Abstract
A connection between diet, obesity and diabetes exists in multiple species and is the basis of an escalating human health problem. The factors responsible provoke both insulin resistance and pancreatic beta cell dysfunction but remain to be fully identified. We report a combination of molecular events in human and mouse pancreatic beta cells, induced by elevated levels of free fatty acids or by administration of a high-fat diet with associated obesity, that comprise a pathogenic pathway to diabetes. Elevated concentrations of free fatty acids caused nuclear exclusion and reduced expression of the transcription factors FOXA2 and HNF1A in beta cells. This resulted in a deficit of GnT-4a glycosyltransferase expression in beta cells that produced signs of metabolic disease, including hyperglycemia, impaired glucose tolerance, hyperinsulinemia, hepatic steatosis and diminished insulin action in muscle and adipose tissues. Protection from disease was conferred by enforced beta cell–specific GnT-4a protein glycosylation and involved the maintenance of glucose transporter expression and the preservation of glucose transport. We observed that this pathogenic process was active in human islet cells obtained from donors with type 2 diabetes; thus, illuminating a pathway to disease implicated in the diet- and obesity-associated component of type 2 diabetes mellitus.
Type 2 diabetes is a disease of impaired glucose homeostasis and insulin action with an etiology that encompasses genetic, cellular and environmental factors1,2. Human type 2 diabetes affects hundreds of millions of people, and cases are predicted to double within 20 years3. This escalation in frequency has been closely associated with a widespread change in the human diet in the presence of obesity. Although only a fraction of overweight and obese individuals are diabetic, the majority of newly diagnosed cases of type 2 diabetes reflect this growing segment of the human population4. A high-fat or Western-style diet leading to obesity is evidently a predisposing factor in disease susceptibility and onset5. Moreover, a link between obesity and diabetes is not restricted to humans. When provided to various animal species, a high-fat diet leads to obesity and induces disease signs that model human type 2 diabetes6–8. Yet it is unclear how diet and obesity trigger diabetic pathophysiology. Genetic variation seems to contribute to only a small fraction of disease cases9.
Insulin resistance is a metabolic hallmark of type 2 diabetes. Insulin action can be impaired by the inheritance of genetic mutations that disrupt essential components in the insulin action pathways10. Although this etiology may represent only a small proportion of type 2 diabetes cases, insulin resistance contributes substantially and broadly to the pathophysiology of diabetes in subjects with this disease11,12. The identification of molecular pathways by which organ-specific and systemic insulin resistance develop in response to diet and obesity is important for understanding the etiology of type 2 diabetes and learning how to prevent this disease.
Pancreatic beta cell dysfunction is also a diagnostic determinant of type 2 diabetes and includes defective insulin secretion exemplified by the loss of glucose-stimulated insulin secretion (GSIS). Normally, beta cells sense elevations of blood glucose by expressing cell surface glucose transporters that enable concentration-dependent glucose transport across the plasma membrane. This is followed by glucokinase action and ultimately calcium-dependent insulin secretion. Loss of GSIS has been linked to impaired beta cell glucose transporter (Glut) expression among animal models, and failure of GSIS has been proposed to further provoke the pathogenic onset of type 2 diabetes in humans13–24.
A model of type 2 diabetes has been reported in mice lacking the GnT-4a glycosyltransferase encoded by the Mgat4a gene25. GnT-4a generates the core β1-4 GlcNAc linkage among the N-glycan structures of some glycoproteins26. GnT-4a function promotes beta cell surface residency of the Slc2a2-encoded glucose transporter-2 (Glut-2) glycoprotein needed for glucose uptake and GSIS, and its deficiency is induced by high-fat diet administration concurrent with the onset of diabetes25. These observations suggest that beta cell GnT-4a glycosylation and glucose transport have important roles in the pathogenesis of diabetes.
We undertook studies to identify factors controlling GnT-4a function and glucose transporter expression and to determine whether deficient GnT-4a protein glycosylation and glucose transport cause disease pathogenesis in the mouse model. We further examined human islets from normal and type 2 diabetes donors for the presence of a similar regulatory and pathogenic process. Our studies reveal a sequence of molecular events that links beta cell–specific GnT-4a glycosylation to the control of glycemia, insulinemia and glucose tolerance as well as hepatic steatosis and systemic insulin resistance. Our findings indicate the presence of a conserved pathogenic pathway to diabetes in mice and humans that is linked to a deficiency of beta cell protein glycosylation and glucose transport.
RESULTS
Control of Mgat4a and Slc2a2 expression by Foxa2 and Hnf1a
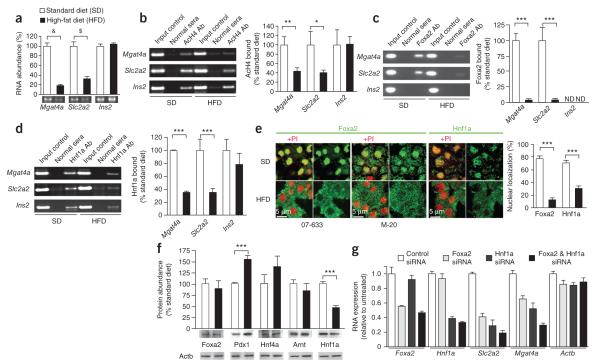
Wild-type (WT) C57BL/6J mice fed a high-fat diet became deficient in Mgat4a and Slc2a2 (also known as Glut2) RNA abundance in pancreatic islet cells, within 3–4 weeks and continuing thereafter, compared with cohorts fed the standard diet (Fig. 1a; ref. 25). We detected reduced acetylation of histone H4 in the promoter regions of the Mgat4a and Slc2a2 genes, suggesting diminished transcription of these alleles (Fig. 1b). Through promoter region DNA sequence analyses of mouse genes Mgat4a and Slc2a2, the orthologous human genes MGAT4A and SLC2A1 (also known as GLUT1) and the human SLC2A2 gene, we identified potential binding sites of multiple transcription factors including mouse and human FOXA2 and HNF A (Supplementary Fig. 1).
Figure 1.
