Abstract
After T4 bacteriophage infection of E. coli a complex series of events take place in the bacterium, including gross inhibition of host transcription and discrete changes in the classes of the genes of T4 that are transcribed. Accompanying these changes in the pattern of transcription one finds T4-induced changes in the RNA polymerase (EC 2.7.7.6; nucleosidetriphosphate:RNA nucleotidyltransferase). The effects of modified polymerase on transcription can be advantageously analyzed in a DNA-directed cell-free system for protein synthesis. In this system gene activity is measured indirectly by the amounts and types of proteins sythesized. In the DNA-directed cell-free system this modified polymerase, like normal polymerase, transcribes T4 DNA with a high efficiency but transcribes bacteriophage lambda and host DNA very poorly. Polymerase reconstruction experiments show that modification of the alpha subunit of the RNA polymerase is sufficient for inhibition of host transcription. Host transcription is also inhibited in vitro by T4 DNA. This latter type of inhibition is presumed to involve competition between host DNA and T4 DNA for some factor essential for transcription. The T4-modified polymerase transcribes from T4 DNA many of the same genes as normal unmodified polymerase; it also shows a capability for transcribing certain "non-early" T4 genes which is enhanced in the presence of protein-containing extracts from T4-infected cells.
Full text
PDF

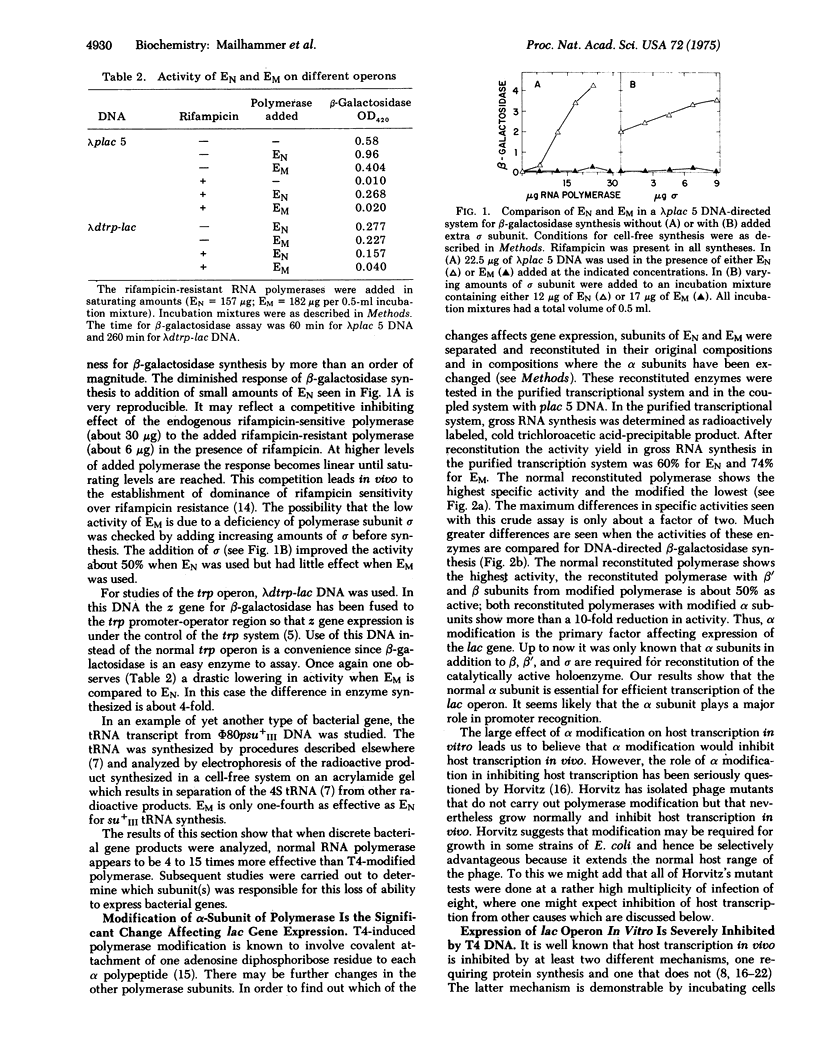
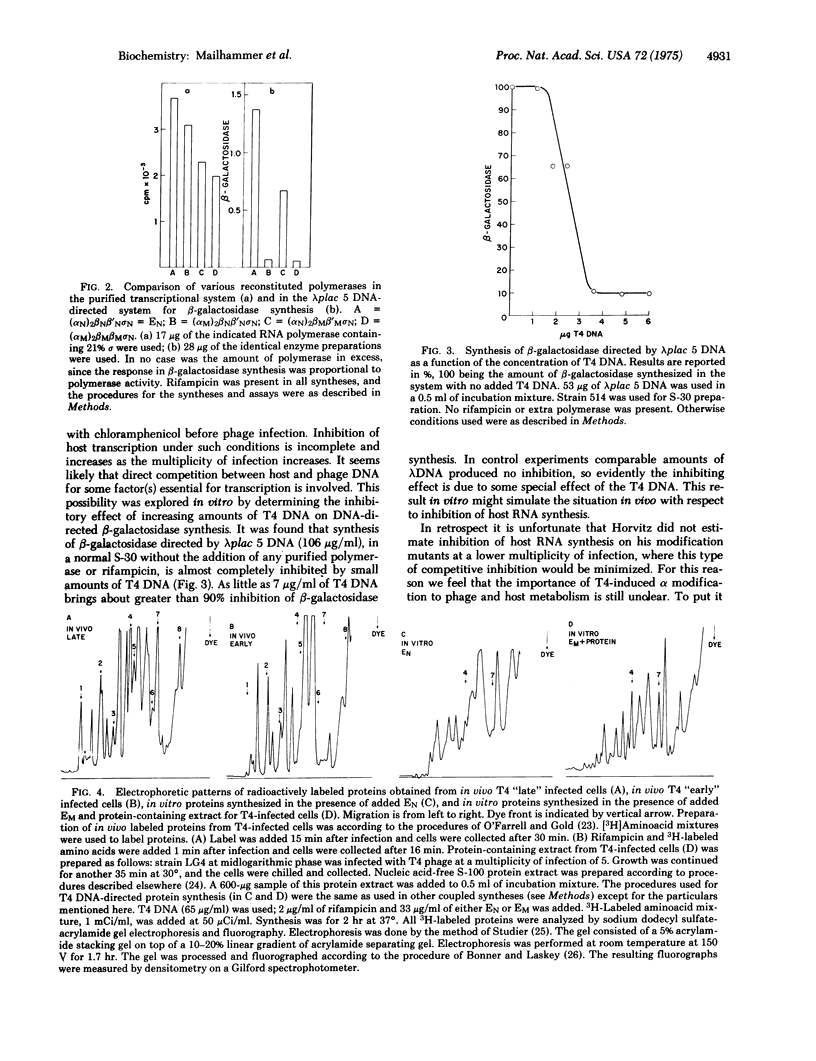

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adesnik M., Levinthal C. RNA metabolism in T4-infected Escherichia coli. J Mol Biol. 1970 Mar 14;48(2):187–208. doi: 10.1016/0022-2836(70)90156-7. [DOI] [PubMed] [Google Scholar]
- Bonner W. M., Laskey R. A. A film detection method for tritium-labelled proteins and nucleic acids in polyacrylamide gels. Eur J Biochem. 1974 Jul 1;46(1):83–88. doi: 10.1111/j.1432-1033.1974.tb03599.x. [DOI] [PubMed] [Google Scholar]
- Boyd D. H., Zillig W. Reference mutations for the beta subunit of RNA polymerase. Mol Gen Genet. 1974 Jun 27;130(4):315–320. doi: 10.1007/BF00333870. [DOI] [PubMed] [Google Scholar]
