Abstract
Purpose
Hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF) are secreted in the cornea in response to injury. In this study, we investigated the HGF- and KGF-mediated effect on the expression of cell cycle and apoptosis controlling proteins, cell survival, and growth in the corneal epithelium to better understand the possible role of their signaling mechanisms in repairing epithelial injuries.
Methods
The cell survival capability of HGF and KGF in epithelial primary cultures was evaluated by using a staurosporine-induced apoptosis model. Apoptosis was quantified with image analysis following nuclear staining with Hoechst fluorescent dye and DNA laddering. Western immunoblotting was used to study the effect of growth factors on the expression of cell cycle- and apoptosis-regulating proteins.
Results
HGF and KGF protected cells from apoptosis for a short duration (10 h), but only KGF exhibited cell survival capability and maintained cell growth for a longer period (24 h). The onset of apoptosis was accompanied by a significant increase in cell cycle inhibitor p27kip. HGF and KGF suppressed p27kip levels in the apoptosis environment; however, KGF- but not HGF-dependent downregulation in p27kip expression was sustained for a longer duration. Inhibition of phosphatidylinositol 3-kinase/Akt activation blocked HGF- and KGF-mediated control of p27kip expression. Further, when compared to HGF, the presence of KGF produced significant downregulation of p53 and poly(adenosine diphosphate-ribose) polymerase, the key proteins involved in apoptosis and blocked the degradation of G1/S cell cycle progression checkpoint protein retinoblastoma. HGF and KGF upregulated the levels of p21cip, cyclins A, D, and E and cyclin-dependent kinases (CDK2 and CDK4) as well, but the KGF-mediated effect on the expression of these molecules lasted longer.
Conclusions
Sustained effect of KGF on cell survival and proliferation could be attributed to its ability to inhibit p53, retinoblastoma, caspases, and p27kip functions in apoptosis and cell cycle arrest and promote the expression of cell cycle progressing molecules for longer duration. Designing therapeutic strategies targeting cell cycle control through KGF may be beneficial for repairing difficult-to-heal corneal epithelial injuries that require sustained growth and cell survival promoting signals.
Introduction
The corneal epithelium is continuously generated to replenish the aged cells that are lost as a result of normal shedding. Due to the cornea’s anatomic location, the cornea surface is frequently subjected to trauma by environmental factors leading to deepithelialization. An intact corneal epithelium is essential for maintaining good vision and protecting against infection. Healing of epithelial wounds in a healthy cornea occurs relatively quickly. However, several factors such as disease state, recurrent erosion, and persistent defects contribute to the poor healing response of the cornea. Providing an environment that enhances epithelial cell proliferation as well as survival is important to overcome delays in healing. Regeneration of the epithelium requires the participation of several entities, including extracellular matrix proteins and growth factors that collectively promote cell adhesion, migration, and proliferation processes [1-5]. To facilitate healing, several intracellular signaling cascades activated in varying degrees by growth factors coordinate cell migration, adhesion, and proliferation processes [6]. In response to injury, several growth factors are released from the stroma and lacrimal gland [7-13]. Two paracrine growth factors, hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF), have been shown to influence corneal epithelial cell metabolism [14-16]. Our laboratory has been investigating various aspects associated with HGF- and KGF-activated signaling in the cornea and the contribution of these signaling cascades to wound healing. Our previous studies and other reports showed that HGF and KGF activate signal mediators phosphatidylinositol 3-kinase (PI-3K)/Akt, p70S6K, and Erk [17-23]. However, it is not clear why these growth factors trigger the activation of the same intracellular signaling cascades to stimulate healing or whether corneal epithelial cells prefer one growth factor over the other to promote different cellular processes involved in wound repair.
Intracellular signaling cascades activated by growth factors trigger the activity of nuclear transcription factors. They promote cell division by exerting their control over the cell cycle [24-28]. Specific interactions between various proteins known as cyclins, cyclin-dependent kinases (CDKs), and cyclin-dependent kinase inhibitors (CDKIs) facilitate the passage of cells through the G1, S, G2, and M phases of the cell cycle for its continued propagation [29-31]. Although HGF- and KGF-mediated stimulation of corneal epithelial cells leads to simultaneous activation of signaling pathways such as PI-3K, p70S6K, and Erk [17-19], the impact of their activation on downstream targets that control the cell cycle is not well understood. The specific effect of HGF and KGF on corneal epithelial cell cycle regulating proteins has not been investigated. Furthermore, previously we found that HGF can rescue epithelial cells from apoptosis [32], but a role for KGF in corneal epithelial cell survival has not yet been identified. Factors that upregulate cell survival and cell cycle progression could influence the rate of wound healing. Better understanding of these mechanisms regulated by growth factors would aid in developing new therapeutic strategies. In this study, we investigated the effects of HGF and KGF on the expression of the corneal epithelial cell cycle controlling machinery under normal growth and an apoptosis-inducing environment. We also evaluated their ability to rescue the cells from apoptosis. Our studies revealed that compared to HGF, KGF exhibits longer-lasting influence on corneal epithelial cell survival and cell growth as well as on the regulation of levels of apoptosis and cell cycle controlling proteins that include p53, poly(adenosine diphosphate-ribose) polymerase (PARP), retinoblastoma protein (Rb), cyclins, CDKs, and cell cycle inhibitor p27kip.
Methods
Materials
Polyclonal or monoclonal antibodies for antip21cip, antip27kip, and phospho Akt (pAkt) were purchased from Upstate Biotechnology (Billercia, MA). Recombinant human HGF and KGF were purchased from R&D Systems (Minneapolis, MN). Antimouse and antirabbit horseradish peroxidase-conjugated secondary antibodies were procured from BD Pharmingen (San Diego, CA). The biotinylated protein markers kit was from New England Bio Labs (Beverly, MA). Rabbit polyclonal antibodies for cyclins A, D, and E and CDK2, CDK4 Rb, and monoclonal antibody for Erk and actin were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Monoclonal anti-PARP and anti-p53 antibodies were procured from Cell Signaling Technology Inc. (Boston, MA) and Zymed Laboratories Inc. (San Francisco, CA) respectively.
Epithelial cell culture
Fresh rabbit eyeballs (Pelfreeze Biologicals, Rogers, AR) were used to prepare the corneal epithelial primary cultures [17]. Rabbit corneal primary epithelial (RCPE) cells were cultured in Dulbecco’s modified Eagle medium with Ham’s F-12 (DMEM/F-12; Life Technologies, Grand Island, NY) containing 10% fetal calf serum (FCS; Atlanta Biologicals, Lawrenceville, GA), and first passage cells were used in all experiments as in previously published studies [32,33]. Cultures grown to 80%–90% confluence were starved overnight in DMEM/F12 containing 0.25% FCS before treatment with various stimulators or inhibitors as indicated in the results.
Induction of apoptosis
Apoptosis was induced in corneal epithelial cells with staurosporine (10 ng/ml) as reported earlier and described below [32]. Staurosporine activates the apoptotic death pathway by affecting the mitochondrial metabolism and is widely used to induce apoptosis in cells. Epithelial cells (90% confluent) were starved overnight (12–16 h) before incubation with staurosporine in DMEM/F12 containing 0.25% FCS for 2 h. Then the medium was removed, and the cells were washed with the same medium without staurosporine and incubated for an additional 2, 8, or 22 h. Next, cultures were analyzed for the presence of apoptotic cells or subjected to analysis for DNA fragmentation as described below.