Dietary regulation of Mgat4a and Slc2a2 gene expression by Foxa2 and Hnf1a in mouse pancreatic islet cells. (a) Abundance of mRNA produced from Mgat4a, Slc2a2 and Ins2 genes in mouse pancreatic islet cells (>90% beta cells) isolated from 18- to 22-week-old WT mice on standard (SD) and high-fat diet (HFD) dietary regimens. (b–d) ChIP and real-time PCR (rtPCR) analysis of acetylated histone H4 (AcH4) bound to promoter regions of the Mgat4a, Slc2a2 and Ins2 genes (b) and binding to these regions by Foxa2 (c) and Hnf1a (d). ND, not detected. (e) In situ localization of Foxa2 and Hnf1a proteins in beta cells from mouse islet tissue in response to diet. Results represent analyses of >24 fields of view consisting of >100 beta cells. Two different antibodies that detect Foxa2 (07-633 and M-20) were used. Image analyses quantified percentage of cellular protein localized to nuclear region. (f) Total islet cell abundance of Foxa2, Pdx1, Hnf4a, Arnt and Hnf1a proteins determined using antibodies specific for each factor. (g) Pancreatic beta cells from mice receiving SD transfected with siRNAs to knock down Foxa2 and Hnf1a, or with a siRNA control, were cultured for 72 h followed by mRNA abundance measurements using rtPCR. Mice for study were normal C57BL/6J mice 18–22 weeks old before initial experimentation. Data in a–f are means ± s.e.m. of triplicate experiments per mouse from ≥12 mice consisting of ≥6 separate littermate pairs. &P = 0.0007; $P = 0.0049; *P = 0.0421; **P = 0.0418; ***P < 0.0001 (Student’s t test, a–f; Bonferroni test after analysis of variance (ANOVA), g).rtPCR measurement of FOXA2 (d) and HNF1A (e) proteins bound to promoter regions of human MGAT4A, SLC2A1, SLC2A2 and INS detected; NA, no addition. (f) Abundance of mRNA from MGAT4A, SLC2A1, SLC2A2 and genes in human islet cells. (g secretion assayed in islet cell cultures containing medium bearing indicated concentrations of glucose. Results in a and c fields of view consisting of >100 beta cells. Data are means ± s.e.m. of triplicate experiments per mouse from six mice fed standard diet and four normal human islet donors. *P = 0.01–0.049; **P = 0.005–0.009; ***P = 0.0004; ****P < 0.0001 (Student’s t test).
Through islet cell chromatin immunoprecipitation (ChIP) analyses from mice receiving the standard diet, we found that Foxa2 and Hnf a proteins were bound to the corresponding promoter regions of the Mgat4a and Slc2a2 genes. This binding was significantly reduced in islets from mice that had received the high-fat diet (Fig. 1c,d) coincident with a marked reduction in nuclear localization of Foxa2 and Hnf a from >80% to 3% and 29%, respectively, and concurrent with increased cytoplasmic localization of both factors (Fig. 1e). In islet cells, our measurements of protein abundance further showed that Foxa2 was unaltered whereas Hnf a was reduced by 50% (Fig. 1f). Together, nuclear exclusion and reduced expression of Hnf a combined to yield an overall nuclear deficiency of >80%, similar to that of Foxa2.
We measured Foxa2 and Hnf a transactivation of the Mgat4a and Slc2a2 genes using siRNAs designed to specifically knock down these two transcription factors in cultured WT mouse islet cells. Both Foxa2 and Hnf a were found to transactivate the Mgat4a and Slc2a2 genes, and the knockdown of both factors diminished Mgat4a and Slc2a2 RNA in mice fed the standard diet to similar levels measured after high-fat diet feeding (Fig. 1g). Foxa2 and Hnf a each contributed to Mgat4a and Slc2a2 transactivation in islets of normal mice fed the standard diet, and this transactivation was impaired in islets of mice fed the high-fat diet.
Palmitic acid recapitulates beta cell dysfunction
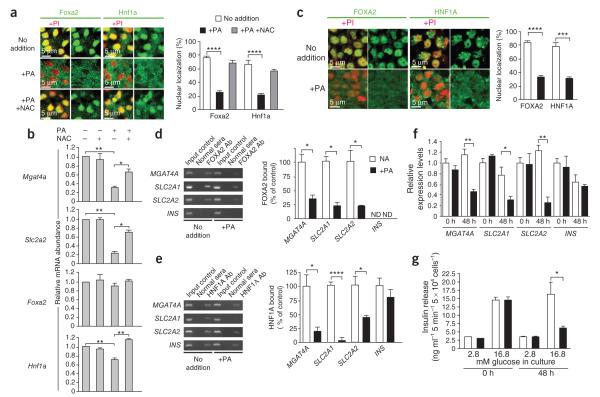
Islet cells were isolated from WT mice fed the standard diet and cultured for 48 h with or without palmitic acid, a lipid used to model the diabetogenic effect of increased free fatty acids. Palmitic acid induced nuclear exclusion of Foxa2 and Hnf a, whereas co-incubation with the antioxidant N-acetylcysteine blocked this effect (Fig. 2a). Palmitic acid treatment further caused the downregulation of Mgat4a and Slc2a2 gene expression, coincident with diminished Hnf a RNA, whereas co-incubation with N-acetylcysteine preserved normal expression (Fig. 2b).
Figure 2.
Effect of palmitic acid on normal mouse and human islet cells. (a) Subcellular localization of Foxa2 and Hnf1a proteins within beta cells from islet tissue measured in response to palmitic acid (PA) and N-acetylcysteine (NAC). Propidium iodide (PI) staining of the nucleus (red) is also included where indicated. (b) mRNA expression of Mgat4a, Slc2a2, Foxa2 and Hnf1a measured by rtPCR from WT islet cells isolated and cultured in medium containing 5 mM glucose for 48 h with or without PA and NAC. Islets in a and b were isolated from normal C57BL/6J mice maintained on standard diet. (c) In situ localization of FOXA2 and HNF1A proteins in beta cells from normal human islet tissue measured with or without PA. (d,e) ChIP and rtPCR measurement of FOXA2 (d) and HNF1A (e) proteins bound to promoter regions of human MGAT4A, SLC2A1, SLC2A2 and INS genes. ND, not detected; NA, no addition. (f) Abundance of mRNA from MGAT4A, SLC2A1, SLC2A2 and genes in human islet cells. (g) Glucose-stimulated insulin secretion assayed in islet cell cultures containing medium bearing indicated concentrations of glucose. Results in a and c represent analyses of >24 fields of view consisting of >100 beta cells. Data are means ± s.e.m. of triplicate experiments per mouse from six mice fed standard diet and four normal human islet donors. *P = 0.01–0.049; **P = 0.005–0.009; ***P = 0.0004; ****P < 0.0001 (Student’s t test).
We similarly studied human islets from healthy nondiabetic donors to measure transcription factor binding, gene expression in response to palmitic acid, and GSIS activity. In the absence of exogenous palmitic acid addition, FOXA2 and HNF A proteins colocalized predominantly with nuclear markers, and the addition of palmitic acid caused nuclear exclusion of FOXA2 and HNF A with a corresponding increase in cytoplasmic localization (Fig. 2c). Both transcription factors were bound to the promoter regions of the human MGAT4A, SLC2A1 and SLC2A2 genes in untreated islet cell cultures from healthy donors. The addition of palmitic acid diminished FOXA2 and HNF A binding and attenuated mRNA expression of MGAT4A, SLC2A1 and SLC2A2, coincident with the loss of GSIS (Fig. 2d–g). The similarities we observed in the responses of normal mouse and human islet cells to palmitic acid suggested that islets from human type 2 diabetes donors may show defects comparable to those of mice rendered diabetic by high-fat diet administration.
