Abstract
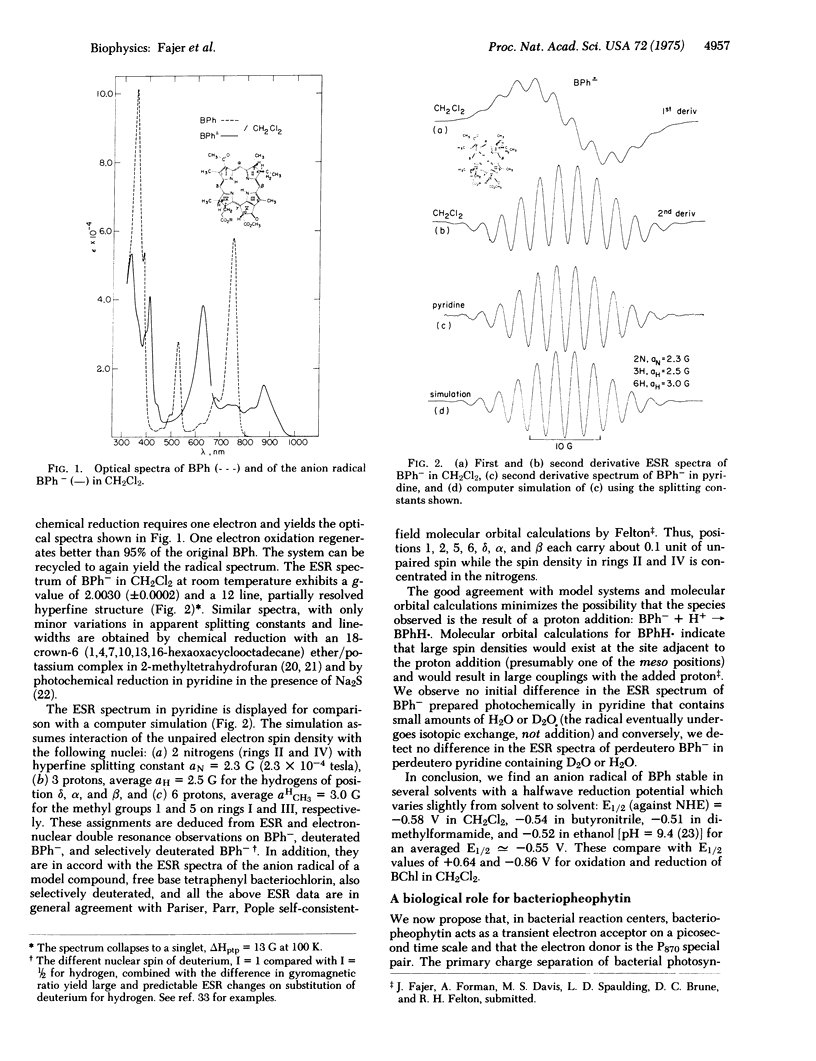
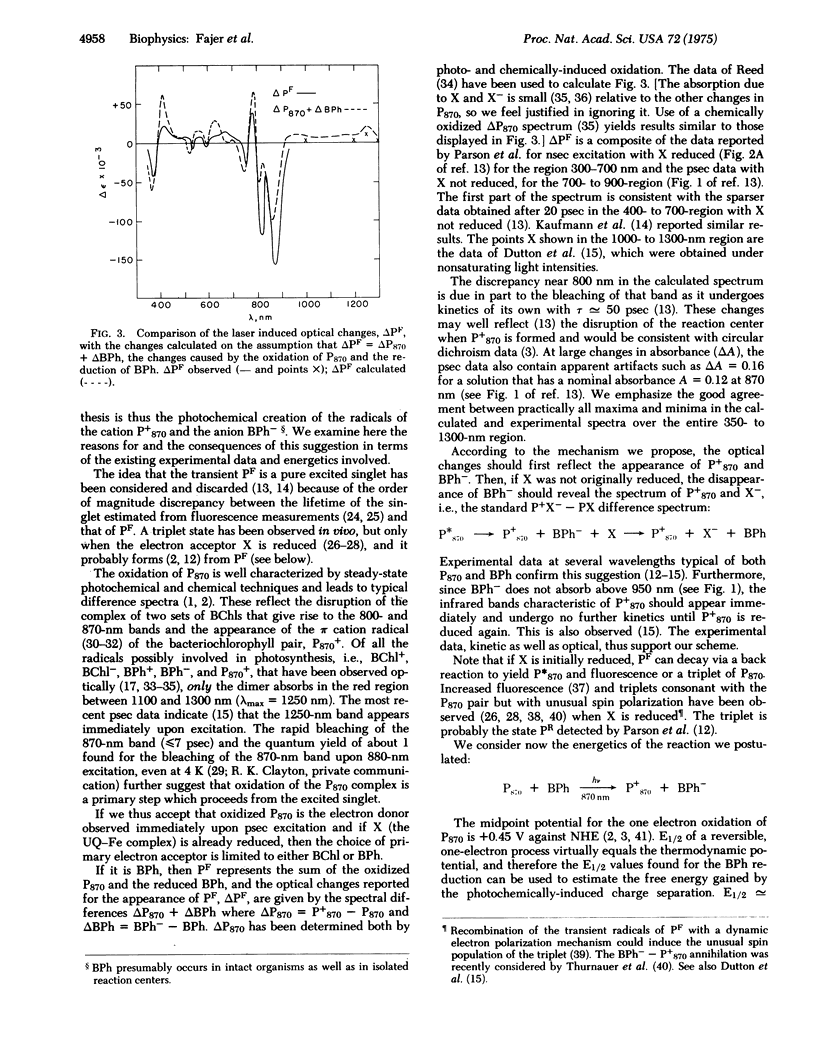
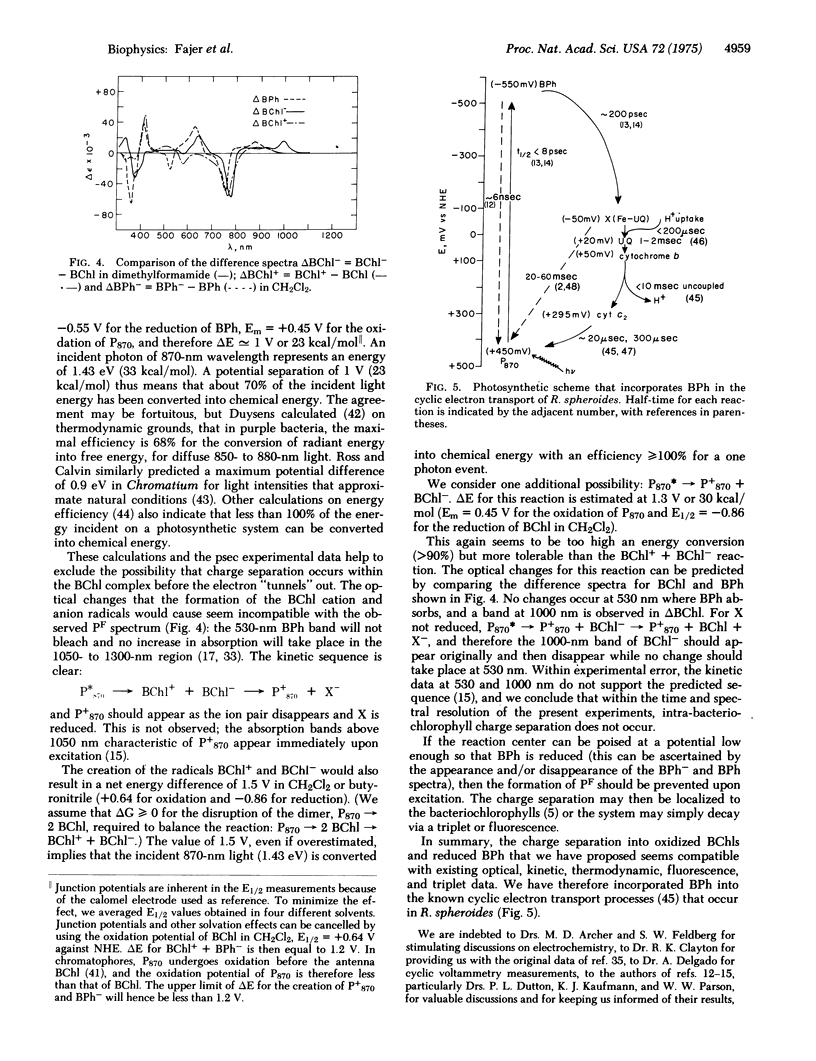
Bacteriopheophytin, the magnesium-free base of bacteriochlorophyll, undergoes reversible one-electron reduction in organic solvents to yield an anionic free radical with characteristic optical and electron spin resonance spectra. The reduction potential of bacteriopheophytin, E1/2 approximately --0.55 V against a normal hydrogen electrode, compared to E1/2 approximately --0.85 V for bacteriochlorophyll, renders it a likely electron acceptor in the primary charge separation of photosynthesis. Comparison of these data with picosecond optical changes recently observed upon pulsed laser excitation of bacterial reaction centers leads us to propose that bacteriopheophytin is indeed a transient electron acceptor and that the primary charge separation of bacterial photosynthesis occurs between the bacteriochlorophyll complex P870 and bacteriopheophytin to yield the radicals of the oxidized chlorophyll dimer cation and reduced pheophytin anion.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Clayton R. K., Fleming H., Szuts E. Z. Photochemical electron transport in photosynthetic reaction centers from Rhodopseudomonas spheroides. II. Interaction with external electron donors and acceptors and a reevaluation of some spectroscopic data. Biophys J. 1972 Jan;12(1):46–63. doi: 10.1016/S0006-3495(72)86070-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K. Primary processes in bacterial photosynthesis. Annu Rev Biophys Bioeng. 1973;2:131–156. doi: 10.1146/annurev.bb.02.060173.001023. [DOI] [PubMed] [Google Scholar]
- Clayton R. K., Straley S. C. Photochemical electron transport in photosynthetic reaction centers. IV. Observations related to the reduced photoproducts. Biophys J. 1972 Oct;12(10):1221–1234. doi: 10.1016/S0006-3495(72)86158-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton R. K., Yau H. F. Photochemical electron transport in photosynthetic reaction centers from Rhodopseudomonas spheroides. I. Kinetics of the oxidation and reduction of P-870 as affected by external factors. Biophys J. 1972 Jul;12(7):867–881. doi: 10.1016/S0006-3495(72)86130-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cogdell R. J., Brune D. C., Clayton R. K. Effects of extraction and replacement of ubiquinone upon the photochemical activity of reaction centers and chromatophores from Rhodopseudomonas spheriodes. FEBS Lett. 1974 Sep 1;45(1):344–347. doi: 10.1016/0014-5793(74)80877-x. