Abstract
Obesity leads to a proinflammatory state with immune responses that include infiltration of adipose tissue with macrophages. These macrophages are believed to alter insulin sensitivity in adipocytes, but the mechanisms that underlie this effect have not been characterized. We have explored the interaction between macrophages and adipocytes in the context of both indirect and direct coculture. Macrophage-secreted factors blocked insulin action in adipocytes via downregulation of GLUT4 and IRS-1, leading to a decrease in Akt phosphorylation and impaired insulin-stimulated GLUT4 translocation to the plasma membrane. GLUT1 was upregulated with a concomitant increase in basal glucose uptake. These changes recapitulate those seen in adipose tissue from insulin-resistant humans and animal models. TNF-α-neutralizing antibodies partially reversed the insulin resistance produced by macrophage-conditioned media. Peritoneal macrophages and macrophage-enriched stromal vascular cells from adipose tissue also attenuated responsiveness to insulin in a manner correlating with inflammatory cytokine secretion. Adipose tissue macrophages from obese mice have an F4/80+CD11b+CD68+CD14− phenotype and form long cellular extensions in culture. Peritoneal macrophages take on similar characteristics in direct coculture with adipocytes and induce proinflammatory cytokines, suggesting that macrophage activation state is influenced by contact with adipocytes. Thus both indirect/secreted and direct/cell contact-mediated factors derived from macrophages influence insulin sensitivity in adipocytes.
Keywords: adipose tissue macrophages, insulin resistance, insulin signaling, obesity
The prevalence of obesity is increasing at an alarming rate along with its associated morbidities such as atherosclerosis and type 2 diabetes (20, 35, 38). The mechanisms that tie obesity to these various health morbidities are largely unknown. One promising hypothesis is that obesity generates a proinflammatory state and promotes inflammatory responses that contribute to the pathogenesis of these diseases (4, 30, 31, 33).
Obesity is associated with elevated levels of circulating proinflammatory cytokines such as plasminogen activator inhibitor-1 (PAI-1), C-reactive protein (CRP), TNF-α, and IL-6 (18). Many of these factors are produced by adipose tissue; circulating levels of TNF-α, IL-6, and monocyte chemoattractant protein-1 (MCP-1) correlate with adiposity and insulin resistance (5, 14, 23), whereas weight loss decreases their levels (14, 15). Moreover, pharmacological and genetic downregulation of inflammatory pathways in mice confer protection from high-fat diet (HFD)-induced insulin resistance (2, 9, 26, 56). Mice deficient in TNF-α are protected from diabetes induced by HFD (50), and blockade of TNF-α activity in obese mice improves insulin sensitivity (23).
The cell types involved in the inflammatory response in obesity are not fully delineated. Recent attention has focused on adipose tissue macrophages (ATMs) as a mediator of inflammatory responses in adipose tissue (53, 54). A significant increase in macrophage content in visceral adipose tissue has been observed in obese humans and mice (7, 8, 10). These macrophages are the predominant source of proinflammatory cytokines such as TNF-α and PAI-1 and may be recruited to adipose tissue via chemoattractants such as MCP-1 (25, 45, 53). Mice with a myeloid-specific knockout of IKKβ are protected from obesity-induced diabetes, demonstrating the importance of macrophages in generating the inflammatory signals that modulate insulin sensitivity (2). Additionally, knockout in mice of factors involved in monocyte/macrophage recruitment, such as MCP-1 and its receptor CCR2, resulted in decreased quantities of ATMs in adipose tissue with obesity and protection from insulin resistance (25, 52), suggesting that ATMs are required for the development of diabetes with obesity.
How macrophages induce insulin resistance in adipocytes has not been established. Secreted factors from macrophage-like cell lines induce an inflammatory response in adipocytes and influence insulin sensitivity (37, 46), but the specific factors involved, and mechanisms by which they exert these effects, remain unknown. We sought to model macrophage and adipocyte interactions in vitro to dissect the mechanisms by which adipocyte function is altered by macrophages. By examining adipocyte insulin signaling under the influence of macrophage-derived factors, we identify alterations in GLUT1, GLUT4, and IRS-1 expression levels and GLUT4 membrane translocation in adipocytes exposed to macrophages. Additionally, we provide evidence that both secreted and cell contact-mediated signals participate in macrophage-adipocyte interactions that contribute to the impairment of adipocyte function.
MATERIALS AND METHODS
Materials and reagents
For immunoblots, anti-phosphotyrosine (4G10) and IRS-1 antibodies were purchased from Upstate (Charlottesville, VA). β-Actin monoclonal antibody, mouse recombinant TNF-α, and lipopolysaccharide (LPS) from Escherichia coli were obtained from Sigma-Aldrich (St. Louis, MO). GLUT1 and GLUT4 antibodies were obtained from Alpha Diagnostics International (San Antonio, TX). Phospho-IRS-1, Akt, and phospho-Akt antibodies were purchased from Cell Signaling (Beverly, MA). Anti-TNF-α neutralizing antibody and ELISA kits were purchased from R&D Systems (Minneapolis, MN). Anti-F4/80 antibodies were from Abcam (Cambridge, MA). CD68 and CD11b antibodies were from Serotec (Raleigh, NC). CD14 antibodies were purchased from BD Biosciences (San Jose, CA).
Animals and animal care
Male C57Bl/6 mice were rendered insulin resistant by feeding an HFD at 8 wk of age consisting of 45% of calories from fat (Research Diets, New Brunswick, NJ) for 10–12 wk. Animals were housed in a specific pathogen-free facility with a 12:12-h light-dark cycle and given free access to food and water. All animal use was in compliance with the Institute of Laboratory Animal Research Guide for Care and Use of Laboratory Animals and approved by the University Committee on Use and Care of Animals at the University of Michigan.
Cell culture
3T3-L1 fibroblasts were propagated and differentiated into adipocytes as described (3). Adipocytes were used in experiments 8–10 days after differentiation. J774A.1 and RAW264 macrophage cell lines were obtained from the American Type Culture Collection (Manassas, VA) and were propagated in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 10% heat-inactivated, endotoxin-free fetal bovine serum (FBS). Peritoneal macrophages were obtained by peritoneal lavage 4 days after intraperitoneal injection with 3 ml of 4% thioglycollate in C57Bl/6 mice. Macrophages were selected by adhesion to plastic followed by 3–4 washes in PBS to remove nonadherent cells. Macrophages were >95% pure by flow cytometry analysis for F4/80 expression. Indirect coculture was performed by growing macrophages (100,000 cells/well) in 0.4 µm cell culture inserts from BD Biosciences (San Jose, CA) and placing them in wells containing differentiated 3T3-L1 adipocytes (8 days after induction of differentiation).
Conditioned media were collected from J774.1 or RAW264 macrophage cultures 16 h after stimulation with LPS at 0.1 ng/ml and sterile filtered (0.45 µm) prior to incubation with adipocytes. TNF-α-neutralizing antibody (R&D Systems, Minneapolis, MN) was added to conditioned media at a final concentration of 0.2 µg/ml.
Direct coculture of macrophages and adipocytes
Peritoneal macrophages were isolated as above. In some experiments the macrophages were labeled by incubation for 5 min with PKH26 (Sigma-Aldrich) at a concentration of 1 µM mixed in Diluent B per manufacturer’s instructions. Cells were washed three times in PBS prior to selection on plastic by adhesion. Labeling efficiency was 100% as assessed by immunofluorescence microscopy. Macrophages were then passaged by scraping with 10 mM EDTA in PBS and added to differentiated adipocytes in 12-well dishes at concentrations of 10,000 and 100,000 cells per well. Macrophages were plated onto empty 12-well dishes as controls. Cells were grown in coculture for 48–72 h prior to analysis.
2-Deoxyglucose uptake assay
Adipocytes were removed from indirect coculture and starved in low-glucose DMEM with 0.5% serum for 3 h prior to insulin stimulation. 2-Deoxyglucose (2-DG) uptake assays were performed as described (24).
