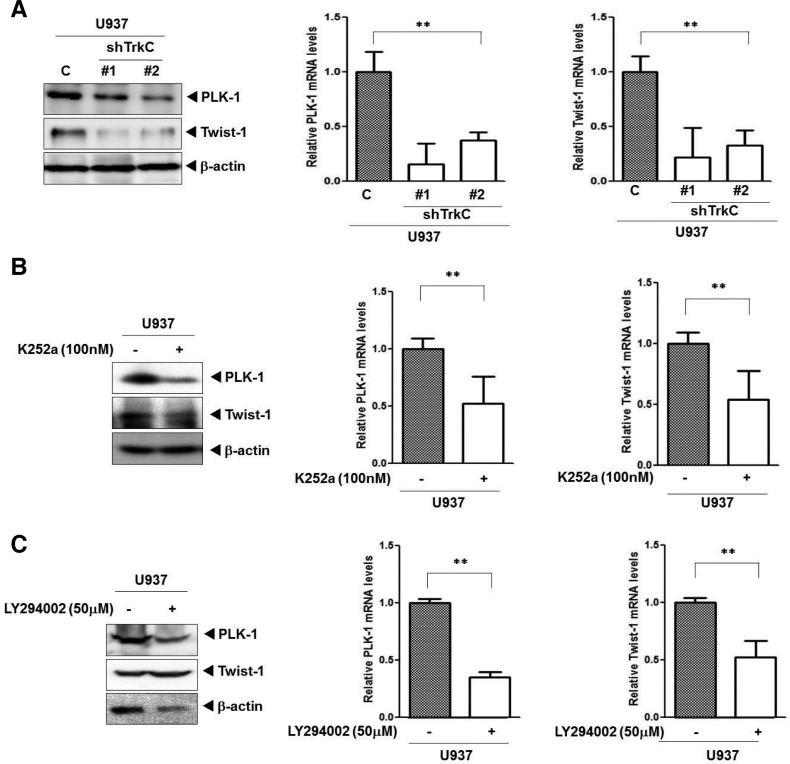
To further understand the functional requirement of endogenous TrkC in leukemia cells, we evaluated whether TrkC regulates PLK-1 and Twist-1, identified as being activated at the beginning of leukemogenesis. In the present study, we found that U937 TrkC-shRNA cells led to systematic decreased expression of PLK-1 and Twist-1 (Fig. 5A) when compared to U937 control-shRNA cells. In addition, ML-1 TrkC-shRNA and Meg01 TrkC-shRNA cells markedly reduced PLK-1 and Twist-1 expression when compared to ML-1 or Meg01 control-shRNA cells (Supplementary Figs. S1A and S2A). Similar results were obtained when U937, ML-1, and Meg01 cells were treated with K252a, an inhibitor of Trk tyrosine kinases. The inhibition of TrkC activation by K252a treatment significantly reduced PLK-1, and Twist-1 expression (Fig. 5B, Supplementary Figs. S1B, and S2B). Next, we examined whether activation of PI3K/Akt/mTOR pathway by TrkC regulates PLK-1 and Twist-1 expression. The inhibition of Akt activation of U937, ML-1, and Meg01 cells by Akt inhibitor (LY294002) significantly reduced PLK-1, and Twist-1 expression (Fig. 5C, Supplementary Figs. S1C, and S2C). These results further suggest that TrkC induces the proliferation and survival of leukemia cells through regulation of Twist-1 and PLK-1.
Fig. 5.
TrkC induces leukemogenesis by upregulating PLK-1 and Twist-1 as downstream targets. (A) The relative expression levels of PLK-1, and Twist-1 was examined with immunoblotting and quantitative RT-PCR in U937 control-shRNA or U937 TrkC-shRNA cells. The data are reported as the mean ± SEM. **P < 0.05 as determined by the Student’s t-test. (B) The relative expression levels of PLK-1, and Twist-1 was examined with immunoblotting and quantitative RT-PCR in U937 cells after K252a treatment. The data are reported as the mean ± SEM. **P < 0.05 as determined by a Student’s t-test. (C) The relative expression levels of PLK-1, and Twist-1 was examined with immunoblotting and quantitative RT-PCR in U937 cells after LY294002 treatment. The data are reported as the mean ± SEM. **P < 0.05 as determined by a Student’s t-test.