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Leigh J. S., Jr, Reed D. W. Primary events in the photosynthetic reaction centre from Rhodopseudomonas spheroides strain R26: triplet and oxidized states of bacteriochlorophyll and the identification of the primary electron acceptor. Biochim Biophys Acta. 1973 Apr 5;292(3):654–664. doi: 10.1016/0005-2728(73)90013-3. [DOI] [PubMed] [Google Scholar]
- Dutton P. L., Wilson D. F. Redox potentiometry in mitochondrial and photosynthetic bioenergetics. Biochim Biophys Acta. 1974 Oct 31;346(2):165–212. doi: 10.1016/0304-4173(74)90008-1. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Dolphin D., Felton R. H. Anion radical of bacteriochlorophyll. J Am Chem Soc. 1973 Apr 18;95(8):2739–2741. doi: 10.1021/ja00789a085. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Dolphin D., Felton R. H. pi-Cation radicals and dications of metalloporphyrins. J Am Chem Soc. 1970 Jun 3;92(11):3451–3459. doi: 10.1021/ja00714a038. [DOI] [PubMed] [Google Scholar]
- Fajer J., Borg D. C., Forman A., Felton R. H., Dolphin D., Vegh L. The cation radicals of free base and zinc bacteriochlorin, bacteriochlorophyll, and bacteriopheophytin. Proc Natl Acad Sci U S A. 1974 Mar;71(3):994–998. doi: 10.1073/pnas.71.3.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feher G., Hoff A. J., Isaacson R. A., Ackerson L. C. ENDOR experiments on chlorophyll and bacteriochlorophyll in vitro and in the photosynthetic unit. Ann N Y Acad Sci. 1975 Apr 15;244:239–259. doi: 10.1111/j.1749-6632.1975.tb41534.x. [DOI] [PubMed] [Google Scholar]
- Feher G., Isaacson R. A., McElroy J. D., Ackerson L. C., Okamura M. Y. On the question of the primary acceptor in bacterial photosynthesis:manganese substituting for iron in reaction centers of Rhodopseudomonas spheroides R-26. Biochim Biophys Acta. 1974 Oct 18;368(1):135–139. doi: 10.1016/0005-2728(74)90104-2. [DOI] [PubMed] [Google Scholar]
- Frenkel A. W. Multiplicity of electron transport reactions in bacterial photosynthesis. Biol Rev Camb Philos Soc. 1970 Nov;45(4):569–616. doi: 10.1111/j.1469-185x.1970.tb01177.x. [DOI] [PubMed] [Google Scholar]