GLUT4 translocation assay
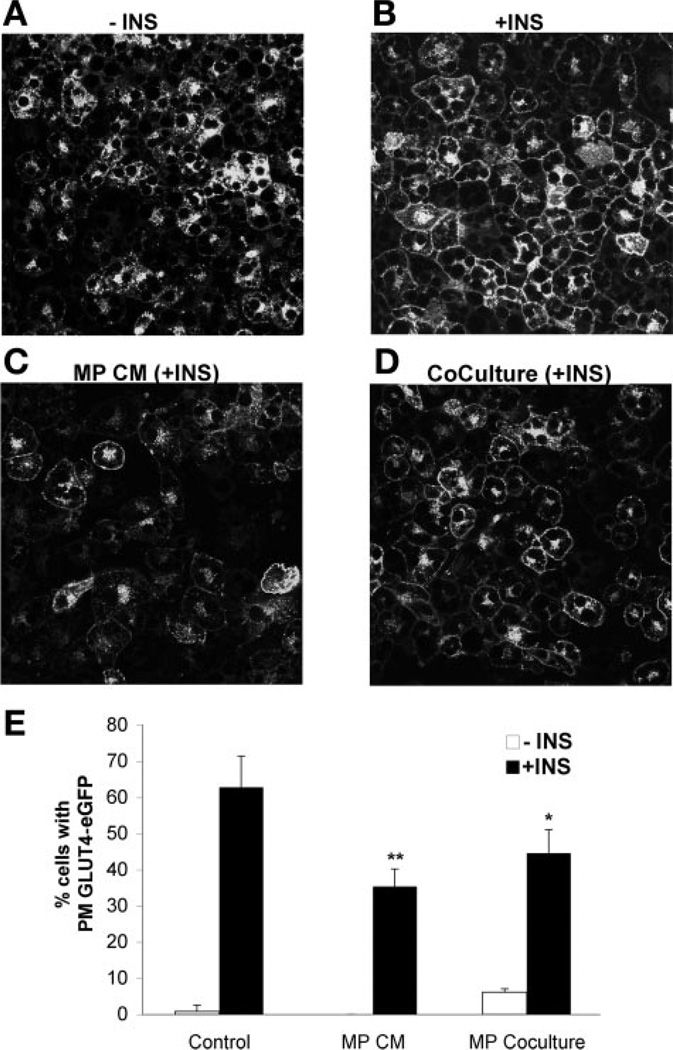
3T3-L1 fibroblasts were infected with a retrovirus containing a Myc-GLUT4-eGFP expression cassette [pMX-GLUT4myc7-GFP(6)]. High Myc-GLUT4-eGFP-expressing fibroblasts were enriched by fluorescence-activated cell sorting prior to differentiation into adipocytes and plating onto glass coverslips. Differentiated adipocytes were exposed to control medium, macrophage-conditioned medium, or macrophage coculture for 24 h and then serum starved in DMEM (5 mM glucose) with 0.5% FBS for 3 h prior to insulin stimulation for 15 min. Cells were placed on ice, washed in cold PBS, and fixed in 4% paraformaldehyde in PBS for 15 min prior to confocal imaging for GFP localization. GLUT4-GFP translocation was assessed by counting the number of cells with plasma membrane GLUT4 staining in four high-power fields (>50 cells/field) for each experimental condition.
Cytokine analysis
Conditioned media were removed from cultures at designated times and centrifuged at 10,000 g for 5 min. Supernatants were then analyzed by ELISA for mouse IL-6, TNF-α, macrophage inflammatory protein-2 (MIP-2), and MCP-1 per the manufacturer’s instructions (R&D Systems).
Stromal vascular fraction isolation and immunofluorescence
Epididymal fat pads from male C57Bl/6 mice fed an HFD were excised and minced in PBS with 0.5% bovine serum albumin (BSA). Collagenase (1 mg/ml; Sigma-Aldrich, St. Louis, MO) was added and incubated at 37°C for 20 min with shaking to generate a uniform cell suspension. The cell suspension was filtered through a 100-µm filter and then spun at 300 g for 5 min. The pellet containing the stromal vascular fraction (SVF) cells was resuspended in growth medium for further experiments. Proper separation of SVF and adipocytes was confirmed by microscopy to ensure that adipocyte fractions were devoid of adherent cells. For growth on cell culture inserts, 100,000 cells were added to each well, and adherent SVF cells were allowed to attach for 2 h at 37°C. Nonadherent cells were washed away by multiple PBS washes, and then inserts were added to adipocyte cultures.
For immunofluorescence, SVF cells were plated onto glass cover-slips for 2 h, and nonadherent cells were removed by three washes with PBS. After 24 h, the cells were fixed by incubation with 10% formalin for 20 min at room temperature. For CD68 antibody staining, cells were permeablized by incubation with 0.5% Triton X-100 in PBS. Cells were blocked in 2% bovine serum albumin in PBS and incubated with primary antibodies diluted in blocking buffer. Alexa 488-conjugated secondary antibodies were incubated at a concentration of 2 µg/ml with or without rhodamine-conjugated phalloidin (Invitrogen, Carlsbad, CA) to label actin structures. Coverslips were mounted on slides with Vectashield (Vector Laboratories, Burlingame, CA) and imaged on a confocal fluorescence microscopy (Olympus IX SLA).
Immunoblotting and immunoprecipitation
Cell lysates were prepared by washing cultured cells twice in ice-cold PBS followed by the addition of lysis buffer [150 mM sodium chloride, 50 mM Tris, pH 8, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 1 mM EDTA, 10 mM sodium fluoride, 1 mM sodium pyrophosphate, 1 mM sodium orthovanadate, and protease inhibitors (Complete minitablets; Roche Applied Science, Indianapolis, IN)]. Cells were lysed at 4°C for 30 min and clarified by centrifugation. For immunoprecipitations, lysates were incubated with 1 µg of anti-IRS-1 or anti-insulin receptor (IR) antibodies overnight at 4°C and immune complexes precipitated with protein A/G-conjugated beads (Santa Cruz Biotechnology, Santa Cruz, CA). Beads were washed with lysis buffer and resuspended in sample buffer. Lysates and immune complexes were separated by SDS-PAGE, transferred to nitrocellulose, immunoblotted with primary antibodies, and followed by species-specific horseradish peroxidase (HRP)-conjugated secondary antibodies. HRP activity was detected by chemiluminescence and quantitated on a phosphoimager (Typhoon; GE Healthcare, Piscataway, NJ).
Statistical analysis
Results are presented as means ± SD or means ± SE as indicated. Statistical analyses were conducted with an unpaired Student’s t-test with significance set at a P value of <0.05.
RESULTS
Macrophage-secreted factors induce insulin resistance in adipocytes
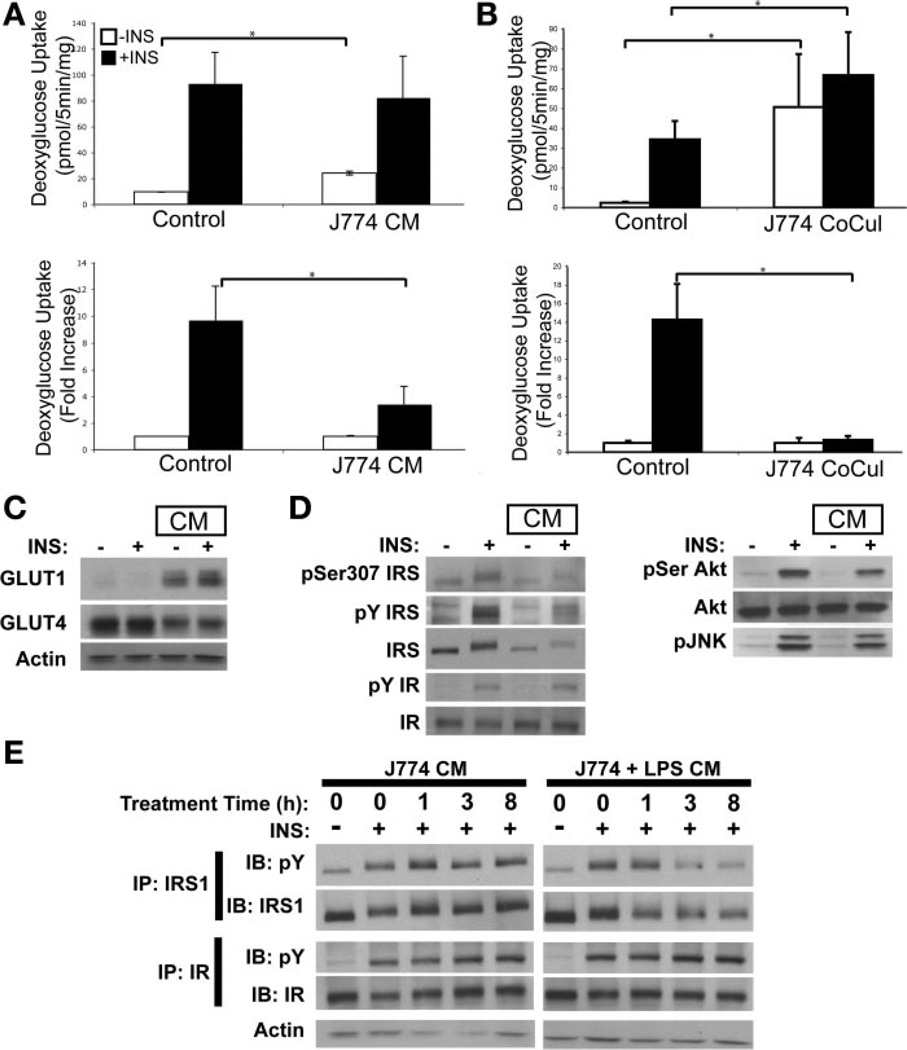
To evaluate the paracrine interactions between macrophages and adipocytes, we evaluated how factors secreted from activated macrophages influence adipocyte insulin responsiveness. As free fatty acids are elevated in obesity (27) and activate macrophages via Toll-like receptor-4 (TLR4) (29), J774 macrophages were activated with the TLR4 ligand lipopolysaccharide (LPS). Macrophage-conditioned media were collected after 16 h of treatment, filtered to remove cells, and then added to differentiated 3T3-L1 adipocytes for 24 h. Insulin responsiveness was assessed by evaluating insulin-stimulated glucose uptake. Direct addition of LPS or unstimulated J774 conditioned medium to adipocytes did not significantly impact glucose uptake (data not shown, and see Fig. 2A). However, exposure to conditioned medium from LPS-activated J774 macrophages significantly increased basal and decreased insulin-stimulated glucose uptake (Fig. 1A). Increased basal and decreased insulin-stimulated glucose uptake were also seen with indirect coculture of J774 cells and adipocytes for 5 days (Fig. 1B), indicating that cross talk between J774 macrophages and adipocytes is sufficient to decrease insulin responsiveness in adipocytes. Similar results were observed in adipocytes treated with conditioned medium from activated RAW264.7 cells, another macrophage model cell line (data not shown), and adipocytes in indirect coculture with RAW264.7 cells.
