Abstract
The Sm family of proteins is closely associated with RNA metabolism throughout all life. These proteins form homomorphic and heteromorphic rings consisting of six or seven subunits with a characteristic central pore, the presence of which is critical for binding U-rich regions of single-stranded RNA. Eubacteria and Archaea typically carry one or two forms of Sm proteins and assemble one homomorphic ring per Sm protein. Eukaryotes typically carry 16 or more Sm proteins that assemble to form heteromorphic rings which lie at the center of a number of critical RNA-associated small nuclear ribonucleoproteins (snRNPs). High Sm protein diversity and heteromorphic Sm rings are features stretching back to the origin of eukaryotes; very deep phylogenetic divisions among existing Sm proteins indicate simultaneous evolution across essentially all existing eukaryotic life. Two basic forms of heteromorphic Sm rings are found in eukaryotes. Fixed Sm rings are highly stable and static and are assembled around an RNA cofactor. Flexible Sm rings also stabilize and chaperone RNA but assemble in the absence of an RNA substrate and, more significantly, associate with and dissociate from RNA substrates more freely than fixed rings. This suggests that the conformation of flexible Sm rings might be modified in some specific manner to facilitate association and dissociation with RNA. Diversification of eukaryotic Sm proteins may have been initiated by gene transfers and/or genome clashes that accompanied the origin of the eukaryotic cell itself, with further diversification driven by a greater need for steric specificity within increasingly complex snRNPs.
Keywords: Sm protein, RNA processing, snRNP, multimeric proteins, trans-splicing, spliceosome
Introduction
The Sm family of proteins, encompassing the Sm and Sm-like (Lsm) proteins (Séraphin 1995), are common participants in RNA metabolism in Eubacteria (Valentin-Hansen et al. 2004), Archaea (Salgado-Garrido et al. 1999; Mura et al. 2001), and eukaryotes (Mattaj and Derobertis 1985). Sm proteins primarily occur as small (∼9–29 kDa) stand-alone proteins lacking other domains (Anantharaman et al. 2002; for an exception see Pillai et al. 2003) that assemble to form characteristic homomorphic or heteromorphic rings containing six or seven proteins. Members of the family are characterized by the conserved bipartite Sm domain or “Sm fold” which functions, at least in part, in binding to neighboring Sm proteins within such rings (Box 1 and Hermann et al. 1995; Séraphin 1995; Khusial et al. 2005). One highly conserved characteristic of Sm rings is the direct interaction of the central pore of the ring with short uracil-rich stretches of RNA, in both prokaryotes (Box 1 and Törö et al. 2001; Schumacher et al. 2002; Mura, Kozhukhovsky et al. 2003; Thore et al. 2003) and eukaryotes (Branlant et al. 1982; Liautard et al. 1982; Urlaub et al. 2001; Khusial et al. 2005). The Sm family in eukaryotes has undergone considerable diversification, with a variety of heteromorphic Sm rings participating within many RNA-processing pathways and snRNP complexes (Anantharaman et al. 2002; Khusial et al. 2005; Wilusz CJ and Wilusz J 2005). Some multidomain proteins involved in RNA processing carry divergent Sm domains but have not been found within Sm rings (Albrecht and Lengauer 2004; Albrecht et al. 2004; Anantharaman and Aravind 2004; Fleischer et al. 2006; Tritschler et al. 2007); here we focus on Sm protein members of Sm rings.
Box 1. Sm protein structure
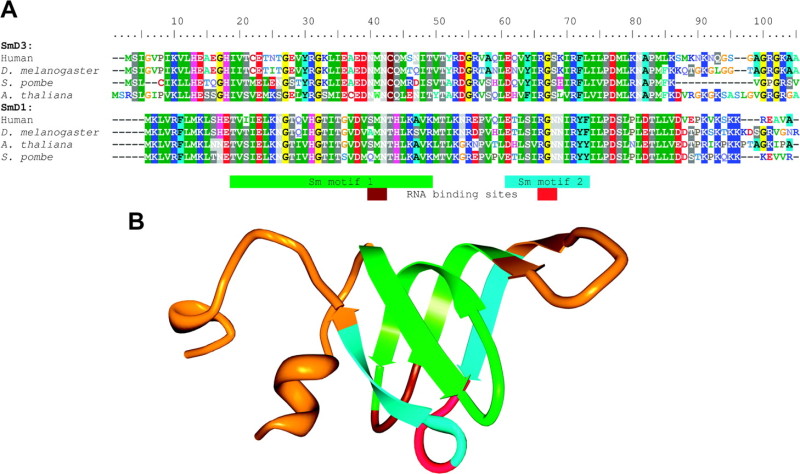 Box 1 FIG.—Sm protein structure. (A) Sequence alignment of Sm proteins SmD1 and SmD3 in human, Drosophila melanogaster, Schizosaccharomyces pombe and Arabidopsis thaliana. RG-rich regions characteristic of the 3′-ends of these particular Sm proteins have been removed. (B) Human SmD1 protein structural model (from PDB ID 1B34, Kambach, Walke, Young, et al. 1999). Green, light blue, brown, and red regions of the model correspond to the Sm motif and RNA-binding site sequences as shown in (A).
Box 1 FIG.—Sm protein structure. (A) Sequence alignment of Sm proteins SmD1 and SmD3 in human, Drosophila melanogaster, Schizosaccharomyces pombe and Arabidopsis thaliana. RG-rich regions characteristic of the 3′-ends of these particular Sm proteins have been removed. (B) Human SmD1 protein structural model (from PDB ID 1B34, Kambach, Walke, Young, et al. 1999). Green, light blue, brown, and red regions of the model correspond to the Sm motif and RNA-binding site sequences as shown in (A).
Sm proteins are small proteins characterized by the presence of the highly conserved Sm fold containing two conserved motifs each of which consists largely of β-strands and an embedded RNA-binding site. The motifs are separated by a “linker” region that varies in length among different Sm proteins (A; Hermann et al. 1995; Khusial et al. 2005). When a single Sm protein is folded, both RNA-binding sites are found within loops located in close proximity on one side of the protein (B). When an entire Sm ring is assembled, these loops are oriented toward the central pore of the ring (Kambach, Walke, Young, et al. 1999).
Box 2. Sm proteins in prokaryotes
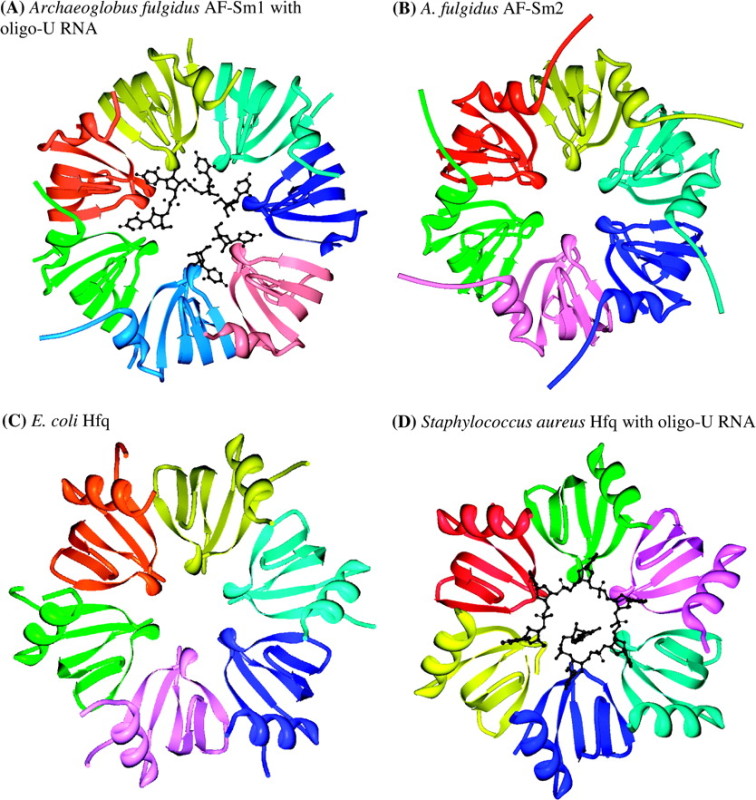 Box 2 FIG.—Prokaryotic Sm rings. (A) and (B) Archaeon Archaeoglobus fulgidus, two distinct Sm proteins: AF-Sm1, PDB 1I5L, complexed with oligo-U RNA (Törö et al. 2001), and AF-Sm2, PDB 1LJO (Törö et al. 2002). (C) Eubacteria Eschericia coli Hfq, PDB 1HK9 (Sauter et al. 2003). (D) Eubacteria Staphylococcus aureus Hfq, PDF 1KQ2, complexed with oligo-U RNA (Schumacher et al. 2002). Monomers are colored individually for clarity but are identical within each ring. All structures from Protein Data Bank (Berman et al. 2000) rendered with Protein Workshop (Moreland et al. 2005). Rings not all on same scale.
Box 2 FIG.—Prokaryotic Sm rings. (A) and (B) Archaeon Archaeoglobus fulgidus, two distinct Sm proteins: AF-Sm1, PDB 1I5L, complexed with oligo-U RNA (Törö et al. 2001), and AF-Sm2, PDB 1LJO (Törö et al. 2002). (C) Eubacteria Eschericia coli Hfq, PDB 1HK9 (Sauter et al. 2003). (D) Eubacteria Staphylococcus aureus Hfq, PDF 1KQ2, complexed with oligo-U RNA (Schumacher et al. 2002). Monomers are colored individually for clarity but are identical within each ring. All structures from Protein Data Bank (Berman et al. 2000) rendered with Protein Workshop (Moreland et al. 2005). Rings not all on same scale.
In prokaryotes, Sm proteins form six- or seven-membered homomeric rings with each member being an identical copy of the protein (Box 2 fig.). For example, Hfq from Escherichia coli forms 6-meric rings (Sauter et al. 2003), whereas the archaeon Archaeoglobus fulgidus has two Sm proteins: Sm1, which forms a homomorphic 7-meric ring, and Sm2, which forms a homomorphic ring containing either six (via crystallography, Törö et al. 2002) or seven (via electron microscopy when complexed with RNA, Achsel et al. 2001) Sm2 proteins. Thus, apart from differences that may be artifacts arising from methods of structural determination, the diversity of Sm rings in prokaryotes is determined on a one-to-one basis by the diversity of Sm proteins (Achsel et al. 2001; Törö et al. 2002).