- KUNTZ I. D., Jr, LOACH P. A., CALVIN M. ABSORPTION CHANGES IN BACTERIAL CHROMATOPHORES. Biophys J. 1964 May;4:227–249. doi: 10.1016/s0006-3495(64)86779-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz J. J., Strain H. H., Harkness A. L., Studier M. H., Svec W. A., Janson T. R., Cope B. T. Esterifying alcohols in the chlorophylls of purple photosynthetic bacteria. A new chlorophyll, bacteriochlorophyll (gg), all-trans-geranylgeranyl bacteriochlorophyllide a. J Am Chem Soc. 1972 Nov 1;94(22):7938–7939. doi: 10.1021/ja00777a054. [DOI] [PubMed] [Google Scholar]
- Kaufmann K. J., Dutton P. L., Netzel T. L., Leigh J. S., Rentzepis P. M. Picosecond kinetics of events leading to reaction center bacteriochlorophyll oxidation. Science. 1975 Jun 27;188(4195):1301–1304. doi: 10.1126/science.188.4195.1301. [DOI] [PubMed] [Google Scholar]
- Ke B., Chaney T. H., Reed D. W. The electrostatic interaction between the reaction-center bacteriochlorophyll derived from Rhodopseudomonas spheroides and mammalian cytochrome c and its effect on light-activated electron transport. Biochim Biophys Acta. 1970 Sep 1;216(2):373–383. doi: 10.1016/0005-2728(70)90229-x. [DOI] [PubMed] [Google Scholar]
- Knox R. S. Thermodynamics and the primary processes of photosynthesis. Biophys J. 1969 Nov;9(11):1351–1362. doi: 10.1016/S0006-3495(69)86457-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leigh J. S., Jr, Dutton P. L. Reaction center bacteriochlorophyll triplet states: redox potential dependence and kinetics. Biochim Biophys Acta. 1974 Jul 25;357(1):67–77. doi: 10.1016/0005-2728(74)90113-3. [DOI] [PubMed] [Google Scholar]
- Loach P. A., Kung M., Hales B. J. Characterization of the phototrap in photosynthetic bacteria. Ann N Y Acad Sci. 1975 Apr 15;244:297–319. doi: 10.1111/j.1749-6632.1975.tb41537.x. [DOI] [PubMed] [Google Scholar]
- Norris J. R., Scheer H., Katz J. J. Models for antenna and reaction center chlorophylls. Ann N Y Acad Sci. 1975 Apr 15;244:260–280. doi: 10.1111/j.1749-6632.1975.tb41535.x. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Clayton R. K., Cogdell R. J. Excited states of photosynthetic reaction centers at low recox potentials. Biochim Biophys Acta. 1975 May 15;387(2):265–278. doi: 10.1016/0005-2728(75)90109-7. [DOI] [PubMed] [Google Scholar]
- Parson W. W., Cogdell R. J. The primary photochemical reaction to bacterial photosynthesis. Biochim Biophys Acta. 1975 Mar 31;416(1):105–149. doi: 10.1016/0304-4173(75)90014-2. [DOI] [PubMed] [Google Scholar]