Fig. 2.
CM and macrophage (MP) coculture impair insulin-stimulated GLUT4 plasma membrane (PM) translocation. A and B: insulin-stimulated GLUT4 translocation in adipocytes. 3T3-L1 adipocytes stably expressing Myc-GLUT4-eGFP fusion protein were serum starved and treated with medium without (A) or with (B) insulin (100 nM for 15 min). Cells were imaged for GFP expression, demonstrating translocation of Myc-GLUT4-GFP to the plasma membrane. C: CM blocks GLUT4 translocation. Myc-GLUT4-eGFP-expressing 3T3-L1 adipocytes were incubated for 24 h with CM. Insulin stimulation was performed as in A. D: coculture with macrophages blocks GLUT4 translocation. RAW264 macrophages (2 × 105 cells/well of 6-well dish) were cocultured with Myc-GLUT4-eGFP-expressing 3T3-L1 adipocytes for 24 h prior to insulin stimulation and imaging GLUT4 translocation. E: quantitation of GLUT4 plasma membrane translocation. Cells with translocation of Myc-GLUT4-eGFP to the plasma membrane were quantitated in 2 independent experiments (>200 cells/experiment) per condition. Data are presented as mean %GLUT4 translocation ± SE. *P < 0.05 vs. control; **P < 0.005 vs. control.
Fig. 1.
Macrophage-conditioned medium (CM) blocks insulin (Ins) action in 3T3-L1 adipocytes. A: insulin-stimulated 2-deoxyglucose (2-DG) uptake in 3T3-L1 adipocytes was examined after exposure to medium harvested from LPS-stimulated J774 macrophages. CM or unconditioned medium (Control) were added to differentiated 3T3-L1 adipocytes for 24 h. 2-DG uptake assessed without (open bars) or with (filled bars) 30 min of insulin (100 nM) stimulation. B: 2-DG uptake with indirect coculture. J774 macrophages were cultured in the upper cell culture insert with differentiated 3T3-L1 adipocytes in the lower chamber for 5 days. 2-DG uptake was assessed with and without insulin stimulation as in A. For A and B, data are expressed as means (SD) of triplicate determinants. Similar results were obtained from 3 independent experiments. *P < 0.05. C: effects of CM on adipocyte glucose transporter expression. Immunoblots (IB) of 3T3-L1 adipocytes lysates with or without treatment with CM for 24 h. Cells were stimulated with insulin (100 nM) for 10 min. Actin was used as loading control. D: effect of CM on insulin signaling in adipocytes. Following insulin stimulation, adipocyte lysates from cells treated with or without CM were probed for insulin receptor (IR), IRS-1, Akt/PKB, and phosphorylated Jun kinase (pJNK). Phosphotyrosine (pY) antibody was used on lysates to identify IR and IRS phosphorylation, and IRS phospho-Ser307-specific antibody was used to probe IRS serine phosphorylation. E: time course of exposure to CM. Adipocytes were treated with CM from unstimulated (J774 CM) and LPS-stimulated J774 cells (J774 + LPS CM) for 0, 1, 3, and 8 h prior to insulin treatment (100 nM for 10 min). Lysates were immunoprecipitated (IP) for IRS-1 and IR using specific antibodies and probed for pY or the immunoprecipitated protein. Actin from lysates was used as loading control.
To examine the mechanism of altered glucose transport, lysates from adipocytes exposed to macrophage-conditioned medium were immunoblotted with antibodies to glucose transport proteins. The GLUT1 glucose transporter was increased with conditioned medium, whereas GLUT4 expression was decreased (Fig. 1C), correlating with the increased basal and decreased insulin-stimulated glucose uptake. This altered pattern of glucose transporter expression is also consistent with changes seen in adipocytes from insulin-resistant obese mice (48).
Insulin signaling components were also examined. Addition of macrophage-conditioned medium to adipocytes decreased adipocyte IRS-1 protein levels with a concomitant decrease in IRS tyrosine phosphorylation (Fig. 1, D and E). Consistent with this change, a mild reduction in Akt phosphorylation was observed after treatment with macrophage-conditioned medium. There was no significant change in basal or insulin-stimulated serine phosphorylation of IRS-1 at Ser307 with macrophage coculture. Additionally, no change in basal or insulin-stimulated JNK phosphorylation was observed. IR protein levels and tyrosine phosphorylation were unaffected (Fig. 1, D and E). Time course analysis of exposure to activated macrophage-conditioned medium revealed that IRS-1 protein levels were decreased by 1 h of treatment with macrophage-conditioned medium (Fig. 1E). Insulin-stimulated IRS tyrosine phosphorylation was decreased by 3 h of treatment, although no changes in IR phosphorylation were observed over this time course.
Macrophage-conditioned medium decreases insulin-stimulated GLUT4 plasma membrane translocation
Although the 2-DG uptake experiments suggest that insulin-stimulated GLUT4 plasma membrane translocation is blocked by macrophages, the induction of GLUT1 and increase in basal glucose uptake with macrophage-conditioned medium and coculture complicate this assessment. Therefore, we examined the effect of macrophage-conditioned medium on insulin-stimulated translocation of GLUT4 to the plasma membrane. 3T3-L1 cells stably expressing a Myc-GLUT4-eGFP fusion protein were differentiated and exposed to macrophage-conditioned medium for 24 h prior to insulin stimulation. Although insulin stimulated efficient GLUT4 plasma membrane translocation in control cells (Fig. 2, A and B), treatment with conditioned medium led to a significant decrease in insulin-stimulated translocation of Myc-GLUT4-eGFP (Fig. 2, C and E). A similar decrease in Myc-GLUT4-eGFP translocation was seen with coculture of macrophages with adipocytes (Fig. 2, D and E). Combined with the 2-DG uptake data, this demonstrates that, in addition to increasing GLUT1 and basal glucose uptake, macrophage-derived signals block insulin-stimulated GLUT4 membrane trafficking in adipocytes and can thus reduce insulin responsiveness.