Hoechst fluorescent staining and quantification of apoptotic cells
Hoechst 33,258 (Molecular Probes, Eugene, OR) stain was used to detect apoptotic cells in live cultures by fluorescence imaging as previously published [32,34]. The protocol of the method is as follows. Cell cultures were washed with phosphate buffer (potassium phosphate monobasic and sodium phosphate dibasic) saline (0.09% sodium chloride, pH 7.4) twice and incubated with 2 μM Hoechst at 37 °C in darkness. After 1 h incubation, the staining solution was removed and cells were washed two times with PBS and the cultures were visualized under the microscope. When taken up by living cells undergoing apoptosis, the dye stains the cell nucleus and manifests bright blue fluorescence whereas normal cell nucleus staining produces a dull blue background. The stained cells emitting fluorescence were visualized in a Zeiss-Axiovision (Oberkochen, Germany) inverted microscope using an ultraviolet (UV) cube. All the images were captured with the same settings using a Zeiss-Axiocam MRC 5 color camera fitted to the microscope. For each experimental condition, images of five to eight different visual fields covering the entire culture dish were taken for analysis and quantification. All the images were processed to count the apoptotic and nonapoptotic cells with the Zeiss-Axiovision 4.1 software program with a fixed set of values for brightness and contrast adjustment. The average cell counts for all images taken from each culture dish for each condition are presented in the results.
Separation of epithelial cell deoxyribonucleic acid and ladder assay
To determine apoptosis, DNA was isolated from RCPE extracts using a Genomic DNA purification Kit (Promega Corp, Madison, WI) as previously performed [32,34]. Briefly, medium from the cell cultures of the apoptosis experiments was removed, and the cells were detached by trypsinization followed by centrifugation. The medium collected before the trypsin treatment was also centrifuged to collect sloughed-off apoptotic cells during the course of the experiment and combined with the cells obtained after the trypsin treatment. The cell nuclei were lysed with nuclear lysis solution. The lysates were treated with DNase-free RNase (Promega; 3 μg) at 37 °C for 15 min. The cellular proteins were then removed by salt precipitation. The DNA was finally precipitated with isopropanol and washed once with 70% ethanol. The DNA was dissolved in 10 mM Tris-HCl buffer (pH 7.4) containing 1 mM EDTA, and the concentration was determined at 260 nm with spectrophotometry. The purified DNA was subjected to agarose (1.8%) gel electrophoresis and the characteristic breakdown/fragmentation of the chromatin was visualized with a UV light after the gel was stained with ethidium bromide. Approximately 4–5 µg DNA was loaded in each sample well. A 100 bp DNA ladder was used as the standard. The approximate quantity of the DNA degradation products formed in each experimental condition was measured with image analysis using the NIH Image J software program. The areas under the peaks present in the integration curves for each condition were added to obtain the total degradation products. Changes in DNA degradation under various conditions are expressed in percentages relative to that obtained with control condition, i.e., treated with staurosporine alone.
Preparation of cell extracts and western immunoblotting
Epithelial cells from various experiments were extracted in lysis buffer (20 mM 2-[4-(2-hydroxyethyl)piperazin-1-yl]ethanesulfonic acid [HEPES], 2 mM Mg2+, 2 mM ethyleneglycol tetraacetic acid [EGTA], 2 mM sodiumorthovanadate, 2 mM dithiothreitol [DTT], 1 mM phenylmethylsulfonyl fluoride [PMSF], 0.1 mM leupeptin, 150 mM NaCl, 1% Triton X-100, and 0.5% NP-40) and centrifuged at 20,000 ×g for 30 min, and the supernatant was collected. All the operations were performed at 4 °C. The protein content of the extracts was determined with the Bio-Rad dye-binding method. The cell extracts (25–35 µg protein) were subjected to sodium dodecyl sulfate–polyacrylamide gel electrophoresis in 9%–15% gels. The proteins in the gels were transferred to nitrocellulose membranes. The membranes were blocked with 5% nonfat dry milk and then probed with specific antibodies as indicated in the results. The blots were incubated at room temperature with primary antibodies for 2 h, washed six times with TBS (20 mM Tris-HCl pH 7.4, 150 mM NaCl, 0.05% Tween-20) and further incubated for 2 h with horseradish peroxidase-conjugated secondary antibodies. The membranes were treated with enhanced chemiluminescence reagent (GE-Amersham, Arlington Heights, IL). The protein bands of interest were identified after the membranes were exposed in the UVP-image analyzer or to X-ray film. Molecular sizes were determined by comparing with the mobility of biotinylated protein markers that were run simultaneously during electrophoresis. All protein extracts transferred to membranes were also blotted with antiactin to show the unchanged levels of actin, the housekeeping protein used during various experimental conditions.
Statistical analysis
The results were analyzed with the Student t test or ANOVA for statistical significance and shown as mean±standard deviation (SD). A p value less than 0.05 was considered statistically significant.
Results
Differential effects of hepatocyte growth factor and keratinocyte growth factor on corneal epithelial cell survival
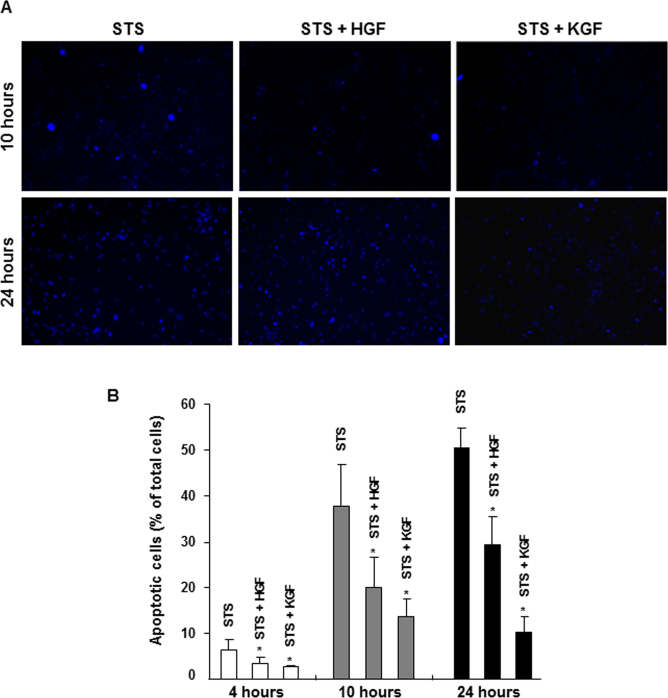
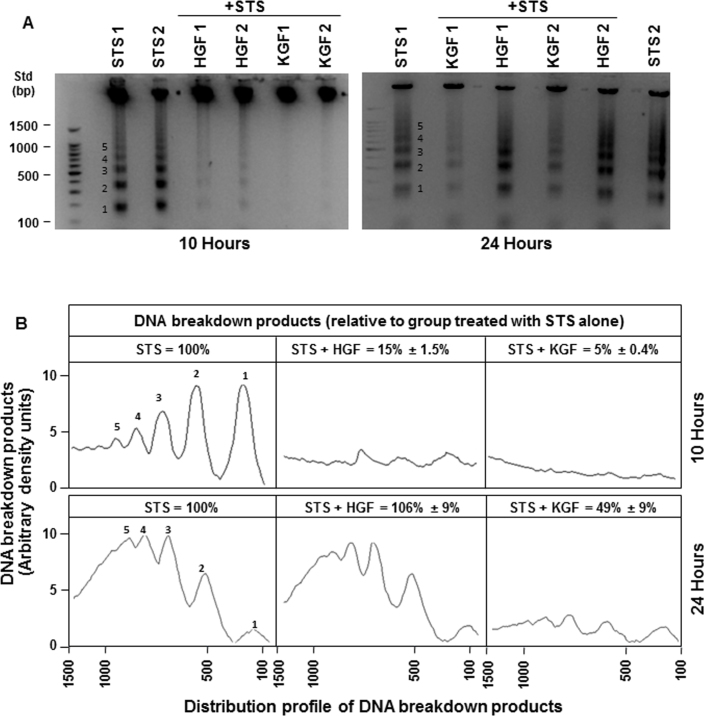
The staurosporine-induced apoptosis model was used to compare the cell survival capability of HGF and KGF. As described in our earlier studies [32], staurosporine produced significant apoptosis in corneal epithelial cells that is evidenced by the increase in intense Hoechst dye positive staining of nuclei due to DNA condensation (Figure 1A, left panels). The percentage of apoptotic nuclei in the cultures increased from 5% to 50% between 4 and 24 h after staurosporine treatment (Figure 1B). The presence of HGF or KGF (20 ng/ml) caused a significant decrease in staurosporine-induced apoptosis in the cultures after 10 h. The percentage of apoptotic cells present was 20% and 7% in the presence of HGF and KGF, respectively, whereas in the staurosporine alone condition, it was 37%. The decrease in apoptosis was larger with KGF than with HGF. Interestingly, by 24 h the effectiveness of HGF in rescuing the cells from apoptosis diminished considerably, while the cell-survival potential of KGF remained unaffected. The percentage of cells treated with HGF showing apoptosis staining was about 30% whereas with KGF it was only 10%. The distribution of apoptotic and normal cells under various conditions is presented in Table 1. The dead cells that slough off from culture plates as a consequence of apoptosis were not accounted for in this analysis, and the cell density of cultures in the absence of HGF or KGF was significantly low, specifically at the 24 h time point. The difference in the cell survival capability of HGF and KGF was also demonstrated by performing DNA laddering assays (Figure 2). In the staurosporine-treated cultures, the amount of DNA breakdown leading to the characteristic ladder appearance due to varying sizes of breakdown products was clearly evident at 10 and 24 h. Similar to the Hoechst positive staining pattern, the presence of HGF and KGF reduced the amount of quantifiable DNA breakdown products by 10 h (Figure 2A) to 5%–15% of what was observed with staurosporine (Figure 2B). After 24 h, the HGF effect decreased almost completely, and the DNA breakdown was similar to that was found in the staurosporine alone condition, while the KGF influence in preventing DNA breakdown persisted. These results clearly show that HGF and KGF exhibit significant differences in their ability to protect corneal epithelial cells. Degradation of DNA or staining for apoptotic cells was not observed in control cultures that were not treated with staurosporine (data not shown).
