Abstract
Iron is a micronutrient essential for almost all organisms: bacteria, plants, and animals. It is a metal that exists in multiple redox states, including the divalent ferrous (Fe2+) and the trivalent ferric (Fe3+) species. The multiple oxidation states of iron make it excellent for electron transfer, allowing iron to be selected during evolution as a cofactor for many proteins involved in central cellular processes including oxygen transport, mitochondrial respiration, and DNA synthesis. However, the redox cycling of ferrous and ferric iron in the presence of H2O2, which is physiologically present in the cells, also leads to the production of free radicals (Fenton reaction) that can attack and damage lipids, proteins, DNA, and other cellular components. To meet the physiological needs of the body, but to prevent cellular damage by iron, the amount of iron in the body must be tightly regulated. Here we review how the liver is the central conductor of systemic iron balance and show that this central role is related to the secretion of a peptide hormone hepcidin by hepatocytes. We then review how the liver receives and integrates the many signals that report the body’s iron needs to orchestrate hepcidin production and maintain systemic iron homeostasis.
Iron in the body
The total body iron content is approximately 3 to 5 g in the average adult human. The greatest amount of iron found in the body is complexed as Fe2+ in heme in the hemoglobin of red blood cells and myoglobin of muscles (2-3 g). Body iron balance is maintained by a daily intake of 1 to 2 mg from dietary iron to compensate for the losses of iron through the sloughing of skin, mucosal cells, and sometimes blood loss. Because 20 to 25 mg of iron are required for red blood cell production and cellular metabolism, the amount of iron absorbed from alimentation is insufficient to meet daily catabolic needs. Thus the bulk of iron for daily body needs is provided by the macrophages that recycle senescent red blood cells. Other organs, primarily the liver, serve as reservoirs of iron. There is no regulated pathway to excrete iron from the body, and thus iron balance is primarily preserved by the regulation of iron absorption from the duodenum and iron recycling from macrophages and other tissue stores.
Absorption of iron from food takes place at the brush border of the enterocytes in the duodenum. The mechanisms of absorption of inorganic iron (through the divalent metal transporter 1 (DMT1) and solute carrier family 11, member 2 (SLC11A2)) and iron complexes to heme have been described in detail in previously published reviews.1,2
Cytosolic iron that is not directly used by the cell is either stored in ferritin or exported into the plasma by the basolateral transmembrane iron exporter, ferroportin/SLC40A13,4 and loaded onto transferrin.
Aged or damaged erythrocytes are permanently degraded by macrophages primarily in the spleen, liver, and bone marrow through erythrophagocytosis and iron is exported from phagocytic vesicles via NRAMP1 (natural resistance–associated macrophages protein 1), a divalent metal transporter homologous to DMT1 expressed at the phagolysosomal membrane.5 Iron is then stored in ferritin and, when needed, can be released back into circulation through ferroportin.
Iron transport to cells and absorption by cells
After export from enterocytes and macrophages, iron circulates in the bloodstream bound to transferrin (Tf)6 molecules for delivery to tissues for utilization or storage.
Cells acquire their iron from plasma iron–bound Tf by receptor-mediated endocytosis through the ubiquitous cell surface transferrin receptor 1 (TfR1).7 TfR1 is a homodimeric membrane glycoprotein that binds 2 molecules of Tf to deliver iron into cells. The holo-Tf complex is then internalized and iron is exported to the cytosol through DMT1 or its homolog Nramp1 in macrophages. Finally, the apo-Tf/TfR1 complex is recycled to the cell surface, where Tf is dissociated from TfR1. Tf returns to the bloodstream and is available to rebind iron, whereas TfR1 remains at the plasma membrane.
TfR2 is a transferrin receptor homolog to TfR1 mainly expressed in hepatocytes. It has been primarily described to mediate iron-Tf uptake in nonhepatoma cell lines in vitro, possibly via receptor-mediated endocytosis, similar to TfR1 but with a lower affinity for Tf.8 However, it is currently unclear whether TfR2 truly has a role in cellular iron uptake in hepatocytes.9 Nevertheless, it is now well characterized that even if TfR2 does not participate directly in iron uptake, it is still required for iron homeostasis because mutations in the TfR2 gene induce the iron overload disorder hereditary hemochromatosis, as discussed later.10
In conditions of iron excess, when Tf becomes largely saturated with iron, another form of iron can be found in plasma, non–transferrin-bound iron (NTBI). The topic of NTBI has been recently reviewed11 but will not be discussed in detail here.
The central role of the liver in systemic iron homeostasis
The liver secretes hepcidin
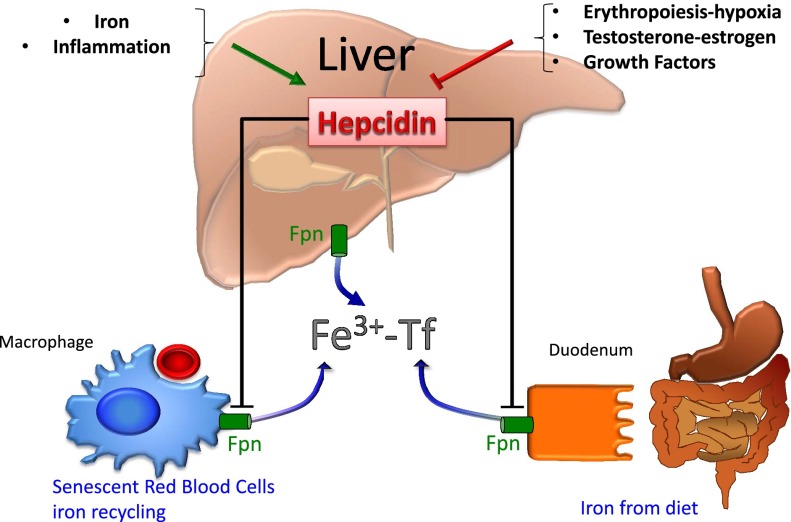
Hepcidin is a small peptide hormone mainly secreted by hepatocytes in the liver (Figure1). Hepcidin is the main regulator of systemic iron homeostasis that blocks the transfer of iron into the plasma from the 3 main sources: dietary absorption in the duodenum, recycling by macrophages, and efflux of stored iron from hepatocytes. Hepcidin was identified simultaneously for its role as an antimicrobial peptide12 and its role in iron homeostasis.13 Although hepatocytes are the main source of circulating hepcidin in the plasma, other cells can also produce hepcidin (such as macrophages14 and adipocytes15) to a much lower extent, but the role of this extrahepatic secretion is still unclear. Because this review is focused on the role of the liver in iron homeostasis, we will only discuss the regulation of hepcidin expression in hepatocytes. The mature form of hepcidin is a 25–amino acid peptide generated from an 84–amino acid prepropeptide after peptidase and furin cleavage.16 In plasma, the mature form of hepcidin circulates associated with α2-macroglobulin17 and albumin,18 although the proportion of this associated form compared with free hepcidin is still uncertain.18
Figure 1.
The liver is the major source of hepcidin production. Iron and inflammation stimulate, whereas erythropoiesis, hormones, and growth factors decrease, hepcidin production. Hepcidin downregulates ferroportin in macrophages, enterocytes, and hepatocytes, leading to decreased iron release into the serum that is subsequently bound to transferrin (Fe3+-Tf).
The effect of hepcidin on iron body balance is related to its direct binding to the principal iron exporter ferroportin at the cell surface, leading to ferroportin internalization and subsequent lysosomal degradation.19 Degradation of ferroportin results in the loss of the ability to export iron and leads to iron sequestration in enterocytes, macrophages, and hepatocytes.