DISCUSSION
TrkC is now believed to play an important part in human cancer; accordingly, a better understanding of the involvement of TrkC in leukemogenesis is obviously the first step in taking full advantage of its use as a target for hematological malignancies. Previous work has demonstrated expression of TrkC is elevated in AML patients and TrkC expression always occurred concomitantly with TrkB (Li et al., 2009). Whereas previous work has demonstrated a clear correlation of TrkC expression pattern in AML patients, how TrkC functionally and mechanistically involve in leukemogenesis are poorly understood. In the present study, we demonstrated that the expression of TrkC is elevated in human leukemia patients and leukemia cell lines. In contrast to previous study (Li et al., 2009), TrkC was significantly upregulated in ALL, AML, and CLL samples when compared to healthy bone marrow samples. These results provide evidence that TrkC may plays an important role in leukemogenesis. Also, inhibition of TrkC expression by shRNA results in significantly slower growth. In addition, the tyrosine kinase activity of TrkC leads to the continuous proliferation of leukemia cells, and plays an important role in the activation of Akt and mTOR expression. Moreover, TrkC induces total mTOR expression at the transcriptional level. Deregulation of the PI3K/Akt/mTOR pathway has been reported in all major types of epithelial cancers (Hawkins et al., 2006; Manning and Cantley, 2007) and leukemia (Park et al., 2010). Many of the events elicited via PI3K/Akt/mTOR pathways have direct effects on survival pathways and hematologic malignancies. Akt activation is induced in the course of signal transduction by growth factors or insulin and is involved in many cellular processes, such as cell growth and survival, glucose metabolism, and transcriptional regulation (Song et al., 2005). Moreover, the signaling pathway involving PI3-kinase, Akt, and mTOR kinases, which is stimulated by survival signals to block apoptosis, also inhibits autophagy. Specifically, when survival signals are insufficient, the PI3K signaling pathway is downregulated and autophagy and/or apoptosis may be induced (Park et al., 2010). In addition, promotion of cell migration requires Akt activation in melanoma (Fenouille et al., 2012) and aberrant regulation of survival pathways can contribute to uncontrolled cell growth and lead to leukemia (Renner et al., 2010), and our data suggest that in leukemia, deregulation of the PI3K/Akt/mTOR pathway can be caused by the overexpression of TrkC.
PLK-1 and Twist-1 have been identified as being activated upon entrance into leukemogenesis. PLK-1 is a serine/threonine kinase that plays an essential role in mitosis, in the maintenance of genomic stability, and in cell cycle progression in normal and neoplastic cells. PLK-1 is also overexpressed in human tumors and has prognostic potential in cancer, indicating its involvement in carcinogenesis and its potential as a therapeutic target (Ikezoe et al., 2009). Moreover, PLK-1 inhibitors counteract cell cycle progression and growth in solid tumors or hematologic malignancies (Renner et al., 2010). Furthermore, PLK-1 expression in leukemia cells is regulated through a PI3K- and p38-dependent pathway (Gleixner et al., 2010; Tang et al., 2008). Twist is upregulated in several types of epithelial cancers, including breast, prostate, and gastric carcinomas (Puisieux et al., 2006). Also, Twist transcriptionally upregulates AKT2 in breast cancer cells, leading to increased migration and invasion (Cheng et al., 2007). Moreover, cells expressing Twist-1 display inefficient p53 upregulation in response to DNA damage induced by γ-irradiation or the genotoxic drug adriamycin, which influences the activation of p53 target genes such as p21Waf1 and Bax, and leads to aberrant cell-cycle regulation and inhibition of apoptosis (Vichalkovski et al., 2010). In addition, Twist leads to the acquisition of stem cell characteristics through EMT, and Twist expression in highly metastatic mammary carcinoma cells specifically induces their ability to metastasize from the mammary gland to the lung. These results suggest that Twist-1 is associated with the metastatic process (Mani et al., 2008). Twist-1 was recently used as a powerful biomarker for early detection of TKI resistance. Specifically, it was found to be overexpressed in CML diagnostic samples of patients who later developed cytogenetic resistance to imatinib, even those without any detectable resistance mechanism (Ikezoe et al., 2009). Our data demonstrated that knockdown of TrkC or treatment with K252a almost completely inhibited PIK1 and Twist-1 expression. In summary, our results suggest that TrkC is a physiologically critical regulator of the growth and survival of leukemia cells. Moreover, TrkC, through its regulation of PLK-1 and Twist-1 by activation of Akt/mTOR pathway, serves as a master enforcer of the fate of leukemia cells, and that inhibition of TrkC may have a notable impact on leukemogenesis. Therefore, the role of TrkC in leukemogenesis deserves further attention.