Neutralizing anti-TNF-α antibodies reverse the blockade of insulin action caused by macrophage-secreted factors
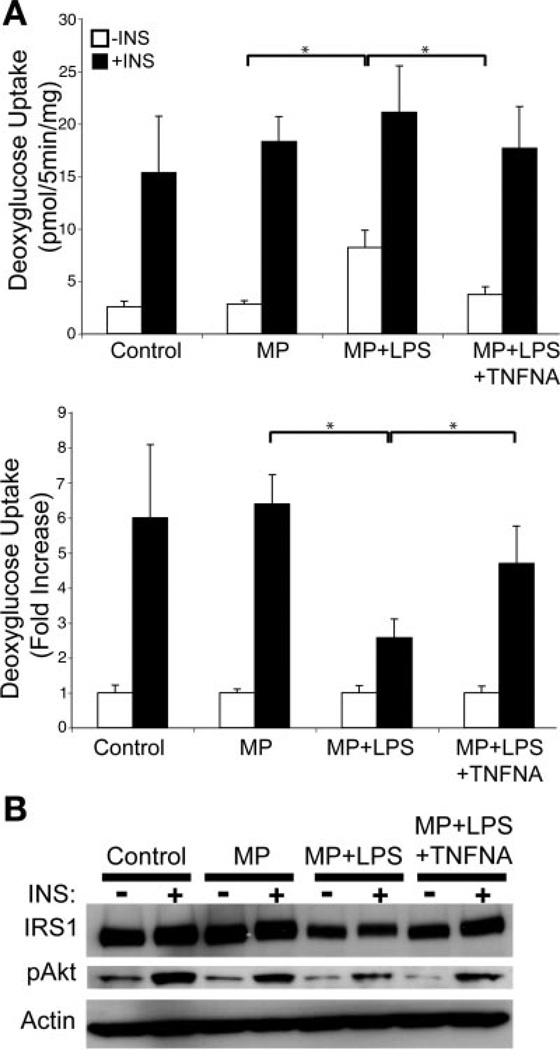
The decreased GLUT4 and IRS-1 expression seen with macrophage-conditioned medium are similar to effects reported from prolonged exposure of adipocytes to TNF-α (22, 41). TNF-α, IL-6, and IL-1β were all significantly elevated in macrophage-conditioned medium compared with adipocyte medium alone, with TNF-α present in the highest concentration (36.3 ± 2 ng/ml TNF-α vs. 3.59 ± 0.45 ng/ml IL-6 vs. 87 ± 6 pg/ml IL-1β). To investigate the contribution of TNF-α to the altered insulin response, 3T3-L1 cells were exposed to macrophage-conditioned medium in the presence or absence of neutralizing anti-TNF-α antibodies. Medium from nonactivated J774 cells had little effect on glucose uptake, as previously observed, whereas conditioned medium from activated macrophages increased basal glucose uptake (Fig. 3A). Anti-TNF-α antibodies reversed the effect of conditioned medium on the basal glucose uptake, whereas non-specific IgG controls had no effect on changes induced by the conditioned medium (data not shown). Additionally, neutralizing antibodies partially restored the phosphorylation of IRS-1 and Akt that was decreased by treatment with conditioned medium (Fig. 3B). These results indicate that TNF-α is a significant contributor to the attenuation of insulin action in this paradigm.
Fig. 3.
TNF-α blockade partially restores insulin sensitivity in adipocytes when exposed to CM. A: insulin-stimulated glucose uptake with CM in the presence or absence of TNF-α neutralizing antibodies (TNFNA). Adipocytes were treated for 16 h with control medium or CM from unstimulated (MP) or LPS-stimulated (MP + LPS) J774 cells. TNF-α neutralizing antibodies were added to the media from stimulated cells prior to addition to adipocytes (MP + LPS + TNFNA). Insulin-stimulated glucose uptake was then assessed. Data are expressed as means (SD) of triplicate determinants. Similar results were obtained from 2 independent experiments. *P < 0.05. B: anti-TNF-α antibodies restore IRS-1 protein levels in adipocytes exposed to CM. Immunoblots of 3T3-L1 adipocyte lysates from CM experiments were probed for IRS-1 expression and Akt serine phosphorylation at Ser473. Similar results were obtained from duplicate experiments, and a representative experiment is shown.
Indirect coculture of primary macrophages and adipose tissue stromal cells with adipocytes attenuates insulin action
Macrophage populations demonstrate significant heterogeneity in their inflammatory capacity and response to environmental stimuli (21). J774 and RAW264 cells are convenient for studying the mechanisms underlying macrophage-adipocyte communication but may not have the same properties as primary macrophages. To investigate the effect of primary macrophage populations on adipocyte function, we employed indirect coculture experiments with peritoneal macrophages (PMs) or adipose tissue SVF cells derived from obese C57Bl/6 mice. The SVF is a mixed population of stromal cells comprising ~40–60% F4/80+CD11b+ macrophages, preadipocytes, and fibroblasts (C. N. Lumeng and A. R. Saltiel, unpublished observations and Ref. 53).
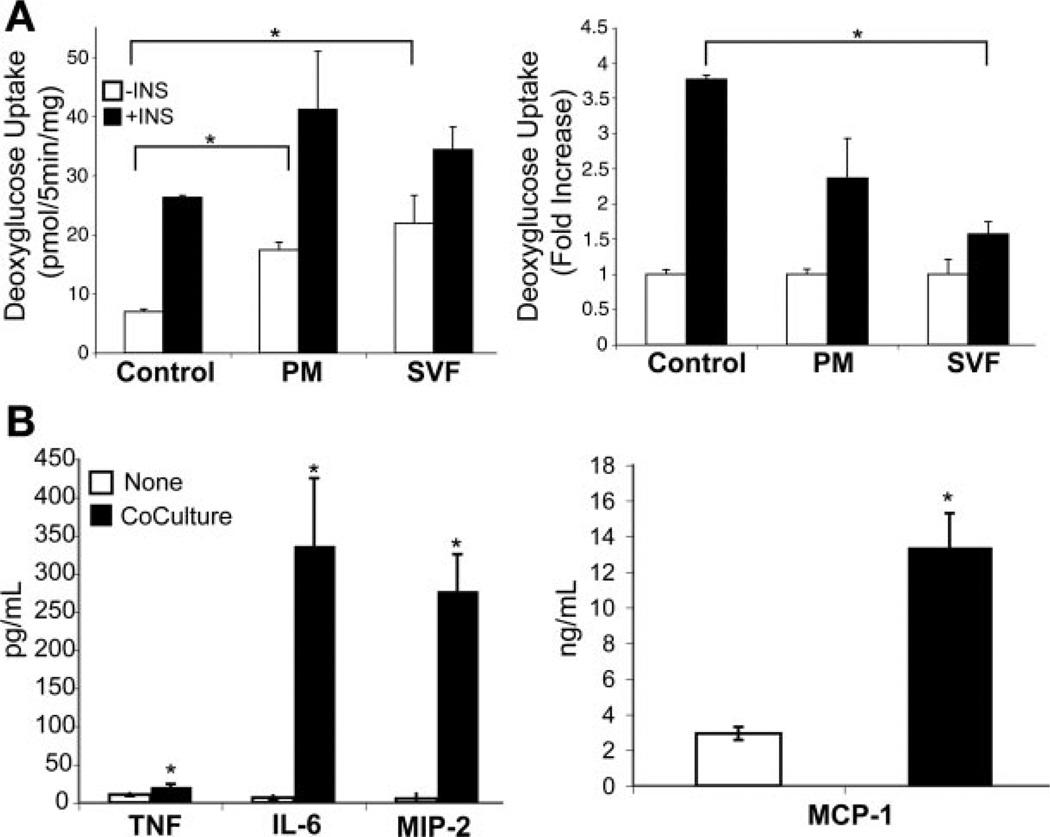
Adherent PMs and SVF cells (105 cells/insert) were cultured in the upper chamber of a permeable cell culture insert with 3T3-L1 adipocytes cultured in the lower chamber. The permeable membrane in this culture system permits secreted factors to pass between the two cell types but does not permit direct cell-cell contact. Coculture with both PMs and SVF cells led to an increase in basal glucose uptake, which decreased the fold change in insulin-stimulated glucose uptake (Fig. 4A). Coculture with SVF cells produced a greater inhibitory effect than did coculture with PMs. Moreover, coculture conditions led to a pronounced secretion of the proinflammatory cytokines IL-6, MIP-2, and MCP-1 in the media and a lesser but statistically significant increase in TNF-α (Fig. 4B).
Fig. 4.
Indirect coculture with primary murine macrophages blocks insulin action in adipocytes. A: insulin-stimulated glucose uptake in 3T3-L1 adipocytes cocultured with peritoneal macrophages (PM) or stromal vascular fraction (SVF) cells from epididymal adipose tissue from C57Bl/6 mice. Cells (105) of PM or SVF were added to cell culture insert, which was cultured over differentiated 3T3-L1 cells in the lower chamber. After 48 h of coculture, glucose uptake was assessed with (filled bars) and without (open bars) insulin stimulation. Data are expressed as means (SD) of triplicate determinants. Similar results were obtained from 2 independent experiments. *P < 0.05. B: cytokine analysis of media from SVF and adipocyte coculture. Media from control 3T3-L1 cultures (open bars) and indirect cocultures of SVF with 3T3-L1 adipocytes (filled bars) were collected and examined for proinflammatory cytokines TNF-α, IL-6, MIP-2, and MCP-1; n = 5 samples/condition. *P < 0.05.
