Abstract
Obesity resistance due to elevated orexin signaling is accompanied by high levels of spontaneous physical activity (SPA). The behavioral and neural mechanisms underlying this observation have not been fully worked out. We determined the contribution of hypothalamic orexin receptors (OXR) to SPA stimulated by orexin A (OXA), whether OXA-stimulated SPA was secondary to arousal and whether voluntary wheel running led to compensations in 24-h SPA. We further tested whether orexin action on dopamine one receptors (DA1R) in the substantia nigra (SN) plays an important role in generation of SPA. To test this, SPA response was determined in lean and obese rats with cannulae targeted towards the rostral lateral hypothalamus (rLH) or SN. Sleep/wake states were also measured in rats with rLH cannula and EEG/EMG radiotelemetry transmitters. SPA in lean rats was more sensitive to antagonism of the orexin 1 receptor (OX1R) and in the early response to the orexin 2 agonist. OXA increased arousal equally in lean and obese rodents, which is discordant from the greater SPA response in lean rats. Obesity resistant rats ran more and wheel running was directly related to 24-h SPA levels. The OX1R antagonist, SB-334867-A, and the DA1R antagonist, SCH3390, in SN more effectively reduced SPA stimulated by OXA in OR rats. These data suggest OXA-stimulated SPA is not secondary to enhanced arousal, propensity for SPA parallels inclination to run and that orexin action on dopaminergic neurons in SN may participate in mediation of SPA and running wheel activity.
Obesity resistance that is accompanied by elevated orexin signaling is also associated with high levels of spontaneous physical activity (SPA) (Kotz et al., 2012). Orexin A (OXA, also referred to as hypocretin 1), a neuropeptide synthesized in discrete areas within the lateral, perifornical, and dorsomedial hypothalamus (de Lecea et al., 1998, Sakurai et al., 1998), is crucial for normal energy homeostasis and arousal. Spontaneous physical activity stimulated by central OXA infusion induces weight loss (Novak and Levine, 2009) while mice lacking OXA are obese and have lower physical activity despite lower energy intake compared to wild-type mice (Hara et al., 2001), highlighting the critical energy balance role of OXA-stimulated SPA. Central OXA infusion also stimulates arousal and lack of endogenous orexin or orexin receptors disrupts sleep/wake patterns, so it has been suggested that orexin-stimulated SPA may be secondary to arousal. Spontaneous physical activity induced by orexin also increases energy expenditure, but the contribution of this and voluntary activity such as wheel running to total daily energy expenditure is unclear in rodents. Thus whether the SPA increase translates into an overall daily increase in energy output remains to be determined since there is the possibility of compensation in one physical activity compartment for changes in another.
It is clear that activation of orexin receptors (OX1R and OX2R) modulates SPA, but the pathway linking orexin neuronal action to SPA remains relatively undefined. The selective OX1R antagonist, SB-334867-A, reduces OXA-stimulated increases in energy expenditure (Kiwaki et al., 2004) and several types of physical activity (Duxon et al., 2001, Jones et al., 2001, Rodgers et al., 2001, Kiwaki et al., 2004). Antagonism of both orexin receptors reduces overall physical activity (Brisbare-Roch et al., 2007, Whitman et al., 2009, Winrow et al., 2011), oxygen consumption (Li and Nattie, 2010) as well orexin B and amphetamine-stimulated physical activity (Bergman et al., 2008, Winrow et al., 2010). In addition, it has been found that dopamine receptor antagonists reduce OXA-stimulated physical activity (Nakamura et al., 2000, Matsuzaki et al., 2002, Kotz et al., 2006), and a large body of work has revealed differences in dopamine neurotransmission between obese and lean rodents (Levin et al., 1986, Yang and Meguid, 1995, Fetissov et al., 2002, Geiger et al., 2008, Waters et al., 2008, Rada et al., 2010, Garland et al., 2011). The sites of action for orexin on dopaminergic neurons have not been fully established, but one possible site is the substantia nigra (SN)(Kotz et al., 2006, Kotz et al., 2008).
Based on our past work showing elevated 24-h SPA, OXA-stimulated SPA, OXR mRNA and protein levels in lean obesity resistant (OR) rats (Novak et al., 2006, Teske et al., 2006, Kotz et al., 2012), we sought to investigate brain and behavior-related mechanisms underlying these results. We hypothesized that in OR rats 1) orexin antagonists in hypothalamus would be more effective at blocking OXA-stimulated SPA; 2) orexin agonists would be more effective in stimulating SPA; 3) that OXA-stimulated arousal would be similar in lean and obese rats, while OXA stimulation of SPA would be greater in lean rats; 4) propensity to run would be greater in lean rats and directly proportional to 24-h SPA level; and 5) OXA-stimulated SPA after infusion into SN would be greater in lean rats and that antagonism of orexin and dopamine receptors would block the induced SPA more effectively in the lean rats.