Several solved structures (e.g., Box 2 fig. A and D) show prokaryotic Sm rings complexed with U-rich RNA encircling one face of the central pore of the ring (PDB IDs: 1I5L, Törö et al. 2001; 1LOJ, Mura, Kozhukhovsky et al. 2003; 1M8V, Thore et al. 2003), with one structure showing RNA encircling as well as penetrating the central pore (1KQ2, Schumacher et al. 2002). Strong affinity for U-rich RNA oligomers has been confirmed in the archaeon A. fulgidus (Törö et al. 2001) and affinity for A-rich regions, including the poly-A tails of transcripts, has been shown for the Sm protein Hfq in the Eubacteria E. coli (Hajnsdorf and Régnier 2000; Schumacher et al. 2002).
Functional roles include participation in a variety of RNA-processing steps. In E. coli, Hfq primarily functions as an RNA chaperone, by binding to A/U–rich regions with sufficient strength to destabilize nearby (transient) secondary structures and thereby promoting correct RNA–RNA complex formation; such actions are implicated in regulation of translation (reviewed in Schumacher et al. 2002).
All evidence to date indicates that though prokaryotes may vary in the number of Sm proteins encoded by their genomes (Sun et al. 2002; Mura, Phillips et al. 2003), a one-to-one in vivo relationship exists between prokaryotic Sm proteins and homomorphic Sm rings (Achsel et al. 2001; Törö et al. 2002). If this is, indeed, true, barring the unlikely discovery of an Sm ring assembly pathway in prokaryotes, Sm proteins within a multi-Sm prokaryote must be considerably more self-selective than eukaryotic Sm proteins, for which empirical data show rather promiscuous binding between Sm folds (Plessel et al. 1997; Collins et al. 2003). This self-selectivity may be enhanced by having one ring assemble spontaneously in vivo, whereas another assembles in the presence of RNA, as is the case with Sm1 and Sm2, respectively, in A. fulgidus (Achsel et al. 2001). Supporting predictions include the exclusive assembly of homomeric Sm rings from mixtures of different Sm proteins from the same prokaryote, enhanced self-assembly kinetics when co-occurring Sm proteins form 7-meric rings versus rings with different numbers of monomers, and reduced selectivity within in vitro and in vivo mixtures of Sm proteins from different prokaryotes, particularly when the Sm proteins originate from more distantly related prokaryotes, for example, Archaea and Eubacteria.
Though the evolutionary diversification of Sm proteins and Sm rings in eukaryotes clearly reflects a general increase in RNA-processing complexity in eukaryotes (Anantharaman et al. 2002), there are several dramatic discontinuities in comparison to prokaryotes. These include the formation of heteromorphic, 7-meric Sm rings; the highly stable, static association of Sm rings with snRNA cofactors; the use of a dedicated pathway for assembling Sm rings with snRNA cofactors; and the central participation of Sm–snRNA complexes within heterogeneous small nuclear ribonucleoproteins (snRNPs) having a variety of eukaryote-specific RNA-processing functions.
The existing “diversification–duplication” model for the evolution of the Sm protein family in eukaryotes proposes two primary steps early in the evolution of eukaryotes (Salgado-Garrido et al. 1999). The protein family first diverged to form a single heterogeneous Sm ring, followed by a large-scale duplication that allowed for the assembly of a second but related Sm ring; these two rings are the “canonical” Sm ring at the heart of the U1, U2, U4, and U5 spliceosomal snRNPs and the Lsm ring at the heart of the U6 spliceosomal snRNP. Subsequent smaller duplications led to the creation of other Sm proteins that participate in other Sm rings.
In this study, we consider the pattern of evolutionary diversification of Sm proteins in eukaryotes. We first examine the phylogenetic structure of Sm proteins across a wide variety of prokaryotes and eukaryotes and identify major evolutionary trends in the family. We find that eukaryotic Sm diversity reached much of its present breadth very early in eukaryotic evolution. We then review Sm structure, diversity and function in prokaryotes and eukaryotes, as well as the pathways for Sm ring assembly in eukaryotes. Based on the functional review and the results of the phylogenetic analysis, we partition Sm rings in eukaryotes into two classes: fixed Sm rings and flexible Sm rings, corresponding roughly to the rather informal nomenclature for “Sm-type” and “Lsm-type” rings. Our fixed and flexible Sm ring class designations reflect not only broad differences in the duration of the Sm ring–RNA cofactor association but also presumed differences in Sm ring–RNA-binding patterns and Sm ring structural stability that underlie differences in Sm ring–RNA associations. Finally, we consider some possible mechanisms for Sm ring evolution in eukaryotes. The analyses presented here provide insights into the evolution of this distinctive, ubiquitous, and critical family of RNA-associated proteins.
Phylogenetic Relationships among Sm Proteins
We examined the evolutionary relationships among Sm proteins across all life by assembling a phylogenetic tree containing 202 Sm proteins from GenBank records. Prokaryotic Sm proteins include those representing Archaea (figures in brackets indicate the number of Sm proteins in the taxa where greater than one): Archaeoglobus fulgidus [2], Thermoplasma volcanium GSS1 [2], Thermoplasma acidophilum DSM 1728 [2], Aeropyrum pernix K1 [2], Pyrobaculum aerophilum IM2 [3], and Methanopyrus kandleri AV19 and Eubacteria: Escherichia coli O157:H7, Staphylococcus aureus ssp. aureus JH1, Clostridium botulinum B strain Eklund 17B, Thermobifida fusca YX, α-proteobacterium HTCC2255 [2], and γ-proteobacterium HTCC2207. We also broadly sampled Sm proteins from eukaryotes, including the diplomonad Giardia lamblia, the parabasalid Trichomonas vaginalis G3, the apicomplexan Plasmodium falciparum 3D7, the cryptomonad Guillardia theta, the amoebozoan Entamoeba histolytica, the chlorarachniophyte Bigelowiella natans, the kinetoplastid Trypanosoma cruzi strain CL Brener, the plant Arabidopsis thaliana, the microsporidian Encephalitozoon cuniculi, the fungus Saccharomyces cerevisiae, and animals, including Drosophila melanogaster, the urochordate Ciona intestinalis, and human. Sm proteins were initially identified by GenBank annotations with the sequence set incrementally expanded via PSI-Blast (Altschul et al. 1997). For species having insufficient GenBank coverage for Sm proteins, the excellent spliceosomal protein sequence database provided by Barbosa-Morais et al. (2006) was used; see their paper for further details of their sources. Multiple sequence alignment was performed using MUSCLE with default settings (Edgar 2004), with a neighbor-joining tree constructed from this alignment using ClustalW with gaps included and 1000 bootstraps (Chenna et al. 2003). Expanded information for the 202 sequences used may be found in supplementary table 1 (Supplementary Material online).
Although the extreme age and high degree of diversification of the Sm protein family has resulted in relatively high uncertainty at more basal nodes within clades, some features are readily apparent in figure 1 and its more detailed version in supplementary figure 1 (Supplementary Material online): 1) the basal positions of archaeal and eubacterial Sm proteins, with archaeal proteins scattered throughout the tree and eubacterial proteins clustering together in a single clade; 2) the extreme depth of the fundamental splits among individual eukaryotic Sm proteins; and 3) the subsequent evolution within individual Sm protein clades following, with some variation, the course of eukaryotic diversification. In this tree, there is relatively little support for any one configuration of deeper branches above clades for individual Sm proteins. The interrelationships among some Sm protein clades shifts somewhat with different compositions of the data set, though some neighbor clade pairs remain relatively robust, for example, Lsm8 + Lsm1, Lsm5 + SmE, and Lsm3 + SmD2 (data not shown). In this tree, some individual proteins are out of place, such as Lsm8, Lsm6, and SmB in S. cerevisiae. This is not surprising, given the great age of individual proteins in this family, but some of these placements may reflect species-specific evolutionary differences in RNA processing, for example, S. cerevisiae contains an unusually depauperate set of introns (Fink 1987).
FIG. 1.—
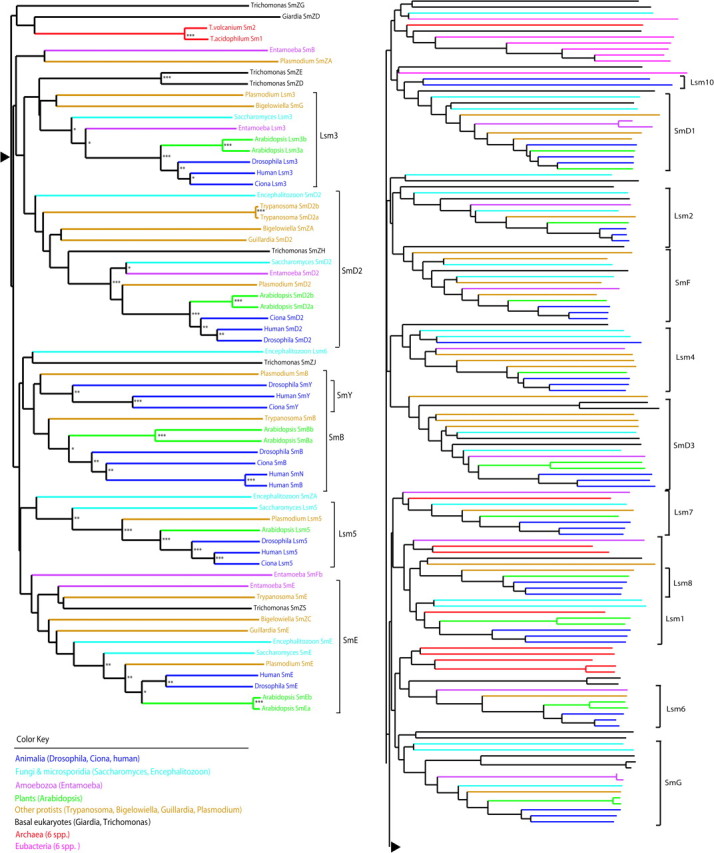
Evolution of Sm family proteins throughout the three domains of life. Bootstrap support “***”≥90%, “**”≥70%, “*”≥50%. A fully annotated version of this figure is available as supplementary figure 1 (Supplementary Material online).