- Burgess R. R. A new method for the large scale purification of Escherichia coli deoxyribonucleic acid-dependent ribonucleic acid polymerase. J Biol Chem. 1969 Nov 25;244(22):6160–6167. [PubMed] [Google Scholar]
- De Crombrugghe B., Adhya S., Gottesman M., Pastan I. Effect of Rho on transcription of bacterial operons. Nat New Biol. 1973 Feb 28;241(113):260–264. doi: 10.1038/newbio241260a0. [DOI] [PubMed] [Google Scholar]
- Ennis H. L. Protein synthesis and the inhibition of host messenger ribonucleic acid production in bacteriophage T4-infected Escherichia coli. Virology. 1970 Mar;40(3):727–733. doi: 10.1016/0042-6822(70)90217-5. [DOI] [PubMed] [Google Scholar]
- Goff C. G. Chemical structure of a modification of the Escherichia coli ribonucleic acid polymerase alpha polypeptides induced by bacteriophage T4 infection. J Biol Chem. 1974 Oct 10;249(19):6181–6190. [PubMed] [Google Scholar]
- Hayward W. S., Green M. H. Inhibition of Escherichia coli and bacteriophage lambda messenger RNA synthesis by T4. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1675–1678. doi: 10.1073/pnas.54.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvitz H. R. Bacteriophage T4 mutants deficient in alteration and modification of the Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):739–750. doi: 10.1016/0022-2836(74)90537-3. [DOI] [PubMed] [Google Scholar]
- Horvitz H. R. Control by bacteriophage T4 of two sequential phosphorylations of the alpha subunit of Escherichia coli RNA polymerase. J Mol Biol. 1974 Dec 25;90(4):727–738. doi: 10.1016/0022-2836(74)90536-1. [DOI] [PubMed] [Google Scholar]
- Ilyina T. S., Ovadis M. I., Mindlin S. Z., Gorlenko Z. M., Khesin R. B. Interaction of RNA polymerase mutations in haploid and merodiploid cells of Escherichia coli K-12. Mol Gen Genet. 1971;110(2):118–133. doi: 10.1007/BF00332643. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Effect of infection with T-even phage on the inducible synthesis of beta-glactosidase in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):453–468. doi: 10.1016/0022-2836(67)90051-4. [DOI] [PubMed] [Google Scholar]
- Kaempfer R. O., Magasanik B. Mechanism of beta-galactosidase induction in Escherichia coli. J Mol Biol. 1967 Aug 14;27(3):475–494. doi: 10.1016/0022-2836(67)90053-8. [DOI] [PubMed] [Google Scholar]