1. EXPERIMENTAL PROCEDURES
1.1 Animals
Male Sprague-Dawley and selectively-bred obesity prone (OP) and OR rats (Charles River, Kingston, NY) were housed individually in either wire-hanging cages or solid-bottom cages in corn cobb bedding with a 12-h light/12-h dark photo-cycle (lights on at 0600 h) in a temperature controlled room (21–22 °C). Rodent chow (Harlan Teklad 8604) and water were allowed ad libitum. Studies were approved by the Institutional Animal Care and Use Committee at the Minneapolis VA Health Care System and the University of Minnesota. Four sets of rats were used. The first set of rats was used for studies one and two. A second set of rats was used for study three and the third for study four. A fourth set of rats was used for studies five and six.
1.2 Surgery
Animals were anesthetized with Ketamine (50 mg/kg) and Xylazine (15 mg/kg) and 26-gauge stainless steel unilateral cannulae (Plastics One, Roanoke, VA) were directed towards the rostral lateral hypothalamus (rLH) or SN as described previously (Kotz et al., 2006, Teske et al., 2006). Stereotaxic coordinates were determined from the rat brain atlas of Paxinos and Watson (Paxinos et al., 1985). The coordinates for the rLH and SN, respectively, were as follows: −2.2 and −5.3 mm posterior, 1.9 and 2.4 mm lateral to bregma, and 7.3 and 7.6 mm below the skull surface. For all cannulations, the incisor bar was set at 3.3 mm below the ear bars. A dummy stylet was placed in the guide cannula that extended to the tip of the cannula after surgery and between injections. Animals were allowed to recover from surgery for at least seven days before experimental trials began. One OP, one OR, and two Sprague-Dawley rats died of unknown cause after surgery.
In study three, animals were implanted with electroencephalogram (EEG) electrodes, electromyogram (EMG) electrodes and a transmitter (F40-EET, Data Sciences International, St. Paul, MN) to allow recording of sleep/wake states by radiotelemetry in addition to the rLH cannula as described previously (Mavanji et al., 2010). After cannula implantation, bilateral surface EEG electrodes (3.1mm posterior and 1.5mm lateral to bregma) (Paxinos et al., 1985) were secured through bur holes to contact the dura to record future cortical EEG. Then two leads were secured in the nuchal muscles to record EMG. The transmitter was placed in a blunt dissected channel across the animals flank. Animals were returned to plastic solid-bottom cages with corn-cob rodent bedding after surgery and remained in the solid-bottom cages through out the duration of this study.
1.3 Drugs
Orexin A (American Peptides, Sunnyvale, CA), the selective OX2R agonist (Ala11, D-Leu15-Orexin B (Asahi et al., 2003) (Tocris, Ellisville, MO), and the selective dopamine 1 receptor (DA1R) antagonist (SCH3390) (Sigma, St. Louis, MO) were dissolved in artificial cerebrospinal fluid. The selective OX1R antagonist (SB-334867-A) was provided as a gift by GlaxoSmithKline and was dissolved in 5% dimethyl sulfoxide in sterile water. Artificial cerebrospinal fluid was used as the vehicle control for studies with OXA and SCH3390. Five percent dimethyl sulfoxide in sterile water was used as the vehicle control for studies with SB-334867-A. All drugs were stored at 4°C for less than 48-h.
1.4 Injections
A volume of 0.5 μl was injected slowly over 30 s with a 33-gauge injector (Plastics One, Roanoke, VA) that extended 1.0 mm beyond the tip of the guide cannula as described previously (Kotz et al., 2002, Kotz et al., 2006, Teske et al., 2006). The injector was left in place an additional 10 s to ensure extrusion from the tip and to minimize distribution of the drug upwards on the cannula tract. After injection, the injector was withdrawn and the stylet replaced. The first set of rats received 16 injections, the second set of rats received 16 injections, and the third set of rats received two injections in total. In previous studies, lack of extensive tissue damage after 50 repeated injections as measured by gliosis around the injection site and light microscopy at 100x was demonstrated (Picker et al., 1999). Cannula placement was verified physiologically based on the reported SPA response to 250pmol/0.5uL OXA in the rLH in OP, OR and Sprague-Dawley rats (Teske et al., 2006) and the SPA response to 500pmol/0.5uL orexin A in the substantia nigra in Sprague-Dawley rats (Kotz et al., 2008). Rats in the current studies were deemed to have correctly placed cannuale if the respective SPA response to OXA was within the mean +/− standard deviation reported in these studies (Kotz et al., 2006, Teske et al., 2006). Based on this physiological verification, 54 out of 58 rats had correctly placed cannulae and these data were included in the analyses. Based on this verification and our previous experience with rLH and SN cannulations (Sweet et al., 1999, Kotz et al., 2002, Thorpe et al., 2005b, Kotz et al., 2006, Teske et al., 2006), we are confident that a high percentage (≈95%) of the animals had correctly placed cannulas. Injections were performed between 0800 and 1100h and at least 48-h elapsed between injections.