Sm Protein Diversification in Eukaryotes
Eukaryotic Sm proteins have undergone considerable diversification in comparison to their prokaryotic ancestors and play a central role in many eukaryote-specific snRNPs. Nearly all in vivo Sm rings have been found to be 7-merous with each of the 7 members being a distinct Sm protein (Fig. 2A). Exceptions to date include two functional complexes containing 6 distinct Sm proteins: the Sm ring selectively binding U8 snoRNA in Xenopus (Tomasevic and Peculis 2002) and the Sm ring binding snR5 RNA in Saccharomyces (Fernandez et al. 2004). Eukaryotic Sm proteins are involved in snRNA stability (Mayes et al. 1999; Liu et al. 2004), interaction with nuclear import factors during snRNP maturation and recycling of spliceosomal multi-snRNP complexes (Palacios et al. 1997; Chan et al. 2003; Liu et al. 2004; Narayanan et al. 2004), stabilization of spliceosome–mRNA complexes (Zhang et al. 2001), and modification of a variety of RNA substrates (Pillai et al. 2003).
FIG. 2.—
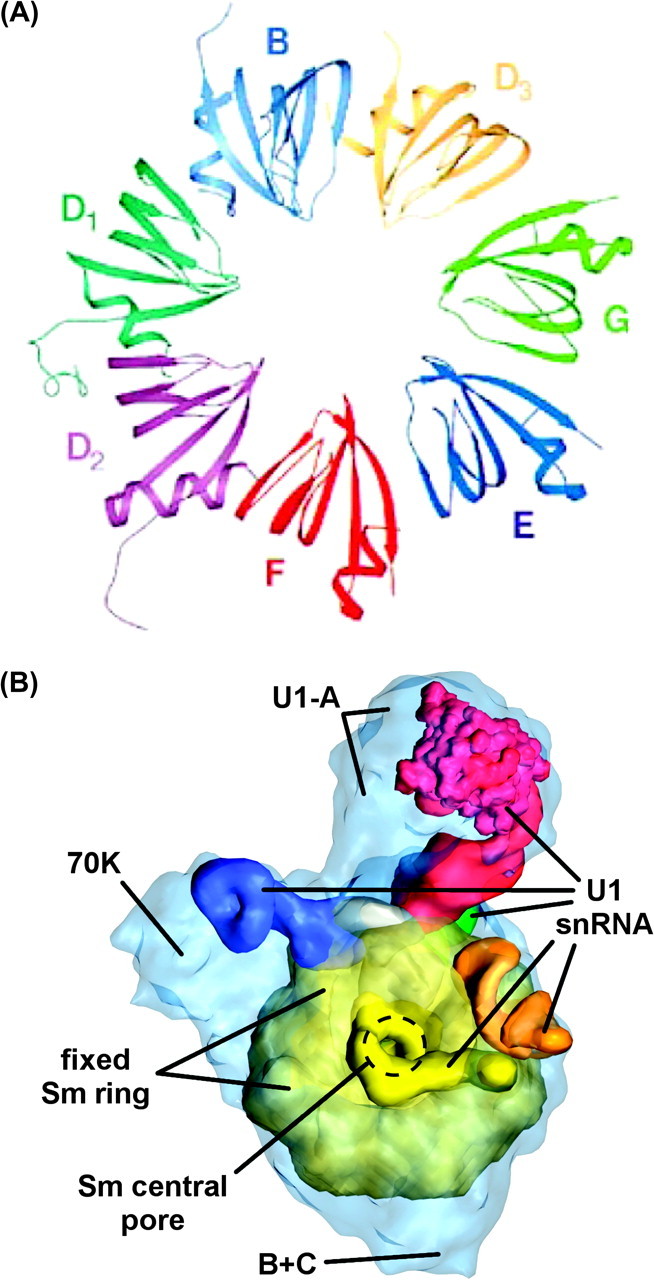
Eukaryotic Sm ring and Sm-anchored snRNP. (A) Human fixed class Sm ring anchoring spliceosomal snRNPs (see text). Reprinted from Kambach, Walke, Young, et al. (1999). ©1999 with permission from Elsevier. (B) Human U1 snRNP. Shown are the fixed Sm ring with its central pore; the U1 snRNA passing through the central pore of the ring with loops I, II, II, and IV and the Sm site in blue, red, green, orange, and yellow, respectively; and the locations of the 70K, U1-A, and B + C set of U1-associated proteins (Stark and Lührmann 2006). Figure kindly provided by H. Stark (Max Planck Institute for Biophysical Chemistry, Göttingen, Germany); labels added by authors.
In this section, we will define and use our fixed and flexible class designations where appropriate. We will consider these class designations more fully in the Discussion.
Fixed Class (Sm-Type) Sm Rings
Fixed class eukaryotic Sm rings form stable, long-term associations with RNA substrates, around which they are assembled via a dedicated pathway. Fixed class Sm rings form largely passive scaffolds around which RNA and protein cofactors assemble in a spatially explicit manner. RNA substrates of fixed Sm rings contain an “Sm site,” which consists of PuAU4−6GPu flanked by two stem–loop structures (Branlant et al. 1982; Liautard et al. 1982; Urlaub et al. 2001; Khusial et al. 2005). Electron microscopy and UV cross-linking studies of the human U1 snRNP suggest that, rather than encircle one face of the Sm ring as in prokaryotes, the Sm site of U1 snRNA circles through the central pore (Fig. 2B and Stark et al. 2001; Urlaub et al. 2001; Stark and Lührmann 2006). The binding configuration of other fixed Sm ring–RNA associations is currently unknown.
The “canonical” fixed class eukaryotic Sm ring is found at the center of the U1, U2, U4, and U5 spliceosomal snRNPs (Fig. 2A). This Sm ring contains monomers in the order SmD1/SmD2, SmF/SmE/SmG, and SmD3/SmB, where the subgroups indicate spontaneously forming dimers and a trimer that are then assembled to form the complete ring (Kambach, Walke, and Nagai 1999; Kambach, Walke, Young, et al. 1999; Raker et al. 1999). This Sm ring is assembled around its various snRNA substrates in the cytoplasm via the Survival of Motor Neurons (SMN) pathway (Meister et al. 2002; Yong, Wan, and Dreyfuss 2004) with components that selectively bind both Sm proteins (Pu et al. 1999; Friesen et al. 2001) and snRNA (Pellizzoni, Yong, and Dreyfuss 2002; Yong, Pellizzoni, et al. 2002; Golembe, Yong, and Dreyfuss 2005). These Sm-snRNA pre-snRNPs are transported into the nucleus complexed with the SMN protein (Fischer et al. 1991; Palacios et al. 1997; Massenet et al. 2002; Narayanan et al. 2004) and are then matured within Cajal bodies (Jády et al. 2003; Kiss 2004; Cioce and Lamond 2005; Liu, Murphy, et al. 2006; Matera and Shpargel 2006; Stanek and Neugebauer 2006; Tycowski et al. 2006). The high selectivity of the SMN pathway ensures correct association of Sm site–containing snRNA with the appropriate fixed Sm ring, but it is not foolproof. RNAs associated with the primate virus Herpesvirus saimiri can outcompete host snRNAs for fixed Sm rings on this same pathway and thereby gain intracellular stability (Golembe, Yong, Battle, et al. 2005).
There are taxa- and tissue-specific variations in the composition of the fixed Sm ring in spliceosomal snRNPs. In trypanosomes, some spliceosomal snRNPs contain variant Sm rings. Trypanosomes’ U2 snRNAs have a divergent Sm site in comparison both to other spliceosomal snRNAs in trypanosomes and to U2 snRNAs in other eukaryotes, and the Sm ring assembled around this Sm site is also divergent (Wang et al. 2006). The SmD3/SmB dimer is replaced by the novel Sm proteins Sm16.5K/Sm15K, the closest sequence homologs of which are SmD3 and SmB, respectively (Wang et al. 2006). In trypanosomes’ U4 snRNP, SmD3 alone is replaced by a novel Sm protein not found in the U2 snRNP (Tkacz et al. 2007). In mammals, there are three variants of SmB (SmB, SmB′, and SmN) that may all substitute for SmB in spliceosomal snRNPs; they are interrelated by alternative splicing and gene duplication (McAllister et al. 1988; McAllister et al. 1989; Chu and Elkon 1991; Griffith et al. 1992; Gray et al. 1999). SmN is most highly expressed in neural tissue, particularly postnatal brain, and its underexpression is associated with Prader–Willi syndrome (Gray et al. 1999; Nicholls and Knepper 2001).
There are other snRNPs that contain fixed Sm rings assembled and matured via the SMN pathway and Cajal bodies. The U7 snRNP (Schümperli and Pillai 2004) performs 3′-end processing of histone mRNAs and is highly conserved among metazoans. The U7 snRNA contains a divergent Sm site bound by an Sm ring similar in composition to that for the spliceosomal snRNPs but with Sm proteins Lsm10/Lsm11 substituted for SmD1/SmD2, with these new Sm proteins derived from SmD1 (or SmD3) and SmD2, respectively. Lsm11, in contrast to the largely passive role played by all other known Sm proteins found in Sm rings, contains an additional domain that is directly involved in histone mRNA processing (Pillai et al. 2003). The divergent Sm site in U7 snRNA is required for the SMN pathway to assemble the divergent Sm ring (Kolev and Steitz 2006), and Cajal bodies are involved in U7 snRNP maturation in the nucleus (Stanek and Neugebauer 2006).
