Abstract
In-depth chemical understanding of complex biological processes hinges upon the ability to systematically perturb individual systems. However, current approaches to study impacts of biologically relevant reactive small molecules involve bathing of the entire cell or isolated organelle with excess amounts, leading to off-target effects. The resultant lack of biochemical specificity has plagued our understanding of how biological electrophiles mediate signal transduction or regulate responses that confer defense mechanisms to cellular electrophilic stress. Here we introduce a target-specific electrophile delivery platform that will ultimately pave the way to interrogate effects of reactive electrophiles on specific target proteins in cells. The new methodology is demonstrated by photoinducible targeted delivery of 4-hydroxynonenal (HNE) to the proteins Keap1 and PTEN. Covalent conjugation of the HNE-precursor to HaloTag® fused to the target proteins enables directed HNE delivery upon photoactivation. The strategy provides proof of concept of selective delivery of reactive electrophiles to individual electrophile-responsive proteins in mammalian cells. It opens a new avenue enabling more precise determination of the pathophysiological consequences of HNE-induced chemical modifications on specific target proteins in cells.
This communication demonstrates a new way to study impacts of reactive small molecules, such as electrophilic lipids, on specific proteins in living cells. A well-established example of a reactive lipid is 4-hydroxynonenal (HNE), a key end-product of polyunsaturated fatty acids (PUFAs) peroxidation.1 HNE can result either from reactive oxygen species-initiated peroxidation of PUFAs, or from lipooxygenases-mediated peroxidation of PUFAs.2 HNE was classified for many years as a non-specific, highly reactive entity implicated in causing neurodegeneration in Alzheimer’s disease.3 However, mounting evidence has highlighted HNE’s cell signaling roles.4 Although these pioneering studies have opened up new lines of investigations, progress is hindered because presently there exists no method to study impact of this reactive Michael acceptor in a temporally controlled or targeted manner (neither to individual proteins nor subcellular compartments).5
Current strategy to probe the effects of electrophilic small molecule modulators, such as HNE, on individual proteins or pathways of interest involves treatment of the entire cell,1–5 oftentimes with non-physiological quantities of the reactive electrophile (Figure 1a). The strategy clearly leads to a large amount of noise due to off-target effects. Whole proteome or isolated organelle incubation with µM–mM HNE also precludes rigorous assessments on HNE’s regulatory capacity where physiological amounts (< 0.1–1 µM) are thought to trigger specific responses.1,6 In fact, there exist >3,400 reports on pathophysiological functions of HNE.7 Myriad regulatory proteins have been implicated to participate in HNE-induced signaling at submicromolar HNE concentrations. However, whether the observed responses stem from on vs. off-target effects of HNE remains unclear.