Figure 1.
Hepatocyte growth factor (HGF)– and keratinocyte growth factor (KGF)–mediated cell survival during staurosporine (STS)-induced apoptosis. Rabbit corneal primary epithelial (RCPE) cell cultures in Dulbecco’s Modified Eagle Medium with Ham’s F-12 (DMEM/F-12)/0.25% fetal calf serum (FCS) were pretreated with 20 ng/ml HGF or KGF for 30 min before STS was added. Cultures were further incubated in the presence of STS (10 ng/ml) for 2 h. Then the medium was removed, and fresh medium containing HGF or KGF but no STS was added, and incubation continued for an additional 2 to 22 h. The cultures were stained with Hoechst fluorescent nuclear staining reagent to visualize cells containing apoptotic nuclei as described in the Methods section, and images were captured (A). The number of apoptotic cells in each experimental condition was quantified and expressed as the percentage of total cells (apoptotic + normal; B). Data shown are mean±standard deviation (SD). *p<0.05, STS alone versus various treatments after 4 or 10 or 24 h.
Table 1. Changes in the population of normal cells in corneal epithelial cultures treated with or without HGF or KGF during apoptosis.
| Time |
Staurosporine |
Staurosporine + HGF |
Staurosporine + KGF |
|||
|---|---|---|---|---|---|---|
| Apoptotic | Normal | Apoptotic | Normal | Apoptotic | Normal | |
|
4 h |
126±49 |
1895±122 |
65±20 |
1875±252 |
56±6 |
2012±125 |
|
10 h |
696±229 |
1168±352 |
394±120 |
1795±109 |
285±65 |
1860±274 |
| 24 h | 535±89 | 539±134 | 604±192 | 1429±221 | 234±76 | 2021±105 |
Data presented are mean ± SD of 3–5 separate experimental samples (p<0.05, staurosporine versus staurosporine + HGF, staurosporine + KGF).
Figure 2.
Hepatocyte growth factor (HGF)– and keratinocyte growth factor (KGF)–mediated inhibition in deoxyribonucleic acid degradation and ladder formation during apoptosis. All treatments were performed as described in Figure 1, and the isolated DNA from cultures, after various treatments, was subjected to agarose gel electrophoresis for DNA ladder assay (A). Quantification of DNA breakdown products shown in A (bands 1–5) was performed by integrating areas under different peaks, corresponding to bands 1 to 5 as depicted in the chart (B). The x-axis indicates the base pair sizes of the DNA breakdown products. The experiments were repeated once with similar.
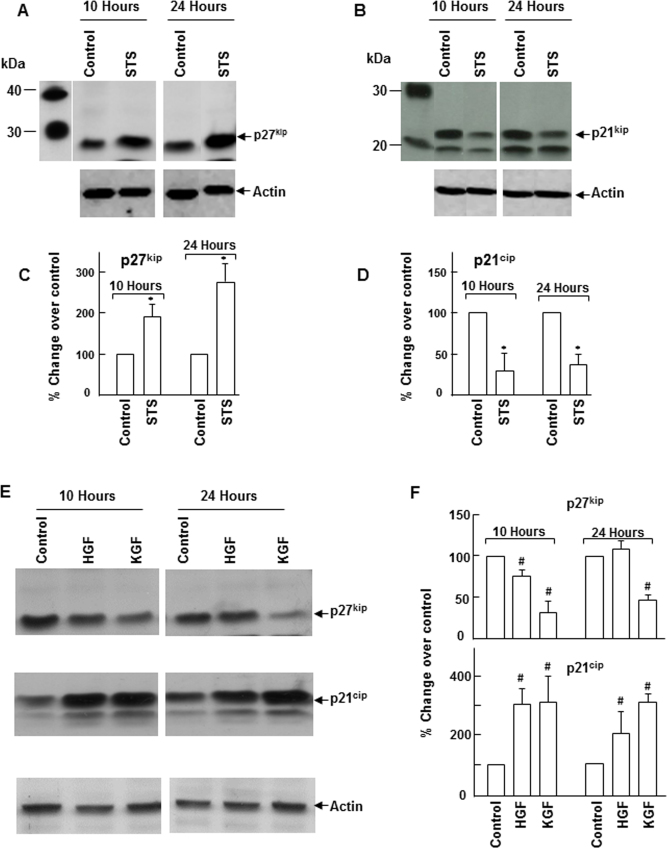
Cell cycle inhibitor p27kip levels upregulated during apoptosis
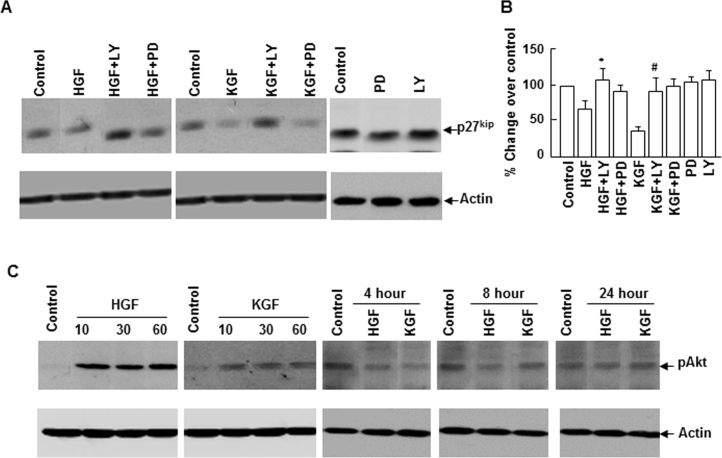
Cells that enter the G0 phase when the cell cycle is arrested either undergo differentiation or are eliminated by apoptosis. Changes in the cell cycle control mechanism that lead to cell cycle arrest could enhance apoptosis. To understand the molecular basis for the differences in the cell survival function of HGF and KGF, we analyzed the status of cell cycle inhibitors p27kip and p21cip in the cultures subjected to apoptosis. As shown in Figure 3, there was a gradual increase in the p27kip level with time that coincided with the onset of apoptosis. When compared to the controls, the level increased by about 200% at 10 h and close to 300% at 24 h after the staurosporine treatment. In contrast to the p27kip response, staurosporine-induced apoptosis caused a significant decrease in p21cip expression. These results indicate that susceptibility of corneal epithelial cells to apoptosis could be related to the increase in the p27kip level that causes cell cycle arrest. Next, we examined the effect of HGF and KGF on the expression of p27kip and p21cip under normal growth conditions. HGF and KGF downregulated p27kip expression in corneal epithelial cells (Figure 4). However, the HGF-mediated effect on p27kip expression was short lived (for 10 h) whereas the effect of KGF was sustained for a longer period (24 h). Interestingly, in the presence of HGF and KGF, the expression of p21cip was not decreased; instead, its levels increased considerably (Figure 3E).