Direct coculture of macrophages in contact with adipocytes blocks insulin action and alters macrophage morphology and activation
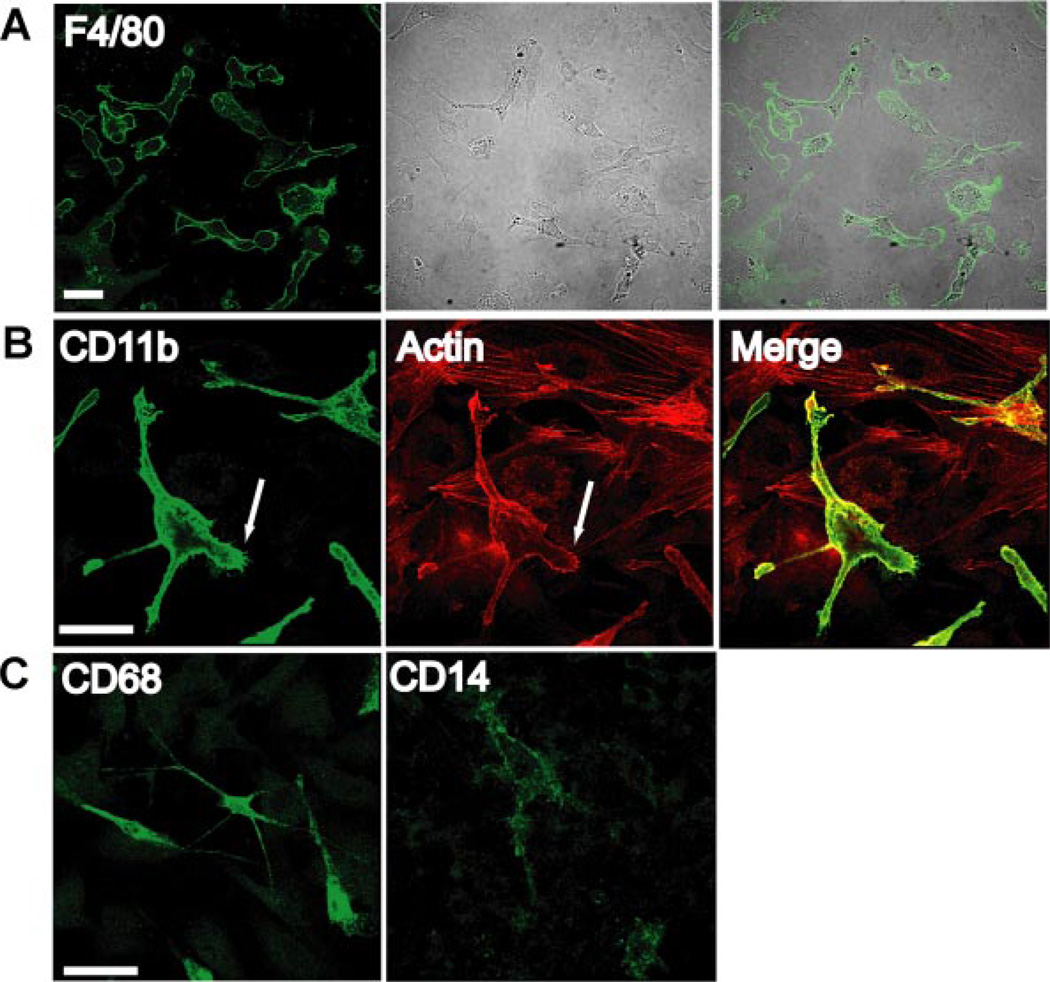
Although indirect coculture permits paracrine interactions between the cell types, it does not allow cell-cell contacts that may influence activation. The importance of cellular contact on adipose tissue macrophage properties is evident in cultures of SVF-derived ATMs from HFD-fed mice. The SVF was isolated from epididymal white adipose tissue from male HFD-fed mice. Adherent cells were then enriched by adhesion. Cells were cultured for 3 days and stained for macrophage-specific markers. F4/80+ ATMs displayed an elongated morphology with long cellular processes (Fig. 5A). The ATMs spread over surrounding fibroblasts and produced actin-enriched filopodia. These cells were also positive for macrophage-specific markers CD11b and CD68 but low in expression of CD14 (Fig. 5, B and C).
Fig. 5.
Adipose tissue macrophage (ATM) morphology and cell surface markers in obese mice. A: SVF cells were isolated from high-fat diet-fed mice and selected by adhesion on coverslips. Cultures were then stained with anti-F4/80 antibodies and visualized by immunofluorescence microscopy and phase contrast microscopy. B: SVF cells stained for macrophage marker CD11b (green) and counterstained with rhodamine-phalloidin (red) to visualize actin-enriched processes and filopodia that extend over fibroblasts (arrow). C: SVF cells stained for CD68 in permeablized cells and CD14 show that ATMs have a CD68+CD14− phenotype. Scale bar, 50 µm for all figures.
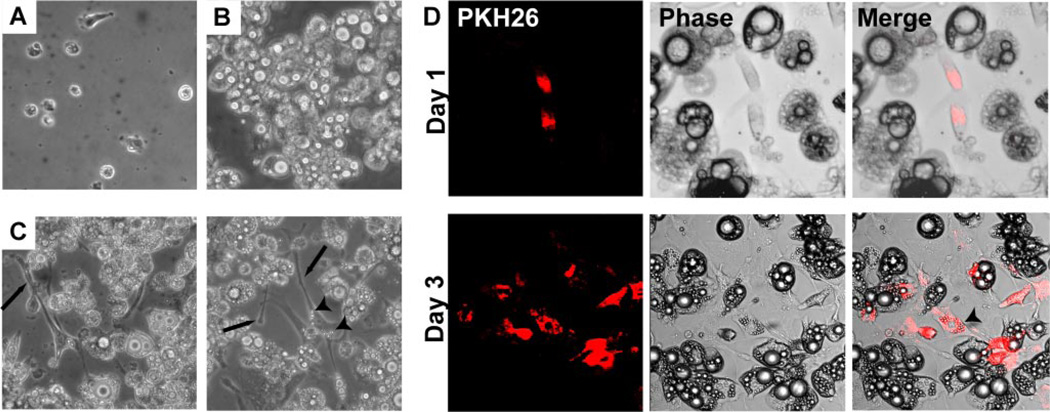
To assess the effect of direct contact between macrophages and adipocytes on insulin sensitivity, PMs were placed onto 3T3-L1 adipocytes at different densities [0, 104 (10k), 105 (100k) cells/well]. PMs cultured alone appeared rounded or showed minimal spreading with short processes (Fig. 6A). Differentiated adipocyte cultures were free of undifferentiated fibroblasts (Fig. 6B). With 72 h of direct coculture, the PMs took on an elongated appearance with long cellular extensions between and along the surrounding adipocytes (Fig. 6C), resembling the morphology of ATMs isolated from obese mice. Many of the macrophages developed increased accumulation of lipid vacuoles in their cytoplasm with coculture.
Fig. 6.
Direct coculture of macrophages with adipocytes alters macrophage morphology. A: phase contrast pictures of thioglycollate PMs in isolated culture. B: phase contrast pictures of differentiated 3T3-L1 adipocytes in isolated culture. C: direct coculture of PMs and adipocytes after 3 days demonstrates that macrophages take on an elongated phenotype with long cellular processes (arrows). Two representative views are shown. Increased accumulation of intracellular lipid (arrowhead) is also seen in macrophages. D: PMs were labeled with PKH26 (red) prior to plating on differentiated 3T3-L1 adipocytes. After 1 day of coculture with adipocytes (top), MPs appear small and oblong. After 3 days of coculture (bottom), PKH26+ macrophages appear more elongated and accumulate intracellular lipid (arrowhead).
To better identify the macrophages in the coculture system, the PMs were labeled prior to plating onto adipocytes by incubation with PKH26, an inert fluorescent dye that is taken up by phagocytic cells (Fig. 6D). This enabled tracking of PKH26+ PMs in the adipocyte cultures. At 24 h of direct coculture with adipocytes, macrophages were rounded in appearance with short extensions. With 72 h of direct coculture, cells were elongated. These observations suggest that PMs in coculture with adipocytes undergo morphological alterations similar to those in ATMs.