Figure 1.
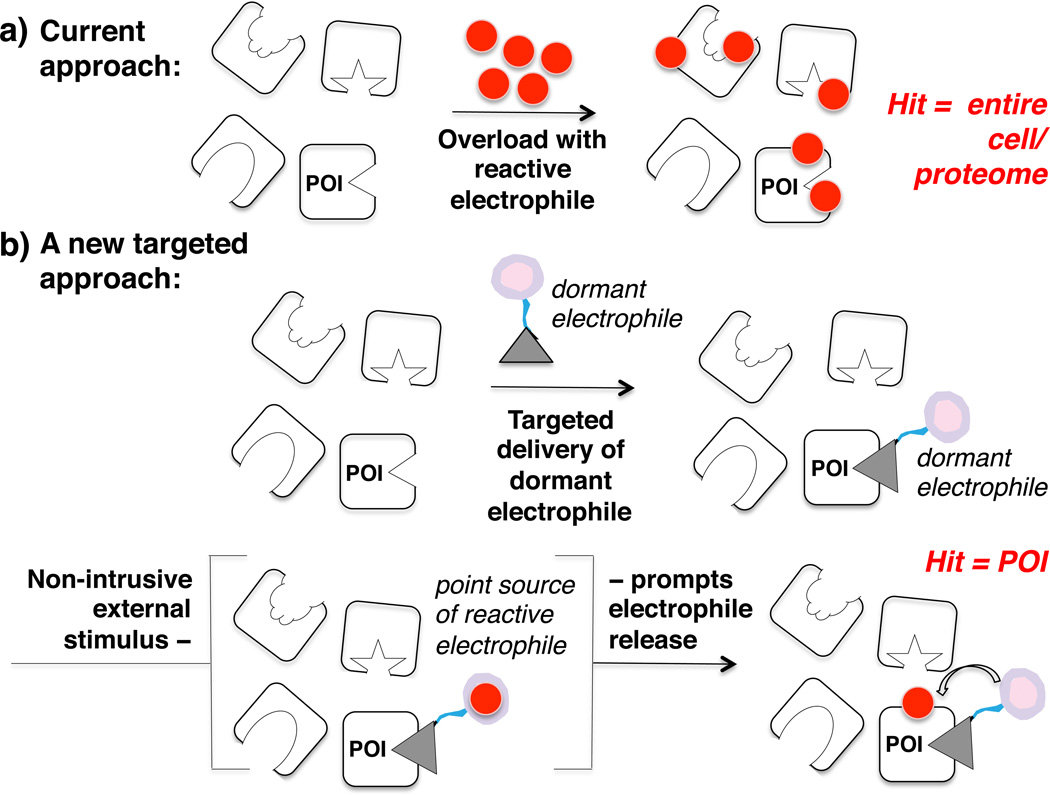
(a) Current approach to study impacts of reactive electrophiles on protein of interest (POI) involves treatment of entire cell/proteome with a reactive entity, unavoidably leading to multiple off-target effects. Red, HNE. (b) A new approach to interrogate with biochemical specificity effects of electrophiles on individual proteins. The strategy focuses on a time-resolved targetspecific delivery of electrophiles to a single POI against the entire cell/proteome. The synthetic delivery platform ‘dormant electrophile’ shown has 3 modules: gray, high affinity/specificity ligand to POI; blue, flexible linker; pink, inert warhead – a caged HNE.
To overcome this loss in biochemical specificity, we conceived a biocompatible chemical platform that would for the first time enable a controlled perturbation of a specific electrophile-responsive protein of interest (POI) with HNE in living cells. The platform is designed such that the amount of reactive electrophile liberated is equivalent to the concentration of POI, conditions that would mimic endogenous signaling directed to one target protein. Shown schematically in Figure 1b, the first step involves a high specificity and affinity ligand (gray triangle) binding the POI. For POIs with no known ligands, known functionalizable protein tags may be fused to the POI. The POI or the tag fused to POI is tethered via a flexible linker (blue line) to a caged HNE (pink sphere). The cell-permeable small molecule should remain bound to the POI while the unbound molecule can be washed out, so that at the point of photoactivated release, HNE is only available in the cell at concentrations similar to POI. At a precise time, a non-intrusive stimulation with external light activates a rapid and localized HNE generation.
Unlike free radicals, longer half-lives of reactive aldehydes such as HNE render them predisposed to diffusion from original sites of production.8 However, because the targeted platform ensures HNE release on or proximal to POI, and because HNE is released in stoichiometric (or lesser) amounts relative to POI, we hypothesized that adduction of an electrophile-responsive POI by the liberated HNE would be sufficiently rapid so that covalent conjugation would offset potential diffusion. Effects arising from any leakage of the liberated HNE to the surroundings are also likely averaged over the entire proteome.