The telomerase snRNP replicates chromosome ends in eukaryotes (Collins 2006), and there is evidence suggestive of metazoan telomerase snRNP hosting a fixed Sm ring at its core. Both human and yeast telomerase snRNA contain an Sm site around which Sm proteins assemble, yet curiously, in both humans and yeast only two Sm proteins have yet been identified, SmB and SmD3 in humans (Fu and Collins 2006), SmD1 and SmD3 in yeast (Seto et al. 1999). Given the composition of other Sm rings, it seems reasonable to expect that additional Sm proteins will eventually be found in these complexes. There is a great deal of divergence in maturation pathways among eukaryotic telomerase snRNPs, for example, ciliate telomerase does not contain Sm proteins (Collins 2006). Yeast telomerase snRNPs have been shown to be assembled in the cytoplasm and imported into the nucleus (Ferrezuelo et al. 2002; Teixeira et al. 2002), and in humans, telomerase components are found in Cajal bodies (Fu and Collins 2006), suggesting that maturation pathways in at least some metazoans are similar to those used by spliceosomal and U7 snRNPs.
A wide variety of eukaryotes process mono- and/or polycistronic transcripts via trans-splicing, in which a fragment of a “leader” RNA is spliced onto each cistron from a specialized splice leader (SL) snRNA found within a specialized SL snRNP (Hastings 2005). All SL snRNAs examined to date contain an Sm site (Mandelboim et al. 2003; Zeiner et al. 2004; Zhang et al. 2007) to which is bound an Sm ring that contains many if not all the same Sm proteins in the fixed Sm ring at the center of other spliceosomal snRNPs (Bruzik et al. 1988; Thomas et al. 1988; Palfi et al. 2000; Tkacz et al. 2007). A very curious consequence of trans-splicing is that the life cycle of the SL snRNP containing the SL snRNA is unusually short in comparison to that of other snRNPs anchored by fixed Sm rings (MacMorris et al. 2007). We will consider SL snRNPs further in the Discussion.
Flexible Class (Lsm-Type) Sm Rings
Flexible class eukaryotic Sm rings differ in several respects from fixed class Sm rings, the primary difference being a more fluid association with RNA substrates. Flexible Sm rings can assemble spontaneously and are stable in the absence of RNA (Achsel et al. 1999; Zaric et al. 2005). RNA substrates of flexible Sm rings lack Sm sites but do have U-rich tracts (Will and Luhrmann 2001). When attached to an RNA substrate, a flexible Sm ring stabilizes and chaperones RNA as does a fixed Sm ring, yet a flexible Sm ring may be more easily associated and dissociated from RNA in some presumably specific manner. A flexible Sm ring may also play a somewhat more active role, in that its presence or absence may signal a transition in the life cycle of an snRNP or RNA substrate.
A characteristic member of the flexible class of Sm rings is the “Lsm ring” at the center of the U6 spliceosomal snRNP, containing the seven proteins Lsm2 through Lsm8 (Achsel et al. 1999; Mayes et al. 1999). Several aspects of the assembly and maturation of the U6 snRNP and its flexible Sm ring differ from that for other spliceosomal snRNPs. In addition to assembling spontaneously in the absence of RNA (Achsel et al. 1999; Zaric et al. 2005), the U6-associated flexible ring appears to be transported into the nucleus as an assembled unit (Will and Luhrmann 2001). The U6 snRNA is transcribed by RNA pol III and is never exported from the nucleus (Kunkel et al. 1986), and assembly of the final U6 snRNP appears to take place entirely within the nucleus (Will and Luhrmann 2001). Though in at least some taxa proteins chaperone pre-U6 snRNP components, for example, the La protein with newly transcribed U6 snRNA in yeast (Xue et al. 2000), both ring assembly and ring–snRNA association steps in U6 snRNP maturation occur without use of the cytoplasmic SMN pathway characteristic of fixed Sm rings.
As details begin to emerge, additional functional differences serve to distinguish the flexible Sm ring at the heart of the U6 snRNP from the fixed Sm ring anchoring the other spliceosomal snRNPs (Karaduman et al. 2006). The flexible Sm ring binds to an U-rich region at the 3′-end of U6 snRNA (Achsel et al. 1999). The extensive base pairing of U4 and U6 snRNAs at the center of the U4/U6 di-snRNP is facilitated by conformational changes in U6 snRNA induced by the ring (Karaduman et al. 2006), and formation of the catalytic center of the spliceosome is critically dependent upon its subsequent dissociation from U6 snRNA (Chan et al. 2003). Following dissociation of the spliceosome, the nuclear retention of U6 snRNA and nuclear regeneration of U6 snRNP are both dependent upon the presence of the flexible Sm ring (Verdone et al. 2004; Spiller et al. 2007).
Other flexible Sm rings have similarly transient associations with their RNA substrates. In yeast, a flexible Sm ring containing the proteins Lsm1 through Lsm7 assembles in the absence of RNA (Zaric et al. 2005) and functions in cytoplasmic mRNA degradation (He and Parker 2000; Tharun et al. 2000; Bergman et al. 2007). The same flexible Sm ring at the center of U6 snRNP (Lsm2 through Lsm8) also plays a role in mRNA degradation within the nucleus (Kufel et al. 2004).
Flexible Sm rings are also common participants in RNA editing pathways, where the fluidity of their association with RNA substrates may be beneficial. Lsm2 through Lsm7, for example, associate with several RNAs: snR5, a box H/ACA snoRNA for guiding site-specific modifications of rRNA (Fernandez et al. 2004); with pre-RNase P RNA (Salgado-Garrido et al. 1999); and perhaps with all rRNA, tRNA, and certain U3 snoRNA precursors (Kufel et al. 2002; Kufel, Allmag, Petfalski, et al. 2003; Kufel, Allmag, Verdone, et al. 2003). A flexible Sm ring composed of a possibly different set of Sm proteins is involved in binding U8 snoRNA which edits rRNA in Xenopus oocytes (Tomasevic and Peculis 2002). It is likely that additional experimental evidence will reveal yet more flexible Sm rings and more variations among species.
Discussion
The basal locations of prokaryotic Sm proteins within the tree provide clear evidence that current eukaryotic Sm proteins are derived from those in prokaryotes (Fig. 1). Since it was first proposed, the diversification–duplication model has been the primary model for the evolution of Sm protein family in eukaryotes (Salgado-Garrido et al. 1999). Our results provide support for some aspects of this model, but there are a number of reasons to believe the picture is considerably more complicated. Consistent with the diversification–duplication model, our phylogenetic analysis revealed that, except for a few more recently derived proteins such as SmY and SmN, the establishment of nearly all existing Sm proteins occurred prior to the last eukaryotic common ancestor (Fig. 1). However, weak support for deeper nodes within the tree makes specific assignment of neighbor relationships among Sm protein clades difficult. A few neighbor clades are robust and largely consistent with the diversification–duplication model (Lsm7 + SmG, Lsm8 + Lsm1, Lsm5 + SmE, Lsm3 + SmD2), but other clades are nested, and neighbor relationships among several clades shift with different data set compositions. It is difficult to reconcile this with the occurrence of a single large-scale duplication event in the family; several partial duplications involving one or a few proteins seem more likely.
The primary novelty in eukaryotic Sm rings is provided by the heteromorphic nature of the Sm ring itself. Although a homomorphic Sm ring as found within prokaryotes can provide spatial specificity for binding and interactions with the pore and faces only within the rotational span of a monomer (Fig. 3A), a heteromorphic Sm ring provides spatial specificity throughout the entire rotational sweep (Fig. 3B). This ensures precise conformational specificity of bound RNA and protein components within the snRNP. This specificity is important for the snRNA, as demonstrated by site-specific pairings of Sm-site uracils in the snRNA with Sm ring pore residues (Hartmuth et al. 1999; Urlaub et al. 2001; Wang et al. 2006) and interactions between snRNA stem–loop secondary structures and faces of the Sm ring (Stark et al. 2001), and for other protein–RNA and protein–protein interactions within the snRNP (Fig. 2B and Urlaub et al. 2000; Dybkov et al. 2006; Stark and Lührmann 2006). The maintenance of consistent three-dimensional structure and snRNA–protein membership of spliceosomal and other snRNPs is certainly dependent upon the heterogeneous nature of the eukaryotic Sm ring. An additional benefit of a heterogeneous Sm ring is that individual Sm proteins are free to develop functional side chains without repetition around the ring (Pillai et al. 2003).
FIG. 3.—
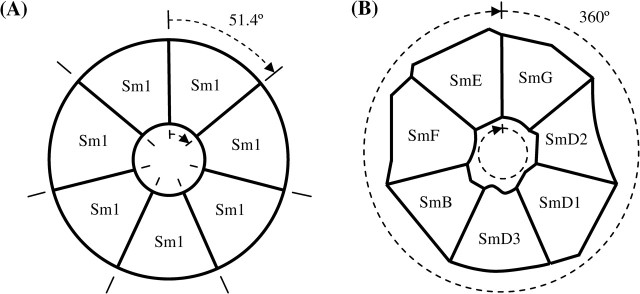
Steric specificity for seven-membered Sm rings. (A) Homomorphic ring typical of prokaryotes. (B) Heteromorphic ring typical of eukaryotes.
With this view, substitutions of Sm proteins within Sm rings central to other snRNPs (e.g., the U7 snRNP, Pillai et al. 2001, 2003) reflect variations in spatial and conformational relationships among the differing RNA and protein cofactors and in the interactions of these partners with pore and faces of the Sm ring itself. The use of the “canonical” eukaryotic Sm ring (Fig. 2A and B) in six spliceosomal snRNPs (U1, U2, U4, U5, U11, and U12 of the minor spliceosome) that are themselves heterogeneous in form and function (Collins and Penny 2005; Russell et al. 2006; Will and Lührmann 2006) indicate that the relationship between snRNP and Sm ring is not exclusive. This suggests a common time of origin for these spliceosomal snRNPs, perhaps coincident with this form of fixed Sm ring.
In support of the necessity of steric specificity in Sm rings, we expect altered spatial conformations of snRNA and proteins within snRNPs anchored by nonstandard Sm rings, that snRNPs anchored by nonstandard Sm rings are more likely to attract novel proteins, that snRNPs containing nonstandard Sm rings may have limited functionality, and that smaller “minimal functional” snRNPs would be formed when anchored by standard Sm rings.