The central role of hepcidin in iron metabolism has been supported by the pathological deregulation of hepcidin expression in humans and mice that results in iron overload or iron deficiency. For conditions in which there is inadequately low hepcidin expression because of mutations in the gene encoding for hepcidin (HAMP) itself,20,21 or in the known positive regulators of hepcidin expression such as bone morphogenetic protein 6 (BMP6, mutations only identified in mouse studies),22,23 hemojuvelin (HFE2, also known as HJV or RGMc),24 mothers against decapentaplegic homolog 4 (SMAD4, confirmed only in mouse studies),25 TFR2,10 or the hemochromatosis protein (HFE),26 the iron exporter ferroportin accumulates at the cell surface of enterocytes and macrophages, leading to unregulated absorption of dietary iron and supraphysiological release of iron from macrophages. This increase of iron export into the plasma rapidly saturates circulating Tf, resulting in an accumulation of NTBI that is easily absorbed by hepatocytes and other tissues, leading to the iron overload disorder known as hereditary hemochromatosis. Although hepatocytes also exhibit higher ferroportin expression under conditions of low hepcidin,27 hepatocytes accumulate iron because the uptake of iron (thought to be a result of the high NTBI levels in plasma) supersedes the increased ferroportin iron export capacity. Conversely, in conditions of hepcidin overexpression, such as is seen in inflammatory conditions,28 artificial hepcidin overexpression,29 and mutations in negative regulator genes of hepcidin such as TMPRSS6,30-32 the result is iron deficiency. Indeed, high hepcidin levels induce degradation of ferroportin that block (1) dietary iron uptake from enterocytes and (2) iron release from macrophages and hepatocytes. In chronic conditions, this iron deficiency can ultimately evolve into an anemia when iron is not sufficiently available for the demands of erythropoiesis (ie, production of red blood cells).
In addition, the central role of the liver in systemic iron homeostasis has also been recently highlighted in a study by Bardou-Jacquet et al.33 They demonstrated that in patients with mutations in the HFE gene, a liver transplant could rescue the low hepcidin phenotype and prevent iron overload.
It is now well characterized that to provide the appropriate amount of iron available for erythropoiesis and cell metabolism, but to prevent toxic iron overload, hepcidin expression by the liver must be tightly regulated. Hepcidin expression is induced by hepatic iron, plasma iron, inflammation, and endoplasmic reticulum stress, whereas it is downregulated by iron deficiency, erythropoiesis, and hypoxia.
The BMP signaling pathway is central for hepcidin regulation
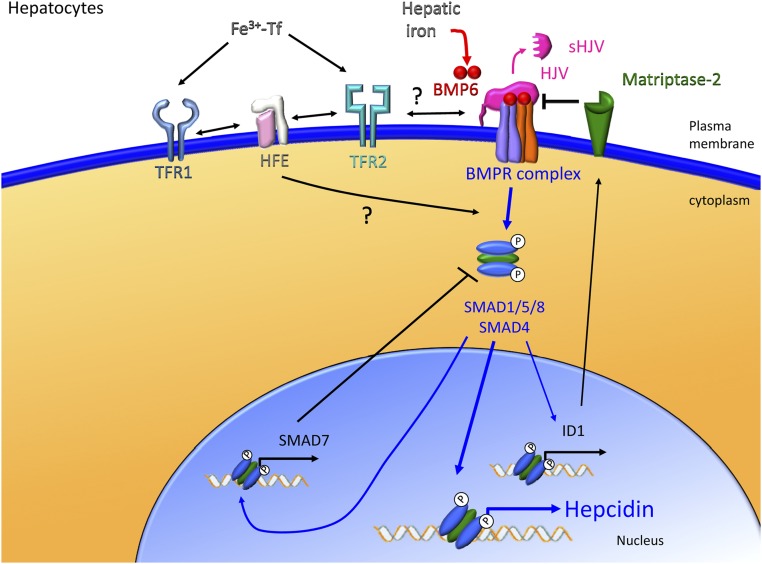
It is now clearly established that the central signaling pathway involved in the regulation of hepcidin expression by iron is the BMP-SMAD pathway.34 The hepcidin promoter contains key BMP-responsive elements, which regulate its transcription.35 Bone morphogenetic proteins (BMPs) represent a large subfamily belonging to the transforming growth factor-β (TGF-β) superfamily of ligands. BMPs mediate many fundamental processes such as embryonic morphogenesis, bone development, and tissue repair.36 Specificity of the BMP-SMAD pathway in the liver and its role in iron homeostasis seems to be dependent on the combination of two factors that are mainly expressed in the liver: an iron-regulated ligand BMP6 and a GPI-membrane-anchor co-receptor hemojuvelin (HJV) (Figure 2).
Figure 2.
The liver responds to iron signals to regulate hepcidin production. Hepatic iron accumulation leads to increased BMP6 production, which is an important ligand for the HJV/BMP receptor complex on the surface of hepatocytes. The activation of the BMP signaling pathway leads to nuclear translocation of SMAD1/5/8 with SMAD4 and subsequent activation of hepcidin transcription. Circulating iron–bound transferrin (Fe3+-Tf) also stimulates hepcidin production by activating the BMP/HJV/SMAD signaling pathway. However, the exact details of the necessary and sufficient interactions of iron-bound transferrin with transferrin receptor 1 (TFR1), transferrin receptor 2 (TFR2), and HFE at the surface of hepatocytes to stimulate hepcidin expression are still unknown. Other modulators such as matriptase-2 are important for regulating iron-induced signals that affect hepcidin production.
All TGF-β superfamily members, including BMPs, share common structural features and a common model of signaling transduction. The active form of BMPs is a disulfide-linked dimeric protein, which is cleaved from a larger precursor protein and secreted. The essential role of BMP6 in hepcidin regulation is highlighted by the inappropriately low level of hepcidin and massive iron overload exhibited in mice lacking BMP6.22,23 However, it is worth noting that the essential role of BMP6 in the regulation of hepcidin expression has not yet been described in humans. Other endogenous BMPs are not able to compensate for the loss of BMP6 in hepcidin regulation (at least in mice) despite the capacity of exogenous BMP2, 4, 5, 7, and 9 to stimulate hepcidin expression.37 Once secreted, BMPs act by binding to 2 distinct receptor types: type I and type II. There are 4 type I receptors (ALK1, ALK2, ALK3, ALK6) and 3 type II receptors (ACTRIIA, ACTRIIB, BMPRII) for the BMP subfamily. For hepcidin regulation in response to iron, the BMP receptors involved are most likely ALK3, ALK2, and ACTRIIA38 because liver-specific deletion of either Alk3 or (to a lesser extent) Alk2 causes iron overload in mice,39 and because ACTRIIA is the predominant type II receptor expressed in human liver.38 Upon BMP binding, the type II receptors phosphorylate the type I receptors, leading to the phosphorylation and activation of specific SMAD proteins. The receptor-regulated SMADs activated in response to BMPs binding to the signaling receptors are SMAD1, 5, and 8. These phosphorylated SMADs in turn bind SMAD4, and the SMAD complex translocates to the nucleus. In the nucleus, the SMAD complex binds to the specific promoter elements of the target genes, including hepcidin to regulate their transcription.36 The importance of the BMP-SMAD pathway in hepcidin expression regulation has also been demonstrated in liver-specific Smad4−/− mice that also present with a massive iron overload similar to the phenotype of hepcidin knockout mice.25
To promote signal transduction under physiological conditions where BMP ligand levels are low, and to generate a specific signal in response to a subset of BMP ligands using a subset of BMP receptors, a BMP co-receptor is required. RGM proteins are the first known family of high-affinity co-receptors that are specific for BMPs. The RGM family is composed of 3 members in mammals; RGMc, also known as HJV, is the one expressed in the liver that is involved in the regulation of hepcidin expression in response to iron. This gene was identified as a hemochromatosis gene in 2004 by a positional cloning strategy for a locus associated with juvenile hemochromatosis in humans.24 However, the link between the regulation of hepcidin by iron and the HJV/BMP-SMAD pathway was established 2 years later by Babitt et al,34 when it was demonstrated that treatment of hepatoma cells with BMPs in combination with HJV overexpression results in the upregulation of hepcidin expression. Interestingly, by surface plasmon resonance, it has been demonstrated that among all RGMs, HJV has the highest affinity for BMP6.40 Global Hjv knockout mice and human patients with HJV mutations do not present with any other phenotype of iron-unrelated BMP functions,41,42 suggesting that HJV has a role that is uniquely nonredundant for iron metabolism regulation. Although HJV is expressed in other tissues such as heart and muscle,24 analysis of HJV tissue–specific knockout mice thus far suggests that HJV expression is predominantly important in hepatocytes.43,44
HJV can be released from cells as soluble HJV (sHJV) and detected in the serum of several species including humans.45,46 In addition, it has been demonstrated that soluble recombinant HJV has the capacity to inhibit the BMP-SMAD signaling pathway and hepcidin expression,37 but the source and function of endogenous sHJV are still poorly understood. In vitro, it has been shown that full-length sHJV can be released into the cell culture media by the action of endogenous phosphatidylinositol-specific phospholipase C (PI-PLC).47 Furin, a pro-protein convertase, can also cleave HJV to generate a smaller fragment of sHJV.48,49 Matriptase-2, encoded by the transmembrane serine protease TMPRSS6, has also been demonstrated to cleave sHJV in in vitro overexpression systems,50,51 and this has been proposed as a mechanism by which mutations in TMPRSS6 lead to hepcidin excess and iron-refractory iron deficiency anemia.