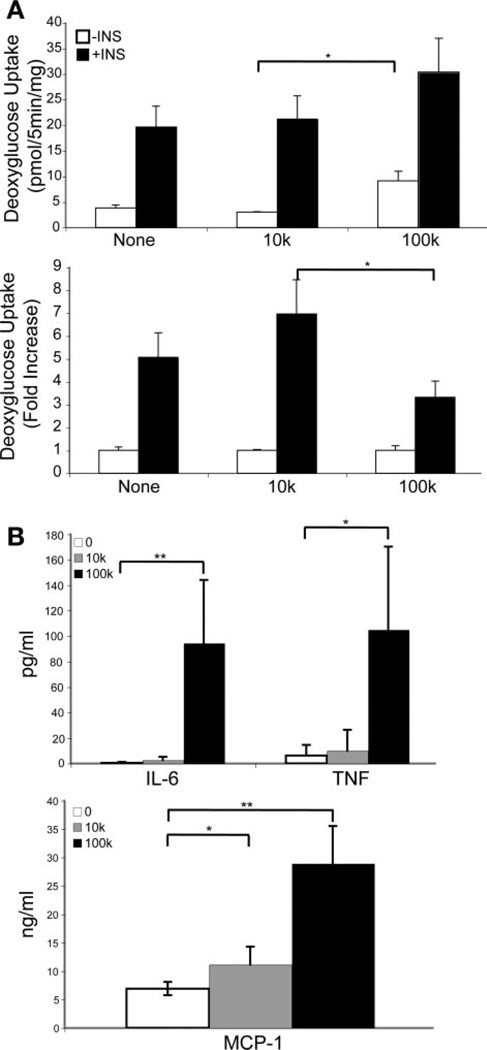
Insulin-stimulated glucose uptake was analyzed in the direct coculture model of macrophages and adipocytes. There was no detectable glucose uptake in macrophage cultures alone (data not shown). However, a dose-dependent increase in basal glucose uptake was observed after addition of macrophages to the adipocyte cultures (Fig. 7A), consistent with previous observations.
Fig. 7.
Direct coculture of macrophages with adipocytes alters insulin sensitivity in a dose-dependent fashion. A: insulin-stimulated 2-DG uptake in direct coculture. Differentiated 3T3-L1 adipocytes were cultured in FBS (None) or after addition of 104 (10k) or 105 (100k) PM. 2-DG uptake was assessed with (filled bars) or without (open bars) 30 min of insulin stimulation. Although 10k PMs had minimal effects on glucose uptake, 100k PMs led to an increase in basal glucose uptake and a decrease in fold insulin stimulation. Data are expressed as means (SD) of triplicate determinants. Similar results were obtained from 2 independent experiments. *P < 0.05. B: cytokine analysis of coculture media. Media from the 3 conditions of coculture were evaluated for cytokine levels of TNF-α, IL-6, (top), and MCP-1 (bottom); n = 3 samples/condition. **P < 0.001; *P < 0.05.
The proinflammatory cytokines TNF-α, IL-6, and MCP-1 were significantly induced with 105 PMs added to the coculture, but not 104, consistent with the glucose uptake data (Fig. 7B). Compared with the indirect coculture experiments in which equal numbers of macrophages (105 cells) were added to adipocytes in similar culture volumes, the levels of TNF-α (105 ± 65.1 vs. 21 ± 3.4 pg/ml, P = 0.025) and MCP-1 (29.0 ± 6.6 vs. 13.4 ± 1.8 ng/ml, P = 0.002) production were greater during direct coculture, suggesting that cell-cell contact between macrophages and adipocytes potentiates the secretion of these proinflammatory factors.
DISCUSSION
The observations that macrophages infiltrate and generate inflammatory responses in adipose tissue have led numerous investigators to reevaluate the mechanisms underlying insulin resistance. We have examined how macrophages and adipocytes interact in vitro and whether macrophages can modify insulin responsiveness and glucose metabolism in adipocytes. Macrophage-secreted factors reduced insulin-stimulated glucose uptake in adipocytes via downregulation of GLUT4 and IRS-1. Consistent with this, insulin-stimulated GLUT4 plasma membrane translocation was attenuated by macrophage-secreted factors. Additionally, GLUT1 transporters were upregulated, correlating with increased basal glucose uptake. Treatment of macrophage-conditioned medium with TNF-α-blocking antibodies partially reversed this inhibitory effect, which was also seen during direct and indirect coculture.
The deleterious effects of macrophage coculture or addition of macrophage-conditioned medium to adipocytes are reminiscent of generalized stress responses. GLUT1 appears to be induced upon conditions of cellular stress such as glucose deprivation in 3T3-L1 adipocytes (28). Increased basal glucose uptake has been observed in isolated adipocytes from several obese animal models and is associated with a decrease in insulin responsiveness (16, 48). Although the mechanisms are unknown, this response may be due to increased GLUT1 mRNA stability, as TNF-α has been shown to induce proteins that bind to the 3′-untranslated region of the GLUT1 mRNA and stabilize the message (32).
The studies described herein show that macrophages induce the loss of GLUT4 and IRS-1 expression in adipocytes, which parallels changes seen in patients with type 2 diabetes (11, 12). The downregulation and decreased membrane translocation of GLUT4 in response to insulin demonstrate that macrophage-derived factors can limit the ability of adipocytes to take up glucose upon insulin stimulation. Although whole body glucose uptake primarily takes place in muscle, proper glucose transport in adipocytes is required for normal glucose regulation, as adipose tissue-specific GLUT4 knockout mice demonstrate profound insulin resistance (1), and overexpression of GLUT4 in adipose tissue can reverse diabetes in mice without GLUT4 in skeletal muscle (13). Therefore, we and others (34) hypothesize that macrophage-adipocyte interactions lead to decreased adipocyte glucose transport that can contribute to the generation of whole body insulin resistance. The exact mechanism for the cross talk between adipose tissue and muscle is unknown but may involve dysregulation of circulating factors such as RBP4 (55).
The macrophage-induced alterations in IRS signaling may be explained by activation of suppressor of cytokine signaling (SOCS)1/3, both of which have been shown to reduce IRS protein levels by targeting it for ubiquitin-mediated proteosomal degradation (42). In our experiments, IRS-1 was degraded within 1 h of exposure to macrophage-conditioned medium. SOCS proteins are activated by a variety of proinflammatory cytokines, such as TNF-α and IL-6, and are required for the blocking effect of TNF-α on insulin action in adipocytes (19, 49). Consistent with this, anti-TNF-α neutralizing antibodies prevented the effects of macrophage-conditioned medium on glucose uptake and IRS-1 downregulation.
Our observations build on previous observations of TNF-α-induced blockade of insulin action in adipocytes (22, 23, 41). Although these studies examined the effects of exogenous addition of TNF-α to adipocytes, we started with analysis of macrophage-derived factors and reveal a role for TNF-α generated in this setting that had been hypothesized but not directly tested. We also observed that TNF-α is one of many proinflammatory cytokines, such as IL-6, MIP-2, and MCP-1, that are induced in macrophages with coculture. In preliminary experiments, we have not observed significant alteration of the macrophage-induced effects with IL-6 neutralizing antibodies (C. N. Lumeng and A. R. Saltiel, unpublished observations).
TNF-α induces the expression of a variety of inflammatory cytokines in adipocytes, including IL-6, PAI-1, MCP-1, and TNF-α itself (51). Therefore, the induction of insulin-inhibitory effects of TNF-α may not be direct. Previous studies suggest that TNF-α and MCP-1 production are coupled, as TNF-α neutralizing antibodies can block MCP-1 production (46). IL-6 and MCP-1 can both render adipocytes insulin resistant (40, 43, 44). There is 100- to 1,000-fold more MCP-1 than TNF-α or IL-6 secreted by adipocytes in coculture with macrophages. MCP-1 knockout mice were protected from HFD-induced diabetes, suggesting a possible direct role for MCP-1 in influencing insulin sensitivity in adipocytes, perhaps in concert with its effects on macrophage recruitment (25).
Macrophages found in SVF cultures produced long extensions and demonstrated an elongated morphology with spreading alongside and over fibroblast cells in the mixed culture of adipose tissue stromal cells. Cells with similar morphology have been reported in stromal vascular cell cultures, although macrophages were not specifically examined in some of those studies (17, 39, 54). Using a variety of known macrophage markers, we identified ATMs in obese mice as CD11b+F4/80+CD68+CD14− cells. When PMs were cocultured with adipocytes, they took on a similar morphology with long cellular processes that interacted with the surface of adipocytes, suggesting that adipocytes may modify macrophage properties. Similar elongation of macrophages and extension of filopodia were described in macrophages upon activation by platelet-activating factor and macrophage colony-stimulating factor-1 (36, 47). Such changes are crucial to macrophage migration and chemotaxis and are thought to be mediated by small GTPases such as Cdc42 and Rho. Direct contact among macrophages and adipocytes enhances inflammatory cytokine production compared with indirect contact, suggesting that cell-cell interactions modify the inflammatory response. This is consistent with a model in which macrophages are activated upon their recruitment into adipose tissue.
These results stress the importance of cellular contact as well as secreted factors in influencing the interaction between macrophages and adipocytes in adipose tissue. Further analysis of the relative contributions of IL-6 and TNF-α, as well as cell-cell contact to the induction of insulin resistance, will be the focus of future attention.