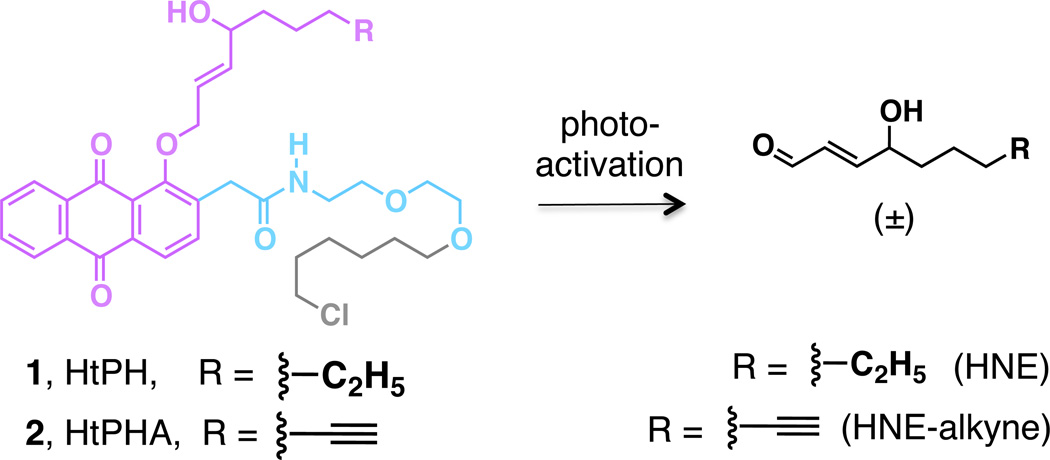
We first set about synthesis of time-resolved targeted HNE delivery systems (Figure 2). To target HNE to a specific POI, HaloTag® genetic encoding strategy9 was selected. This enzyme is an engineered bacterial dehalogenase which is able to react with chloroalkanes to form a covalent enzyme-bound intermediate, via SN2 attack of an Asp at an alkylchloride function. However, unlike in the wild type dehalogenase, the intermediate O-alkyl ester is hydrolytically stable.9 We chose HaloTag® because (1) O-alkyl ester bond formation between the Asp residue of Halo and the chloroalkane ligand is irreversible and highly specific. (2) A range of sterically demanding groups are tolerated on the chloroalkane ligand, demonstrated by synthetic fluorophores.9 (3) Expression vectors encoding 20,000 human and 15,000 mouse genes with HaloTag® are commercially available,10 thus in principle, any desired POI can be investigated using this platform. In the design of the HNE-delivery module, we were encouraged by the previously reported 1-alkoxyanthraquinone-based aldehyde caging strategy where photoinducible HNE release has been demonstrated in aqueous methanol.11 We thus aimed to establish our new approach using this photocaged-HNE system. HaloTag PreHNE (HtPH) (1) and HaloTag PreHNE-alkyne (HtPHA) (2) (Figure 2) were thus synthesized (Scheme S1–2), and evaluated for the time-dependent HNE liberation upon photoactivation (Figure S1). Photolysis (4 W, 365 nm) promoted HNE release that reached saturation within 30 min at 37 °C for both HtPH and HtPHA (Figure S1b).
Figure 2.
Photoinducible HNE-targeting on HaloTag fusion proteins made possible by HaloTag PreHNE, HtPH (1, R = CH2CH3) and HaloTag PreHNE-alkyne, HtPHA (2, R = C2H). Also see Figure S1. Color code refers to the schematic representation of dormant electrophile in Figure 1b.
Keap1 (Kelch-like, ECH-associated protein 1), a key electrophile-sensing regulatory protein in humans, was chosen to test the idea of selective targeting. Keap1 is a central regulator of a major antioxidant signaling pathway that responds to electrophilic stress and promotes cell survival.12 Keap1 is highly susceptible, by virtue of its nucleophilic cysteines, to post-translational modifications by reactive electrophiles including HNE.13 These chemical modulations are thought to in turn influence downstream cell signaling.