Neogenin, a protein of the deleted in colon cancer (DCC) family has been shown to be able to interact with HJV52 and matriptase-253 and may play a role in iron metabolism because the hypomorphic neogenin mouse has iron accumulation in the liver.54 Neogenin has been suggested in some studies,55 but not others,38 to affect HJV secretion and BMP-SMAD signaling. The exact role and function of neogenin in iron metabolism remains to be fully elucidated. Further support that the HJV/BMP pathway plays a role in hepcidin regulation comes from work showing that SMAD7, an inhibitory SMAD protein that mediates a negative feedback loop to both TGF-β and BMP signaling, serves as an inhibitor of hepcidin expression.56
Tissue iron levels are sensed by the liver to regulate hepcidin
Liver BMP6-SMAD signaling is stimulated by iron administration57,58 and, importantly, hepcidin regulation by iron is dependent on the BMP6-SMAD pathway because BMP6-SMAD pathway inhibitors prevent hepcidin induction by iron.59 There appear to be multiple mechanisms by which iron stimulates liver BMP6-SMAD signaling. First, there is a tight correlation between liver iron content and liver Bmp6 mRNA expression in mice,57,60 suggesting that hepatic iron loading regulates Bmp6 mRNA expression. Although the mechanism for this regulation is still unclear, it does not appear to involve the hemochromatosis proteins HFE, TfR2, or HJV because liver Bmp6 mRNA is appropriately increased by iron loading in mice with mutations in these genes.61,62 Interestingly, it was recently reported that although nonparenchymal cells and hepatocytes produce BMP6 mRNA under basal conditions, only nonparenchymal cells increase BMP6 mRNA expression in iron overload conditions, and this increase does not correlate with increased intracellular iron content of these nonparenchymal cells.63 However, another study did show an increase in Bmp6 protein detected by immunohistochemistry in the hepatocytes of mice with iron overload owing to a high iron diet or Hfe inactivation.64 Future studies will be needed to understand the exact mechanism by which hepatic iron loading leads to increased liver Bmp6 mRNA expression.
However, as described by Ramos and al,65 despite the absence of Bmp6, Bmp6−/− mice still have a partial increase in hepcidin expression in response to higher tissue iron loading, suggesting the involvement of others proteins beside Bmp6 in the regulation of hepcidin expression.
Circulating iron levels are sensed by the liver to regulate hepcidin
Hepcidin expression by hepatocytes is also stimulated by an increase in circulating iron via mechanisms that are distinct from hepcidin induction by tissue iron stores. Increases in serum iron, in the absence of hepatic iron loading, do not stimulate BMP6 expression,58 but instead stimulate downstream SMAD1/5/8 phosphorylation by as yet unknown mechanisms. Although the signaling pathway is not fully characterized, it likely involves HFE, TFR2, and HJV.65 The crucial role of HFE and TFR2 in hepcidin regulation by iron is illustrated by the hepcidin deficiency and iron overload that is induced by HFE and TFR2 mutations in humans (hemochromatosis) and mice. In addition, Hfe−/− and Tfr2−/− mice fail to increase hepcidin expression in response to an increase of circulating Tf-iron but maintain the ability to increase hepcidin in response to an increase of hepatic iron, suggesting that the specific role of these 2 proteins to regulate hepcidin expression is mainly in response to circulating Tf-iron.65 There is also evidence that the regulation of hepcidin expression by HFE and TfR2 involves the SMAD pathway because humans and mice lacking HFE, TfR2, or both, exhibit reduced SMAD1/5/8 phosphorylation relative to iron and BMP6 levels.58,61,66-68 The exact link between Tf-iron, HFE, TFR2, and the SMAD pathway is still unclear. HFE has been shown to bind to TFR1,69 and Tf-iron competes with HFE for binding to TFR1 because of overlapping binding sites on TfR1.70 It has been suggested that in the presence of high Tf saturation, HFE is released from TFR1 and interacts with TFR2 (via domains that differ from those involved in HFE/TfR1 interaction71,72) to activate hepcidin transcription. An in vitro overexpression study has recently suggested that in response to Tf-iron, HFE and TFR2 could be part of the HJV-BMP receptor complex to regulate hepcidin.73 Whether this interaction occurs in vivo in the liver has not yet been verified.
Iron deficiency and the regulation of hepcidin expression
Although iron stimulates hepcidin expression, iron deficiency inhibits hepcidin expression through a mechanism that may involve matriptase-2, as suggested by Zhang et al.74 Encoded by the gene named TMPRSS6, matriptase-2 belongs to the family of type II transmembrane serine proteases. Matriptase-2 shares a number of structural features with the other members of the family: a short cytoplasmic domain, a type II transmembrane sequence, a central region with several modular structural domains including 2 CUB (complement factor C1s/C1r, urchin embryonic growth factor, bone morphogenetic protein) domains and 3 low-density lipoprotein–receptor tandem repeats, and, finally, a C-terminal catalytic domain with all the typical features of serine proteinases.75
Despite the identification of human matriptase-2 cDNA in 200275 and exploration of a suspected role as a tumor suppressor, its true physiological role remained unclear until 2008, when 3 laboratories demonstrated that matriptase-2 is essential for iron homeostasis.30-32 Two studies in mice showed that the absence of matriptase-2 resulted in an inappropriately high level of hepcidin, leading to ferroportin degradation and the inhibition of iron absorption, and finally resulting in severe microcytic anemia.31,32 A third study described that in humans, iron-refractory iron deficiency anemia is caused by mutations in the TMPRSS6 gene, leading to a severe microcytic anemia with a high urinary hepcidin level.30 Together these results indicate that matriptase-2 is a potent inhibitor of hepcidin expression in response to iron deficiency.
In parallel, genome-wide association studies identified common TMPRSS6 single nucleotide polymorphisms (such as A736V) associated with iron status,76 erythrocyte level,77 and hemoglobin level,78 suggesting that TMPRSS6 is crucial in the control of iron homeostasis and normal erythropoiesis. The polymorphism A736V has also been shown to be a modifier of iron overload in hereditary hemochromatosis patients.79 This result is also supported in mice models where Tmprss6 is known to be a genetic modifier of the Hfe-hemochromatosis phenotype.80,81
It has been proposed by Silvestri et al that matriptase-2 could regulate hepcidin expression by cleaving HJV to decrease BMP-SMAD signaling. Indeed, the authors demonstrated that in vitro matriptase-2 does interact with HJV at the membrane cell surface and has the capacity to cleave HJV, preventing the induction of hepcidin expression during BMP2 treatment.50 Moreover, interplay between matriptase-2 and the BMP-HJV-SMAD signaling pathway to regulate hepcidin expression is supported by double mutant mouse models Hjv−/− Tmprss6−/−,82,83 and Bmp6−/− Tmprss6−/−,84 which exhibit a low hepcidin mRNA level similar to that of the simple Hjv−/− or Bmp6−/− single knockout mice, suggesting at least a genetic interaction between TMPRSS6 and the BMP6-HJV-SMAD pathway. However, 2 recent studies performed in vivo provide results that do not support a role for HJV in TMPRSS6 action. Analysis of Tmprss6−/− mice reveals that compared with wild-type mice, HJV levels at the cell membrane surface are unexpectedly decreased85 and soluble HJV in the serum is unchanged,46 suggesting that HJV may not be the natural substrate of matriptase-2 in vivo. In addition, Gibert et al provided evidence in a zebrafish model suggesting that matriptase-2 could regulate hepcidin expression independently of HJV, because knockdown of matriptase-2 leads to high levels of the Hamp transcript in an Hjv-independent manner.86 Thus, more work is needed to fully understand the mechanism of hepcidin regulation by matriptase-2.
Study of the regulation of matriptase-2 expression has recently demonstrated a link between matriptase-2 and hepcidin expression regulation in response to BMP6 and hepatic iron.87 Injection of BMP6 or administration of an iron-enriched diet each induced an upregulation of Tmprss6 expression in mice. In addition, the authors demonstrated that in vitro, this regulation is dependent on expression of Id1 mRNA, a transcript upregulated by the BMP-SMAD pathway and iron. Modulation of TMPRSS6 expression could serve as a negative feedback inhibitor to avoid excessive hepcidin increases by iron to help maintain tight homeostatic balance of systemic iron levels.