Figure 3.
Changes in the levels of p27kip and p21cip during apoptosis. A, B: Apoptosis was induced in rabbit corneal primary epithelial (RCPE) cell cultures with staurosporine (STS) as described in the Methods section. E: Subconfluent (80%–90%) RCPE cultures were starved overnight with Dulbecco’s Modified Eagle Medium with Ham’s F-12 (DMEM/F-12)/0.25% fetal calf serum (FCS) and then treated with 20 ng/ml HGF or KGF for 10 or 24 h. Levels of p27kip and p21cip in cellular extracts at different time points were determined with western immunoblotting using specific antibodies. Quantification of p27kip and p21cip levels in apoptosis cultures (C–D) and HGF- and KGF-treated cultures (F) was performed with densitometry. Data are representative of results obtained from three similar experiments. Data shown are mean±standard deviation (SD). *p<0.05, STS versus corresponding control at 10 or 24 h, #p<0.05, control versus HGF or KGF at 10 or 24 h.
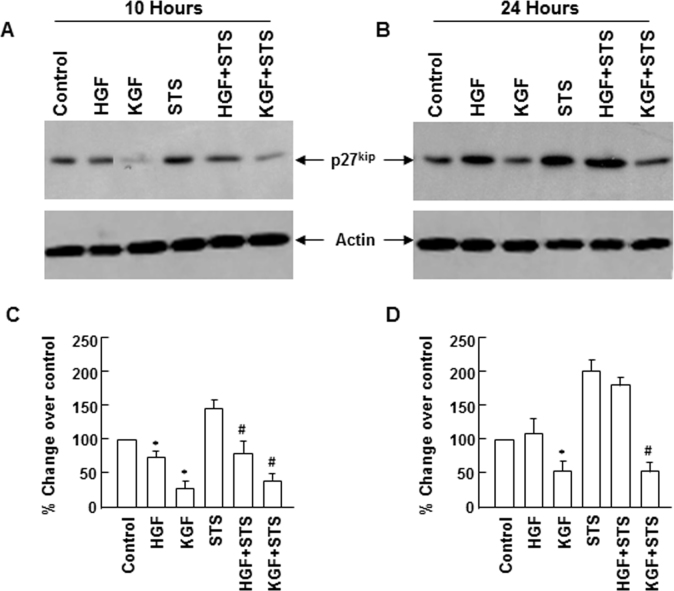
Figure 4.
Downregulation of p27kip expression by hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF) during apoptosis. Rabbit corneal primary epithelial (RCPE) cell cultures in Dulbecco’s Modified Eagle Medium with Ham’s F-12 (DMEM/F-12)/0.25% fetal calf serum (FCS) were pretreated with 20 ng/ml HGF or KGF for 30 min before staurosporine (STS) was added. Cultures were further incubated in the presence of STS (10 ng/ml) for 2 h. Then the medium was removed, and fresh medium containing HGF or KGF but no STS was added, and incubation continued for 8 h (A) to 22 h (B). Levels of p27kip in cellular extracts at different time points were determined with western immunoblotting using monoclonal p27kip antibody and quantified with densitometry (C and D). Similar results were obtained in two additional experiments. Data shown are mean±standard deviation (SD). *p<0.05, control versus HGF or KGF, #p<0.05 STS versus STS+HGF or STS+KGF.
Downregulation of p27kip during hepatocyte growth factor– and keratinocyte growth factor–mediated promotion of cell survival
We evaluated the effect of HGF and KGF on p27kip expression in conditions that protected epithelial cells from apoptosis. Similar to the effect observed in normal growth conditions, HGF and KGF blocked p27kip expression that was upregulated 10 h after apoptosis was induced (Figure 4). After 24 h, KGF, but not HGF, retained the ability to control the expression of p27kip, and its level decreased considerably with KGF, whereas in the presence of HGF, the p27kip level remained elevated. These results indicate that downregulation of p27kip may be important in providing protection against apoptosis and the sustained cell survival property of KGF could be attributed to its capacity to suppress the expression of p27kip for a prolonged period.
PI-3K/Akt-1 and Erk signaling cascades play a vital role in cell survival in many mammalian tissues. Our previous studies demonstrated that HGF and KGF stimulate these signal mediators in epithelial cells. To evaluate the involvement of the PI-3K/Akt-1 and Erk cascades in HGF- and KGF-mediated regulation on p27kip, we determined the effect of PI-3K/Akt and Erk inhibition on p27kip expression. Inhibition of PI-3K/Akt activation by LY294002 blocked KGF as well as HGF influence in suppressing p27kip levels. However, Erk inhibition by PD98059 did not significantly alter the effect of KGF or HGF on p27kip (Figure 5A). The basal levels of p27kip were unaffected in the presence of LY294002 or PD98059. Further, to corroborate the activation of Akt-1, we evaluated its phosphorylation status (pAkt formation) in cells stimulated with HGF or KGF for varying times, and the pAkt level was significantly higher up to 60 min of stimulation (Figure 5C). Although the cell survival effect of HGF and KGF and their influence on p27kip expression lasted for several hours, activation of Akt was not observed in cells stimulated for 4 h or more. These results imply that continuous activation of Akt-1 is not required for cell survival or p27kip downregulation promoted by HGF and KGF.
Figure 5.
Phosphatidylinositol 3-kinase (PI-3K)//Akt inhibition suppresses hepatocyte growth factor (HGF)– and keratinocyte growth factor (KGF)–mediated effect on p27kip expression. Rabbit corneal primary epithelial (RCPE) cell growth cultures at 80%–90% confluence were starved overnight with Dulbecco’s Modified Eagle Medium with Ham’s F-12 (DMEM/F-12)/0.25% fetal calf serum (FCS) and then pretreated with PI-3K/Akt inhibitor (LY294002, 20 μM) and Erk inhibitor (PD98059, 20 μM) for 30 min before 20 ng/ml HGF) or KGF was added. Cultures were incubated for various time points as indicated, and the levels of (A) p27kip and (C) phospho Akt (pAkt) in cellular extracts were determined with western immunoblotting. Densitometric quantification of p27kip levels is presented in B. Data shown are mean±standard deviation (SD). p<0.05, *HGF versus LY+HGF, #KGF versus LY+KGF.