ACKNOWLEDGMENTS
We thank the University of Michigan Immunology Core for help with ELISA assays and the Flow Cytometry Core Facility with assistance in fluorescence-activated cell sorting. We are grateful to Dr. Harvey Lodish for providing the Myc-GLUT4-eGFP viral constructs, and Dr. Shian Huey Chiang for preparing 3T3-L1 adipocytes overexpressing this construct.
GRANTS
This research was supported by a training grant from the National Institute of Child Health and Human Development to the Department of Pediatrics and Communicable Diseases (T32-HD-007513-07) and National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-60591 to A. R. Saltiel.
REFERENCES
- 1.Abel ED, Peroni O, Kim JK, Kim YB, Boss O, Hadro E, Minnemann T, Shulman GI, Kahn BB. Adipose-selective targeting of the GLUT4 gene impairs insulin action in muscle and liver. Nature. 2001;409:729–733. doi: 10.1038/35055575. [DOI] [PubMed] [Google Scholar]
- 2.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–198. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 3.Baumann CA, Ribon V, Kanzaki M, Thurmond DC, Mora S, Shigematsu S, Bickel PE, Pessin JE, Saltiel AR. CAP defines a second signalling pathway required for insulin-stimulated glucose transport. Nature. 2000;407:202–207. doi: 10.1038/35025089. [DOI] [PubMed] [Google Scholar]
- 4.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 5.Bluher M, Fasshauer M, Tonjes A, Kratzsch J, Schon MR, Paschke R. Association of interleukin-6, C-reactive protein, interleukin-10 and adiponectin plasma concentrations with measures of obesity, insulin sensitivity and glucose metabolism. Exp Clin Endocrinol Diabetes. 2005;113:534–537. doi: 10.1055/s-2005-872851. [DOI] [PubMed] [Google Scholar]
- 6.Bogan JS, McKee AE, Lodish HF. Insulin-responsive compartments containing GLUT4 in 3T3-L1 and CHO cells: regulation by amino acid concentrations. Mol Cell Biol. 2001;21:4785–4806. doi: 10.1128/MCB.21.14.4785-4806.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bouloumie A, Curat CA, Sengenes C, Lolmede K, Miranville A, Busse R. Role of macrophage tissue infiltration in metabolic diseases. Curr Opin Clin Nutr Metab Care. 2005;8:347–354. doi: 10.1097/01.mco.0000172571.41149.52. [DOI] [PubMed] [Google Scholar]
- 8.Bruun JM, Helge JW, Richelsen B, Stallknecht B. Diet and exercise reduce low-grade inflammation and macrophage infiltration in adipose tissue but not in skeletal muscle in severely obese subjects. Am J Physiol Endocrinol Metab. 2006;290:E961–E967. doi: 10.1152/ajpendo.00506.2005. [DOI] [PubMed] [Google Scholar]
- 9.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–190. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancello R, Henegar C, Viguerie N, Taleb S, Poitou C, Rouault C, Coupaye M, Pelloux V, Hugol D, Bouillot JL, Bouloumie A, Barbatelli G, Cinti S, Svensson PA, Barsh GS, Zucker JD, Basdevant A, Langin D, Clement K. Reduction of macrophage infiltration and chemoattractant gene expression changes in white adipose tissue of morbidly obese subjects after surgery-induced weight loss. Diabetes. 2005;54:2277–2286. doi: 10.2337/diabetes.54.8.2277. [DOI] [PubMed] [Google Scholar]
- 11.Carvalho E, Jansson PA, Axelsen M, Eriksson JW, Huang X, Groop L, Rondinone C, Sjostrom L, Smith U. Low cellular IRS 1 gene and protein expression predict insulin resistance and NIDDM. FASEB J. 1999;13:2173–2178. doi: 10.1096/fasebj.13.15.2173. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho E, Jansson PA, Nagaev I, Wenthzel AM, Smith U. Insulin resistance with low cellular IRS-1 expression is also associated with low GLUT4 expression and impaired insulin-stimulated glucose transport. FASEB J. 2001;15:1101–1103. [PubMed] [Google Scholar]
- 13.Carvalho E, Kotani K, Peroni OD, Kahn BB. Adipose-specific over-expression of GLUT4 reverses insulin resistance and diabetes in mice lacking GLUT4 selectively in muscle. Am J Physiol Endocrinol Metab. 2005;289:E551–E561. doi: 10.1152/ajpendo.00116.2005. [DOI] [PubMed] [Google Scholar]
- 14.Christiansen T, Richelsen B, Bruun JM. Monocyte chemoattractant protein-1 is produced in isolated adipocytes, associated with adiposity and reduced after weight loss in morbid obese subjects. Int J Obes Relat Metab Disord. 2005;29:146–150. doi: 10.1038/sj.ijo.0802839. [DOI] [PubMed] [Google Scholar]
- 15.Clement K, Viguerie N, Poitou C, Carette C, Pelloux V, Curat CA, Sicard A, Rome S, Benis A, Zucker JD, Vidal H, Laville M, Barsh GS, Basdevant A, Stich V, Cancello R, Langin D. Weight loss regulates inflammation-related genes in white adipose tissue of obese subjects. FASEB J. 2004;18:1657–1669. doi: 10.1096/fj.04-2204com. [DOI] [PubMed] [Google Scholar]
- 16.Czech MP, Richardson DK, Becker SG, Walters CG, Gitomer W, Heinrich J. Insulin response in skeletal muscle and fat cells of the genetically obese Zucker rat. Metabolism. 1978;27:1967–1981. doi: 10.1016/s0026-0495(78)80013-4. [DOI] [PubMed] [Google Scholar]
- 17.El-Ghalbzouri A, Van Den Bogaerdt AJ, Kempenaar J, Ponec M. Human adipose tissue-derived cells delay re-epithelialization in comparison with skin fibroblasts in organotypic skin culture. Br J Dermatol. 2004;150:444–454. doi: 10.1046/j.1365-2133.2004.05830.x. [DOI] [PubMed] [Google Scholar]
- 18.Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 19.Fasshauer M, Kralisch S, Klier M, Lossner U, Bluher M, Klein J, Paschke R. Insulin resistance-inducing cytokines differentially regulate SOCS mRNA expression via growth factor- and Jak/Stat-signaling pathways in 3T3-L1 adipocytes. J Endocrinol. 2004;181:129–138. doi: 10.1677/joe.0.1810129. [DOI] [PubMed] [Google Scholar]
- 20.Geiss LS, Pan L, Cadwell B, Gregg EW, Benjamin SM, Engelgau MM. Changes in incidence of diabetes in U.S. adults, 1997–2003. Am J Prev Med. 2006;30:371–377. doi: 10.1016/j.amepre.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 21.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–964. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 22.Hotamisligil GS, Murray DL, Choy LN, Spiegelman BM. Tumor necrosis factor alpha inhibits signaling from the insulin receptor. Proc Natl Acad Sci USA. 1994;91:4854–4858. doi: 10.1073/pnas.91.11.4854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259:87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 24.Inoue M, Chiang SH, Chang L, Chen XW, Saltiel AR. Compartmentalization of the exocyst complex in lipid rafts controls Glut4 vesicle tethering. Mol Biol Cell. 2006;17:2303–2311. doi: 10.1091/mbc.E06-01-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kanda H, Tateya S, Tamori Y, Kotani K, Hiasa K, Kitazawa R, Kitazawa S, Miyachi H, Maeda S, Egashira K, Kasuga M. MCP-1 contributes to macrophage infiltration into adipose tissue, insulin resistance, and hepatic steatosis in obesity. J Clin Invest. 2006;116:1494–1505. doi: 10.1172/JCI26498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim JK, Kim YJ, Fillmore JJ, Chen Y, Moore I, Lee J, Yuan M, Li ZW, Karin M, Perret P, Shoelson SE, Shulman GI. Prevention of fat-induced insulin resistance by salicylate. J Clin Invest. 2001;108:437–446. doi: 10.1172/JCI11559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsari C, Jensen MD. Free fatty acid metabolism in human obesity. J Lipid Res. 2006;47:1643–1650. doi: 10.1194/jlr.R600011-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Kumar A, Xiao YP, Laipis PJ, Fletcher BS, Frost SC. Glucose deprivation enhances targeting of GLUT1 to lipid rafts in 3T3-L1 adipocytes. Am J Physiol Endocrinol Metab. 2004;286:E568–E576. doi: 10.1152/ajpendo.00372.2003. [DOI] [PubMed] [Google Scholar]