Another mechanism to lower hepcidin expression in response to iron deprivation is to decrease the BMP6-SMAD pathway activity, as is suggested by the decrease of Bmp6 mRNA observed in this condition.57
Inflammatory signals are sensed by the liver to regulate hepcidin
Inflammation caused by infection, autoimmune disease, or cancers stimulates the synthesis of many proinflammatory cytokines, such as interferon-γ, interleukin-1, and interleukin-6 (IL-6), leading to a stimulation of hepcidin expression by the liver as well as white blood cells (Figure 3).88,89 This induction of hepcidin production causes hypoferremia and accumulation of iron in macrophages. This early host defense mechanism might be an advantage against specific infections such as malaria90 by preventing the iron availability for pathogens. However, this innate immune response is also a disadvantage for the host in conditions of chronic inflammation. Indeed, the iron sequestration also limits the availability of iron for erythropoiesis and contributes to the anemia of chronic disease.91
Figure 3.
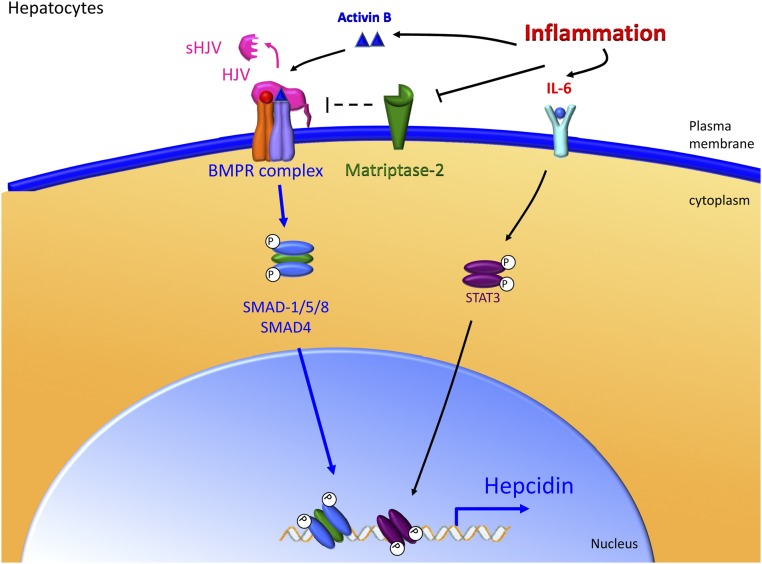
The liver responds to inflammatory signals to regulate hepcidin production. Inflammatory signals such as IL-6 and Activin B stimulate the production of hepcidin by the liver. IL-6 increases phospho-STAT3 levels and subsequently stimulates transcription of hepcidin. Activin B may stimulate the BMP receptor complex directly. Matriptase-2 may also regulate inflammatory stimulation of hepcidin. Importantly, the proximal BMP-responsive element in the hepcidin promoter is necessary for the full effect of STAT-3 stimulation on hepcidin transcription.
The signaling pathways controlling hepcidin expression in hepatocytes under inflammatory condition are now well characterized. Inflammation stimulates IL-6 production that in turn activates the Janus kinase 2/signal transducer and activator of transcription 3 (STAT3) pathway, leading to the phosphorylation of STAT3. Translocation of phosphorylated STAT3 into the nucleus and binding to the canonical STAT3 binding site in the proximal hepcidin promoter results in upregulation of hepcidin mRNA expression.92,93 Interestingly, interplay between the STAT3 pathway and the BMP-SMAD pathway is required to regulate hepcidin expression in response to inflammation. Thus mice lacking SMAD4 (common mediator of the BMP-SMAD pathway) lose the ability to induce hepcidin expression in response to IL-6 injection.25 Moreover, in cells, the presence of the proximal BMP response element, in addition to the STAT3 response element on the hepcidin promoter, is crucial to induce hepcidin expression during IL-6 treatment.35 Indeed, increased liver SMAD1/5/8 phosphorylation has been observed in mice with anemia of inflammation in addition to increased STAT3 phosphorylation.94 More recently, Besson-Fournier et al have suggested that the BMP/TGF-β superfamily ligand participating in the SMAD1/5/8 pathway activation in response to LPS injection in mice is Activin B.95
Meynard et al have recently highlighted an additional layer of crosstalk between the BMP-SMAD pathway and the IL-6–STAT3 pathway. Those authors demonstrated that LPS or IL-6 injection in mice causes a downregulation of matriptase-2 expression, which may be part of the regulatory mechanism leading to the increase of hepcidin expression under inflammatory conditions.96
Erythropoiesis and hypoxia are sensed by the liver to regulate hepcidin
Because erythropoiesis depends on iron availability in the body, erythroid activity and iron absorption need to be coordinated. This coordination occurs through the downregulation of hepcidin expression in hepatocytes,97,98 which results in an increase of iron absorption. The regulatory mechanism of hepcidin inhibition by erythropoiesis is still unclear, but it has recently been established that bone marrow cells are involved.99 Growth differentiation factor 15 (GDF15) and twisted gastrulation-1 (TWSG1) are potentials mediators of bone marrow signaling because their expression is stimulated under ineffective erythropoiesis conditions.100,101 However, a nonredundant role for these proteins as erythroid regulators is excluded. Indeed, GDF15 is not required to balance iron homeostasis in response to blood loss,102 and the involvement of TWSG1 has only been reported in thalassemia.101 Further studies are still needed to identify the erythroid regulator of hepcidin expression and to characterize the molecular mechanism of its hepcidin regulation in hepatocytes.
Under hypoxia, hepcidin is also reduced. In mice, stabilization of HIF1 and HIF2 (hypoxia-inducible factors) both inhibit hepcidin expression.103,104 A direct regulation of hepcidin expression by the binding of HIF on hepcidin promoter was suggested by a study published by Peyssonaux et al,103 but has not been confirmed by subsequent studies.105 In vivo, hypoxia stimulates erythropoietin production that in turn could decrease hepcidin expression. This hypothesis is supported by recent findings showing that hepatic HIF2 is not directly involved in hepcidin repression but contributes to the repression of hepcidin through erythropoietin-mediated increased erythropoiesis.104
Signaling pathways that downregulate hepcidin in the liver
Several factors have been shown to downregulate hepcidin expression, in particular epidermal growth factor (EGF), hepatocyte growth factor,106 tumor necrosis factor-α,107 and estrogen.108 The role, if any, of these pathways in iron homeostasis remains unknown. In addition, recently, 2 laboratories showed independently that testosterone downregulates hepcidin,109,110 explaining the difference seen in tissue iron loading between male and female animals. Latour et al also demonstrated that the hepcidin regulation by testosterone was mediated through EGFR. Alcohol is also an inhibitor of hepcidin expression. This inhibition is dependent on both HIF-1α and HIF-2α, which leads to a decrease of C/EBPα,111 a transcription factor critical in maintaining basal hepcidin expression.112 In the same manner, it is now well established that hepatitis C infection results in a decrease of hepcidin expression and that this regulation occurs through a destabilization of C/EBPα and STAT3 on the hepcidin promoter.113
Conclusion
To provide the appropriate amount of iron available for erythropoiesis and cell metabolism, but to prevent toxic iron overload, hepcidin expression by the liver must be tightly regulated. The liver is the central organ that senses a variety of signals related to iron, oxygenation, and erythropoiesis for the purpose of regulating hepcidin expression. HJV/BMP/SMAD and IL6/STAT3 seem to be the main pathways involved in the regulation of hepcidin expression in response to multiple stimuli. However, the important pathway modulating hepcidin in response to erythropoiesis and hypoxia in hepatocytes is still unknown and needs to be elucidated. The liver interprets and integrates these disparate signals to regulate the production of hepcidin mRNA and subsequently systemic iron homeostasis.
The identification of the liver as a central regulator of iron homeostasis and especially the discovery of hepcidin and its regulation were important steps for the development of new promising therapeutic strategies for the management of iron disorders. All the strategies for the treatment of anemia of chronic disease or anemia of inflammation have been recently well summarized in the review by Sun et al,91 such as antihepcidin antibodies, short interference RNA and antisense oligonucleotides against hepcidin, BMP6-HJV-SMAD pathway inhibitors, and IL-6 pathway inhibitors.