Keratinocyte growth factor– but not hepatocyte growth factor–mediated influence on apoptosis and cell cycle regulating machinery is sustained
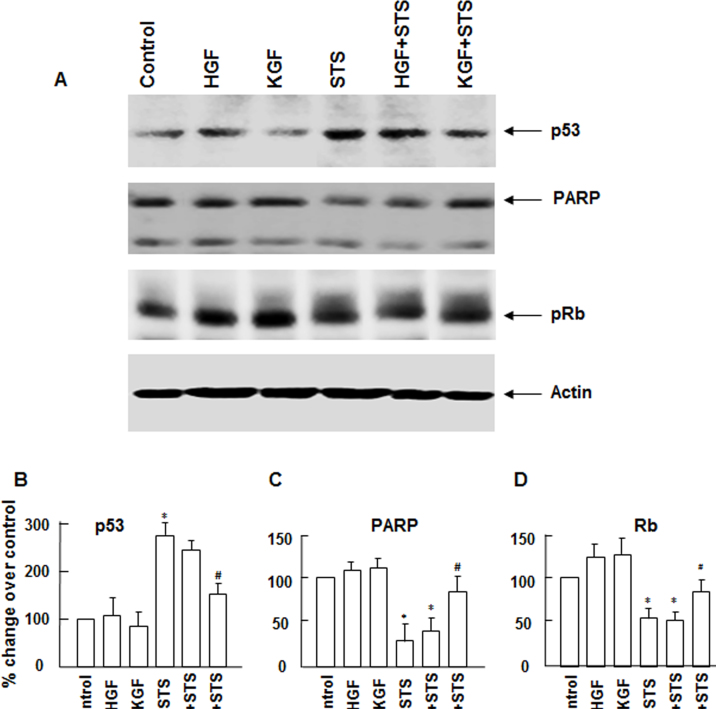
Although we found that KGF, but not HGF, produces sustained downregulation of p27kip that could possibly lead to increased cell survival and ell cycle progression, to provide further evidence why KGF is a better protector of apoptosis than HGF and understand the possible mechanistic differences in their action, we evaluated the influence of KGF and HGF on cell survival and cell cycle controlling proteins PARP, p53, and Rb. As shown in Figure 6, 24 h after apoptosis was induced, the presence of KGF prevented the degradation of PARP whereas the presence of HGF did not have a significant effect on blocking PARP degradation. PARP is a DNA-repairing enzyme that prevents DNA damage leading to apoptosis. Inducing apoptosis leads to the activation of caspases that degrade many cellular enzymes and proteins required for cell survival including PARP [35,36]. Further, we assessed the effect of HGF and KGF on the tumor suppressor proteins Rb and p53 that regulate cell cycle and apoptosis. In response to DNA-damaging agents, p53 levels rise, and p53 promotes the activation of many apoptosis-causing genes [37]. We observed an increase in the p53 protein level in cultures in response to the staurosporine treatment. However, in apoptosis cultures in the presence of KGF, we did not find a significant increase in the p53 level when compared to HGF. Rb, which serves as the G1/S cell cycle phase progression restricting protein and promotes cell cycle progression following mitogenic stimulation [37], can also be degraded in response to death stimuli [38,39]. Although Rb levels were stable in normal growth cultures in the presence of HGF and KGF, the degradation of Rb was more pronounced in apoptosis cultures in the presence of HGF compared to KGF. These results suggest that KGF’s ability to block the activation of caspases that suppress PARP and Rb degradation and downregulate p53 levels could contribute to KGF’s longer-lasting antiapoptosis and proliferative activity.
Figure 6.
Changes in the expression of apoptosis and cell cycle regulating proteins during Staurosporine (STS)-induced apoptosis in the presence of hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF). Rabbit corneal primary epithelial (RCPE) cell cultures in Dulbecco’s Modified Eagle Medium with Ham’s F-12 (DMEM/F-12)/0.25% fetal calf serum (FCS) were pretreated with 20 ng/ml HGF or (KGF) for 30 min before STS was added. Cultures were further incubated in the presence of STS (10 ng/ml) for 2 h. Then the medium was removed, and fresh medium containing HGF or KGF but no STS was added, and incubation continued for 22 h. Levels of and poly(adenosine diphosphate-ribose) polymerase (PARP), p53, and Rb proteins cellular extracts were determined with western immunoblotting by using antibodies specific for each protein (A). Experiments were performed two to three times. Quantification of different proteins is shown in bar diagrams (B–D). Data shown are mean±standard deviation (SD). *p<0.05, control versus STS, #p<0.05, STS versus STS+KGF in in (B); *p<0.05, control versus STS or STS+HGF, #p<0.05, STS versus STS+KGF in (C and D).
Differential effects hepatocyte growth factor and keratinocyte growth factor on the expression of cyclins and cyclin-dependent kinases
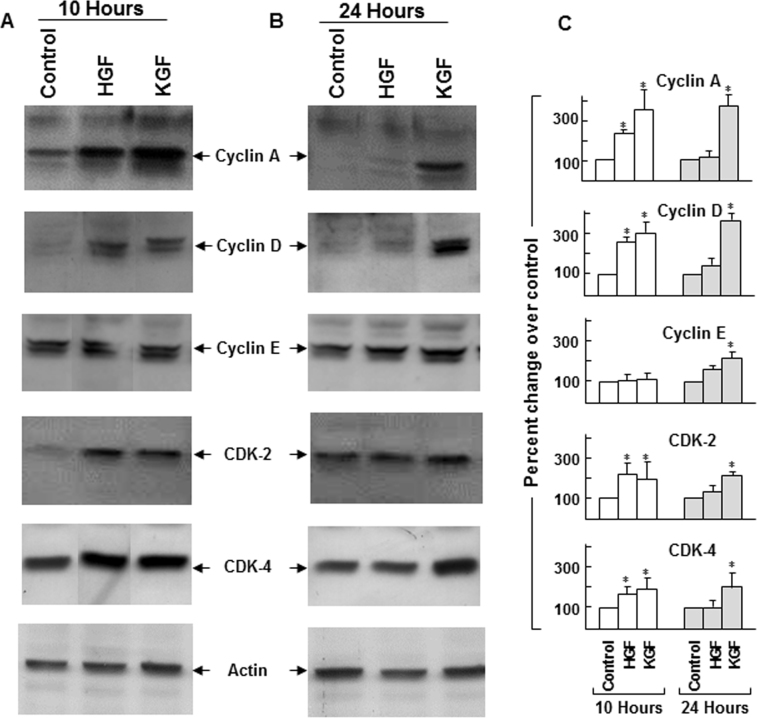
In the normal physiologic state, continuous regeneration and growth of the corneal epithelium depend on a balanced modulation of cell cycle progression and programmed cell death. Since HGF and KGF exhibited differential effects in maintaining the levels of Rb and p53 and cell cycle inhibitor p27kip, we investigated their effect on cell cycle promoting proteins, the cyclins and CDKs. As shown in Figure 7, the presence of HGF and KGF resulted in the upregulation of cyclins A, D, and E as well as CDK2 and CDK4 expression. Further, similar to the pattern observed with the control of p21cip and p27kip, KGF-dependent regulation of analyzed cyclins and CDKs was also sustained for a prolonged period (24 h) compared to HGF. These results show that in addition to preventing apoptosis, HGF- and KGF-mediated control of the expression of cyclins and CDKs may be important for cell cycle progression and new cell generation.
Figure 7.
Changes in the expression of cell cycle progressing cyclins and cyclin-dependent kinases in the presence of hepatocyte growth factor (HGF) and keratinocyte growth factor (KGF). Rabbit corneal primary epithelial (RCPE) cell growth cultures at 80%–90% confluence were starved overnight with Dulbecco’s Modified Eagle Medium with Ham’s F-12 (DMEM/F-12)/0.25% fetal calf serum (FCS) and then treated with 20 ng/ml HGF or KGF for 10 h (A) or 24 h (B). Levels of cyclins A, D, and E, and cyclin-dependent kinase 2 (CDK2) and CDK4 in cellular extracts were determined with western immunoblotting by using polyclonal antibodies specific for each protein. Data presented represent results obtained from two to three similar experiments. Quantification of cell cycle proteins is shown in bar diagrams (C). Data shown are mean±standard deviation (SD). *p<0.05, control versus HGF or KGF at 10 or 24 h.