Human islet cell dysfunction in type 2 diabetes
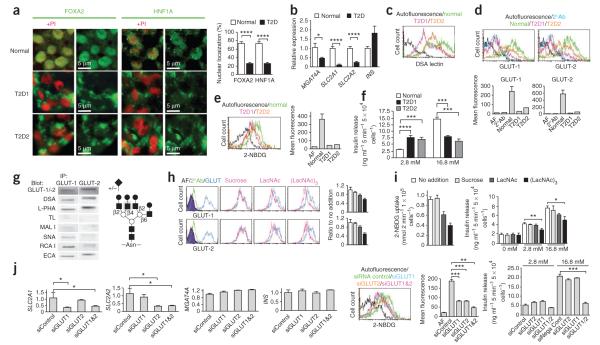
In analyses of six normal human donor islets, we found that FOXA2 and HNF A predominantly localized with the cell nucleus, whereas we observed their nuclear exclusion by >70% in the islet cells of two independent human donors with type 2 diabetes (Fig. 3a). In addition, expression of SLC2A1 and SLC2A2 mRNAs encoding the human GLUT and GLUT2 glucose transporters was decreased by 70–90% in human type 2 diabetes islets, and MGAT4A mRNA expression was reduced by 60% (Fig. 3b). The relatively modest decrease of MGAT4A RNA abundance translated into a 0- to 50-fold reduction of the GnT– 4a glycan product measured as β1-4GlcNAc glycan linkages present at the cell surface using DSA lectin binding (Fig. 3c). GLUT is the predominant glucose transporter expressed in normal human islet cells27; however, we detected both GLUT and GLUT2 glycoproteins. In our findings, islet cells from donors with type 2 diabetes were deficient in cell surface expression of both GLUT and GLUT2 glycoproteins, with an 80–90% reduction on average (Fig. 3d). Furthermore, human type 2 diabetes islet cells had very low glucose transport activity and lacked the GSIS response (Fig. 3e,f).
Figure 3.
Analyses of human islets from normal donors and donors with type 2 diabetes. (a) Subcellular localization of FOXA2 and HNF1A proteins in human beta cells from normal donors and donors with type 2 diabetes (T2D). Results represent analyses of six normal islet samples and two T2D islet samples. Propidium iodide (PI) staining of the nucleus (red) is included where indicated. (b) Abundance of mRNA produced from MGAT4A, SLC2A1, SLC2A2 and INS genes in the designated human islet cells. (c) Islet cell surface abundance of the DSA lectin-binding glycan produced by the GnT-4a glycosyltransferase. (d) Islet cell surface expression of human GLUT-1 and GLUT-2 glucose transporters. (e) Glucose transport activity of the indicated human islets measured using the fluorescent glucose analog 2-NBDG. (f) Glucose-stimulated insulin secretion assayed in islet cell cultures containing medium bearing the indicated concentrations of glucose. (g) Left, GLUT-1 and GLUT-2 glycoproteins were immunoprecipitated (IP) from normal human islet cell extracts followed by electrophoresis and analyses with the indicated antibodies and lectins. Right, deduced tetra-antennary N-glycan structure residing on both human islet cell GLUT-1 and GLUT-2 bearing undersialylated glycan branch termini (+/−). Gray circle, core β1-4GlcNAc linkage produced by GnT-4a. (h) Normal human islet cells were cultured with or without the indicated glycans (10 mM) for 2 h before analyses of cell surface GLUT-1 and GLUT-2 expression by flow cytometry. (i) Fluorescent glucose analog (2-NBDG) transport (left) and GSIS activity (right) measured among islet cells treated in h. The results in h and i represent analyses of three islet cell samples from normal human donors. (j) siRNA knockdown of GLUT1 and GLUT2 mRNA in normal human islet cell cultures measured at 72 h (left four graphs). Glucose analog 2-NBDG transport and GSIS activity were also measured at 72 h. Data are expressed as means ± s.e.m. from six normal human islet donors and two human donors with T2D, unless otherwise stated. *P = 0.01–0.04; **P = 0.002–0.005; ***P = 0.0002–0.0005; ****P < 0.0001 (Student’s t test).
Human GnT-4a glycosylation and glucose transporter expression
Downregulation of human islet cell surface GLUT and GLUT2 glycoproteins associated with the loss of GSIS in type 2 diabetes may involve the lack of sufficient GnT-4a activity, as has been observed in the mouse25. The results of lectin-binding analyses of human GLUT- and GLUT-2 N-glycans from healthy donors were consistent with the presence of an undersialylated tetra-antennary structure bearing the core β1-4GlcNAc glycan linkage produced by GnT-4a (Fig. 3g). This tetra-antennary N-glycan exists on the Glut-2 glycoprotein of mouse beta cells and has been proposed to contribute to formation of a lectin ligand that controls the rate of glucose transporter endocytosis from the cell surface and subsequent degradation25. Support for this model in humans was gained after the addition of the lectin ligand N-acetyllactosamine to normal islet cell cultures, which led to a rapid decline in cell surface expression of both GLUT- and GLUT-2 glycoproteins, indicating that both are stabilized at the cell surface by a lectin-ligand interaction bearing similar binding specificity (Fig. 3h). Decreases in cell surface glucose transporter expression were proportional to reductions in glucose transport and GSIS (Fig. 3i).
We compared the roles of GLUT- and GLUT-2 in glucose transport and GSIS in normal human islet cell cultures by applying siRNAs designed to knock down RNA expression from the corresponding genes. We measured a significant decrease in mRNA expression involving the corresponding glucose transporters (Fig. 3j). However, only the addition of both SLC2A1 and SLC2A2 siRNAs led to the elimination of GSIS, and this also coincided with the largest impairment of glucose transport (Fig. 3j). These findings indicate that endogenous expression of either GLUT- or GLUT-2 is sufficient to maintain GSIS in human beta cells, and are also consistent with studies reporting that expression of either human SLC2A1 or SLC2A2 genes can re-establish GSIS activity in Glut-2 deficient mouse beta cells28. Our findings further support the view that diminished cell surface expression of GLUT- and GLUT-2 observed in human islets from donors with type 2 diabetes is responsible for the loss of GSIS.
Beta cell GnT-4a protects against diabetes
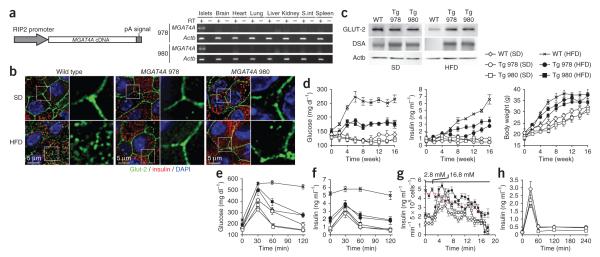
We evaluated the impact of diminished GnT-4a glycosylation on glucose transporter expression and the onset of disease signs in the high-fat diet–induced mouse model of type 2 diabetes. Transgenic mice were generated bearing constitutive expression of the human MGAT4A gene in beta cells and not in other cell types or tissues surveyed, including the brain (Fig. 4a). In WT mice receiving the high-fat diet, beta cell Glut-2 is mostly internalized into endosomes and lysosomes, as has been observed25. In contrast, Glut-2 protein localization was fully retained at the beta cell surface in MGAT4A transgenic littermates (Fig. 4b). The preservation of beta cell surface Glut-2 expression in MGAT4A transgenic mice on a high-fat diet coincided with preservation of GnT-4a activity evidenced by the retention of DSA lectin binding (Fig. 4c).