Supplementary Material
Acknowledgments
This work was supported by a National Research Foundation of Korea (NRF) grant funded by the government of the Republic of Korea (Ministry of Education, Science and Technology) (NRF-2010-0002525 and NRF-2012R1A2A2A01002728 to Jin, W.). The English in this document has been checked by at professional editor (Nurisco company).
Note:
Supplementary information is available on the Molecules and Cells website (www.molcells.org).
REFERENCES
- Chen-Tsai CP, Colome-Grimmer M, Wagner RF., Jr (2004). Correlations among neural cell adhesion molecule, nerve growth factor, and its receptors, TrkA, TrkB, TrkC, and p75, in perineural invasion by basal cell and cutaneous squamous cell carcinomas. Dermatol Surg. 30, 1009–1016 [DOI] [PubMed] [Google Scholar]
- Cheng GZ, Chan J, Wang Q, Zhang W, Sun CD, Wang LH. (2007). Twist transcriptionally up-regulates AKT2 in breast cancer cells leading to increased migration, invasion, and resistance to paclitaxel. Cancer Res. 67, 1979–1987 [DOI] [PubMed] [Google Scholar]
- Christiansen DH, Andersen MK, Desta F, Pedersen-Bjergaard J. (2005). Mutations of genes in the receptor tyrosine kinase (RTK)/RAS-BRAF signal transduction pathway in therapy-related myelodysplasia and acute myeloid leukemia. Leukemia 19, 2232–2240 [DOI] [PubMed] [Google Scholar]
- Cosset E, Hamdan G, Jeanpierre S, Voeltzel T, Sagorny K, Hayette S, Mahon FX, Dumontet C, Puisieux A, Nicolini FE, et al. (2011). Deregulation of TWIST-1 in the CD34+ compartment represents a novel prognostic factor in chronic myeloid leukemia. Blood 117, 1673–1676 [DOI] [PubMed] [Google Scholar]
- Dong F, Brynes RK, Tidow N, Welte K, Lowenberg B, Touw IP. (1995). Mutations in the gene for the granulocyte colony-stimulating-factor receptor in patients with acute myeloid leukemia preceded by severe congenital neutropenia. N Engl J Med. 333, 487–493 [DOI] [PubMed] [Google Scholar]
- Eguchi M, Eguchi-Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, Kudoh S, Tanaka K, Setoyama M, Nagamura F, et al. (1999). Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25). Blood 93, 1355–1363 [PubMed] [Google Scholar]
- Evangelisti C, Ricci F, Tazzari P, Tabellini G, Battistelli M, Falcieri E, Chiarini F, Bortul R, Melchionda F, Pagliaro P, et al. (2011). Targeted inhibition of mTORC1 and mTORC2 by active-site mTOR inhibitors has cytotoxic effects in T-cell acute lymphoblastic leukemia. Leukemia 25, 781–791 [DOI] [PubMed] [Google Scholar]
- Fenouille N, Tichet M, Dufies M, Pottier A, Mogha A, Soo JK, Rocchi S, Mallavialle A, Galibert MD, Khammari A, et al. (2012). The epithelial-mesenchymal transition (EMT) regulatory factor SLUG (SNAI2) is a downstream target of SPARC and AKT in promoting melanoma cell invasion. PLoS One 7, e40378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gleixner KV, Ferenc V, Peter B, Gruze A, Meyer RA, Hadzijusufovic E, Cerny-Reiterer S, Mayerhofer M, Pickl WF, Sillaber C, et al. (2010). Polo-like kinase 1 (Plk1) as a novel drug target in chronic myeloid leukemia: overriding imatinib resistance with the Plk1 inhibitor BI 2536. Cancer Res. 70, 1513–1523 [DOI] [PubMed] [Google Scholar]
- Grotzer MA, Janss AJ, Fung K, Biegel JA, Sutton LN, Rorke LB, Zhao H, Cnaan A, Phillips PC, Lee VM, et al. (2000). TrkC expression predicts good clinical outcome in primitive neuroectodermal brain tumors. J Clin Oncol. 18, 1027–1035 [DOI] [PubMed] [Google Scholar]