What might have been some of the early events leading from homomorphic Sm rings in prokaryotes to heteromorphic Sm rings in eukaryotes? The initial diversification may not have originated in prokaryotes; none of the proteins in the Archaea we examined with multiple (and self-selective) Sm proteins (P. aerophilum, A. pernix, T. volcanium, T. acidophilum, A. fulgidus) fell near each other in our tree (supplementary Fig. 1, Supplementary Material online). The “seeding” of Sm protein diversity may have occurred via contact between long-isolated Sm proteins that would have lost the “niche-exclusionary” ability to avoid binding with each other's Sm folds. Such contact could have occurred via lateral transfer among Archaea or between Archaea and Eubacteria or could have coincided with genome clashes and/or gene transfers proposed to have accompanied the endosymbiotic origin of the eukaryotic cell itself (Koonin 2006; Martin and Koonin 2006). The evolved self-affinity necessary for the formation of the original homomorphic rings would still be in place, but competition from a novel Sm fold would have resulted in the formation of heteromorphic Sm rings. Such heteromorphic rings could have remained functional, for two reasons: 1) the novel rings would have continued to bind U-rich RNA, much as this ability is maintained in novel in vitro Sm complexes in yeast (Collins et al. 2003) and 2) because of low rotational specificity in the original homomorphic rings, any protein–protein or protein–RNA interactions required for function likely did not require the entire homomorphic ring and would have been able to occur against the original monomers (Mikulecky et al. 2004). This could be tested by examining the in vitro behavior of novel mixtures of Sm proteins from closely and distantly related prokaryotes.
Once established, a heteromorphic Sm ring could have provided a template for further diversification, by allowing for greater steric specificity and thus greater complexity of Sm ring–associated snRNPs. Novel substitutions can occur with comparatively high frequency in heteromorphic Sm rings, as evidenced by clade-, species-, and even tissue-specific substitutions within eukaryotic Sm rings.
Finally, the heptameric structure of Sm rings could have accelerated the process of diversification. Consider a homomorphic heptamer, A7. A single substitution creates a 6 + 1 ring, A6B. For this structure to be stabilized, neighbor–neighbor relationships should evolve some specificity, but 7 is prime; there is no small multiple that eases this transition. In a hexamer, this could occur in pairs (AB)3 or (with an additional Sm protein) in triplets (ABC)2. Polymerization of such small subunits into novel hexamers and octamers has been observed in vitro for eukaryotic Sm proteins (Zaric et al. 2005). Thus, the most stable seven-member heteromorphic ring may be one that is entirely heteromorphic, with no repeated subunits.
Why do spliceosomal snRNPs contain two distinct Sm rings? The flexible Sm ring at the heart of the U6 spliceosomal snRNP is highly conserved in eukaryotes (Séraphin 1995; Mayes et al. 1999; Salgado-Garrido et al. 1999; Liu et al. 2004). There may be a functional basis derived from the formation of the U4/U6 di-snRNP, which then forms a tri-snRNP with the U5 snRNP in which RNA–RNA, RNA–protein and protein–protein contacts are all important (Vidal et al. 1999; Chan et al. 2003; Karaduman et al. 2006; Liu, Rauhut, et al. 2006). Especially during the formation of the U4/U6 di-snRNP, proper interactions may depend upon each snRNP containing entirely nonoverlapping sets of Sm proteins. The stabilization of these interactions was an early event in the evolution of the spliceosome (Collins and Penny 2005).
Fixed and Flexible Sm Rings
We have proposed nomenclature for classes of Sm rings––fixed (abbreviated “Fix”) and flexible (“Flex”) as replacements for the informal classes of Sm-type and Lsm-type, respectively––that is both reflective of the different functional roles played by each class and evocative of the manner in which they are associated and dissociated from their RNA cofactors (table 1). Our nomenclature is also free from potential confusions that may arise when an Sm ring such as that at the center of the metazoan U7 snRNP is composed of both “Sm”-prefixed and ”Lsm”-prefixed protein monomers (Pillai et al. 2003). Some of these characteristics are shared by Sm proteins in Archaea, with one ring forming only in the presence of RNA and one forming spontaneously (Achsel et al. 2001). Proteins involved in fixed and flexible rings do not form monophyletic groups (Fig. 1), thus it may be these functional classes for Sm rings, broadly defined and suitably diversified, which have been maintained throughout the evolution of the Sm protein family, rather than the specific protein composition of the rings.
Table 1.
Characteristics distinguishing fixed from flexible Sm rings
| Trait | Fixed Sm ring | Flexible Sm ring |
| Example ring | SmB/D3/G/E/F/D2/D1 found in U1, U2, U4, U5 snRNP | Lsm8/4/7/5/6/3/2 found in U6 snRNP |
| Alternate rings with Sm protein substitutions? | Yes | Yes |
| RNA has Sm site? | Yes | No |
| RNA-binding configuration | Passes through pore (?) | Encircles one face of pore (?) |
| Dedicated assembly pathway? | Yes | No |
| Assembles spontaneously in vitro? | Not without RNA | Yes |
| Dissociates from RNA during normal operation? | No | Yes |
One currently somewhat speculative characteristic that distinguishes fixed from flexible eukaryotic Sm rings is the manner in which each class binds to associated RNA (table 1). The U1 snRNA appears to pass through the central pore of the fixed Sm ring at the center of the U1 snRNP (Fig. 2B, see also Stark et al. 2001; Urlaub et al. 2001; Stark and Lührmann 2006). Such a configuration would likely confer additional stability onto the fixed Sm ring–snRNA association, while increasing the cost of dissociation. In comparison, flexible Sm rings might be more easily dissociated from their RNA cofactors if the RNA circles around one face of the central pore without passing through, as appears to be common in prokaryotic Sm–RNA associations.
The Sm ring that anchors the SL snRNP in organisms that use trans-splicing is an anomalous fixed class ring with rapid association and dissociation from its RNA substrate. The steps required for this are 1) assembly of a fixed Sm ring around SL snRNA, 2) maturation of the SL snRNP, 3) participation of the SL snRNP in trans-splicing, 4) removal of non-Sm proteins from the SL snRNP, and 5) dissociation of the fixed Sm ring from the “spent” SL snRNA. Details of most of these steps are unknown (MacMorris et al. 2007). In trypanosomes, the fixed Sm ring at the center of the SL snRNP appears to be identical in protein composition to the one at the center of the U1, U4, and U5 snRNPs, so some particular feature of the SL snRNP allows this rapid turnover. In trypanosomes and other organisms with trans-splicing, there may be a dedicated pathway that recognizes spent SL snRNPs; though it seems likely to exist, evidence of such a pathway has not yet been found.
SL snRNPs represent a sort of functional intermediate between the longer term associations of fixed Sm rings and the more transient associations typical of flexible Sm rings. For the evolution of trans-splicing, these stumbling blocks would have to be removed. The use of a fixed Sm ring in SL snRNPs may indicate that the SL snRNP is derived from one of the other spliceosomal snRNPs and that trans-splicing is itself derived from ancestral cis-splicing, perhaps multiple times in several eukaryotic lineages (Nilsen 2001; Hastings 2005). The maintenance of the fixed Sm ring at the center of all SL snRNPs may represent simply a contingent characteristic or may indicate the need to have a fixed Sm ring at the heart of an SL snRNP, perhaps to provide greater stability during interactions and reconfigurations involving other spliceosomal snRNPs.
Recently, a number of multidomain RNA-associated proteins found in a wide range of eukaryotes contain divergent but identifiable Sm domains (Albrecht and Lengauer 2004; Albrecht et al. 2004; Tadauchi et al. 2004; Fleischer et al. 2006; Yang et al. 2006; Tritschler et al. 2007). Unlike Lsm11, which contains an additional domain functional in processing of histone mRNAs (Pillai et al. 2003), none of these proteins have been observed in any form of Sm ring. However, they may yet be found in Sm rings or may interact via their Sm domains to form novel multimeric complexes (Fleischer et al. 2006).
In conclusion, the evolutionary diversification of Sm rings coincided with the evolution of eukaryotes. We do not find much specific support for the diversification–duplication model for the development of separate fixed and flexible Sm rings. Fixed Sm rings such as those anchoring the majority of spliceosomal snRNPs and the U7 snRNP are stable, passive, noncatalytic, spatially specific protein scaffolds around which RNA and proteins that are active within snRNPs can organize. Flexible Sm rings share many of these characteristics but can be more freely associated and dissociated from RNA substrates. As further details concerning the diversity of RNA processing in eukaryotes are found, it is likely that additional forms of both fixed and flexible rings will be discovered.
Supplementary Material
Supplementary Table 1 and Figure 1 are available at Molecular Biology and Evolution online (http://www.mbe.oxfordjournals.org/).
Acknowledgments
This work was supported by National Science Foundation grants DBI-0434671 to D.G.S. and MCB-0342431 to M.L. D.G.S. thanks D. A. Campbell for helpful discussions and two anonymous reviewers for helpful comments that greatly improved the manuscript.