Figure 4.
Enforced beta cell–specific GnT-4a glycosylation prevents loss of Glut-2 expression and inhibits onset of disease signs including hyperglycemia and failure of GSIS. (a) Human MGAT4A cDNA was incorporated into a transgene vector that conferred beta cell–specific expression in multiple tissues of two separate founder lines, 978 and 980. Transgene expression was detected using vector-specific primers. (b) Pancreatic beta cell histology from WT and MGAT4A transgenic littermates that received either standard diet (SD) or high-fat diet (HFD) for the preceding 10 weeks. Antibody binding and visualization of GLUT-2 (green), insulin (red) and DNA (DAPI-blue). (c) GLUT-2 protein abundance and glycosylation in beta cells from WT or MGAT4A (Tg) littermates as in b were analyzed by blotting GLUT-2 immunoprecipitates with antibody to GLUT-2 or DSA lectin. Single analysis shown represents three independent experiments with different littermates. (d) Blood glucose (left), blood insulin (center) and body weight (right) were measured (unfasted) every 2 weeks for up to 16 weeks of HFD administration (e,f). In fasted mice receiving SD or the HFD for 10 weeks, glucose was injected into the intraperitoneal space before analysis of glucose tolerance (e) and insulin release (f). (g) GSIS activity was analyzed ex vivo by perifusion in islet cells isolated from mice that received either SD or HFD for the preceding 4 weeks. Glucose concentration was increased from 2.8 mM to 16.8 mM at the time indicated. Data are mean ± s.e.m. of three independent studies of beta cells from distinct littermates. Data from WT beta cells, red line. (h) Insulin secretion response to l-arginine injection measured in fasting WT and MGAT4A Tg mice receiving SD. Data are means ± s.e.m. in triplicate experiments per mouse, from six or seven mice of each genotype unless otherwise stated.
Compared to WT mice with hyperglycemia and hyperinsulinemia, MGAT4A transgenic littermates maintained much lower concentrations of blood glucose and insulin, whereas both groups became obese (Fig. 4d and Supplementary Table 1). Fasted MGAT4A transgenic mice had markedly greater glucose tolerance (Fig. 4e), whereas insulinemia was decreased and GSIS was preserved (Fig. 4f). Ex vivo perifusion studies of islet cells also showed the preservation of GSIS activity afforded by constitutive beta cell GnT-4a expression (Fig. 4g), whereas arginine-induced insulin release was unaffected by the MGAT4A transgene (Fig. 4h). Circulating free fatty acid and triglyceride concentrations in MGAT4A transgenic mice were markedly reduced in comparison to WT littermates (Supplementary Table 1), indicating that enforced beta cell GnT-4a protein glycosylation diminished multiple and systemic disease signs.
Beta cell GnT-4a increases insulin sensitivity and prevents steatosis
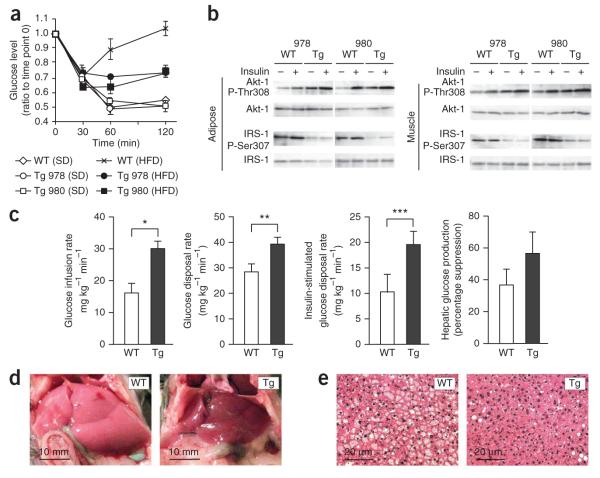
Insulin tolerance tests in mice administered the high-fat diet indicated greater glucose clearance in MGAT4A transgenic mice than in WT mice (Fig. 5a). We further analyzed signatures of insulin action in adipose and muscle tissue, involving the phosphorylation of Akt-1 Thr308 and IRS-1 Ser307 (refs. 29,30). The presence of protein-linked phosphate was considerably greater on Akt-1 Thr308 and substantially lower on IRS-1 Ser307 in both adipose and muscle tissue of MGAT4A transgenic mice compared with WT mice, consistent with the likelihood of higher systemic insulin sensitivity (Fig. 5b). We therefore further analyzed insulin action using euglycemic-hyperinsulinemic clamps (Fig. 5c). The glucose infusion rate, indicative of whole-body insulin sensitivity, was significantly greater in MGAT4A transgenic mice than in their WT littermates. Furthermore, the glucose disposal rate, reflecting combined tissue ability to uptake glucose, and the insulin-stimulated glucose disposal rate, indicating muscle-specific insulin sensitivity, were similarly higher in MGAT4A transgenic mice, indicating greater muscle insulin sensitivity. Insulin suppression of hepatic glucose production to detect inhibition of liver gluconeogenesis by insulin was variably increased, in some cohorts more profoundly than in others.
Figure 5.
Beta cell–specific GnT-4a protein glycosylation promotes systemic insulin sensitivity and inhibits development of hepatic steatosis. (a) Insulin challenge response measured in 16–18 week old littermates of indicated genotypes after 10 weeks of standard diet (SD) or high-fat diet (HFD) administration. (b) Phosphorylation of Akt-1 at Thr308 a nd IRS-1 at Ser307 in equivalent cellular protein preparations from adipose and muscle tissue of mice as in a after 2 min of insulin perfusion through inferior vena cava. (c) Euglycemic and hyperinsulinemic clamp studies after 10 weeks on HFD compared measurements of glucose infusion rate, glucose disposal rate, insulin-stimulated glucose disposal rate, and suppression of hepatic glucose production in response to insulin. The analyses included nine WT and seven Tg mice. (d,e) Liver tissue observed macroscopically (d) and by histological analysis stained with H&E (e). *P = 0.0017; **P = 0.0235; ***P = 0.0401 (single-tail t test). Data are means ± s.e.m. from three or more littermates unless otherwise indicated.
Hepatic steatosis was evident in WT mice that received the high-fat diet. In contrast, the livers of MGAT4A transgenic mice administered the high-fat diet notably lacked signs of steatosis (Fig. 5d,e). Together, these findings indicate that enforced beta cell GnT-4a glycosylation imparted a systemic influence substantially improving insulin action in adipose and muscle tissue and conferring protection from the development of steatosis.