For iron disorders associated with an iron overload induced by a low hepcidin state, such as hemochromatosis or β-thalassemia, new therapeutic strategies are also been studied.114,115 Minihepcidins, small druglike hepcidin agonists, are a new promising approach for the prevention and treatment of iron overload that have been tested in hepcidin-deficient mice. In this mouse model, administration of large doses of minihepcidins could prevent iron overload.
With lower doses, there is a more moderate effect and partial redistribution of iron from the liver to the spleen.116 Another strategy currently under study is the activation of the hepcidin pathway by the inhibition of TMPRSS6 expression to inhibit hepcidin expression. Indeed, 2 groups have recently demonstrated that inhibition of Tmprss6 expression by injection of silencing RNA formulated in lipid nanoparticles117 or antisense oligonucleotides118 into Hfe−/− mice and HBB(th3/+) mice achieved knockdown of Tmprss6 expression, leading to an elevation in hepcidin level. These therapies were both efficient in reducing liver iron concentrations in both mouse models.
Because increasing hepcidin does not cause the removal or excretion of iron, these potential new treatments are not likely to be sufficient to replace current treatments (ie, iron chelation and phlebotomy) in patients. They may rather be considered as an additional treatment to be used in combination with current strategies to decrease the frequency of iron chelation or phlebotomy, which have undesirable side effects.
Acknowledgments
This study was supported by in part by National Institutes of Health grant R01 DK087727, a Howard Goodman Fellowship from the Massachusetts General Hospital (J.L.B.); and National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases grants R01 DK069533 and R01 DK071837 (H.Y.L.).
Authorship
Contribution: D.M. wrote the initial draft of the review and generated the figures; J.L.B. edited and helped write the review; and H.Y.L. edited and wrote key sections of the review.
Conflict-of-interest disclosure: J.L.B. and H.Y.L. have ownership interest in a start-up company, Ferrumax Pharmaceuticals, which has licensed technology from the Massachusetts General Hospital based on the work cited here and in prior publications.
Correspondence: Herbert Y. Lin, FASN, Massachusetts General Hospital, 185 Cambridge Street, CPZN-8216, Boston, MA 02114; e-mail: lin.herbert@mgh.harvard.edu.
References
- 1.Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry. 2012;51(29):5705–5724. doi: 10.1021/bi300752r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hentze MW, Muckenthaler MU, Galy B, Camaschella C. Two to tango: regulation of Mammalian iron metabolism. Cell. 2010;142(1):24–38. doi: 10.1016/j.cell.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 3.McKie AT, Marciani P, Rolfs A, et al. A novel duodenal iron-regulated transporter, IREG1, implicated in the basolateral transfer of iron to the circulation. Mol Cell. 2000;5(2):299–309. doi: 10.1016/s1097-2765(00)80425-6. [DOI] [PubMed] [Google Scholar]
- 4.Donovan A, Brownlie A, Zhou Y, et al. Positional cloning of zebrafish ferroportin1 identifies a conserved vertebrate iron exporter. Nature. 2000;403(6771):776–781. doi: 10.1038/35001596. [DOI] [PubMed] [Google Scholar]
- 5.Soe-Lin S, Apte SS, Andriopoulos B, Jr, et al. Nramp1 promotes efficient macrophage recycling of iron following erythrophagocytosis in vivo. Proc Natl Acad Sci USA. 2009;106(14):5960–5965. doi: 10.1073/pnas.0900808106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambert LA. Molecular evolution of the transferrin family and associated receptors. Biochim Biophys Acta. 2012;1820(3):244–255. doi: 10.1016/j.bbagen.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Aisen P. Transferrin receptor 1. Int J Biochem Cell Biol. 2004;36(11):2137–2143. doi: 10.1016/j.biocel.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 8.Kawabata H, Yang R, Hirama T, et al. Molecular cloning of transferrin receptor 2. A new member of the transferrin receptor-like family. J Biol Chem. 1999;274(30):20826–20832. doi: 10.1074/jbc.274.30.20826. [DOI] [PubMed] [Google Scholar]
- 9.Herbison CE, Thorstensen K, Chua AC, et al. The role of transferrin receptor 1 and 2 in transferrin-bound iron uptake in human hepatoma cells. Am J Physiol Cell Physiol. 2009;297(6):C1567–C1575. doi: 10.1152/ajpcell.00649.2008. [DOI] [PubMed] [Google Scholar]
- 10.Camaschella C, Roetto A, Calì A, et al. The gene TFR2 is mutated in a new type of haemochromatosis mapping to 7q22. Nat Genet. 2000;25(1):14–15. doi: 10.1038/75534. [DOI] [PubMed] [Google Scholar]
- 11.Brissot P, Ropert M, Le Lan C, Loréal O. Non-transferrin bound iron: a key role in iron overload and iron toxicity. Biochim Biophys Acta. 2012;1820(3):403–410. doi: 10.1016/j.bbagen.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 12.Park CH, Valore EV, Waring AJ, Ganz T. Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem. 2001;276(11):7806–7810. doi: 10.1074/jbc.M008922200. [DOI] [PubMed] [Google Scholar]
- 13.Pigeon C, Ilyin G, Courselaud B, et al. A new mouse liver-specific gene, encoding a protein homologous to human antimicrobial peptide hepcidin, is overexpressed during iron overload. J Biol Chem. 2001;276(11):7811–7819. doi: 10.1074/jbc.M008923200. [DOI] [PubMed] [Google Scholar]
- 14.Liu XB, Nguyen NB, Marquess KD, Yang F, Haile DJ. Regulation of hepcidin and ferroportin expression by lipopolysaccharide in splenic macrophages. Blood Cells Mol Dis. 2005;35(1):47–56. doi: 10.1016/j.bcmd.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 15.Bekri S, Gual P, Anty R, et al. Increased adipose tissue expression of hepcidin in severe obesity is independent from diabetes and NASH. Gastroenterology. 2006;131(3):788–796. doi: 10.1053/j.gastro.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 16.Valore EV, Ganz T. Posttranslational processing of hepcidin in human hepatocytes is mediated by the prohormone convertase furin. Blood Cells Mol Dis. 2008;40(1):132–138. doi: 10.1016/j.bcmd.2007.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peslova G, Petrak J, Kuzelova K, et al. Hepcidin, the hormone of iron metabolism, is bound specifically to alpha-2-macroglobulin in blood. Blood. 2009;113(24):6225–6236. doi: 10.1182/blood-2009-01-201590. [DOI] [PubMed] [Google Scholar]
- 18.Itkonen O, Stenman UH, Parkkinen J, Soliymani R, Baumann M, Hämäläinen E. Binding of hepcidin to plasma proteins. Clin Chem. 2012;58(7):1158–1160. doi: 10.1373/clinchem.2012.186916. [DOI] [PubMed] [Google Scholar]
- 19.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 20.Roetto A, Papanikolaou G, Politou M, et al. Mutant antimicrobial peptide hepcidin is associated with severe juvenile hemochromatosis. Nat Genet. 2003;33(1):21–22. doi: 10.1038/ng1053. [DOI] [PubMed] [Google Scholar]
- 21.Nicolas G, Bennoun M, Devaux I, et al. Lack of hepcidin gene expression and severe tissue iron overload in upstream stimulatory factor 2 (USF2) knockout mice. Proc Natl Acad Sci USA. 2001;98(15):8780–8785. doi: 10.1073/pnas.151179498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meynard D, Kautz L, Darnaud V, Canonne-Hergaux F, Coppin H, Roth MP. Lack of the bone morphogenetic protein BMP6 induces massive iron overload. Nat Genet. 2009;41(4):478–481. doi: 10.1038/ng.320. [DOI] [PubMed] [Google Scholar]
- 23.Andriopoulos B, Jr, Corradini E, Xia Y, et al. BMP6 is a key endogenous regulator of hepcidin expression and iron metabolism. Nat Genet. 2009;41(4):482–487. doi: 10.1038/ng.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Papanikolaou G, Samuels ME, Ludwig EH, et al. Mutations in HFE2 cause iron overload in chromosome 1q-linked juvenile hemochromatosis. Nat Genet. 2004;36(1):77–82. doi: 10.1038/ng1274. [DOI] [PubMed] [Google Scholar]