Discussion
Regulation of cell survival and cell cycle is important for the normal physiology of the corneal epithelium and during the healing of epithelial injuries. Although the growth of new cells is accomplished through the control of the cell cycle, their survival is also important for restoring the multilayer organization of the epithelium. In diseases such as diabetes and dry eye condition, the corneal epithelial wound healing process could be delayed as a result of the decreased survival of the cells [40,41]. Cell loss due to apoptosis is also an issue with the corneal epithelium in certain chemical injuries, UVB radiation, and infections [42-45]. Factors that enhance the survival property provide better protection to corneal epithelial cells as these factors confer resistance to apoptosis. In a previous study, we described HGF’s ability to rescue corneal epithelial cells from apoptosis [32]. Here, we have demonstrated that KGF also functions as a cell survival factor. Further, we identified significant differences in their potential to serve as cell survival factors and regulators of cell cycle progression. Controlled expression of the cyclins and CDKs and interactions between specific cyclins and CDKs is necessary for cell cycle progression. The proteins p21cip and p27kip are members of the Cip/Kip family that function as cell cycle inhibitors [46,47]. They suppress the interactions between cyclins and CDKs, and thus cause cell cycle arrest. Progression or arrest of the cell cycle is dictated by the relative levels of various inhibitors, cyclins and CDKs. An increase in the level of p21cip or p27kip results in cell cycle arrest, and mitogens facilitate cell cycle progression by suppressing their expression [29-31]. Although cell cycle progression is required for proliferation, cell cycle arrest leads to the G0 phase where cells undergo differentiation if needed or remain dormant until they receive mitogenic stimuli or are otherwise eliminated by apoptosis. We found an increase in the levels of p53 and p27kip in the environment that caused apoptosis. This rise in p27kip indicates the onset of cell cycle arrest [48] that could make the corneal epithelium more susceptible to apoptosis. Under such conditions, we have also observed an increase in p53 upregulation and DNA degradation. The mitogens HGF or KGF are expected to promote cell division by relieving the hold of inhibitors on cell cycle progression. Under the normal growth environment, we found a significant decrease in the levels of p27kip in corneal epithelial cells in the presence of HGF and KGF. Their ability to influence the regulation of p53 and p27kip level is also evident in the apoptosis environment. The cell survival property identified with HGF or KGF appears related to their capacity to decrease the expression of these key molecules. The greater protection provided by KGF, but not HGF, over a longer period may also be attributed to its relative ability to downregulate p53 and p27kip.
HGF and KGF receptor activation leads to the stimulation of PI-3K/Akt and Erk kinase signaling. These signaling mechanisms have been shown to be involved in many physiologic processes such as cell migration, proliferation, and apoptosis. In this study, we established that the PI-3K/Akt signaling cascade controls the expression of p27kip. Inhibition of HGF- and KGF-mediated PI-3K activation abrogated the effect of the growth factors on p27kip expression. These results support our view that suppression of p27kip levels may be necessary for cell survival and one of the effects of PI-3K signaling in cell survival is to control the levels of p27kip. PI-3K signaling leads to the activation of Akt (pAkt). Phosphorylation of p27kip by pAkt earmarks it for nuclear export and degradation upon ubiquitination [49]. Suppression of apoptosis is associated with stimulation of cell cycle progression caused by a decrease in the expression of the cell cycle inhibitor.
To compensate the cell loss or death in adverse conditions such as epithelial injuries or in disease states, an increase in cell proliferation is necessary. An upturn in cell cycle progression would lead to increased cell division and generation of new cells. We found that the expression of cyclins A, D, and E, and CDK2 and CDK4 is regulated by HGF and KGF in growth cultures (Figure 6). However, KGF influence on increasing their levels was sustained for a longer duration compared to HGF, suggesting that the mitogenic effects of KGF could last longer than those of HGF. Further, we observed a moderate increase in the G0-G1 /S cell cycle phase progression gatekeeper protein Rb in the presence of KGF. Rb phosphorylation on several sites by cyclin-CDK2/4 following mitogenic stimulation causes the dissociation of the Rb-E2F complex [37]. The release of the E2F transcription factor leads to cell cycle progression. KGF may elicit a stronger effect in Rb phosphorylation due to the sustained expression of the G0–G1/S phase promoting cyclins (cyclin D and cyclin E) as well as CDKs (CDK4 and CDK2). Further studies are needed to evaluate such possibilities. A decrease in the degradation of Rb in the presence of KGF may also contribute to its growth-promoting ability for a longer period compared to HGF. The long-lasting effects of KGF on cell cycle progression may assume significance and be relevant in stressful cellular environments or in disease states. This view is further supported by the observation of a considerably higher number of normal cells in the presence of KGF than in HGF at 24 h after apoptosis was induced (Table 1). In addition, in contrast to p27kip, the expression of another cell cycle inhibitor p21cip increased significantly in the presence of HGF and KGF. Further, although the p27kip levels increased during apoptosis, there was a concomitant decrease in p21cip (Figure 3) suggesting that this protein may not work in concert with p27kip to arrest the cell cycle and may not contribute to apoptosis. Under our experimental conditions, p21cip in corneal epithelial cells may aid in G1 cell cycle phase progression. Several reports showed an increase in p21cip expression in the presence of growth factors. The role of p21cip in other cellular processes beyond cell cycle arrest has been investigated in many mammalian systems. Involvement of p21cip in proliferation, cell survival, cell shape, and hypertrophy that occur in response to injury or stress has been reported in several cell types. In myeloid cells, p21cip positively affected proliferation and promoted colony growth [50]. Dupont et al. showed that p21cip functions as a positive effector of insulin-like growth factor 1-induced cell proliferation in human breast cancer cells [51]. Inhibition of p53-induced apoptosis by p21cip has also been reported in vascular smooth muscle cells [52]. It also protected human colorectal cancer cells from apoptosis caused by prostaglandin A2 [53]. Further studies are needed to ascertain the consequences of p21cip upregulation with HGF and KGF in the corneal epithelium.
Although HGF and KGF are ligands for receptor tyrosine kinases and activate similar cellular signal pathways, in this study we found that these growth factors exert differential effects on corneal epithelial cell survival, cell cycle protein expression, and cell growth. The quantitative outcome of cellular processes accomplished by the action of these ligands likely depends on the intensity of signal strength generated by activating the intracellular signaling cascades triggered by them. In that regard, KGF, when compared to HGF evinced a stronger effect in preventing PARP degradation likely due to suppression of caspase activation and downregulation of p53. It is also possible that cross-talk that occurs between multiple signal pathways upon their activation, diverts more of the HGF receptor-generated signals to other cellular mechanisms such as migration, and dilutes the effects of HGF on cell survival and cell cycle control compared to KGF. HGF is also known as a scatter factor due to its role in cell migration in many tissues. Our earlier studies showed that p38 and Erk kinases activated by HGF participate in corneal epithelial cell migration [54].
In conclusion, this study clearly shows similarities as well as differences between HGF- and KGF-mediated effects on cell survival and cell cycle progression in corneal epithelial cells. Regarding rescuing cells from apoptotic insult and increasing the levels of cell cycle controlling proteins for promoting proliferation, HGF is as effective as KGF for a shorter length of time but not for a prolonged period. The long-lasting effect of KGF in cell survival and growth may be attributed to KGF’s influence on decreasing the levels of cell cycle inhibitor p27kip and transcription factor p53 and preventing the degradation of PARP and Rb as well as sustaining the expression of cell cycle promoting cyclins and CDKs. In view of the growing interest in cell-type specific treatment methods, this knowledge is important and relevant for the development of new strategies for managing ocular surface injuries. Therapeutic approaches targeting the control of corneal epithelial cell survival and cell cycle machinery through KGF may be worthy of consideration.
Acknowledgments
This research was supported by the funds from the Ph.D graduate program of Department of Pharmaceutical Sciences (SP, Ph.D student), College of Pharmacy, South Dakota State University. We also acknowledge the financial support from South Dakota State 2010-Translational Cancer Research Center (GC) and the National Institute of Health EY06635 (HEPB).