Diminished beta cell glucose transport in diabetes
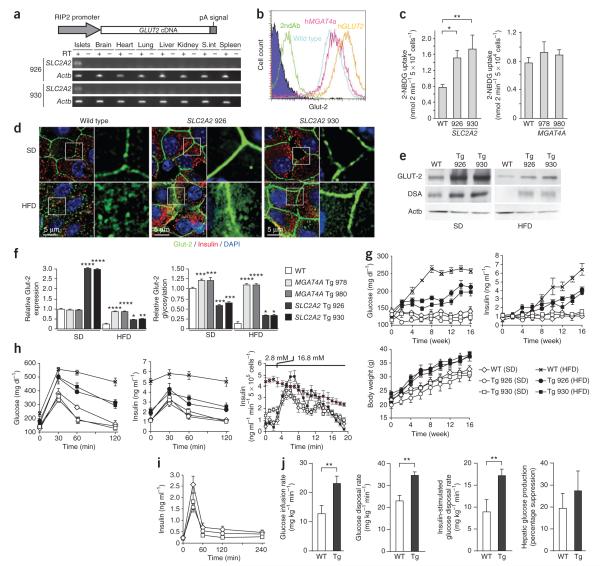
Disease protection afforded by GnT-4a may involve maintenance of beta cell glucose sensing. To test this hypothesis, we generated transgenic mice bearing constitutive beta cell–specific expression of the human SLC2A2 gene (Fig. 6a). The SLC2A2 transgene led to greater beta cell GLUT-2 glycoprotein expression and glucose transport as compared with beta cells from WT and MGAT4A transgenic mice that received the standard diet (Fig. 6b,c). Both SLC2A2 transgenic lines showed retention of glucose transporter glycoprotein expression at the beta cell plasma membrane after high-fat diet administration, although we also noted an accompanying increase in cytosolic localization (Fig. 6d). In addition, GLUT-2 glycoprotein abundance was diminished in beta cells of SLC2A2 transgenic mice after high-fat diet treatment, and compared with MGAT4A transgenic mice. Nevertheless, overall expression was greater than that in WT counterparts and the GLUT-2 molecules remaining were enriched in glycoproteins modified by GnT-4a activity (Fig. 6e).
Figure 6.
Enforced beta cell–specific expression of GnT-4a substrate GLUT-2 mitigates diet- and obesity-induced diabetes. (a) Human SLC2A2 cDNA was incorporated into a transgene vector that conferred beta cell–specific expression in multiple tissues of two separate founder lines, 926 and 930. (b) Beta cell surface expression of GLUT-2 analyzed by flow cytometry from WT, MGAT4A Tg (978), and SLC2A2 Tg (926) mice receiving standard diet (SD). (c) Glucose analog 2-NBDG transport into cultured islet cells isolated from WT mice and Tg littermates fed SD. (d) Pancreatic beta cell histology of WT and MGAT4A Tg littermates that received either SD or high-fat diet (HFD) for the preceding 10 weeks. Antibody binding and visualization of Glut-2 (green), insulin (red) and DNA (blue). (e) Islet cell Glut-2 and GLUT-2 immunoprecipitates were analyzed for Glut-2 abundance and DSA lectin binding from WT or SLC2A2 Tg littermates administered indicated dietary stimuli. Single analysis shown represents three independent experiments with different littermates. (f) Histogram of GLUT-2 protein abundance (left) and GLUT-2 glycosylation (right) was calculated from results in e. (g) Blood glucose (left), blood insulin (right) and body weight (bottom) were measured in unfasted mice every 2 weeks for up to 16 weeks of indicated diet administration. (h) In fasted mice that had received SD or HFD for 10 weeks, glucose tolerance tests measured blood glucose (left) and insulin release (middle). GSIS activity was analyzed ex vivo by perifusion (bottom) in islet cells isolated from mice that received either SD or HFD for preceding 4 weeks. Glucose concentration was increased from 2.8 mM to 16.8 mM at time indicated. Data from WT beta cells, red line. (i) Insulin secretion response to l-arginine injection in fasting WT and SLC2A2 Tg mice receiving SD. (j) Euglycemic and hyperinsulinemic clamp studies after 10 weeks on HFD compared measurements of glucose infusion rate, glucose disposal rate, insulin-stimulated glucose disposal rate and hepatic suppression of gluconeogenesis. The clamp studies included eight WT and eight SLC2A2 Tg littermates. Data plotted throughout are means ± s.e.m. At least six or seven mice including littermates were studied in each experiment unless otherwise stated. *P = 0.01–0.04; **P = 0.001–0.09; ***P = 0.0001–0.0009; ****P < 0.0001 (Student’s t test, c, f and g; single-tail t test, j).
We compared results and found that the SLC2A2 transgene conferred an intermediate degree of improvement between WT and MGAT4A transgenic mice. For example, glucose transporter protein abundance decreased in SLC2A2 transgenic mice after high-fat diet administration, in contrast with a much lower level observed in WT litteramates and the complete retention of endogenous Glut-2 expression observed in MGAT4A transgenic mice (Fig. 6f). In analyses of glucose transporter glycosylation, the high expression of GLUT-2 protein produced by the SLC2A2 transgene seemed to surpass the capacity of endogenous GnT-4a activity to glycosylate the increased amount of Glut-2 (Fig. 6f). Glucose transporter glycosylation by GnT-4a remained optimal in islet cells of MGAT4A transgenic mice receiving the high-fat diet, whereas corresponding SLC2A2 transgenic beta cells showed a decrease in GLUT-2 glycosylation among mice fed the high-fat diet, consistent with the downmodulation of endogenous Mgat4a expression, diminished GnT-4a activity and decreased GLUT-2 abundance (Fig. 6f).
The SLC2A2 transgene likewise provided an intermediate reduction in hyperglycemia and hyperinsulinemia, whereas the development of obesity was unaffected (Fig. 6g). Nevertheless, an intraperitoneal glucose tolerance test indicated markedly greater glucose tolerance and retention of GSIS (Fig. 6h). We further noted preservation of GSIS in ex vivo islet cell perifusion studies (Fig. 6h), and insulin release in response to arginine was unaltered by the SLC2A2 transgene (Fig. 6i). In addition, hyperinsulinemic-euglycemic clamp studies of SLC2A2 transgenic mice indicated significantly greater systemic insulin sensitivity (Fig. 6j). Upon examination of liver sections, however, we found no significant protection from liver steatosis (data not shown), in contrast with our findings in MGAT4A transgenic mice. The intermediate and lesser impact of enforced GLUT-2 expression in beta cells, as compared with GnT-4a expression, is consistent with the assignment of GnT-4a activity as the limiting factor in optimal glucose transporter glycosylation and retention at the beta cell surface.
These findings, spanning both mouse and human, can be incorporated into a molecular and pathogenic pathway that includes a key role of pancreatic beta cell GnT-4a glycosylation and glucose transport in the origin and severity of disease signs including insulin resistance that are together diagnostic of type 2 diabetes (Supplementary Fig. 2).