- 25.Wang RH, Li C, Xu X, et al. A role of SMAD4 in iron metabolism through the positive regulation of hepcidin expression. Cell Metab. 2005;2(6):399–409. doi: 10.1016/j.cmet.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 26.Feder JN, Gnirke A, Thomas W, et al. A novel MHC class I-like gene is mutated in patients with hereditary haemochromatosis. Nat Genet. 1996;13(4):399–408. doi: 10.1038/ng0896-399. [DOI] [PubMed] [Google Scholar]
- 27.Ramey G, Deschemin JC, Durel B, Canonne-Hergaux F, Nicolas G, Vaulont S. Hepcidin targets ferroportin for degradation in hepatocytes. Haematologica. 2010;95(3):501–504. doi: 10.3324/haematol.2009.014399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Babitt JL, Lin HY. Molecular mechanisms of hepcidin regulation: implications for the anemia of CKD. Am J Kidney Dis. 2010;55(4):726–741. doi: 10.1053/j.ajkd.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicolas G, Bennoun M, Porteu A, et al. Severe iron deficiency anemia in transgenic mice expressing liver hepcidin. Proc Natl Acad Sci USA. 2002;99(7):4596–4601. doi: 10.1073/pnas.072632499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Folgueras AR, de Lara FM, Pendas AM, et al. Membrane-bound serine protease matriptase-2 (Tmprss6) is an essential regulator of iron homeostasis. Blood. 2008;112(6):2539–2545. doi: 10.1182/blood-2008-04-149773. [DOI] [PubMed] [Google Scholar]
- 32.Du X, She E, Gelbart T, et al. The serine protease TMPRSS6 is required to sense iron deficiency. Science. 2008;320(5879):1088–1092. doi: 10.1126/science.1157121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bardou-Jacquet E, Philip J, Lorho R, et al. Liver transplantation normalizes serum hepcidin level and cures iron metabolism alterations in HFE hemochromatosis. doi: 10.1002/hep.26570. [published online ahead of print June 14, 2013]. Hepatology. doi: 10.1002/hep.25670. [DOI] [PubMed] [Google Scholar]
- 34.Babitt JL, Huang FW, Wrighting DM, et al. Bone morphogenetic protein signaling by hemojuvelin regulates hepcidin expression. Nat Genet. 2006;38(5):531–539. doi: 10.1038/ng1777. [DOI] [PubMed] [Google Scholar]
- 35.Casanovas G, Mleczko-Sanecka K, Altamura S, Hentze MW, Muckenthaler MU. Bone morphogenetic protein (BMP)-responsive elements located in the proximal and distal hepcidin promoter are critical for its response to HJV/BMP/SMAD. J Mol Med (Berl) 2009;87(5):471–480. doi: 10.1007/s00109-009-0447-2. [DOI] [PubMed] [Google Scholar]
- 36.Corradini E, Babitt JL, Lin HY. The RGM/DRAGON family of BMP co-receptors. Cytokine Growth Factor Rev. 2009;20(5-6):389–398. doi: 10.1016/j.cytogfr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babitt JL, Huang FW, Xia Y, Sidis Y, Andrews NC, Lin HY. Modulation of bone morphogenetic protein signaling in vivo regulates systemic iron balance. J Clin Invest. 2007;117(7):1933–1939. doi: 10.1172/JCI31342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xia Y, Babitt JL, Sidis Y, Chung RT, Lin HY. Hemojuvelin regulates hepcidin expression via a selective subset of BMP ligands and receptors independently of neogenin. Blood. 2008;111(10):5195–5204. doi: 10.1182/blood-2007-09-111567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinbicker AU, Bartnikas TB, Lohmeyer LK, et al. Perturbation of hepcidin expression by BMP type I receptor deletion induces iron overload in mice. Blood. 2011;118(15):4224–4230. doi: 10.1182/blood-2011-03-339952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu Q, Sun CC, Lin HY, Babitt JL. Repulsive guidance molecule (RGM) family proteins exhibit differential binding kinetics for bone morphogenetic proteins (BMPs). PLoS ONE. 2012;7(9):e46307. doi: 10.1371/journal.pone.0046307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Niederkofler V, Salie R, Arber S. Hemojuvelin is essential for dietary iron sensing, and its mutation leads to severe iron overload. J Clin Invest. 2005;115(8):2180–2186. doi: 10.1172/JCI25683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang FW, Pinkus JL, Pinkus GS, Fleming MD, Andrews NC. A mouse model of juvenile hemochromatosis. J Clin Invest. 2005;115(8):2187–2191. doi: 10.1172/JCI25049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gkouvatsos K, Wagner J, Papanikolaou G, Sebastiani G, Pantopoulos K. Conditional disruption of mouse HFE2 gene: maintenance of systemic iron homeostasis requires hepatic but not skeletal muscle hemojuvelin. Hepatology. 2011;54(5):1800–1807. doi: 10.1002/hep.24547. [DOI] [PubMed] [Google Scholar]
- 44.Chen W, Huang FW, de Renshaw TB, Andrews NC. Skeletal muscle hemojuvelin is dispensable for systemic iron homeostasis. Blood. 2011;117(23):6319–6325. doi: 10.1182/blood-2010-12-327957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brasse-Lagnel C, Poli M, Lesueur C, et al. Immunoassay for human serum hemojuvelin. Haematologica. 2010;95(12):2031–2037. doi: 10.3324/haematol.2010.022129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen W, Sun CC, Chen S, Meynard D, Babitt JL, Lin HY. A novel validated enzyme-linked immunosorbent assay to quantify soluble hemojuvelin in mouse serum. Haematologica. 2013;98(2):296–304. doi: 10.3324/haematol.2012.070136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kuninger D, Kuns-Hashimoto R, Kuzmickas R, Rotwein P. Complex biosynthesis of the muscle-enriched iron regulator RGMc. J Cell Sci. 2006;119(Pt 16):3273–3283. doi: 10.1242/jcs.03074. [DOI] [PubMed] [Google Scholar]
- 48.Silvestri L, Pagani A, Camaschella C. Furin-mediated release of soluble hemojuvelin: a new link between hypoxia and iron homeostasis. Blood. 2008;111(2):924–931. doi: 10.1182/blood-2007-07-100677. [DOI] [PubMed] [Google Scholar]
- 49.Lin L, Nemeth E, Goodnough JB, Thapa DR, Gabayan V, Ganz T. Soluble hemojuvelin is released by proprotein convertase-mediated cleavage at a conserved polybasic RNRR site. Blood Cells Mol Dis. 2008;40(1):122–131. doi: 10.1016/j.bcmd.2007.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Silvestri L, Pagani A, Nai A, De Domenico I, Kaplan J, Camaschella C. The serine protease matriptase-2 (TMPRSS6) inhibits hepcidin activation by cleaving membrane hemojuvelin. Cell Metab. 2008;8(6):502–511. doi: 10.1016/j.cmet.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Maxson JE, Chen J, Enns CA, Zhang AS. Matriptase-2- and proprotein convertase-cleaved forms of hemojuvelin have different roles in the down-regulation of hepcidin expression. J Biol Chem. 2010;285(50):39021–39028. doi: 10.1074/jbc.M110.183160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang AS, West AP, Jr, Wyman AE, Bjorkman PJ, Enns CA. Interaction of hemojuvelin with neogenin results in iron accumulation in human embryonic kidney 293 cells. J Biol Chem. 2005;280(40):33885–33894. doi: 10.1074/jbc.M506207200. [DOI] [PubMed] [Google Scholar]
- 53.Enns CA, Ahmed R, Zhang AS. Neogenin interacts with matriptase-2 to facilitate hemojuvelin cleavage. J Biol Chem. 2012;287(42):35104–35117. doi: 10.1074/jbc.M112.363937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee DH, Zhou LJ, Zhou Z, et al. Neogenin inhibits HJV secretion and regulates BMP-induced hepcidin expression and iron homeostasis. Blood. 2010;115(15):3136–3145. doi: 10.1182/blood-2009-11-251199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhang AS, Yang F, Meyer K, et al. Neogenin-mediated hemojuvelin shedding occurs after hemojuvelin traffics to the plasma membrane. J Biol Chem. 2008;283(25):17494–17502. doi: 10.1074/jbc.M710527200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mleczko-Sanecka K, Casanovas G, Ragab A, et al. SMAD7 controls iron metabolism as a potent inhibitor of hepcidin expression. Blood. 2010;115(13):2657–2665. doi: 10.1182/blood-2009-09-238105. [DOI] [PubMed] [Google Scholar]