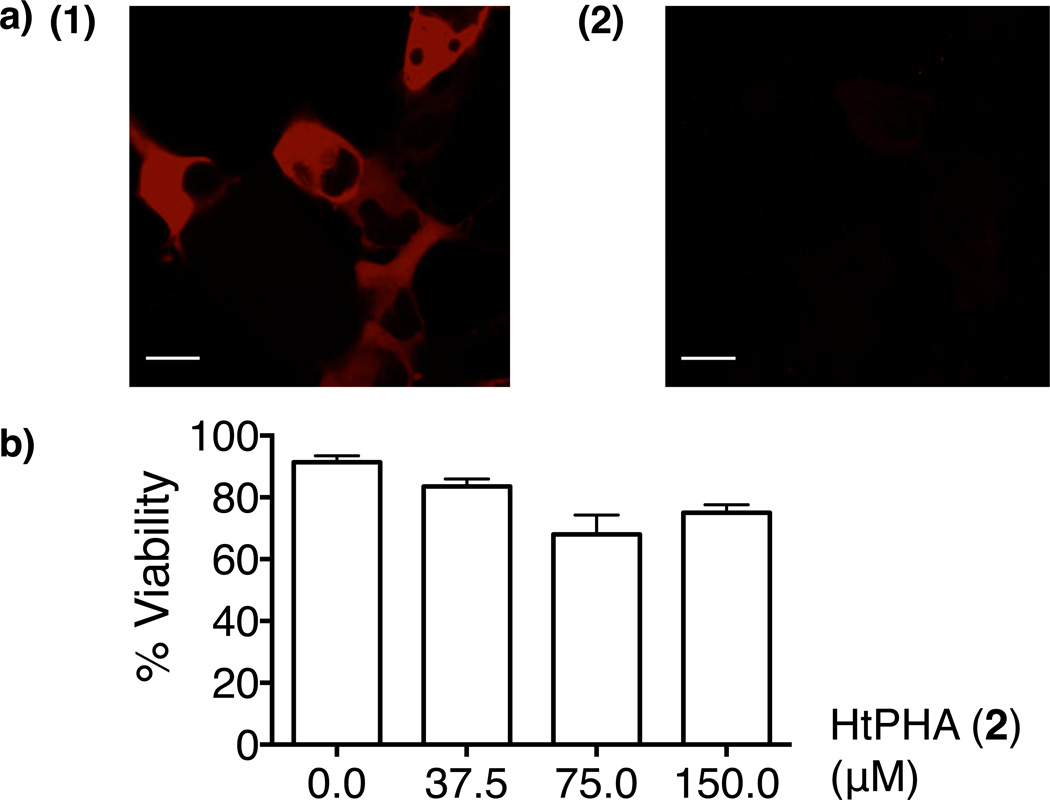
Using HaloKeap1 as a model protein, we first showed the designed HNE-delivery platforms were biocompatible. To demonstrate cell-permeability and affinity of HtPH (1) to HaloKeap1, we used a commercial chloroalkane ligand-conjugated TMR dye (HaloTag® TMR) that binds selectively to Halo-tagged proteins labeling them with the TMR red dye. Initially, treatment of COS-1 cells overexpressing HaloKeap1 with HaloTag® TMR resulted in cytosolic localization of the red dye post wash out (Figure 3a-1). Since Keap1 localizes to the cytosol,12 this is consistent with TMR selectively labeling the functional HaloKeap1 fusion protein. Addition of 50 µM HtPH (1) for 1 h prior to addition of HaloTag® TMR prevented labeling of the COS-1 cells overexpressing HaloKeap1 (Figure 3a-2). This result showed that HtPH (1) can saturate to the Halo domain within HaloKeap1 in the cells rendering, to the limit of detection, all HaloKeap1 unable to be dye-labeled. This observation also suggests that previously unrecognized strategy to deliver HNE to a subcellular compartment such as cytosol can be possible. In addition, analysis of cell morphology and flow cytometry dye exclusion assays of COS-1 cells subsequent to HtPH (1) and HtPHA (2) treatments and propidium iodide staining, showed both of our compounds had a low effect on viability over this period (Figure 3b).