References
- 1.Yu FS, Yin J, Xu K, Huang J. Growth factors and corneal epithelial wound healing. Brain Res Bull. 2010;81:229–35. doi: 10.1016/j.brainresbull.2009.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu L, Reinach PS, Kao WY. Corneal Epithelial Wound Healing. Exp Biol Med (Maywood) 2001;226:653–64. doi: 10.1177/153537020222600711. [DOI] [PubMed] [Google Scholar]
- 3.Zieske JD. Extracellular matrix and wound healing. Curr Opin Ophthalmol. 2001;12:237–41. doi: 10.1097/00055735-200108000-00001. [DOI] [PubMed] [Google Scholar]
- 4.Nishida T, Tanaka T. Extracellular matrix and growth factors in corneal wound healing. Curr Opin Ophthalmol. 1996;7:2–11. doi: 10.1097/00055735-199608000-00002. [DOI] [PubMed] [Google Scholar]
- 5.Wilson SE, Mohan RR, Mohan RR, Ambrósio R, Jr, Hong J, Lee J. The corneal wound healing response: cytokine-mediated interaction of the epithelium, stroma, and inflammatory cells. Prog Retin Eye Res. 2001;20:625–37. doi: 10.1016/s1350-9462(01)00008-8. [DOI] [PubMed] [Google Scholar]
- 6.Chi C, Trinkaus-Randall V. New insights in wound response and repair of epithelium. J Cell Physiol. 2013;228:925–9. doi: 10.1002/jcp.24268. [DOI] [PubMed] [Google Scholar]
- 7.Imanishi J, Kamiyama K, Iguchi I, Kita M, Sotozono C, Kinoshita S. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Prog Retin Eye Res. 2000;19:113–29. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 8.Wilson SE, Chen L, Mohan RR, Liang Q, Lin J. Expression of HGF, KGF, EGF, and receptor messenger in corneal epithelial cells after wounding. Exp Eye Res. 1999;68:377–97. doi: 10.1006/exer.1998.0603. [DOI] [PubMed] [Google Scholar]
- 9.Grierson I, Heathcote L, Hiscott P, Hogg P, Briggs M, Hagan S. Hepatocyte growth factor/scatter factor in the eye. Prog Retin Eye Res. 2000;19:779–802. doi: 10.1016/s1350-9462(00)00015-x. [DOI] [PubMed] [Google Scholar]
- 10.Klenkler B, Sheardown H, Jones L. Growth factors in the tear film: role in tissue maintenance, wound healing, and ocular pathology. Ocul Surf. 2007;5:228–39. doi: 10.1016/s1542-0124(12)70613-4. [DOI] [PubMed] [Google Scholar]
- 11.Tervo T, Vesaluoma M, Bennett GL, Schwall R, Helena M, Liang Q, Wilson SE. Tear hepatocyte growth factor (HGF) availability increases markedly after excimer laser surface ablation. Exp Eye Res. 1997;64:501–4. doi: 10.1006/exer.1996.0226. [DOI] [PubMed] [Google Scholar]
- 12.Zoukhri D. Mechanisms involved in injury and repair of the murine lacrimal gland: role of programmed cell death and mesenchymal stem cells. Ocul Surf. 2010;8:60–9. doi: 10.1016/s1542-0124(12)70070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Baldwin HC, Marshall J. Growth factors in corneal wound healing following refractive surgery: A review. Acta Ophthalmol Scand. 2002;80:238–47. doi: 10.1034/j.1600-0420.2002.800303.x. [DOI] [PubMed] [Google Scholar]
- 14.McBain VA, Forrester JV, McCaig CD. HGF, MAPK, and a small physiological electric field interact during corneal epithelial cell migration. Invest Ophthalmol Vis Sci. 2003;44:540–7. doi: 10.1167/iovs.02-0570. [DOI] [PubMed] [Google Scholar]
- 15.Yang H, Sun X, Wang Z, Ning G, Zhang F, Kong J, Lu L, Reinach PS. EGF stimulated growth by enhancing capacitative calcium entry in corneal epithelial cells. J Membr Biol. 2003;194:47–58. doi: 10.1007/s00232-003-2025-9. [DOI] [PubMed] [Google Scholar]
- 16.Lee JS, Liu J. Hong J1, Wilson SE. Differential expression analysis by gene array of cell cycle modulators in human corneal epithelial cells stimulated with epidermal growth factor (EGF), hepatocyte growth factor (HGF), or keratinocyte growth factor (KGF). Curr Eye Res. 2001;23:69–76. doi: 10.1076/ceyr.23.1.69.5421. [DOI] [PubMed] [Google Scholar]
- 17.Chandrasekher G, Kakazu A, Bazan HE. HGF- and KGF-induced activation of PI-3K/p70 S6 kinase pathway in corneal epithelial cells: its relevance in wound healing. Exp Eye Res. 2001;73:191–202. doi: 10.1006/exer.2001.1026. [DOI] [PubMed] [Google Scholar]
- 18.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–97. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]
- 19.Kakazu A, Sharma G, Bazan HE. Association of protein tyrosine phosphatases (PTPs)-1B with c-Met receptor and modulation of corneal epithelial wound healing. Invest Ophthalmol Vis Sci. 2008;49:2927–35. doi: 10.1167/iovs.07-0709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharma GD, Kakazu A, Bazan HE. Protein kinase C alpha and epsilon differentially modulate hepatocyte growth factor-induced epithelial proliferation and migration. Exp Eye Res. 2007;85:289–97. doi: 10.1016/j.exer.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liang Q, Mohan RR, Chen L, Wilson SE. Signaling by HGF and KGF in corneal epithelial cells: Ras/MAP kinase and Jak-STAT pathways. Invest Ophthalmol Vis Sci. 1998;39:1329–38. [PubMed] [Google Scholar]
- 22.Cheng CC, Wang DY, Kao MH, Chen JK. The growth-promoting effect of KGF on limbal epithelial cells is mediated by upregulation of DeltaNp63alpha through the p38 pathway. J Cell Sci. 2009;122:4473–80. doi: 10.1242/jcs.054791. [DOI] [PubMed] [Google Scholar]
- 23.Ottino P, Taheri F, Bazan HE. Growth factor-induced proliferation in corneal epithelial cells is mediated by 12(S)-HETE. Exp Eye Res. 2003;76:613–22. doi: 10.1016/s0014-4835(03)00003-4. [DOI] [PubMed] [Google Scholar]
- 24.Luo Y, Cheng Z, Dixon CJ, Hall JF, Taylor E, Boarder MR. Endosomal signaling of epidermal growth factor receptors contributes to EGF-stimulated cell cycle progression in primary hepatocytes. Eur J Pharmacol. 2011;654:173–80. doi: 10.1016/j.ejphar.2010.11.038. [DOI] [PubMed] [Google Scholar]
- 25.Devi TS, Singh LP, Hosoya K, Terasaki T. GSK-3β/CREB axis mediates IGF-1-induced ECM/adhesion molecule expression, cell cycle progression and monolayer permeability in retinal capillary endothelial cells: Implications for diabetic retinopathy. Biochim Biophys Acta. 2011;xxx:1080–88. doi: 10.1016/j.bbadis.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 26.Han J, Tsukada Y, Hara E, Kitamura N, Tanaka T. Hepatocyte growth factor induces redistribution of p21(CIP1) and p27(KIP1) through ERK-dependent p16(INK4a) up-regulation, leading to cell cycle arrest at G1 in HepG2 hepatoma cells. J Biol Chem. 2005;280:31548–56. doi: 10.1074/jbc.M503431200. [DOI] [PubMed] [Google Scholar]
- 27.Buckley S, Driscoll B, Anderson KD, Warburton D. Cell cycle in alveolar epithelial type II cells: integration of Matrigel and KGF. Am J Physiol. 1997;273:L572–80. doi: 10.1152/ajplung.1997.273.3.L572. [DOI] [PubMed] [Google Scholar]