- 57.Kautz L, Meynard D, Monnier A, et al. Iron regulates phosphorylation of Smad1/5/8 and gene expression of Bmp6, Smad7, Id1, and Atoh8 in the mouse liver. Blood. 2008;112(4):1503–1509. doi: 10.1182/blood-2008-03-143354. [DOI] [PubMed] [Google Scholar]
- 58.Corradini E, Meynard D, Wu Q, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu PB, Hong CC, Sachidanandan C, et al. Dorsomorphin inhibits BMP signals required for embryogenesis and iron metabolism. Nat Chem Biol. 2008;4(1):33–41. doi: 10.1038/nchembio.2007.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Corradini E, Meynard D, Wu Q, et al. Serum and liver iron differently regulate the bone morphogenetic protein 6 (BMP6)-SMAD signaling pathway in mice. Hepatology. 2011;54(1):273–284. doi: 10.1002/hep.24359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Corradini E, Garuti C, Montosi G, et al. Bone morphogenetic protein signaling is impaired in an HFE knockout mouse model of hemochromatosis. Gastroenterology. 2009;137(4):1489–1497. doi: 10.1053/j.gastro.2009.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kautz L, Meynard D, Besson-Fournier C, et al. BMP/Smad signaling is not enhanced in Hfe-deficient mice despite increased Bmp6 expression. Blood. 2009;114(12):2515–2520. doi: 10.1182/blood-2009-02-206771. [DOI] [PubMed] [Google Scholar]
- 63.Enns CA, Ahmed R, Wang J, et al. Increased iron loading induces Bmp6 expression in the non-parenchymal cells of the liver independent of the BMP-signaling pathway. PLoS ONE. 2013;8(4):e60534. doi: 10.1371/journal.pone.0060534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kautz L, Besson-Fournier C, Meynard D, Latour C, Roth MP, Coppin H. Iron overload induces BMP6 expression in the liver but not in the duodenum. Haematologica. 2011;96(2):199–203. doi: 10.3324/haematol.2010.031963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ramos E, Kautz L, Rodriguez R, et al. Evidence for distinct pathways of hepcidin regulation by acute and chronic iron loading in mice. Hepatology. 2011;53(4):1333–1341. doi: 10.1002/hep.24178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ryan JD, Ryan E, Fabre A, Lawless MW, Crowe J. Defective bone morphogenic protein signaling underlies hepcidin deficiency in HFE hereditary hemochromatosis. Hepatology. 2010;52(4):1266–1273. doi: 10.1002/hep.23814. [DOI] [PubMed] [Google Scholar]
- 67.Wallace DF, Summerville L, Crampton EM, Frazer DM, Anderson GJ, Subramaniam VN. Combined deletion of Hfe and transferrin receptor 2 in mice leads to marked dysregulation of hepcidin and iron overload. Hepatology. 2009;50(6):1992–2000. doi: 10.1002/hep.23198. [DOI] [PubMed] [Google Scholar]
- 68.Poli M, Luscieti S, Gandini V, et al. Transferrin receptor 2 and HFE regulate furin expression via mitogen-activated protein kinase/extracellular signal-regulated kinase (MAPK/Erk) signaling. Implications for transferrin-dependent hepcidin regulation. Haematologica. 2010;95(11):1832–1840. doi: 10.3324/haematol.2010.027003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Feder JN, Penny DM, Irrinki A, et al. The hemochromatosis gene product complexes with the transferrin receptor and lowers its affinity for ligand binding. Proc Natl Acad Sci USA. 1998;95(4):1472–1477. doi: 10.1073/pnas.95.4.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lebrón JA, West AP, Jr, Bjorkman PJ. The hemochromatosis protein HFE competes with transferrin for binding to the transferrin receptor. J Mol Biol. 1999;294(1):239–245. doi: 10.1006/jmbi.1999.3252. [DOI] [PubMed] [Google Scholar]
- 71.Goswami T, Andrews NC. Hereditary hemochromatosis protein, HFE, interaction with transferrin receptor 2 suggests a molecular mechanism for mammalian iron sensing. J Biol Chem. 2006;281(39):28494–28498. doi: 10.1074/jbc.C600197200. [DOI] [PubMed] [Google Scholar]
- 72.Chen J, Chloupková M, Gao J, Chapman-Arvedson TL, Enns CA. HFE modulates transferrin receptor 2 levels in hepatoma cells via interactions that differ from transferrin receptor 1-HFE interactions. J Biol Chem. 2007;282(51):36862–36870. doi: 10.1074/jbc.M706720200. [DOI] [PubMed] [Google Scholar]
- 73.D’Alessio F, Hentze MW, Muckenthaler MU. The hemochromatosis proteins HFE, TfR2, and HJV form a membrane-associated protein complex for hepcidin regulation. J Hepatol. 2012;57(5):1052–1060. doi: 10.1016/j.jhep.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 74.Zhang AS, Anderson SA, Wang J, et al. Suppression of hepatic hepcidin expression in response to acute iron deprivation is associated with an increase of matriptase-2 protein. Blood. 2011;117(5):1687–1699. doi: 10.1182/blood-2010-06-287292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Velasco G, Cal S, Quesada V, Sánchez LM, López-Otín C. Matriptase-2, a membrane-bound mosaic serine proteinase predominantly expressed in human liver and showing degrading activity against extracellular matrix proteins. J Biol Chem. 2002;277(40):37637–37646. doi: 10.1074/jbc.M203007200. [DOI] [PubMed] [Google Scholar]
- 76.Tanaka T, Roy CN, Yao W, et al. A genome-wide association analysis of serum iron concentrations. Blood. 2010;115(1):94–96. doi: 10.1182/blood-2009-07-232496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Benyamin B, Ferreira MA, Willemsen G, et al. Common variants in TMPRSS6 are associated with iron status and erythrocyte volume. Nat Genet. 2009;41(11):1173–1175. doi: 10.1038/ng.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chambers JC, Zhang W, Li Y, et al. Genome-wide association study identifies variants in TMPRSS6 associated with hemoglobin levels. Nat Genet. 2009;41(11):1170–1172. doi: 10.1038/ng.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Valenti L, Fracanzani AL, Rametta R, et al. Effect of the A736V TMPRSS6 polymorphism on the penetrance and clinical expression of hereditary hemochromatosis. J Hepatol. 2012;57(6):1319–1325. doi: 10.1016/j.jhep.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 80.Finberg KE, Whittlesey RL, Andrews NC. Tmprss6 is a genetic modifier of the Hfe-hemochromatosis phenotype in mice. Blood. 2011;117(17):4590–4599. doi: 10.1182/blood-2010-10-315507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lee P, Hsu MH, Welser-Alves J, Peng H. Severe microcytic anemia but increased erythropoiesis in mice lacking Hfe or Tfr2 and Tmprss6. Blood Cells Mol Dis. 2012;48(3):173–178. doi: 10.1016/j.bcmd.2011.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Finberg KE, Whittlesey RL, Fleming MD, Andrews NC. Downregulation of Bmp/Smad signaling by Tmprss6 is required for maintenance of systemic iron homeostasis. Blood. 2010;115(18):3817–3826. doi: 10.1182/blood-2009-05-224808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Truksa J, Gelbart T, Peng H, Beutler E, Beutler B, Lee P. Suppression of the hepcidin-encoding gene Hamp permits iron overload in mice lacking both hemojuvelin and matriptase-2/TMPRSS6. Br J Haematol. 2009;147(4):571–581. doi: 10.1111/j.1365-2141.2009.07873.x. [DOI] [PubMed] [Google Scholar]
- 84.Lenoir A, Deschemin JC, Kautz L, et al. Iron-deficiency anemia from matriptase-2 inactivation is dependent on the presence of functional Bmp6. Blood. 2011;117(2):647–650. doi: 10.1182/blood-2010-07-295147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Krijt J, Fujikura Y, Ramsay AJ, Velasco G, Nečas E. Liver hemojuvelin protein levels in mice deficient in matriptase-2 (Tmprss6). Blood Cells Mol Dis. 2011;47(2):133–137. doi: 10.1016/j.bcmd.2011.04.009. [DOI] [PubMed] [Google Scholar]