1.5 SPA measurement
SPA was measured using customized, high precision racks of infrared activity sensors (Med Associates, St. Albans, Vermont) placed around a square acrylic cage (17.0″ × 17.0″) as previously described (Teske et al., 2006). Briefly, ambulation was detected by two I/R arrays in the x and y-axes and vertical movement was detected by a third elevated x array and thus movement was simultaneously detected in all three axes. From the SPA measurements, the sum of time spent ambulating (locomotor activity) and vertical (rearing or standing) were calculated and will be referred to as “time spent moving”. Prior to the start of the studies, animals were acclimated to SPA chambers for 140 min on three separate occasions. Continuous SPA was measured for 140 min post-injection. Since handling involved in the injection procedure results in increased SPA for up to 20 min post-injection independent of treatment, the first 20 min of data post-injection data were excluded in the data analysis (Kotz et al., 2002). Thus, the data analyses were performed on time spent moving measured in the 20–140-min post-injection period. Data are reported in the 1-h and 2-h time periods.
1.6 EEG/EMG recording and determination of behavioral states
EEG and EMG were recorded and behavioral states determined as described previously (Mavanji et al., 2010). The EEG/EMG recordings were facilitated with the implanted transmitter with EEG/EMG leads, that transmit EEG/EMG by radiotelemetry to a receiver (PhysioTel RPC-1). Electroencephalogram signals (1.0–30.0 Hz bandpass) and EMG signals (30–100.0 Hz bandpass) were amplified, filtered, recorded, digitized and visualized with a Data Exchange Matrix and Dataquest A.R.T 4.1 software (Data Sciences International, St. Paul, MN). Sleep/wake behavioral states were manually scored in consecutive 10 s epochs based on EEG/EMG waveforms. The EEG/EMG data were classified into one of the following four behavioral states as described previously (Mavanji et al., 2010): (1) slow-wave sleep (SWS); (2) rapid eye movement sleep (REM); (3) quiet wake; and (4) active wake (AW). Percent time spent in each state was calculated from the scored data. Data are reported in the 1-h time period.
1.7 Wheel running
Wheel revolutions were determined in running wheels (Model 80850, width 4.3″, diameter 14″ and run distance 1.10 m/revolution, Layafette Instruments, Lafayette, IN) that were attached by a tunnel to solid-bottom cages (Model 80852, 19″ × 10.5″ × 8″) with corn cobb bedding. Wheel revolutions were counted with an optical sensor (Model 86060), downloaded to a PC computer with a USB computer interface (Model 86056A) and visualized with AWM software (Layafette Instruments, Lafayette, IN). Food and water were available ad libitum.
1.8 Specific experimental designs
1.8.1 Study 1. Effect of the OX1R antagonist and orexin A in the rLH on SPA in 3–4 month old rats
The OX1R antagonist (250, 650, 1050 pmol/0.5 μl) or vehicle was injected into the rLH in a randomly assigned latin-square counter-balanced design (OP, N = 10; OR, N = 9; Sprague-Dawley, n = 8). Twenty minutes later OXA (250 pmol/0.5 μl) or vehicle was injected into the rLH.
1.8.2 Study 2. Effect of the OX2R agonist in the rLH on SPA in 3–4 month old rats
The OX2R agonist (250, 650, 1050 pmol/0.5 μl) or vehicle was injected into the rLH (OP, N = 10; OR, N = 8; Sprague-Dawley, N = 8). One OR rat died of unknown cause during this study. Mean body weight was calculated from body weights taken on the first day of study one and the final day of study two.
1.8.3 Study 3. Effect of orexin A into the rLH on arousal in 3–4 month old rats
Orexin A (500 pmol/0.5 μL) or vehicle was injected into the rLH in a separate set of OP and OR rats (N = 6/group) and EEG/EMG was recorded for 140 minutes post-injection. Data were manually scored to determine time spent in sleep/wake states and reported as the percentage of time spent in each sleep/wake state. In our previous work, we demonstrated that doses below 500 pmol of OXA failed to increase SPA (Teske et al., 2006) in OP rats. Thus the 500 pmol dose of OXA was chosen since we were interested in determining whether OXA-simulating arousal was secondary to OXA-stimulated increases in SPA. Sprague-Dawley rats were excluded in this study and the next based on our finding that Sprague-Dawley and OP rats had similar behavioral profiles (Teske et al., 2006, Teske and Kotz, 2009).