- 29.Lee JY, Sohn KH, Rhee SH, Hwang D. Saturated fatty acids, but not unsaturated fatty acids, induce the expression of cyclooxygenase-2 mediated through Toll-like receptor 4. J Biol Chem. 2001;276:16683–16689. doi: 10.1074/jbc.M011695200. [DOI] [PubMed] [Google Scholar]
- 30.Lee YH, Pratley RE. The evolving role of inflammation in obesity and the metabolic syndrome. Curr Diab Rep. 2005;5:70–75. doi: 10.1007/s11892-005-0071-7. [DOI] [PubMed] [Google Scholar]
- 31.Linton MF, Fazio S. Macrophages, inflammation, and atherosclerosis. Int J Obes Relat Metab Disord. 2003;27(Suppl 3):S35–S40. doi: 10.1038/sj.ijo.0802498. [DOI] [PubMed] [Google Scholar]
- 32.McGowan KM, Police S, Winslow JB, Pekala PH. Tumor necrosis factor-alpha regulation of glucose transporter (GLUT1) mRNA turnover. Contribution of the 3′-untranslated region of the GLUT1 message. J Biol Chem. 1997;272:1331–1337. doi: 10.1074/jbc.272.2.1331. [DOI] [PubMed] [Google Scholar]
- 33.Murdolo G, Smith U. The dysregulated adipose tissue: a connecting link between insulin resistance, type 2 diabetes mellitus and atherosclerosis. Nutr Metab Cardiovasc Dis. 2006;16(Suppl 1):S35–S38. doi: 10.1016/j.numecd.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 34.Neels JG, Olefsky JM. Inflamed fat: what starts the fire? J Clin Invest. 2006;116:33–35. doi: 10.1172/JCI27280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ogden CL, Carroll MD, Curtin LR, McDowell MA, Tabak CJ, Flegal KM. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295:1549–1555. doi: 10.1001/jama.295.13.1549. [DOI] [PubMed] [Google Scholar]
- 36.Peppelenbosch M, Boone E, Jones GE, van Deventer SJ, Haegeman G, Fiers W, Grooten J, Ridley AJ. Multiple signal transduction pathways regulate TNF-induced actin reorganization in macrophages: inhibition of Cdc42-mediated filopodium formation by TNF. J Immunol. 1999;162:837–845. [PubMed] [Google Scholar]
- 37.Permana PA, Menge C, Reaven PD. Macrophage-secreted factors induce adipocyte inflammation and insulin resistance. Biochem Biophys Res Commun. 2006;341:507–514. doi: 10.1016/j.bbrc.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Poirier P, Giles TD, Bray GA, Hong Y, Stern JS, Pi-Sunyer FX, Eckel RH. Obesity and cardiovascular disease: pathophysiology, evaluation, and effect of weight loss: an update of the 1997 American Heart Association Scientific Statement on Obesity and Heart Disease from the Obesity Committee of the Council on Nutrition, Physical Activity, and Metabolism. Circulation. 2006;113:898–918. doi: 10.1161/CIRCULATIONAHA.106.171016. [DOI] [PubMed] [Google Scholar]
- 39.Rehman J, Traktuev D, Li J, Merfeld-Clauss S, Temm-Grove CJ, Bovenkerk JE, Pell CL, Johnstone BH, Considine RV, March KL. Secretion of angiogenic and antiapoptotic factors by human adipose stromal cells. Circulation. 2004;109:1292–1298. doi: 10.1161/01.CIR.0000121425.42966.F1. [DOI] [PubMed] [Google Scholar]
- 40.Rotter V, Nagaev I, Smith U. Interleukin-6 (IL-6) induces insulin resistance in 3T3-L1 adipocytes and is, like IL-8 and tumor necrosis factor-alpha, overexpressed in human fat cells from insulin-resistant subjects. J Biol Chem. 2003;278:45777–45784. doi: 10.1074/jbc.M301977200. [DOI] [PubMed] [Google Scholar]
- 41.Ruan H, Lodish HF. Insulin resistance in adipose tissue: direct and indirect effects of tumor necrosis factor-alpha. Cytokine Growth Factor Rev. 2003;14:447–455. doi: 10.1016/s1359-6101(03)00052-2. [DOI] [PubMed] [Google Scholar]
- 42.Rui L, Yuan M, Frantz D, Shoelson S, White MF. SOCS-1 and SOCS-3 block insulin signaling by ubiquitin-mediated degradation of IRS1 and IRS2. J Biol Chem. 2002;277:42394–42398. doi: 10.1074/jbc.C200444200. [DOI] [PubMed] [Google Scholar]
- 43.Sartipy P, Loskutoff DJ. Monocyte chemoattractant protein 1 in obesity and insulin resistance. Proc Natl Acad Sci USA. 2003;100:7265–7270. doi: 10.1073/pnas.1133870100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sell H, Dietze-Schroeder D, Kaiser U, Eckel J. Monocyte chemotactic protein-1 is a potential player in the negative cross-talk between adipose tissue and skeletal muscle. Endocrinology. 2006;147:2458–2467. doi: 10.1210/en.2005-0969. [DOI] [PubMed] [Google Scholar]
- 45.Skurk T, Herder C, Kraft I, Muller-Scholze S, Hauner H, Kolb H. Production and release of macrophage migration inhibitory factor from human adipocytes. Endocrinology. 2005;146:1006–1011. doi: 10.1210/en.2004-0924. [DOI] [PubMed] [Google Scholar]
- 46.Suganami T, Nishida J, Ogawa Y. A paracrine loop between adipocytes and macrophages aggravates inflammatory changes: role of free fatty acids and tumor necrosis factor alpha. Arterioscler Thromb Vasc Biol. 2005;25:2062–2068. doi: 10.1161/01.ATV.0000183883.72263.13. [DOI] [PubMed] [Google Scholar]
- 47.Sumita C, Yamane M, Matsuda T, Maeda M, Nariai T, Fujio Y, Azuma J. Platelet activating factor induces cytoskeletal reorganization through Rho family pathway in THP-1 macrophages. FEBS Lett. 2005;579:4038–4042. doi: 10.1016/j.febslet.2005.06.022. [DOI] [PubMed] [Google Scholar]
- 48.Talior I, Yarkoni M, Bashan N, Eldar-Finkelman H. Increased glucose uptake promotes oxidative stress and PKC-δ activation in adipocytes of obese, insulin-resistant mice. Am J Physiol Endocrinol Metab. 2003;285:E295–E302. doi: 10.1152/ajpendo.00044.2003. [DOI] [PubMed] [Google Scholar]
- 49.Ueki K, Kondo T, Kahn CR. Suppressor of cytokine signaling 1 (SOCS-1) and SOCS-3 cause insulin resistance through inhibition of tyrosine phosphorylation of insulin receptor substrate proteins by discrete mechanisms. Mol Cell Biol. 2004;24:5434–5446. doi: 10.1128/MCB.24.12.5434-5446.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Uysal KT, Wiesbrock SM, Marino MW, Hotamisligil GS. Protection from obesity-induced insulin resistance in mice lacking TNF-alpha function. Nature. 1997;389:610–614. doi: 10.1038/39335. [DOI] [PubMed] [Google Scholar]
- 51.Wang B, Trayhurn P. Acute and prolonged effects of TNF-alpha on the expression and secretion of inflammation-related adipokines by human adipocytes differentiated in culture. Pflügers Arch. 2006;452:418–427. doi: 10.1007/s00424-006-0055-8. [DOI] [PubMed] [Google Scholar]
- 52.Weisberg SP, Hunter D, Huber R, Lemieux J, Slaymaker S, Vaddi K, Charo I, Leibel RL, Ferrante AW. CCR2 modulates inflammatory and metabolic effects of high-fat feeding. J Clin Invest. 2005;116:115–124. doi: 10.1172/JCI24335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weisberg SP, McCann D, Desai M, Rosenbaum M, Leibel RL, Ferrante AW., Jr Obesity is associated with macrophage accumulation in adipose tissue. J Clin Invest. 2003;112:1796–1808. doi: 10.1172/JCI19246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu H, Barnes GT, Yang Q, Tan G, Yang D, Chou CJ, Sole J, Nichols A, Ross JS, Tartaglia LA, Chen H. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112:1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yang Q, Graham TE, Mody N, Preitner F, Peroni OD, Zabolotny JM, Kotani K, Quadro L, Kahn BB. Serum retinol binding protein 4 contributes to insulin resistance in obesity and type 2 diabetes. Nature. 2005;436:356–362. doi: 10.1038/nature03711. [DOI] [PubMed] [Google Scholar]
- 56.Yuan M, Konstantopoulos N, Lee J, Hansen L, Li ZW, Karin M, Shoelson SE. Reversal of obesity- and diet-induced insulin resistance with salicylates or targeted disruption of Ikkbeta. Science. 2001;293:1673–1677. doi: 10.1126/science.1061620. [DOI] [PubMed] [Google Scholar]