- 86.Gibert Y, Lattanzi VJ, Zhen AW, et al. BMP signaling modulates hepcidin expression in zebrafish embryos independent of hemojuvelin. PLoS ONE. 2011;6(1):e14553. doi: 10.1371/journal.pone.0014553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Meynard D, Vaja V, Sun CC, et al. Regulation of TMPRSS6 by BMP6 and iron in human cells and mice. Blood. 2011;118(3):747–756. doi: 10.1182/blood-2011-04-348698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Nemeth E, Rivera S, Gabayan V, et al. IL-6 mediates hypoferremia of inflammation by inducing the synthesis of the iron regulatory hormone hepcidin. J Clin Invest. 2004;113(9):1271–1276. doi: 10.1172/JCI20945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Nicolas G, Chauvet C, Viatte L, et al. The gene encoding the iron regulatory peptide hepcidin is regulated by anemia, hypoxia, and inflammation. J Clin Invest. 2002;110(7):1037–1044. doi: 10.1172/JCI15686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Portugal S, Carret C, Recker M, et al. Host-mediated regulation of superinfection in malaria. Nat Med. 2011;17(6):732–737. doi: 10.1038/nm.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sun CC, Vaja V, Babitt JL, Lin HY. Targeting the hepcidin-ferroportin axis to develop new treatment strategies for anemia of chronic disease and anemia of inflammation. Am J Hematol. 2012;87(4):392–400. doi: 10.1002/ajh.23110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wrighting DM, Andrews NC. Interleukin-6 induces hepcidin expression through STAT3. Blood. 2006;108(9):3204–3209. doi: 10.1182/blood-2006-06-027631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Verga Falzacappa MV, Vujic Spasic M, Kessler R, Stolte J, Hentze MW, Muckenthaler MU. STAT3 mediates hepatic hepcidin expression and its inflammatory stimulation. Blood. 2007;109(1):353–358. doi: 10.1182/blood-2006-07-033969. [DOI] [PubMed] [Google Scholar]
- 94.Theurl I, Schroll A, Nairz M, et al. Pathways for the regulation of hepcidin expression in anemia of chronic disease and iron deficiency anemia in vivo. Haematologica. 2011;96(12):1761–1769. doi: 10.3324/haematol.2011.048926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Besson-Fournier C, Latour C, Kautz L, et al. Induction of activin B by inflammatory stimuli up-regulates expression of the iron-regulatory peptide hepcidin through Smad1/5/8 signaling. Blood. 2012;120(2):431–439. doi: 10.1182/blood-2012-02-411470. [DOI] [PubMed] [Google Scholar]
- 96.Meynard D, Sun CC, Chen W, et al. Inflammation regulates TMPRSS6 expression via STAT5.; Abstract presented at Fifth Congress of the International BioIron Society (IBIS). June 14-18, 2013. London, United Kingdom. [Google Scholar]
- 97.Vokurka M, Krijt J, Sulc K, Necas E. Hepcidin mRNA levels in mouse liver respond to inhibition of erythropoiesis. Physiol Res. 2006;55(6):667–674. doi: 10.33549/physiolres.930841. [DOI] [PubMed] [Google Scholar]
- 98.Pak M, Lopez MA, Gabayan V, Ganz T, Rivera S. Suppression of hepcidin during anemia requires erythropoietic activity. Blood. 2006;108(12):3730–3735. doi: 10.1182/blood-2006-06-028787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sasaki Y, Noguchi-Sasaki M, Yasuno H, Yorozu K, Shimonaka Y. Erythropoietin stimulation decreases hepcidin expression through hematopoietic activity on bone marrow cells in mice. Int J Hematol. 2012;96(6):692–700. doi: 10.1007/s12185-012-1217-4. [DOI] [PubMed] [Google Scholar]
- 100.Tanno T, Bhanu NV, Oneal PA, et al. High levels of GDF15 in thalassemia suppress expression of the iron regulatory protein hepcidin. Nat Med. 2007;13(9):1096–1101. doi: 10.1038/nm1629. [DOI] [PubMed] [Google Scholar]
- 101.Tanno T, Porayette P, Sripichai O, et al. Identification of TWSG1 as a second novel erythroid regulator of hepcidin expression in murine and human cells. Blood. 2009;114(1):181–186. doi: 10.1182/blood-2008-12-195503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Casanovas G, Spasic MV, Casu C, et al. The murine growth differentiation factor 15 is not essential for systemic iron homeostasis in phlebotomized mice. Haematologica. 2013;98(3):444–447. doi: 10.3324/haematol.2012.069807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peyssonnaux C, Zinkernagel AS, Schuepbach RA, et al. Regulation of iron homeostasis by the hypoxia-inducible transcription factors (HIFs). J Clin Invest. 2007;117(7):1926–1932. doi: 10.1172/JCI31370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mastrogiannaki M, Matak P, Mathieu JR, et al. Hepatic hypoxia-inducible factor-2 down-regulates hepcidin expression in mice through an erythropoietin-mediated increase in erythropoiesis. Haematologica. 2012;97(6):827–834. doi: 10.3324/haematol.2011.056119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Volke M, Gale DP, Maegdefrau U, et al. Evidence for a lack of a direct transcriptional suppression of the iron regulatory peptide hepcidin by hypoxia-inducible factors. PLoS ONE. 2009;4(11):e7875. doi: 10.1371/journal.pone.0007875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Goodnough JB, Ramos E, Nemeth E, Ganz T. Inhibition of hepcidin transcription by growth factors. Hepatology. 2012;56(1):291–299. doi: 10.1002/hep.25615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Shanmugam NK, Ellenbogen S, Trebicka E, et al. Tumor necrosis factor α inhibits expression of the iron regulating hormone hepcidin in murine models of innate colitis. PLoS ONE. 2012;7(5):e38136. doi: 10.1371/journal.pone.0038136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hou Y, Zhang S, Wang L, et al. Estrogen regulates iron homeostasis through governing hepatic hepcidin expression via an estrogen response element. Gene. 2012;511(2):398–403. doi: 10.1016/j.gene.2012.09.060. [DOI] [PubMed] [Google Scholar]
- 109.Latour C, Kautz L, Besson-Fournier C, et al. Testosterone perturbs systemic iron balance through activation of EGFR signaling in the liver and repression of hepcidin. doi: 10.1002/hep.26648. [published online ahead of print August 1, 2013]. Hepatology. doi: 10.1002/hep.26648. [DOI] [PubMed] [Google Scholar]
- 110.Guo W, Bachman E, Li M, et al. Testosterone administration inhibits hepcidin transcription and is associated with increased iron incorporation into red blood cells. Aging Cell. 2013;12(2):280–291. doi: 10.1111/acel.12052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Anderson ER, Taylor M, Xue X, et al. The hypoxia-inducible factor-C/EBPα axis controls ethanol-mediated hepcidin repression. Mol Cell Biol. 2012;32(19):4068–4077. doi: 10.1128/MCB.00723-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Courselaud B, Pigeon C, Inoue Y, et al. C/EBPalpha regulates hepatic transcription of hepcidin, an antimicrobial peptide and regulator of iron metabolism. Cross-talk between C/EBP pathway and iron metabolism. J Biol Chem. 2002;277(43):41163–41170. doi: 10.1074/jbc.M202653200. [DOI] [PubMed] [Google Scholar]
- 113.Miura K, Taura K, Kodama Y, Schnabl B, Brenner DA. Hepatitis C virus-induced oxidative stress suppresses hepcidin expression through increased histone deacetylase activity. Hepatology. 2008;48(5):1420–1429. doi: 10.1002/hep.22486. [DOI] [PubMed] [Google Scholar]
- 114.Finberg KE. Striking the target in iron overload disorders. J Clin Invest. 2013;123(4):1424–1427. doi: 10.1172/JCI68889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Camaschella C. Treating iron overload. N Engl J Med. 2013;368(24):2325–2327. doi: 10.1056/NEJMcibr1304338. [DOI] [PubMed] [Google Scholar]
- 116.Ramos E, Ruchala P, Goodnough JB, et al. Minihepcidins prevent iron overload in a hepcidin-deficient mouse model of severe hemochromatosis. Blood. 2012;120(18):3829–3836. doi: 10.1182/blood-2012-07-440743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schmidt PJ, Toudjarska I, Sendamarai AK, et al. An RNAi therapeutic targeting Tmprss6 decreases iron overload in Hfe–/– mice and ameliorates anemia and iron overload in murine beta-thalassemia intermedia. Blood. 2013;121(7):1200–1208. doi: 10.1182/blood-2012-09-453977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Guo S, Casu C, Gardenghi S, et al. Reducing TMPRSS6 ameliorates hemochromatosis and β-thalassemia in mice. J Clin Invest. 2013;123(4):1531–1541. doi: 10.1172/JCI66969. [DOI] [PMC free article] [PubMed] [Google Scholar]