- Manley J., Reiness G., Zubay G., Gefter M. L. Cell-free synthesis of SU + 3 tyrosyl transfer RNA: characterization of the 4S product. Arch Biochem Biophys. 1973 Jul;157(1):50–54. doi: 10.1016/0003-9861(73)90388-3. [DOI] [PubMed] [Google Scholar]
- Nomura M., Witten C., Mantei N., Echols H. Inhibition of host nucleic acid synthesis by bacteriophage T4: effect of chloramphenicol at various multiplicities of infection. J Mol Biol. 1966 May;17(1):273–278. doi: 10.1016/s0022-2836(66)80107-9. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. Z., Gold L. M. Bacteriophage T4 gene expression. Evidence for two classes of prereplicative cistrons. J Biol Chem. 1973 Aug 10;248(15):5502–5511. [PubMed] [Google Scholar]
- Schachner M., Seifert W., Zillig W. A correlation of changes in host and T 4 bacteriophage specific RNA synthesis with changes of DNA-dependent RNA polymerase in Escherichia coli infected with bacteriophage T 4 . Eur J Biochem. 1971 Oct 26;22(4):520–528. doi: 10.1111/j.1432-1033.1971.tb01572.x. [DOI] [PubMed] [Google Scholar]
- Stevens A. New small polypeptides associated with DNA-dependent RNA polymerase of Escherichia coli after infection with bacteriophage T4. Proc Natl Acad Sci U S A. 1972 Mar;69(3):603–607. doi: 10.1073/pnas.69.3.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Walter G., Seifert W., Zillig W. Modified DNA-dependent RNA polymerase from E. coli infected with bacteriophage T4. Biochem Biophys Res Commun. 1968 Feb 15;30(3):240–247. doi: 10.1016/0006-291x(68)90441-5. [DOI] [PubMed] [Google Scholar]
- Yang H., Zubay G. A possible termination factor for transcription in Escherichia coli. Biochem Biophys Res Commun. 1974 Feb 4;56(3):725–731. doi: 10.1016/0006-291x(74)90665-2. [DOI] [PubMed] [Google Scholar]
- Zillig W., Zechel K., Halbwachs H. J. A new method of large scale preparation of highly purified DNA-dependent RNA-polymerase from E. coli. Hoppe Seylers Z Physiol Chem. 1970 Feb;351(2):221–224. doi: 10.1515/bchm2.1970.351.1.221. [DOI] [PubMed] [Google Scholar]
- Zubay G. In vitro synthesis of protein in microbial systems. Annu Rev Genet. 1973;7:267–287. doi: 10.1146/annurev.ge.07.120173.001411. [DOI] [PubMed] [Google Scholar]