DISCUSSION
Genetics, diet and obesity contribute to the current epidemic of human type 2 diabetes. A substantial proportion of the human population seems predisposed to a combination of these factors, perhaps analogous to different mouse strains that are resistant or susceptible to diet- and obesity-induced diabetes3. Our findings show that the extent of disruption of beta cell glycosylation and glucose transport by diet and obesity directly contributes to disease onset and severity in susceptible mice, illuminating a pathogenic pathway that encompasses lipotoxicity and glucotoxicity32. This pathway seems to be conserved in normal human islet cells and is activated in islets from donors with type 2 diabetes. A pathogenic tipping point in this pathway may occur when elevated free fatty acid (FFA) concentrations impair the expression and function of FOXA2 and HNF A transcription factors sufficiently in beta cells to deplete GnT-4a glycosylation and glucose transporter expression. The resulting dysfunction of beta cells leads to impaired glucose tolerance and failure of GSIS and further contributes to hyperglycemia, hepatic steatosis and systemic insulin resistance. Preservation of beta cell GnT-4a glycosylation and glucose transporter expression breaks this pathogenic cycle and its link to diet and obesity.
The nuclear exclusion and functional attenuation of transcription factors Foxa2 and Hnf a induced by high-fat diet in mice was similarly observed in islet cells from human donors with type 2 diabetes as well as in mouse and human islet cell cultures augmented with the FFA palmitic acid. The linkage of palmitic acid abundance to diminished GnT-4a activity and glucose transport can further explain how elevated FFA concentrations contribute to beta cell dysfunction and the onset of diabetes. FFAs induce the activation of one or more beta cell G protein–coupled FFA receptor such as GPR40, GPR119 and GPR120 (ref. 33). Although FFAs seem to promote beta cell function in some contexts, the chronic elevation of FFAs increases mitochondrial oxidation and reactive oxygen species and has been observed in beta cell cultures with diminished GSIS and reduced glucose transporter expression34,35. Our observations that the antioxidant N-acetylcysteine inhibits FFA-induced attenuation of Foxa2 and Hnf a function are consistent with those findings. Nuclearcytoplasmic shuttling of Foxa2 has been observed in hepatocytes, where attenuation of Foxa2 contributes to the onset of steatosis and insulin resistance36. In addition, Foxa2 inactivation has been biochemically linked to phosphorylation at Thr 56, independent of nuclear exclusion, and involving the Akt kinase, which can be activated in response to insulin or stimuli that induce oxidative stress in beta cells37,38. Whereas both Foxa2 and Hnf a contribute to the expression of GnT-4a and the glucose transporters in beta cells, a complete absence of Foxa2 engineered by genetic ablation can alone cause failure of GSIS39,40. Our findings support the role of Foxa2 and Hnf a as key metabolic regulators of energy homeostasis pathways that include beta cell responses to nutritional cues.
The connections established among beta cell HNF A function and the expression of GnT-4a and the glucose transporters suggest that the pathogenesis of diet- and obesity-associated type 2 diabetes may occur by a similar mechanism to that operating in mature onset diabetes of the young subtype 3 (MODY3), the most common form of human MODY41. Hnf a transactivates the mouse Slc2a2 gene42 and we have found that expression of the human MGAT4A gene is also maintained by HNF A function. The presence of multiple HNF A-binding sites in the promoter regions of human MGAT4A, SLC2A1 and SLC2A2 genes is consistent with the possibility that singular or combined decreases in human GnT-4a and glucose transporter expression in beta cells may be responsible for the loss of GSIS in MODY3.
The altered expression of hundreds of genes has been reported in comparisons of human islet cells from normal donors and donors with type 2 diabetes43. In those studies and in ours reported here, the MGAT4A gene is substantially and similarly downregulated, although the decrease is relatively modest and might not garner attention when the expression of other genes is altered by tenfold or more. Yet this 60% reduction of MGAT4A RNA expression was associated with a 0- to 50-fold decrease of core β1-4GlcNAc N-glycan linkages produced by GnT-4a activity. This outcome may reflect various mechanisms that can regulate glycosyltransferases, including altered intracellular localization, competition with endogenous acceptor substrates and variable access to donor substrates44.
Multiple cell types express GnT-4a with high expression in the pancreas of normal rodents and humans. Nevertheless, beta cell–specific transgene expression markedly diminished signs of high-fat diet– induced diabetes in the presence of endogenous GnT-4a expression. In beta cells, multiple glycoprotein substrates of GnT-4a exist, including the insulin-like growth factor-1 receptor and insulin receptor-α subunit. However, the turnover of these substrates was unaffected by the deficiency of GnT-4a activity and core β1-4GlcNAc glycan linkages25. The glucose transporters of beta cells are thus unique among glycoproteins analyzed in requiring GnT-4a glycosylation to sustain an extended half-life at the cell surface, suggesting a stabilizing role of lectin-ligand binding that may involve the galectins45,46. This specificity of action may reflect a combinatorial role of protein and glycan sequences in determining biological function, analogous to enzymatic phosphorylation, which dictates different functional outcomes on different proteins44.
The impact of enforced beta cell–specific expression of MGAT4A and SLC2A2 on metabolic abnormalities including GSIS and insulin resistance was notable in this study. Constitutive beta cell expression of GnT-4a or Glut-2 preserved considerable systemic insulin sensitivity, indicating that beta cell function influences insulin action on these peripheral target tissues. Beta cells may accomplish this by one or more mechanisms. The retention of beta cell GSIS may achieve a more effective delivery of insulin, precisely timed to glucose excursions to meet tissue and body needs. Without such a ‘natural’ delivery system, chronic insulin exposure by some current treatment modalities may desensitize insulin-signaling pathways or provide improperly timed signals. For example, the optimization of insulin administration can better normalize blood glucose concentration and increase peripheral insulin sensitivity in subjects with type 2 diabetes, and the degree of glycemic control achieved by insulin treatment in subjects with type diabetes is a major determinant of the degree of insulin sensitivity retained in peripheral tissues in vivo47–49. The marked reduction in hyperglycemia we observed from enforced expression of either GnT-4a or Glut-2 indicates how effective preservation of beta cell glucose transport can be in improving glycemia. In turn, normalizing glucose homeostasis could also promote peripheral insulin action by attenuating the effects of glucolipotoxicity, which impair insulin signaling. For instance, the severity of hyperglycemia is a factor in protein kinase C–mediated inhibition of insulin signaling50.
Our findings do not address whether retention of GSIS is protective in the onset of diabetes; however, loss of GSIS is a marker of beta cell dysfunction in type 2 diabetes, and retention of GSIS is associated with disease protection. The beta cell could produce and secrete multiple glycoprotein factors that depend upon GnT-4a glycosylation for normal activity and half-life and that, along with insulin, directly contribute to glucose homeostasis and peripheral insulin action. Nevertheless, we predict that the severe decrease in glucose transport and intracellular glucose availability causes metabolic alterations in beta cells that may connect our findings to further downstream events that contribute to disease etiology. For example, the reduction of glucose transporter expression may reach a threshold at which glucose transport instead of glucokinase activity becomes rate limiting in the formation of glucose-6-phosphate needed for maintaining various beta cell functions.