- Haferlach T, Kohlmann A, Wieczorek L, Basso G, Kronnie GT, Bene MC, De Vos J, Hernandez JM, Hofmann WK, Mills KI, et al. (2010). Clinical utility of microarray-based gene expression profiling in the diagnosis and subclassification of leukemia: report from the International Microarray Innovations in Leukemia Study Group. J Clin Oncol. 28, 2529–2537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins PT, Anderson KE, Davidson K, Stephens LR. (2006). Signalling through class I PI3Ks in mammalian cells. Biochem Soc Trans. 34, 647–662 [DOI] [PubMed] [Google Scholar]
- Hisaoka M, Sheng WQ, Tanaka A, Hashimoto H. (2002). Gene expression of TrkC (NTRK3) in human soft tissue tumours. J Pathol. 197, 661–667 [DOI] [PubMed] [Google Scholar]
- Huang EJ, Reichardt LF. (2003). Trk receptors: roles in neuronal signal transduction. Ann Rev Biochem. 72, 609–642 [DOI] [PubMed] [Google Scholar]
- Ikezoe T, Yang J, Nishioka C, Takezaki Y, Tasaka T, Togitani K, Koeffler HP, Yokoyama A. (2009). A novel treatment strategy targeting polo-like kinase 1 in hematological malignancies. Leukemia 23, 1564–1576 [DOI] [PubMed] [Google Scholar]
- Jin W, Yun C, Kim HS, Kim SJ. (2007). TrkC binds to the bone morphogenetic protein type II receptor to suppress bone morphogenetic protein signaling. Cancer Res. 67, 9869–9877 [DOI] [PubMed] [Google Scholar]
- Jin W, Kim GM, Kim MS, Lim MH, Yun C, Jeong J, Nam JS, Kim SJ. (2010). TrkC plays an essential role in breast tumor growth and metastasis. Carcinogenesis 31, 1939–1947 [DOI] [PubMed] [Google Scholar]
- Jin W, Lee JJ, Kim MS, Son BH, Cho YK, Kim HP. (2011). DNA methylation-dependent regulation of TrkA, TrkB, and TrkC genes in human hepatocellular carcinoma. Biochem Biophys Res Commun. 406, 89–95 [DOI] [PubMed] [Google Scholar]
- Kiyoi H, Towatari M, Yokota S, Hamaguchi M, Ohno R, Saito H, Naoe T. (1998). Internal tandem duplication of the FLT3 gene is a novel modality of elongation mutation which causes constitutive activation of the product. Leukemia 12, 1333–1337 [DOI] [PubMed] [Google Scholar]
- Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. (1998). A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 18, 184–187 [DOI] [PubMed] [Google Scholar]
- Li Z, Beutel G, Rhein M, Meyer J, Koenecke C, Neumann T, Yang M, Krauter J, von Neuhoff N, Heuser M, et al. (2009). High-affinity neurotrophin receptors and ligands promote leukemogenesis. Blood 113, 2028–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Schwaller J, Kutok J, Cain D, Aster JC, Williams IR, Gilliland DG. (2000). Signal transduction and transforming properties of the TEL-TRKC fusions associated with t(12;15) (p13;q25) in congenital fibrosarcoma and acute myelogenous leukemia. EMBO J. 19, 1827–1838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, Brooks M, Reinhard F, Zhang CC, Shipitsin M, et al. (2008). The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133, 704–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning BD, Cantley LC. (2007). AKT/PKB signaling: navigating downstream. Cell 129, 1261–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor LM, McCune BK, Graff JR, McDowell PR, Romans KE, Yancopoulos GD, Ball DW, Baylin SB, Nelkin BD. (1999). Roles of trk family neurotrophin receptors in medullary thyroid carcinoma development and progression. Proc Natl Acad Sci USA 96, 4540–4545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, Jeung HK, Lee ST, Lee MH, Hahn JS, Ko YW. (2003). Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia 17, 995–997 [DOI] [PubMed] [Google Scholar]
- Padua RA, Guinn BA, Al-Sabah AI, Smith M, Taylor C, Pettersson T, Ridge S, Carter G, White D, Oscier D, et al. (1998). RAS, FMS and p53 mutations and poor clinical outcome in myelodysplasias: a 10-year follow-up. Leukemia 12, 887–892 [DOI] [PubMed] [Google Scholar]