- Prince R. C., Dutton P. L. A kinetic completion of the cyclic photosynthetic electron pathway of Rhodopseudomonas sphaeroides: cytochrome b-cytochrome c2 oxidation-reduction. Biochim Biophys Acta. 1975 Jun 17;387(3):609–613. doi: 10.1016/0005-2728(75)90101-2. [DOI] [PubMed] [Google Scholar]
- Reed D. W. Isolation and composition of a photosynthetic reaction center complex from Rhodopseudomonas spheroides. J Biol Chem. 1969 Sep 25;244(18):4936–4941. [PubMed] [Google Scholar]
- Rockley M. G., Windsor M. W., Cogdell R. J., Parson W. W. Picosecond detection of an intermediate in the photochemical reaction of bacterial photosynthesis. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2251–2255. doi: 10.1073/pnas.72.6.2251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross R. T., Calvin M. Thermodynamics of light emission and free-energy storage in photosynthesis. Biophys J. 1967 Sep;7(5):595–614. doi: 10.1016/S0006-3495(67)86609-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slooten L. Electron acceptors in reaction center preparations from photosynthetic bacteria. Biochim Biophys Acta. 1972 Aug 17;275(2):208–218. doi: 10.1016/0005-2728(72)90042-4. [DOI] [PubMed] [Google Scholar]
- Slooten L. Reaction center preparations of Rhodopseudomonas spheroides: energy transfer and structure. Biochim Biophys Acta. 1972 Feb 28;256(2):452–466. doi: 10.1016/0005-2728(72)90074-6. [DOI] [PubMed] [Google Scholar]
- Thurnauer M. C., Katz J. J., Norris J. R. The triplet state in bacterial photosynthesis: Possible mechanisms of the primary photo-act. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3270–3274. doi: 10.1073/pnas.72.9.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uphaus R. A., Norris J. R., Katz J. J. Triplet states in photosynthesis. Biochem Biophys Res Commun. 1974 Dec 11;61(3):1057–1063. doi: 10.1016/0006-291x(74)90262-9. [DOI] [PubMed] [Google Scholar]
- Wraight C. A., Leigh J. S., Dutton P. L., Clayton R. K. The triplet state of reaction center bacteriochlorophyll: determination of a relative quantum yeild. Biochim Biophys Acta. 1974 Mar 26;333(3):401–408. doi: 10.1016/0005-2728(74)90123-6. [DOI] [PubMed] [Google Scholar]
- Zankel K. L., Reed D. W., Clayton R. K. Fluorescence and photochemical quenching in photosynthetic reaction centers. Proc Natl Acad Sci U S A. 1968 Dec;61(4):1243–1249. doi: 10.1073/pnas.61.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]