Protection from disease conferred by GnT-4a and beta cell glucose transport was consistent with the assignment of beta cell GnT-4a glycosylation as a limiting factor. Both GnT-4a and the glucose transporters are highly regulated in pancreatic beta cells. Their expression is rapidly lost in primary beta cell cultures, and immortalized beta cell lines lose GSIS activity. Dietary stimuli leading to obesity trigger this process with the inactivation of transcription factors that normally sustain beta cell GnT-4a protein glycosylation and glucose transport, thereby promoting the onset of a disease pathway implicated in the diet- and obesity-associated component of type 2 diabetes mellitus. The molecules that impinge upon this pathway to sustain beta cell GnT-4a activity and glucose transporter expression may suggest new therapeutic targets to achieve effective prevention and treatment of diabetes.
Supplementary Material
ACKNOWLEDGMENTS
This research was funded primarily by US National Institutes of Health (NIH) grant DK48247 with additional support from GM62116 and CA71932 (J.D.M.). Further funding was obtained from DK033651, DK074868, T32 DK007494, DK063491 and the Eunice Kennedy Shriver National Institute of Child Health and Human Development–NIH through cooperative agreement of U54 HD 012303-25 as part of the specialized Cooperative Centers Program in Reproduction and Infertility Research (J.M.O.) and the Japan Diabetes Foundation and Suntory Institute for Bioorganic Research (SUNBOR grant; K.O.).
Footnotes
METHODS Methods and any associated references are available in the online version of the paper at http://www.nature.com/naturemedicine/.
Note: Supplementary information is available on the Nature Medicine website.
AUTHOR CONTRIBUTIONS K.O. conducted the majority of experiments and helped write the manuscript. M.Z.C. and J.M.O. carried out the hyperinsulinemic-euglycemic clamp studies and helped write the manuscript. J.D.M. conceived of and supervised the project and wrote the manuscript.
COMPETING FINANCIAL INTERESTS The authors declare no competing financial interests.
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
References
- 1.Olefsky JM, Courtney CH. Type 2 diabetes mellitus: etiology, pathogenesis and natural history. In: DeGroot LJ, et al., editors. Endocrinology. 5th edn W.B. Saunders; 2005. pp. 1093–1117. [Google Scholar]
- 2.Weir GC, Leahy JL. Pathogenesis of non-insulin dependent (type II) diabetes mellitus. In: Joslin EP, Kahn CR, Weir GC, editors. Joslin’s Diabetes Mellitus. Lea and Febiger; 1994. pp. 240–264. Ch. 14. [Google Scholar]
- 3.Smyth S, Heron A. Diabetes and obesity: the twin epidemics. Nat. Med. 2006;12:75–80. doi: 10.1038/nm0106-75. [DOI] [PubMed] [Google Scholar]
- 4.Korner J, Woods SC, Woodworth KA. Regulation of energy homeostasis and heath consequences in obesity. Am. J. Med. 2009;122:S12–S18. doi: 10.1016/j.amjmed.2009.01.003. [DOI] [PubMed] [Google Scholar]
- 5.Parillo M, Ricardi G. Diet composition and the risk of type 2 diabetes: epidemiological and clinical evidence. Br. J. Nutr. 2004;92:7–19. doi: 10.1079/BJN20041117. [DOI] [PubMed] [Google Scholar]
- 6.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes. 1988;37:1163–1167. doi: 10.2337/diab.37.9.1163. [DOI] [PubMed] [Google Scholar]
- 7.Kahn SE. The relative contribution of insulin resistance and beta-cell dysfunction to the pathophysiology of type 2 diabetes. Diabetologia. 2003;46:3–19. doi: 10.1007/s00125-002-1009-0. [DOI] [PubMed] [Google Scholar]
- 8.Winzell MS, Ahren B. The high-fat diet-fed mouse: a model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes. 2004;53(suppl. 3):S215–S219. doi: 10.2337/diabetes.53.suppl_3.s215. [DOI] [PubMed] [Google Scholar]
- 9.Leroith D, Accili D. Mechanisms of disease: using genetically altered mice to study concepts of type 2 diabetes. Nat. Clin. Pract. Endocrinol. Metab. 2008;4:164–172. doi: 10.1038/ncpendmet0729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saltiel AR, Kahn CR. Insulin signaling and the regulation of glucose and lipid metabolism. Nature. 2001;414:799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 11.Schenk S, Saberi M, Olefsky JM. Insulin sensitivity: modulation by nutrients and inflammation. J. Clin. Invest. 2008;118:2992–3002. doi: 10.1172/JCI34260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reaven GM. The insulin resistance syndrome: definition and dietary approaches to treatment. Annu. Rev. Nutr. 2005;25:391–406. doi: 10.1146/annurev.nutr.24.012003.132155. [DOI] [PubMed] [Google Scholar]
- 13.Johnson JH, et al. Underexpression of beta cell high Km glucose transporters in noninsulin-dependent diabetes. Science. 1990;250:546–549. doi: 10.1126/science.2237405. [DOI] [PubMed] [Google Scholar]
- 14.Orci L, et al. Evidence that down-regulation of β-cell glucose transporters in non-insulin-dependent diabetes may be the cause of diabetic hyperglycemia. Proc. Natl. Acad. Sci. USA. 1990;87:9953–9957. doi: 10.1073/pnas.87.24.9953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orci L, et al. Reduced β-cell glucose transporter in new onset diabetic rats. J. Clin. Invest. 1990;86:1615–1622. doi: 10.1172/JCI114883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thorens B, Weir G, Leahy JL, Lodish HF, Bonner-Weir S. Reduced expression of the liver/beta-cell glucose transporter isoform in glucose-insensitive pancreatic beta cells of diabetic rats. Proc. Natl. Acad. Sci. USA. 1990;87:6492–6496. doi: 10.1073/pnas.87.17.6492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Unger RH. Diabetic hyperglycemia: link to impaired glucose transport in pancreatic beta cells. Science. 1991;251:1200–1205. doi: 10.1126/science.2006409. [DOI] [PubMed] [Google Scholar]
- 18.Valera A, et al. Expression of GLUT2 antisense RNA in β cells of transgenic mice leads to diabetes. J. Biol. Chem. 1994;269:28543–28546. [PubMed] [Google Scholar]
- 19.Gremlich S, Roduit R, Thorens B. Dexamethasone induces posttranslational degradation of GLUT2 and inhibition of insulin secretion in isolated pancreatic β cells. J. Biol. Chem. 1997;272:3216–3222. doi: 10.1074/jbc.272.6.3216. [DOI] [PubMed] [Google Scholar]
- 20.Guillam MT, et al. Early diabetes and abnormal postnatal pancreatic islet development in mice lacking Glut-2. Nat. Genet. 1997;17:327–330. doi: 10.1038/ng1197-327. [DOI] [PubMed] [Google Scholar]