References
- Achsel T, Brahms H, Kastner B, Bachi A, Wilm M, Lührmann R. A doughnut-shaped heteromer of human Sm-like proteins binds to the 3′-end of U6 snRNA, thereby facilitating U4/U6 duplex formation in vitro. EMBO J. 1999;18:5789–5802. doi: 10.1093/emboj/18.20.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Achsel T, Stark H, Lührmann R. The Sm domain is an ancient RNA-binding motif with oligo(U) specificity. Proc Natl Acad Sci USA. 2001;98:3685–3689. doi: 10.1073/pnas.071033998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht M, Golatta M, Wullner U, Lengauer T. Structural and functional analysis of ataxin-2 and ataxin-3. Eur J Biochem. 2004;271:3155–3170. doi: 10.1111/j.1432-1033.2004.04245.x. [DOI] [PubMed] [Google Scholar]
- Albrecht M, Lengauer T. Novel Sm-like proteins with long C-terminal tails and associated methyltransferases. FEBS Lett. 2004;569:18–26. doi: 10.1016/j.febslet.2004.03.126. [DOI] [PubMed] [Google Scholar]
- Altschul S, Madden T, Schaffer A, Zhang J, Zhang Z, Miller W, Lipman D. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman V, Aravind L. Novel conserved domains in proteins with predicted roles in eukaryotic cell-cycle regulation, decapping and RNA stability. BMC Genomics. 2004;5:45. doi: 10.1186/1471-2164-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anantharaman V, Koonin EV, Aravind L. Comparative genomics and evolution of proteins involved in RNA metabolism. Nucleic Acids Res. 2002;30:1427–1464. doi: 10.1093/nar/30.7.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa-Morais NL, Carmo-Fonseca M, Aparício S. Systematic genome-wide annotation of spliceosomal proteins reveals differential gene family expansion. Genome Res. 2006;16:66–77. doi: 10.1101/gr.3936206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman N, Moraes KCM, Anderson JR, Zaric B, Kambach C, Schneider RJ, Wilusz CJ, Wilusz J. Lsm proteins bind and stabilize RNAs containing 5′ poly(A) tracts. Nat Struct Mol Biol. 2007;14:824–831. doi: 10.1038/nsmb1287. [DOI] [PubMed] [Google Scholar]
- Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The Protein Data Bank. Nucleic Acids Res. 2000;28:235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branlant C, Krol A, Ebel JP, Lazar E, Haendler B, Jacob M. U2 RNA shares a structural domain with U1, U4, and U5 RNAs. EMBO J. 1982;1:1259–1265. doi: 10.1002/j.1460-2075.1982.tb00022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzik JP, Vandoren K, Hirsh D, Steitz JA. Trans splicing involves a novel form of small nuclear ribonucleoprotein particles. Nature. 1988;335:559–562. doi: 10.1038/335559a0. [DOI] [PubMed] [Google Scholar]
- Chan SP, Kao DI, Tsai WY, Cheng SC. The Prp19p-associated complex in spliceosome activation. Science. 2003;302:279–282. doi: 10.1126/science.1086602. [DOI] [PubMed] [Google Scholar]
- Chenna R, Sugawara H, Koike T, Lopez R, Gibson TJ, Higgins DG, Thompson JD. Multiple sequence alignment with the Clustal series of programs. Nucleic Acids Res. 2003;31:3497–3500. doi: 10.1093/nar/gkg500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu JL, Elkon KB. The small nuclear ribonucleoproteins, SmB and B′, are products of a single gene. Gene. 1991;97:311–312. doi: 10.1016/0378-1119(91)90069-n. [DOI] [PubMed] [Google Scholar]
- Cioce M, Lamond AI. Cajal bodies: a long history of discovery. Annu Rev Cell Dev Biol. 2005;21:105–131. doi: 10.1146/annurev.cellbio.20.010403.103738. [DOI] [PubMed] [Google Scholar]
- Collins BM, Cubeddu L, Naidoo N, Harrop SJ, Kornfeld GD, Dawes IW, Curmi PMG, Mabbutt BC. Homomeric ring assemblies of eukaryotic Sm proteins have affinity for both RNA and DNA: crystal structure of an oligomeric complex of yeast SmF. J Biol Chem. 2003;278:17291–17298. doi: 10.1074/jbc.M211826200. [DOI] [PubMed] [Google Scholar]
- Collins K. The biogenesis and regulation of telomerase holoenzymes. Nat Rev Mol Cell Biol. 2006;7:484–494. doi: 10.1038/nrm1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L, Penny D. Complex spliceosomal organization ancestral to extant eukaryotes. Mol Biol Evol. 2005;22:1053. doi: 10.1093/molbev/msi091. [DOI] [PubMed] [Google Scholar]
- Dybkov O, Will CL, Deckert J, Behzadnia N, Hartmuth M, Luhrmann R. U2 snRNA-protein contacts in purified human 17S U2 snRNPs and in spliceosomal A and B complexes. Mol Cell Biol. 2006;26:2803–2816. doi: 10.1128/MCB.26.7.2803-2816.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernandez CF, Pannone BK, Chen XG, Fuchs G, Wolin SL. An Lsm2-Lsm7 complex in Saccharomyces cerevisiae associates with the small nucleolar RNA snR5. Mol Biol Cell. 2004;15:2842–2852. doi: 10.1091/mbc.E04-02-0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrezuelo F, Steiner B, Aldea M, Futcher B. Biogenesis of yeast telomerase depends on the importin Mtr10. Mol Cell Biol. 2002;22:6046–6055. doi: 10.1128/MCB.22.17.6046-6055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink GR. Pseudogenes in yeast. Cell. 1987;49:5–6. doi: 10.1016/0092-8674(87)90746-x. [DOI] [PubMed] [Google Scholar]
- Fischer U, Darzynkiewicz E, Tahara SM, Dathan NA, Lührmann R, Mattaj IW. Diversity in the signals required for nuclear accumulation of U snRNPs and variety in the pathways of nuclear transport. J Cell Biol. 1991;113:705–714. doi: 10.1083/jcb.113.4.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischer TC, Weaver CM, McAfee KJ, Jennings JL, Link AJ. Systematic identification and functional screens of uncharacterized proteins associated with eukaryotic ribosomal complexes. Genes Dev. 2006;20:1294–1307. doi: 10.1101/gad.1422006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friesen WJ, Massenet S, Paushkin S, Wyce A, Dreyfuss G. SMN, the product of the spinal muscular atrophy gene, binds preferentially to dimethylarginine-containing protein targets. Mol Cell. 2001;7:1111–1117. doi: 10.1016/s1097-2765(01)00244-1. [DOI] [PubMed] [Google Scholar]
- Fu D, Collins K. Human telomerase and Cajal body ribonucleoproteins share a unique specificity of Sm protein association. Genes Dev. 2006;20:531–536. doi: 10.1101/gad.1390306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembe TJ, Yong J, Dreyfuss G. Specific sequence features, recognized by the SMN complex, identify snRNAs and determine their fate as snRNPs. Mol Cell Biol. 2005;25:10989–11004. doi: 10.1128/MCB.25.24.10989-11004.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembe TJ, Yong JS, Battle DJ, Feng WQ, Wan LL, Dreyfuss G. Lymphotropic Herpesvirus saimiri uses the SMN complex to assemble Sm cores on its small RNAs. Mol Cell Biol. 2005;25:602–611. doi: 10.1128/MCB.25.2.602-611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray TA, Smithwick MJ, Schaldach MA, Martone DL, Graves JA, McCarrey JR, Nicholls RD. Concerted regulation and molecular evolution of the duplicated SNRPB′/B and SNRPN loci. Nucleic Acids Res. 1999;27:4577–4584. doi: 10.1093/nar/27.23.4577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffith AJ, Schmauss C, Craft J. The murine gene encoding the highly conserved Sm B protein contains a nonfunctional alternative 3′ splice site. Gene. 1992;114:195–201. doi: 10.1016/0378-1119(92)90574-9. [DOI] [PubMed] [Google Scholar]
- Hajnsdorf E, Régnier P. Host factor Hfq of Escherichia coli stimulated elongation of poly(A) tails by poly(A) polymerase I. Proc Natl Acad Sci USA. 2000;97:1501–1505. doi: 10.1073/pnas.040549897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmuth K, Raker VA, Huber J, Branlant C, Luhrmann R. An unusual chemical reactivity of Sm site adenosines strongly correlates with proper assembly of core U snRNP particles. J Mol Biol. 1999;285:133–147. doi: 10.1006/jmbi.1998.2300. [DOI] [PubMed] [Google Scholar]
- Hastings KEM. SL trans-splicing: easy come or easy go? Trends Genet. 2005;21:240–247. doi: 10.1016/j.tig.2005.02.005. [DOI] [PubMed] [Google Scholar]
- He WH, Parker R. Functions of Lsm proteins in mRNA degradation and splicing. Curr Opin Cell Biol. 2000;12:346–350. doi: 10.1016/s0955-0674(00)00098-3. [DOI] [PubMed] [Google Scholar]
- Hermann H, Fabrizio P, Raker VA, Foulaki K, Hornig H, Brahms H, Luhrmann R. snRNP Sm proteins share two evolutionarily conserved sequence motifs which are involved in Sm protein-protein interactions. EMBO J. 1995;14:2076–2088. doi: 10.1002/j.1460-2075.1995.tb07199.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jády BE, Darzacq X, Tucker KE, Matera AG, Bertrand E, Kiss T. Modification of Sm small nuclear RNAs occurs in the nucleoplasmic Cajal body following import from the cytoplasm. EMBO J. 2003;22:1878–1888. doi: 10.1093/emboj/cdg187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kambach C, Walke S, Nagai K. Structure and assembly of the spliceosomal small nuclear ribonucleoprotein particles. Curr Opin Struct Biol. 1999;9:222–230. doi: 10.1016/s0959-440x(99)80032-3. [DOI] [PubMed] [Google Scholar]
- Kambach C, Walke S, Young R, Avis JM, De la Fortelle E, Raker VA, Lührmann R, Li J, Nagai K. Crystal structures of two Sm protein complexes and their implications for the assembly of the spliceosomal snRNPs. Cell. 1999;96:375–387. doi: 10.1016/s0092-8674(00)80550-4. [DOI] [PubMed] [Google Scholar]
- Karaduman R, Fabrizio P, Hartmuth K, Urlaub H, Lührmann R. RNA structure and RNA-protein interactions in purified yeast U6 snRNPs. J Mol Biol. 2006;356:1248–1262. doi: 10.1016/j.jmb.2005.12.013. [DOI] [PubMed] [Google Scholar]