Figure 3.
(a) Designed ligand is cell-permeable. COS-1 cells transiently overexpressing cytosolic HaloKeap1 were treated with either (1) 5 µM HaloTag® TMR alone, or (2) 50 µM HtPH (1, Figure 2) for 1 h prior to 5 µM HaloTag® TMR. Scale bars, 20 µM. (b) Designed ligand is non-cytotoxic. Representative data from flow cytometry dye-exclusion assays on viability of HtPHA (2, Figure 2) treated (3 h) COS-1 cells. Error bars are SD (N=3).
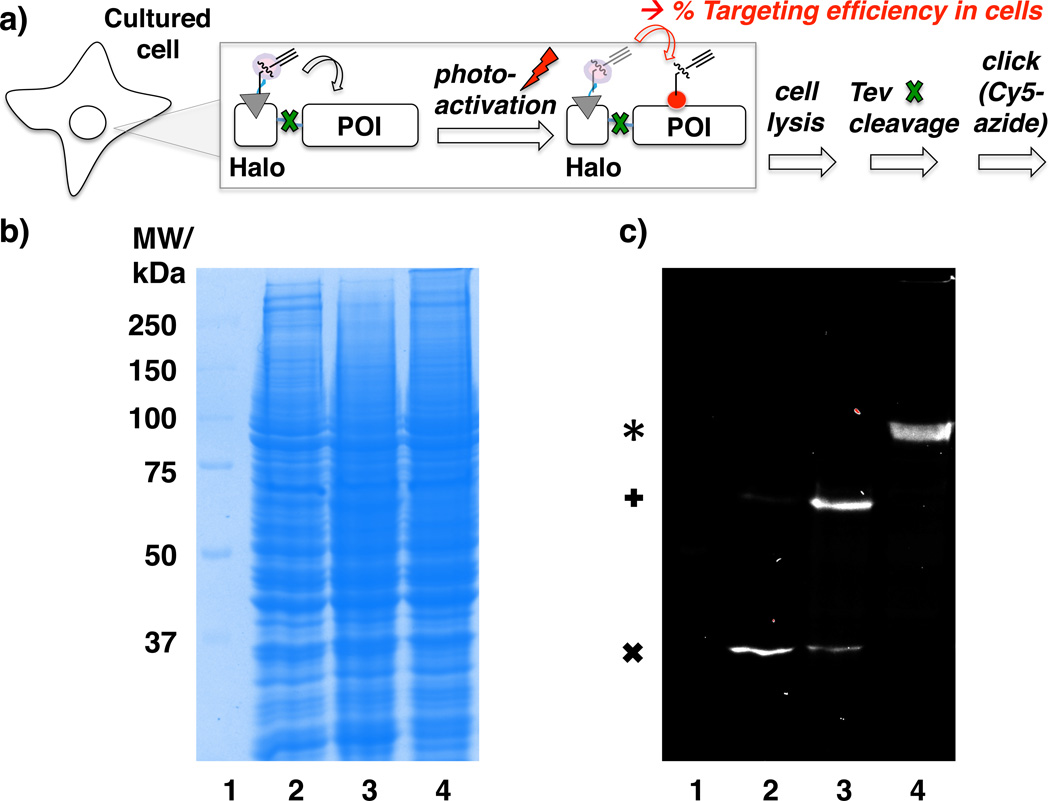
Our next step was to test the possibility of targeted delivery of HNE in cells. To enable quantitation of % targeting efficiency in cells, we used a simple set up that allows separation of the recognition domain (Halo) from the fused target protein (Keap1) (Figure 4a). We made modifications to the HaloKeap1 construct such that a Tev protease recognition site was placed in between the two domains (‘Halo–Keap1’, Figure S2). The positioning of the Tev site permits selective Tev-protease-mediated cleavage and separation of Halo and Keap1 subsequent to cell lysis, thus allowing Keap1-targeted in-cell delivery of HNE-alkyne from HtPHA (2) to be assessed using click chemistry.14 The physical separation of the HaloTag from the POI is necessary because alkyne function reports on the presence of both HNE-precursor covalently bound to Halo as well as the liberated HNE (Figure 4a). The Tev-cleavage set up enables quantitation of % targeting efficiency, where the Cy5 signal intensity on the Halo band after the Tev-cleavage from the sample not exposed to light serves as an input signal, while that on POI (Keap1) band after the Tev-cleavage from the sample exposed to light is the output.