- Park S, Chapuis N, Tamburini J, Bardet V, Cornillet-Lefebvre P, Willems L, Green A, Mayeux P, Lacombe C, Bouscary D. (2010). Role of the PI3K/AKT and mTOR signaling pathways in acute myeloid leukemia. Haematologica 95, 819–828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux A, Valsesia-Wittmann S, Ansieau S. (2006). A twist for survival and cancer progression. Br J Cancer 94, 13–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner AG, Creancier L, Dos Santos C, Fialin C, Recher C, Bailly C, Kruczynski A, Payrastre B, Manenti S. (2010). A functional link between polo-like kinase 1 and the mammalian target-of-rapamycin pathway?. Cell Cycle 9, 1690–1696 [DOI] [PubMed] [Google Scholar]
- Satoh F, Mimata H, Nomura T, Fujita Y, Shin T, Sakamoto S, Hamada Y, Nomura Y. (2001). Autocrine expression of neurotrophins and their receptors in prostate cancer. Int J Urol. 8, S28–34 [DOI] [PubMed] [Google Scholar]
- Segal RA. (2003). Selectivity in neurotrophin signaling: theme and variations. Ann Rev Neurosci. 26, 299–330 [DOI] [PubMed] [Google Scholar]
- Segal RA, Goumnerova LC, Kwon YK, Stiles CD, Pomeroy SL. (1994). Expression of the neurotrophin receptor TrkC is linked to a favorable outcome in medulloblastoma. Proc Natl Acad Sci USA 91, 12867–12871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimada A, Taki T, Tabuchi K, Tawa A, Horibe K, Tsuchida M, Hanada R, Tsukimoto I, Hayashi Y. (2006). KIT mutations, and not FLT3 internal tandem duplication, are strongly associated with a poor prognosis in pediatric acute myeloid leukemia with t(8;21): a study of the Japanese Childhood AML Cooperative Study Group. Blood 107, 1806–1809 [DOI] [PubMed] [Google Scholar]
- Song G, Ouyang G, Bao S. (2005). The activation of Akt/PKB signaling pathway and cell survival. J Cell Mol Med. 9, 59–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stommel JM, Kimmelman AC, Ying H, Nabioullin R, Ponugoti AH, Wiedemeyer R, Stegh AH, Bradner JE, Ligon KL, Brennan C, et al. (2007). Coactivation of receptor tyrosine kinases affects the response of tumor cells to targeted therapies. Science 318, 287–290 [DOI] [PubMed] [Google Scholar]
- Tang J, Yang X, Liu X. (2008). Phosphorylation of Plk1 at Ser326 regulates its functions during mitotic progression. Oncogene 27, 6635–6645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, Becker L, Carneiro F, MacPherson N, Horsman D, et al. (2002). Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell 2, 367–376 [DOI] [PubMed] [Google Scholar]
- Vichalkovski A, Gresko E, Hess D, Restuccia DF, Hemmings BA. (2010). PKB/AKT phosphorylation of the transcription factor Twist-1 at Ser42 inhibits p53 activity in response to DNA damage. Oncogene 29, 3554–3565 [DOI] [PubMed] [Google Scholar]
- Wai DH, Knezevich SR, Lucas T, Jansen B, Kay RJ, Sorensen PH. (2000). The ETV6-NTRK3 gene fusion encodes a chimeric protein tyrosine kinase that transforms NIH3T3 cells. Oncogene 19, 906–915 [DOI] [PubMed] [Google Scholar]
- Xue G, Restuccia DF, Lan Q, Hynx D, Dirnhofer S, Hess D, Ruegg C, Hemmings BA. (2012). Akt/PKB-mediated phosphorylation of Twist1 promotes tumor metastasis via mediating cross-talk between PI3K/Akt and TGF-beta signaling axes. Cancer Discov. 2, 248–259 [DOI] [PubMed] [Google Scholar]
- Yamashiro DJ, Liu XG, Lee CP, Nakagawara A, Ikegaki N, McGregor LM, Baylin SB, Brodeur GM. (1997). Expression and function of Trk-C in favourable human neuroblastomas. Eur J Cancer 33, 2054–2057 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.