- Khusial P, Plaag R, Zieve GW. LSm proteins form heptameric rings that bind to RNA via repeating motifs. Trends Biochem Sci. 2005;30:522–528. doi: 10.1016/j.tibs.2005.07.006. [DOI] [PubMed] [Google Scholar]
- Kiss T. Biogenesis of small nuclear RNPs. J Cell Sci. 2004;117:5949–5951. doi: 10.1242/jcs.01487. [DOI] [PubMed] [Google Scholar]
- Kolev NG, Steitz JA. In vivo assembly of functional U7 snRNP requires RNA backbone flexibility within the Sm-binding site. Nat Struct Mol Biol. 2006;13:347–353. doi: 10.1038/nsmb1075. [DOI] [PubMed] [Google Scholar]
- Koonin EV. The origin of introns and their role in eukaryogenesis: a compromise solution to the introns-early versus introns-late debate? Biol Direct. 2006;1:22. doi: 10.1186/1745-6150-1-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Allmang C, Petfalski E, Beggs J, Tollervey D. Lsm proteins are required for normal processing and stability of ribosomal RNAs. J Biol Chem. 2003;278:2147–2156. doi: 10.1074/jbc.M208856200. [DOI] [PubMed] [Google Scholar]
- Kufel J, Allmang C, Verdone L, Beggs J, Tollervey D. A complex pathway for 3′ processing of the yeast U3 snoRNA. Nucleic Acids Res. 2003;31:6788–6797. doi: 10.1093/nar/gkg904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Allmang C, Verdone L, Beggs JD, Tollervey D. Lsm proteins are required for normal processing of pre-tRNAs and their efficient association with La-homologous protein Lhp1p. Mol Cell Biol. 2002;22:5248–5256. doi: 10.1128/MCB.22.14.5248-5256.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kufel J, Bousquet-Antonelli C, Beggs JD, Tollervey D. Nuclear pre-mRNA decapping and 5′ degradation in yeast require the Lsm2-8p complex. Mol Cell Biol. 2004;24:9646–9657. doi: 10.1128/MCB.24.21.9646-9657.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel GR, Maser RL, Calvet JP, Pederson T. U6 small nuclear RNA is transcribed by RNA polymerase III. Proc Natl Acad Sci USA. 1986;83:8575–8579. doi: 10.1073/pnas.83.22.8575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liautard JP, Sri-Widada J, Brunel C, Jeanteur P. Structural organization of ribonucleoproteins containing small nuclear RNAs from HeLa cells: proteins interact closely with a similar structural domain of U1, U2, U4 and U5 small nuclear RNAs. J Mol Biol. 1982;162:623–643. doi: 10.1016/0022-2836(82)90392-8. [DOI] [PubMed] [Google Scholar]
- Liu JL, Murphy C, Buszczak M, Clatterbuck S, Goodman R, Gall JG. The Drosophila melanogaster Cajal body. J Cell Biol. 2006;172:875–884. doi: 10.1083/jcb.200511038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Liang XH, Uliel S, Belahcen M, Unger R, Michaeli S. Identification and functional characterization of Lsm proteins in Trypanosoma brucei. J Biol Chem. 2004;279:18210–18219. doi: 10.1074/jbc.M400678200. [DOI] [PubMed] [Google Scholar]
- Liu S, Rauhut R, Vornlocher H-P, Lührmann R. The network of protein-protein interactions within the human U4/U6.U5 tri-snRNP. RNA. 2006;12:1418–1430. doi: 10.1261/rna.55406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacMorris M, Kumar M, Lasda E, Larsen A, Kraemer B, Blumenthal T. A novel family of C. elegans snRNPs contains proteins associated with trans-splicing. RNA. 2007;13:511–520. doi: 10.1261/rna.426707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandelboim M, Barth S, Biton M, Liang XH, Michaeli S. Silencing of Sm proteins in Trypanosoma brucei by RNA interference captured a novel cytoplasmic intermediate in spliced leader RNA biogenesis. J Biol Chem. 2003;278:51469. doi: 10.1074/jbc.M308997200. [DOI] [PubMed] [Google Scholar]
- Martin W, Koonin EV. Introns and the origin of nucleus-cytosol compartmentalization. Nature. 2006;440:41–45. doi: 10.1038/nature04531. [DOI] [PubMed] [Google Scholar]
- Massenet S, Pellizzoni L, Paushkin S, Mattaj IW, Dreyfuss G. The SMN complex is associated with snRNPs throughout their cytoplasmic assembly pathway. Mol Cell Biol. 2002;22:6533–6541. doi: 10.1128/MCB.22.18.6533-6541.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matera AG, Shpargel KB. Pumping RNA: nuclear bodybuilding along the RNP pipeline. Curr Opin Cell Biol. 2006;18:317–324. doi: 10.1016/j.ceb.2006.03.005. [DOI] [PubMed] [Google Scholar]
- Mattaj IW, Derobertis EM. Nuclear segregation of U2 snRNA requires binding of specific snRNP proteins. Cell. 1985;40:111–118. doi: 10.1016/0092-8674(85)90314-9. [DOI] [PubMed] [Google Scholar]
- Mayes AE, Verdone L, Legrain P, Beggs JD. Characterization of Sm-like proteins in yeast and their association with U6 snRNA. EMBO J. 1999;18:4321–4331. doi: 10.1093/emboj/18.15.4321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G, Amara SG, Lerner MR. Tissue-specific expression and cDNA cloning of small nuclear ribonucleoprotein-associated polypeptide N. Proc Natl Acad Sci USA. 1988;85:5296–5300. doi: 10.1073/pnas.85.14.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAllister G, Robyshemkovitz A, Amara SG, Lerner MR. cDNA sequence of the rat U snRNP-associated protein N: description of a potential Sm epitope. EMBO J. 1989;8:1177–1181. doi: 10.1002/j.1460-2075.1989.tb03489.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meister G, Eggert C, Fischer U. SMN-mediated assembly of RNPs: a complex story. Trends Cell Biol. 2002;12:472–478. doi: 10.1016/s0962-8924(02)02371-1. [DOI] [PubMed] [Google Scholar]
- Mikulecky PJ, Kaw MK, Brescia CC, Takach JC, Sledjeski DD, Feig AL. Escherichia coli Hfq has distinct interaction surfaces for DsrA, rpoS and poly(A) RNAs. Nat Struct Mol Biol. 2004;11:1206–1214. doi: 10.1038/nsmb858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland J, Gramada A, Buzko O, Zhang Q, Bourne P. The Molecular Biology Toolkit (MBT): a modular platform for developing molecular visualization applications. BMC Bioinformatics. 2005;6:21. doi: 10.1186/1471-2105-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura C, Cascio D, Sawaya MR, Eisenberg DS. The crystal structure of a heptameric archaeal Sm protein: implications for the eukaryotic snRNP core. Proc Natl Acad Sci USA. 2001;98:5532–5537. doi: 10.1073/pnas.091102298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura C, Kozhukhovsky A, Gingery M, Phillips M, Eisenberg D. The oligomerization and ligand-binding properties of Sm-like archaeal proteins (SmAPs) Protein Sci. 2003;12:832–847. doi: 10.1110/ps.0224703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mura C, Phillips M, Kozhukhovsky A, Eisenberg D. Structure and assembly of an augmented Sm-like archaeal protein 14-mer. Proc Natl Acad Sci USA. 2003;100:4539–4544. doi: 10.1073/pnas.0538042100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narayanan U, Achsel T, Luhrmann R, Matera AG. Coupled in vitro import of U snRNPs and SMN, the spinal muscular atrophy protein. Mol Cell. 2004;16:223–234. doi: 10.1016/j.molcel.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Nicholls RD, Knepper JL. Genome organization, function and imprinting in Prader-Willi and Angelman syndromes. Annu Rev Genomics Hum Genet. 2001;2:153–175. doi: 10.1146/annurev.genom.2.1.153. [DOI] [PubMed] [Google Scholar]
- Nilsen TW. Evolutionary origin of SL-addition trans-splicing: still an enigma. Trends Genet. 2001;17:678. doi: 10.1016/s0168-9525(01)02499-4. [DOI] [PubMed] [Google Scholar]
- Palacios I, Hetzer M, Adam SA, Mattaj IW. Nuclear import of U snRNPs requires importin β. EMBO J. 1997;16:6783–6792. doi: 10.1093/emboj/16.22.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi Z, Lucke S, Lahm H-W, Lane WS, Kruft V, Bragado-Nilsson E, Seraphin B, Bindereif A. The spliceosomal snRNP core complex of Trypanosoma brucei: cloning and functional analysis reveals seven Sm protein constituents. Proc Natl Acad Sci USA. 2000;97:8967–8972. doi: 10.1073/pnas.150236097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellizzoni L, Yong J, Dreyfuss G. Essential role for the SMN complex in the specificity of snRNP assembly. Science. 2002;298:1775–1779. doi: 10.1126/science.1074962. [DOI] [PubMed] [Google Scholar]
- Pillai RS, Grimmler M, Meister G, Will CL, Luhrmann R, Fischer U, Schumperli D. Unique Sm core structure of U7 snRNPs: assembly by a specialized SMN complex and the role of a new component, Lsm11, in histone RNA processing. Genes Dev. 2003;17:2321–2333. doi: 10.1101/gad.274403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai RS, Will CL, Luhrmann R, Schumperli D, Muller B. Purified U7 snRNPs lack the Sm proteins D1 and D2 but contain Lsm10, a new 14 kDa Sm D1-like protein. EMBO J. 2001;20:5470–5479. doi: 10.1093/emboj/20.19.5470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plessel G, Lührmann R, Kastner B. Electron microscopy of assembly intermediates of the snRNP core: morphological similarities between the RNA-free (E.F.G) protein heteromer and the intact snRNP core. J Mol Biol. 1997;265:87–94. doi: 10.1006/jmbi.1996.0713. [DOI] [PubMed] [Google Scholar]
- Pu WT, Krapivinsky GB, Krapivinsky L, Clapham DE. pICln inhibits snRNP biogenesis by binding core spliceosomal proteins. Mol Cell Biol. 1999;19:4113–4120. doi: 10.1128/mcb.19.6.4113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raker VA, Hartmuth K, Kastner B, Luhrmann R. Spliceosomal U snRNP core assembly: Sm proteins assemble onto an Sm site RNA nonanucleotide in a specific and thermodynamically stable manner. Mol Cell Biol. 1999;19:6554–6565. doi: 10.1128/mcb.19.10.6554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell AG, Charette JM, Spencer DF, Gray MW. An early evolutionary origin for the minor spliceosome. Nature. 2006;443:863–866. doi: 10.1038/nature05228. [DOI] [PubMed] [Google Scholar]