- 28.Paranjpe S, Bowen WC, Bell AW, Nejak-Bowen K, Luo JH, Michalopoulos GK. Cell cycle effects resulting from inhibition of hepatocyte growth factor and its receptor c-Met in regenerating rat livers by RNA interference. Hepatology. 2007;45:1471–7. doi: 10.1002/hep.21570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sherr CJ. The Pezcoller lecture: cancer cell cycle revisited. Cancer Res. 2000;60:3689–95. [PubMed] [Google Scholar]
- 30.Roberts JM. Evolving ideas about cyclins. Cell. 1999;98:129–32. doi: 10.1016/s0092-8674(00)81007-7. [DOI] [PubMed] [Google Scholar]
- 31.Ekholm SV, Reed SI. Regulation of G(1) cyclin-dependent kinases in the mammalian cell cycle. Curr Opin Cell Biol. 2000;12:676–84. doi: 10.1016/s0955-0674(00)00151-4. [DOI] [PubMed] [Google Scholar]
- 32.Kakazu A, Chandrasekher G, Bazan HE. HGF protects corneal epithelial cells from apoptosis by the PI-3K/Akt-1/Bad- but not the ERK1/2-mediated signaling pathway. Invest Ophthalmol Vis Sci. 2004;45:3485–92. doi: 10.1167/iovs.04-0372. [DOI] [PubMed] [Google Scholar]
- 33.Chandrasekher G, Ma X, Lallier TE, Bazan HE. Delay of corneal epithelial wound healing and induction of keratocyte apoptosis by platelet-activating factor. Invest Ophthalmol Vis Sci. 2002;43:1422–8. [PubMed] [Google Scholar]
- 34.Chandrasekher G, Sailaja D. Phosphatidylinositol 3-kinase (PI-3K)/Akt but not PI-3K/p70 S6 kinase signaling mediates IGF-1-promoted lens epithelial cell survival. Invest Ophthalmol Vis Sci. 2004;45:3577–88. doi: 10.1167/iovs.04-0279. [DOI] [PubMed] [Google Scholar]
- 35.Finkel E. The mitochondrion: is it central to apoptosis? Science. 2001;292:624–6. doi: 10.1126/science.292.5517.624. [DOI] [PubMed] [Google Scholar]
- 36.Soldani C, Scovassi AI. Poly (ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–8. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]
- 37.Sherr CJ, McCormick FM. The RB and p53 pathways in cancer. Cancer Cell. 2002;2:103–12. doi: 10.1016/s1535-6108(02)00102-2. [DOI] [PubMed] [Google Scholar]
- 38.Tan X, Wang JY. The caspase-RB connection in cell death. Trends Cell Biol. 1998;8:116–20. doi: 10.1016/s0962-8924(97)01208-7. [DOI] [PubMed] [Google Scholar]
- 39.Fattman CL, Delach SM, Dou QP, Johnson DE. Sequential two-step cleavage of the retinoblastoma protein by caspase-3/-7 during etoposide-induced apoptosis. Oncogene. 2001;20:2918–26. doi: 10.1038/sj.onc.1204414. [DOI] [PubMed] [Google Scholar]
- 40.Kojima T, Higuchi A, Goto E, Matsumoto Y, Dogru M, Tsubota K. Autologous serum eye drops for the treatment of dry eye diseases. Cornea. 2008;27(Suppl 1):S25–30. doi: 10.1097/ICO.0b013e31817f3a0e. [DOI] [PubMed] [Google Scholar]
- 41.Xu K, Yu FS. Impaired epithelial wound healing and EGFR signaling pathways in the corneas of diabetic rats. Invest Ophthalmol Vis Sci. 2011;52:3301–8. doi: 10.1167/iovs.10-5670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang L, Payton R, Dai W, Lu L. Hyperosmotic stress-induced ATF-2 activation through Polo-like kinase 3 in human corneal epithelial cells. J Biol Chem. 2011;286:1951–8. doi: 10.1074/jbc.M110.166009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen Z, Tong L, Li Z, Yoon KC, Oi H, Farley W, Li DQ, Pflugfelder SC. Hyperosmolarity induced cornification of human corneal epithelial cells is regulated by JNK MAPK. Invest Ophthalmol Vis Sci. 2008;49:539–49. doi: 10.1167/iovs.07-0569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pauloin T, Dutot M, Joly F, Warnet JM, Rat P. High molecular weight hyaluronan decreases UVB-induced apoptosis and inflammation in human epithelial corneal cells. Mol Vis. 2009;15:577–83. [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Li H, Wang J, Dong Z, Mian S, Yu FS. Role of EGFR transactivation in preventing apoptosis in Pseudomonas aeruginosa-infected human corneal epithelial cells. Invest Ophthalmol Vis Sci. 2004;45:2569–76. doi: 10.1167/iovs.03-1323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence, and age-related diseases. Proc Natl Acad Sci USA. 2000;97:4291–6. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Samuelsson MK, Pazirandeh A, Okret S. A pro-apoptotic effect of the CDK inhibitor p57(Kip2) on staurosporine-induced apoptosis in HeLa cells. Biochem Biophys Res Commun. 2002;296:702–9. doi: 10.1016/s0006-291x(02)00912-9. [DOI] [PubMed] [Google Scholar]
- 48.Yoshida K, Nakayama K, Nagahama H, Harada T, Harada C, Imaki J, Matsuda A, Yamamoto K, Ito M, Ohno S, Nakayama K. Involvement of p27(KIP1) degradation by Skp2 in the regulation of proliferation in response to wounding of corneal epithelium. Invest Ophthalmol Vis Sci. 2002;43:364–70. [PubMed] [Google Scholar]
- 49.Shin I, Yakes FM, Rojo F, Shin NY, Bakin AV, Baselga J, Arteaga CL. PKB/Akt mediates cell-cycle progression by phosphorylation of p27(Kip1) at threonine 157 and modulation of its cellular localization. Nat Med. 2002;8:1145–52. doi: 10.1038/nm759. [DOI] [PubMed] [Google Scholar]
- 50.Braun SE, Mantel C, Rosenthal M, Cooper S, Liu L, Robertson KA, Hromas R, Broxmeyer HE. A positive effect of p21cip1/waf1 in the colony formation from murine myeloid progenitor cells as assessed by retroviral-mediated gene transfer. Blood Cells Mol Dis. 1998;24:138–48. doi: 10.1006/bcmd.1998.0181. [DOI] [PubMed] [Google Scholar]
- 51.Dupont J, Karas M, LeRoith D. The cyclin-dependent kinase inhibitor p21CIP/WAF is a positive regulator of insulin-like growth factor I-induced cell proliferation in MCF-7 human breast cancer cells. J Biol Chem. 2003;278:37256–64. doi: 10.1074/jbc.M302355200. [DOI] [PubMed] [Google Scholar]
- 52.Gorospe M, Cirielli C, Wang X, Seth P, Capogrossi MC, Holbrook NJ. p21(Waf1/Cip1) protects against p53-mediated apoptosis of human melanoma cells. Oncogene. 1997;14:929–35. doi: 10.1038/sj.onc.1200897. [DOI] [PubMed] [Google Scholar]
- 53.Gorospe M, Wang X, Guyton KZ, Holbrook NJ. Protective role of p21(Waf1/Cip1) against prostaglandin A2-mediated apoptosis of human colorectal carcinoma cells. Mol Cell Biol. 1996;16:6654–60. doi: 10.1128/mcb.16.12.6654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sharma GD, He J, Bazan HE. p38 and ERK1/2 coordinate cellular migration and proliferation in epithelial wound healing: evidence of cross-talk activation between MAP kinase cascades. J Biol Chem. 2003;278:21989–97. doi: 10.1074/jbc.M302650200. [DOI] [PubMed] [Google Scholar]