Figure 4.
(a) Directed HNE targeting strategy demonstrated by selective Keap1 targeting in living cells. COS-1 cells transiently expressing DsRed and Halo–Keap1 (Figure S2) were treated with 25 µM HtPHA (2) for 2.5 h. Subsequent to exposure to a 4 W 365 nm lamp (20 min), cells were lysed, lysate was treated with Tev protease, followed by Cy5-Click assay reagents. This set up enables quantitation of % targeting efficiency: Cy5 signal intensity on Halo band prior to light exposure serves as the input, and that on the Keap1 band post photo-uncaging and Tev-cleavage serves as the output. (b) A representative resultant SDS-PAGE with Coomassie staining. (c) In-gel Cy5-fluorescence analysis of the same SDS-PAGE. Lane 1, MW ladder; Lane 2, Cells not exposed to light; Lane 3, Cells exposed to light; Lane 4, No Tev cleavage in lysate. *, Halo–Keap1 (104 kDa); +, Keap1 (70 kDa); x, Halo (33 kDa). From independent replicates, average Cy5 signal intensity transfer from Halo band before light exposure, to Keap1 band after light exposure, is quantitated to be 55 ± 13%.
The cells were treated with 25 µM HtPHA (2) for 2.5 h and exposed to 4 W, 365 nm lamp for 20 min. Cell lysis was followed by Tev cleavage to separate Halo and Keap1 (Figure 4a). Click coupling of Cy5-azide dye to the terminal alkyne was subsequently performed. The alkyne moiety could either exist within the unreacted HtPHA (2) conjugated to Halo, within released HNE-alkyne adducted with Cys-rich Keap1, or within liberated HNE-alkyne conjugated to other cellular proteins. The data showed efficient transfer of Cy5 fluorescence signal to a band corresponding to MW of Keap1 (Figure 4c, lane 3). There was negligible fluorescence observed in the bulk cellular proteins. In controls with either no light (lane 2) or no Tev (lane 4) treatment, the signal respectively located to Halo or Halo–Keap1. We also showed that delivery of HNE to Keap1 required an interaction between HtPHA (2) and Halo domain by addition of an unsubstituted chloroalkane ligand (3) (Scheme S1) (i.e., without the pink warhead in Figure 1b) to the cells prior to addition of HtPHA (2). This procedure would block the Halo domain binding site similarly to the experiment shown in Figure 3a and render Halo unable to bind to HtPHA (2). Under these conditions, no Cy5 signal was observed (Figure S4b, lane 7). As a corollary, the use of PreHNE-alkyne (PHA) (4) (Scheme S3), which does not have a chloroalkane recognition motif, showed no Cy5-fluorescence (Figure S4b, lane 5).