- 21.Guillam M-T, Dupraz P, Thorens B. Glucose uptake, utilization, and signaling in GLUT2-null islets. Diabetes. 2000;49:1485–1491. doi: 10.2337/diabetes.49.9.1485. [DOI] [PubMed] [Google Scholar]
- 22.Reimer MK, Ahrén B. Altered β-cell distribution of pdx-1 and GLUT2 after a short-term challenge with a high-fat diet in C57BL/6J mice. Diabetes. 2002;51:S138–S143. doi: 10.2337/diabetes.51.2007.s138. [DOI] [PubMed] [Google Scholar]
- 23.Del Guerra S, et al. Functional and molecular defects of pancreatic islets in human type 2 diabetes. Diabetes. 2005;54:727–735. doi: 10.2337/diabetes.54.3.727. [DOI] [PubMed] [Google Scholar]
- 24.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840–846. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 25.Ohtsubo K, et al. Dietary and genetic control of glucose transporter 2 glycosylation promotes insulin secretion in suppressing diabetes. Cell. 2005;123:1307–1321. doi: 10.1016/j.cell.2005.09.041. [DOI] [PubMed] [Google Scholar]
- 26.Minowa MT, et al. cDNA cloning and expression of bovine UDP-N-acetylglucosamine:α1,3-D-mannoside β1,4-N-acetylglucosaminyltransferase IV. J. Biol. Chem. 1998;273:11556–11562. doi: 10.1074/jbc.273.19.11556. [DOI] [PubMed] [Google Scholar]
- 27.De Vos A, et al. Human and rat beta cells differ in glucose transporter but not glucokinase gene expression. J. Clin. Invest. 1995;96:2489–2495. doi: 10.1172/JCI118308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Thorens B, Guillam MT, Beermann F, Burcelin R, Jaqueet M. Transgenic overexpression of GLUT1 or GLUT2 in pancreatic beta cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J. Biol. Chem. 2000;275:23751–23758. doi: 10.1074/jbc.M002908200. [DOI] [PubMed] [Google Scholar]
- 29.Alessi DR, Cohen P. Mechanism of activation and function of protein kinase B. Curr. Opin. Genet. Dev. 1998;8:55–62. doi: 10.1016/s0959-437x(98)80062-2. [DOI] [PubMed] [Google Scholar]
- 30.White MF. IRS proteins and the common path to diabetes. Am. J. Physiol. Endocrinol. Metab. 2002;283:E413–E422. doi: 10.1152/ajpendo.00514.2001. [DOI] [PubMed] [Google Scholar]
- 31.Rossmeisl M, Rim JS, Koza RA, Kozak LP. Variation in type 2 diabetes-related traits in mouse strains susceptible to diet-induced obesity. Diabetes. 2003;52:1958–1966. doi: 10.2337/diabetes.52.8.1958. [DOI] [PubMed] [Google Scholar]
- 32.Poitout V, Robertson RP. Glucolipotoxicity: fuel excess and beta-cell dysfunction. Endocr. Rev. 2008;29:351–366. doi: 10.1210/er.2007-0023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kebede MA, Alquier T, Latour MG, Poitout V. Lipid receptors and islet function: therapeutic implications? Diabetes Obes. Metab. 2009;11(suppl. 4):10–20. doi: 10.1111/j.1463-1326.2009.01114.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Unger RH. Lipotoxicity in the pathogenesis of obesity-dependent NIDDM. Genetic and clinical implications. Diabetes. 1995;44:863–870. doi: 10.2337/diab.44.8.863. [DOI] [PubMed] [Google Scholar]
- 35.Gremlich S, Bonny C, Waeber G, Thorens B. Fatty acids decrease IDX-1 expression in rat pancreatic islets and reduce GLUT2, glucokinase, insulin, and somatostatin levels. J. Biol. Chem. 1997b;272:30261–30269. doi: 10.1074/jbc.272.48.30261. [DOI] [PubMed] [Google Scholar]
- 36.Wolfrum C, Asilmaz E, Luca E, Friedman JM, Stoffel M. Foxa2 regulates lipid metabolism and ketogenesis in the liver during fasting and in diabetes. Nature. 2004;432:1027–1032. doi: 10.1038/nature03047. [DOI] [PubMed] [Google Scholar]
- 37.Howell JJ, Stoffel M. Nuclear export-independent inhibition of Foxa2 by insulin. J. Biol. Chem. 2009;284:24816–24824. doi: 10.1074/jbc.M109.042135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen YW, et al. The role of phosphoinositide 3-kinase/Akt signaling in low-dose mercury-induced mouse pancreatic beta-cell dysfunction in vitro and in vivo. Diabetes. 2006;55:1614–1624. doi: 10.2337/db06-0029. [DOI] [PubMed] [Google Scholar]
- 39.Lantz KA, et al. Foxa2 regulates multiple pathways of insulin secretion. J. Clin. Invest. 2004;114:512–520. doi: 10.1172/JCI21149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao N, et al. Foxa1 and Foxa2 maintain the metabolic and secretory features of the mature beta-cell. Mol. Endocrinol. 2010;24:1594–1604. doi: 10.1210/me.2009-0513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mitchell SM, Frayling TM. The role of transcription factors in maturity-onset diabetes of the young. Mol. Genet. Metab. 2002;77:35–43. doi: 10.1016/s1096-7192(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 42.Fukui K, et al. The HNF-1 target collection controls insulin exocytosis by SNARE complex formation. Cell Metab. 2005;2:373–384. doi: 10.1016/j.cmet.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 43.Gunton JE, et al. Loss of ARNT/HIF1beta mediates altered gene expression and pancreatic-islet dysfunction in human type 2 diabetes. Cell. 2005;122:337–349. doi: 10.1016/j.cell.2005.05.027. [DOI] [PubMed] [Google Scholar]
- 44.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126:855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 45.Rapoport EM, Kurmyshkina OV, Bovin NV. Mammalian galectins: structure, carbohydrate specificity, and functions. Biochemistry (Mosc.) 2008;73:393–405. doi: 10.1134/s0006297908040032. [DOI] [PubMed] [Google Scholar]
- 46.Rabinovich GA, Toscano MA, Jackson SS, Vasta GR. Functions of cell surface galectin-glycoprotein lattices. Curr. Opin. Struct. Biol. 2007;17:513–520. doi: 10.1016/j.sbi.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Scarlett JA, Gray RS, Griffin J, Olefsky JM, Kolterman OG. Insulin treatment reverses the insulin resistance of type II diabetes mellitus. Diabetes Care. 1982;5:353–363. doi: 10.2337/diacare.5.4.353. [DOI] [PubMed] [Google Scholar]
- 48.Revers RR, Kolterman OG, Scarlett JA, Gray RS, Olefsky JM. Lack of in vivo insulin resistance in controlled insulin-dependent, type I, diabetic patients. J. Clin. Endocrinol. Metab. 1984;58:353–358. doi: 10.1210/jcem-58-2-353. [DOI] [PubMed] [Google Scholar]
- 49.Garvey WT, Olefsky JM, Griffin J, Hamman RF, Kolterman OG. The effect of insulin treatment on insulin secretion and insulin action in type II diabetes mellitus. Diabetes. 1985;34:222–234. doi: 10.2337/diab.34.3.222. [DOI] [PubMed] [Google Scholar]
- 50.Pillay TS, Xiao S, Olefsky JM. Glucose-induced phosphorylation of the insulin receptor. Functional effects and characterization of phosphorylation sites. J. Clin. Invest. 1996;97:613–620. doi: 10.1172/JCI118457. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.