- Salgado-Garrido J, Bragado-Nilsson E, Kandels-Lewis S, Séraphin B. Sm and Sm-like proteins assemble in two related complexes of deep evolutionary origin. EMBO J. 1999;18:3451–3462. doi: 10.1093/emboj/18.12.3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter C, Basquin J, Suck D. Sm-like proteins in Eubacteria: the crystal structure of the Hfq protein from Escherichia coli. Nucleic Acids Res. 2003;31:4091–4098. doi: 10.1093/nar/gkg480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher MA, Pearson RF, Moller T, Valentin-Hansen P, Brennan RG. Structures of the pleiotropic translational regulator Hfq and an Hfq-RNA complex: a bacterial Sm-like protein. EMBO J. 2002;21:3546–3556. doi: 10.1093/emboj/cdf322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schümperli D, Pillai R. The special Sm core structure of the U7 snRNP: far-reaching significance of a small nuclear ribonucleoprotein. Cell Mol Life Sci. 2004;61:2560–2570. doi: 10.1007/s00018-004-4190-0. [DOI] [PubMed] [Google Scholar]
- Séraphin B. Sm and Sm-like proteins belong to a large family: identification of proteins of the U6 as well as the U1, U2, U4 and U5 snRNPs. EMBO J. 1995;14:2089–2098. doi: 10.1002/j.1460-2075.1995.tb07200.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seto AG, Zaug AJ, Sobel SG, Wolin SL, Cech TR. Saccharomyces cerevisiae telomerase is an Sm small nuclear ribonucleoprotein particle. Nature. 1999;401:177–180. doi: 10.1038/43694. [DOI] [PubMed] [Google Scholar]
- Spiller MP, Boon KL, Reijns MAM, Beggs JD. The Lsm2-8 complex determines nuclear localization of the spliceosomal U6 snRNA. Nucleic Acids Res. 2007;35:923–929. doi: 10.1093/nar/gkl1130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanek D, Neugebauer KM. The Cajal body: a meeting place for spliceosomal snRNPs in the nuclear maze. Chromosoma. 2006;115:343–354. doi: 10.1007/s00412-006-0056-6. [DOI] [PubMed] [Google Scholar]
- Stark H, Dube P, Luehrmann R, Kastner B. Arrangement of RNA and proteins in the spliceosomal U1 small nuclear ribonucleoprotein particle. Nature. 2001;409:539–542. doi: 10.1038/35054102. [DOI] [PubMed] [Google Scholar]
- Stark H, Lührmann R. Cryo-electron microscopy of spliceosomal components. Annu Rev Biophys Biomol Struct. 2006;35:435–457. doi: 10.1146/annurev.biophys.35.040405.101953. [DOI] [PubMed] [Google Scholar]
- Sun X, Zhulin I, Wartell RM. Predicted structure and phyletic distribution of the RNA-binding protein Hfq. Nucleic Acids Res. 2002;30:3662–3671. doi: 10.1093/nar/gkf508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tadauchi T, Inada T, Matsumoto K, Irie K. Posttranscriptional regulation of HO expression by the Mkt1-Pbp1 Complex. Mol Cell Biol. 2004;24:3670–3681. doi: 10.1128/MCB.24.9.3670-3681.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira MT, Forstemann K, Gasser SM, Lingner J. Intracellular trafficking of yeast telomerase components. EMBO Rep. 2002;3:652–659. doi: 10.1093/embo-reports/kvf133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tharun S, He WH, Mayes AE, Lennertz P, Beggs JD, Parker R. Yeast Sm-like proteins function in mRNA decapping and decay. Nature. 2000;404:515–518. doi: 10.1038/35006676. [DOI] [PubMed] [Google Scholar]
- Thomas JD, Conrad RC, Blumenthal T. The C. elegans trans-spliced leader RNA is bound to Sm and has a trimethylguanosine cap. Cell. 1988;54:533–539. doi: 10.1016/0092-8674(88)90075-x. [DOI] [PubMed] [Google Scholar]
- Thore S, Mayer C, Sauter C, Weeks S, Suck D. Crystal structures of the Pyrococcus abyssi Sm core and its complex with RNA: common features of RNA binding in Archaea and Eukarya. J Biol Chem. 2003;278:1239–1247. doi: 10.1074/jbc.M207685200. [DOI] [PubMed] [Google Scholar]
- Tkacz ID, Lustig Y, Stern MZ, Biton M, Salmon-Divon M, Das A, Bellofatto V, Michaeli S. Identification of novel snRNA-specific Sm proteins that bind selectively to U2 and U4 snRNAs in Trypanosoma brucei. RNA. 2007;13:30–43. doi: 10.1261/rna.174307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasevic N, Peculis BA. Xenopus LSm proteins bind U8 snoRNA via an internal evolutionarily conserved octamer sequence. Mol Cell Biol. 2002;22:4101–4112. doi: 10.1128/MCB.22.12.4101-4112.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Törö I, Basquin J, Teo-Dreher H, Suck D. Archaeal Sm proteins form heptameric and hexameric complexes: crystal structures of the Sm1 and Sm2 proteins from the hyperthermophile Archaeoglobus fulgidus. J Mol Biol. 2002;320:129–142. doi: 10.1016/S0022-2836(02)00406-0. [DOI] [PubMed] [Google Scholar]
- Törö I, Thore S, Mayer C, Basquin J, Séraphin B, Suck D. RNA binding in an Sm core domain: x-ray structure and functional analysis of an archaeal Sm protein complex. EMBO J. 2001;20:2293–2303. doi: 10.1093/emboj/20.9.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tritschler F, Eulalio A, Truffault V, Hartmann MD, Helms S, Schmidt S, Coles M, Izaurralde E, Weichenrieder O. A divergent Sm fold in EDC3 proteins mediates DCPI binding and P-body targeting. Mol Cell Biol. 2007;27:8600–8611. doi: 10.1128/MCB.01506-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tycowski KT, Kolev NG, Conrad NK, Fok V, Steitz JA. The ever-growing world of small nuclear ribonucleoproteins. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA world. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2006. pp. 327–368. [Google Scholar]
- Urlaub H, Hartmuth K, Kostka S, Grelle G, Luhrmann R. A general approach for identification of RNA-protein cross-linking sites within native human spliceosomal small nuclear ribonucleoproteins (snRNPs): analysis of RNA-protein contacts in native U1 and U4/U6.U5 snRNPs. J Biol Chem. 2000;275:41458–41468. doi: 10.1074/jbc.M007434200. [DOI] [PubMed] [Google Scholar]
- Urlaub H, Raker VA, Kostka S, Luhrmann R. Sm protein-Sm site RNA interactions within the inner ring of the spliceosomal snRNP core structure. EMBO J. 2001;20:187–196. doi: 10.1093/emboj/20.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentin-Hansen P, Eriksen M, Udesen C. The bacterial Sm-like protein Hfq: a key player in RNA transactions. Mol Microb. 2004;51:1525–1533. doi: 10.1111/j.1365-2958.2003.03935.x. [DOI] [PubMed] [Google Scholar]
- Verdone L, Galardi S, Page D, Beggs JD. Lsm proteins promote regeneration of pre-mRNA splicing activity. Curr Biol. 2004;14:1487–1491. doi: 10.1016/j.cub.2004.08.032. [DOI] [PubMed] [Google Scholar]
- Vidal VPI, Verdone L, Mayes AE, Beggs JD. Characterization of U6 snRNA-protein interactions. RNA. 1999;5:1470–1481. doi: 10.1017/s1355838299991355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang PP, Palfi Z, Preusser C, Lucke S, Lane WS, Kambach C, Bindereif A. Sm core variation in spliceosomal small nuclear ribonucleoproteins from Trypanosoma brucei. EMBO J. 2006;25:4513–4523. doi: 10.1038/sj.emboj.7601328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Will CL, Luhrmann R. Spliceosomal UsnRNP biogenesis, structure and function. Curr Opin Cell Biol. 2001;13:290–301. doi: 10.1016/s0955-0674(00)00211-8. [DOI] [PubMed] [Google Scholar]
- Will CL, Lührmann R. Spliceosome structure and function. In: Gesteland RF, Cech TR, Atkins JF, editors. The RNA world. Cold Spring Harbor (NY): Cold Spring Harbor Laboratory Press; 2006. pp. 369–400. [Google Scholar]
- Wilusz CJ, Wilusz J. Eukaryotic Lsm proteins: lessons from bacteria. Nat Struct Mol Biol. 2005;12:1031–1036. doi: 10.1038/nsmb1037. [DOI] [PubMed] [Google Scholar]
- Xue DH, Rubinson DA, Pannone BK, Yoo CJ, Wolin SL. U snRNP assembly in yeast involves the La protein. EMBO J. 2000;19:1650–1660. doi: 10.1093/emboj/19.7.1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang WH, Yu JH, Gulick T, Bloch KD, Bloch DB. RNA-associated protein 55 (RAP55) localizes to mRNA processing bodies and stress granules. RNA. 2006;12:547–554. doi: 10.1261/rna.2302706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong JS, Pellizzoni L, Dreyfuss G. Sequence-specific interaction of U1 snRNA with the SMN complex. EMBO J. 2002;21:1188–1196. doi: 10.1093/emboj/21.5.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yong JS, Wan LL, Dreyfuss G. Why do cells need an assembly machine for RNA-protein complexes? Trends Cell Biol. 2004;14:226–232. doi: 10.1016/j.tcb.2004.03.010. [DOI] [PubMed] [Google Scholar]
- Zaric B, Chami M, Remigy H, Engel A, Ballmer-Hofer K, Winkler FK, Kambach C. Reconstitution of two recombinant LSm protein complexes reveals aspects of their architecture, assembly, and function. J Biol Chem. 2005;280:16066–16075. doi: 10.1074/jbc.M414481200. [DOI] [PubMed] [Google Scholar]
- Zeiner GM, Foldynova S, Sturm NR, Lukes J, Campbell DA. SmD1 is required for spliced leader RNA biogenesis. Eukaryot Cell. 2004;3:241–244. doi: 10.1128/EC.3.1.241-244.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Abovich N, Rosbash M. A biochemical function for the Sm complex. Mol Cell. 2001;7:319–329. doi: 10.1016/s1097-2765(01)00180-0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Hou YB, Miranda L, Campbell DA, Sturm NR, Gaasterland T, Lin SJ. Spliced leader RNA trans-splicing in dinoflagellates. Proc Natl Acad Sci USA. 2007;104:4618–4623. doi: 10.1073/pnas.0700258104. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