The data from independent replicates revealed that 55 ± 13% of Cy5 signal was transferred from Halo to Keap1 subsequent to photoactivation (Figure 4c and S4). The fact that PreHNE-alkyne is present in stoichiometric amounts to Keap1 prior to light exposure, as suggested by the blocking experiments (Figure 3a, Figure S4b, lane 7, Figure S5b, lane 4), further indicates that the 55% ± 13% of the total POI in the proteome is HNEylated using the targeted approach. The data is consistent with directed delivery of HNE from Halo to Keap1. We note that the observed selective targeting was not due to artifacts from high levels of overexpression of Halo–Keap1 since SDS-PAGE analysis (Figure 4b) showed many bands of equal or greater intensity to Keap1 in the lysate, underscoring that delivery is specific to Keap1. We also note that because of the bicistronic expression system (Figure S2) used in our gene delivery, DsRed protein was simultaneously overexpressed at either equal or higher concentrations than Keap1.15 No Cy5 signal was observed around 28 kDa, MW of DsRed (Figure 4c), consistent with absence of non-specific targeting.
We next decided to test the concept on tumor suppressor protein PTEN (phosphatase and tensin homolog), an upstream antagonist of Akt-oncogenic signaling.16 This protein represents a sterner test of our methodology because unlike Keap1, reports on sensitivity of PTEN to electrophiles such as HNE are limited.17 HNE-directed targeting to Halo–PTEN (Figure S3) expressed in HEK cells was found to yield a similar outcome (Figure S5). As in Keap1, results from control experiments with Halo–PTEN suggested targeted delivery. The lower targeting efficiency of 28 ± 10% likely reflects the lower electrophile sensitivity of PTEN over Keap1. We also chose to compare our method to conventional approach of bathing the cells with HNE. HNE-alkyne (HA) (5) (Scheme S4) was synthesized and added to HEK cells at 25 µM, a concentration previously used to study on PTEN signaling triggered by HNE.17b Unsurprisingly, global treatment showed non-specific targeting by the reactive enal (Figure S5b, lane 5).
In summary, we have been able to demonstrate selective delivery of a reactive, biologically relevant small molecule to specific electrophile-responsive proteins of interest. The strategy was demonstrated in living cells on targeting Keap1 and PTEN proteins with HNE. The fact that HNE adduction was observed on two different molecular weight proteins rules out delivery to a contaminating protein present in the cells which can take up liberated HNE. Our approach offers a way to selectively perturb a single POI in the POI’s microenvironment with a reactive electrophile, with absolute spatiotemporal control. Although an initial step, this methodology opens doors to understand how various reactive small molecule electrophiles modulate non-enzyme-mediated post-translational modifications underlying redox-linked cell signaling processes that are governed by electrophile sensing Keap1 or other redoxregulatory proteins. The methodology is not limited to probing the effects of reactive aldehydes such as HNE. Direct targeting effects of other reactive and transient entities such as NO and H2O24b,18 are also currently being studied in our laboratory.
Supplementary Material
ACKNOWLEDGMENT
Y.A. thanks the Howard and Abby Milstein Foundation. M.J.C.L acknowledges the Howard Hughes Medical Institute for an international predoctoral student fellowship. We thank Dr. Anthony Condo Jr. for help with LDI mass characterizations on compounds 1, 2 and 4 which were carried out at the Cornell Center for Materials Research Shared Facilities supported through NSF MRSEC program (DMR-1120296). Cornell Biotech Resource Center Imaging Facility is acknowledged for the use of the confocal fluorescence microscope.
Funding Sources
This research was supported by the Cornell University junior faculty startup funds and the Milstein New Faculty Fellowship (to Y.A).
Footnotes
ASSOCIATED CONTENT
Supporting Information. Experimental details. Small-molecule synthesis procedure and characterization data. Additional biochemical data. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interests.
REFERENCES
- 1.Schopfer FJ, Cipollina C, Freeman BA. Chem. Rev. 2011;111:5997. doi: 10.1021/cr200131e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schneider C, Porter NA, Brash AR. J. Biol. Chem. 2008;28:15539. doi: 10.1074/jbc.R800001200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butterfield DA, Drake J, Pocernich C, Castegna A. Trends. Mol. Med. 2001;7:548. doi: 10.1016/s1471-4914(01)02173-6. [DOI] [PubMed] [Google Scholar]
- 4.(a) Forman HJ, Fukuto JM, Miller T, Zhang H, Rinna A, Levy S. Arch. Biochem. Biophys. 2008;477:183. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jacob C, Winyard PG, editors. Redox Signaling and Regulation in Biology and Medicine. Weinheim: Wiley; 2009. [Google Scholar]
- 5.(a) Liebler DC. Chem. Res. Toxicol. 2008;21:117. doi: 10.1021/tx700235t. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jacobs AT, Marnett LJ. Acc. Chem. Res. 2010;43:673. doi: 10.1021/ar900286y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siems W, Grune T. Mol. Asp. Med. 2003;24:167. doi: 10.1016/s0098-2997(03)00011-6. [DOI] [PubMed] [Google Scholar]
- 7. [Last accessed Aug 30 2013]; www.ncbi.nlm.nih.gov/pubmed.
- 8.Butterfield DA, Stadtman ER. Adv. Cell Aging Gerontol. 1997;2:161. [Google Scholar]
- 9.Los GV. ACS Chem. Biol. 2008;3:373. doi: 10.1021/cb800025k. [DOI] [PubMed] [Google Scholar]
- 10.See, for example: [Last accessed Aug 30 2013]; http://www.genecopoeia.com/tech/halo-tag/.
- 11.Brinson RG, Jones PB. Org. Lett. 2004;6:3767. doi: 10.1021/ol048478l. [DOI] [PubMed] [Google Scholar]
- 12.(a) Kensler TW, Wakayayashi N, Biswal S. Annu. Rev. Pharmacol. Toxicol. 2007;47:89. doi: 10.1146/annurev.pharmtox.46.120604.141046. [DOI] [PubMed] [Google Scholar]; (b) Hur W, Gray NS. Curr. Opin. Chem. Biol. 2011;15:162. doi: 10.1016/j.cbpa.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 13.See, for example: Levonen A-L, Landar A, Ramachandran A, Ceaser EK, Dickinson DA, Zanoni G, Morrow JD, Darley-Usmar VM. Biochem. J. 2004;378:373. doi: 10.1042/BJ20031049. Kode A, Rajendrasozhan S, Caito S, Yang S-R, Megson IL, Rahman I. Am. J. Physiol. Lung. Cell. Mol. Physiol. 2007;294:L478. doi: 10.1152/ajplung.00361.2007.
- 14.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. Angew. Chem., Int. Ed. 2002;41:2596. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 15.(a) Yen HC, Xu Q, Chou DM, Zhao Z, Elledge SJ. Science. 2008;322:918. doi: 10.1126/science.1160489. [DOI] [PubMed] [Google Scholar]; (b) Pan Y, Long MJC, Lin H-C, Hedstrom L, Xu B. Chem. Sci. 2012;3:3495. [Google Scholar]
- 16.Manning BD, Cantley LC. Cell. 2007;129:1261. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.(a) Covey TM, Edes K, Coombs GS, Virshup DM, Fitzpatrick FA. PLos One. 2010;5:e13545. doi: 10.1371/journal.pone.0013545. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Shearn CT, Smathers RL, Stewart BJ, Fritz KS, Galligan JJ, Hail N, Jr, Petersen DR. Mol. Pharm. 2011;79:941. doi: 10.1124/mol.110.069534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Halliwell BB, Gutteridge JMC. Free Radicals in Biology and Medicine. 4th Ed. Oxford: Oxford University Press; 2007. [Google Scholar]; (b) Paulsen CE, Carroll KS. ACS Chem. Biol. 2010;5:47. doi: 10.1021/cb900258z. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Murphy MP, Holmgren A, Larsson N-G, Halliwell B, Chang CJ, Kalyanaraman B, Rhee SG, Thornalley PJ, Patridge L, Gems D, Nyström T, Belousov V, Schumacker PT, Winterbourn CC. Cell. Metab. 2011;13:361. doi: 10.1016/j.cmet.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.