1.8.4 Study 4. Propensity to run on a running wheel in 6-week old rats
A separate set of 6-week old OP and OR rats (N = 6/group) were transferred to solid-bottom cages attached to a running wheel (Layafette Instruments, Lafayette, IN) that were identical in size to their home cage. Rodents were given ad libitum access to the running wheel without prior training. Food and water were available ad libitum. Twenty-four h food intake was determined by comparing the difference between pre-measured food pellets before and the remaining food pellets after a 24-h period. Food spillage was not collected in these cages as it was not possible in the solid bottom cage setting with bedding and the primary endpoint was wheel running activity and body composition. Based on our past experience, spillage in OR and Sprague-Dawley rats at this age is likely less than 0.77g per day. This is based on studies in 3-month old Sprague-Dawely rats in solid bottom cages with perforated floors, which permits spillage collelction. Then rodents were placed in the SPA chambers for a 24-h acclimation period followed by a 24-h test period. Food and water were available ad libitum. We have reported previously significant differences in 24-h food intake between OP and OR rats at this age (Teske et al., 2006), and like, 24-h food intake, spillage can vary across groups. To minimize the effect of this missing information on the data analysis, food intake is expressed as a ratio of 24-h food and lean mass however, we acknowledge that similar to the concerns over normalizing energy expenditure for lean mass, this method of normalizing food intake values for lean mass can also be problematic (Arch et al., 2006, Butler and Kozak, 2010, Even and Nadkarni, 2012, Tschop et al., 2012). Body composition (EchoMRI, Echo Medical Systems, Houston, TX) was determined as described previously (Nixon et al., 2010, Teske et al., 2012) before rodents were given access to the running wheels.
1.8.5 Study 5. Effect of the OX1R antagonist and orexin A into the SN on SPA in 5 month old rats
The OX1R antagonist (1050 pmol/0.5 μl) or vehicle was injected into the SN 20 minutes before OXA (250 pmol/0.5 μl) or vehicle was injected into the SN in a separate set of rats (N = 7/group). One OR died of unknown cause during this study. Mean body weight was calculated from body weights taken on the first day of study five and the final day of study six.
1.8.6 Study 6. Effect of the DA1R antagonist and orexin A into the SN on SPA in 5 month old rats
The DA1R antagonist SCH3390 (30 pmol/0.5 μl) or vehicle was injected into the SN 20 minutes before OXA (250 pmol/0.5 μl) or vehicle was injected into the SN (OP: N=7; OR: N=10; Sprague-Dawley: N = 7). Two OP and one Sprague-Dawley rat lost cannulae during this study. Study six was performed before study five.
1.9 Statistical analyses
Data were analyzed with Prism 5.0b (GraphPad Software, Inc., San Diego, CA), and an alpha level of 0.05 was used for all statistical tests. Data were expressed as mean ± SEM. Spontaneous physical activity and sleep/wake data (studies 1–3, 5, 6) were analyzed by two-factor repeated-measures ANOVA. When significant main effects or an interaction were observed, the data were separated by phenotype and then analyzed by one-factor repeated-measures ANOVA followed by Fisher’s PLSD test to compare individual treatment means within each phenotype. To determine differences within a give treatment across phenotypes, data were analyzed by ANOVA followed by Fisher’s PLSD test. Data in the 1-h, 1–2 and 2-h post-injection time periods were analyzed separately. Main effects for group and treatment were not significant in the 1–2 h period for studies 1, 2, 3, 5 and 6 (data not shown). Hence, results in the two-hour period were due to effects that occurred in the one-hour period. We report data from the two-hour period when results from the one and two-hour periods were similar as defined as being both statistically significant. Two rats were statistical outliers (greater than twice the standard deviation) in study six and there data were excluded. Body weight, percent fat mass, food intake and running wheel revolutions were analyzed by ANOVA or t-test followed by Fisher’s PLSD test to determine difference between phenotypes. Linear regression was used to analyze the relationship between running wheel revolutions and 24-h time spent moving.
2. RESULTS
Obesity resistant rats weighed significantly less than both OP and/or Sprague-Dawley rats (Table 1). Obesity resistant rats had significantly less body fat despite similar food intake compared to OP rats (Table 1).
TABLE 1.
Body weight, body composition and food intake.
| Phenotype | |||
|---|---|---|---|
| Obesity prone | Obesity resistant | Sprague-Dawley | |
| Body weight (g) | |||
| 1 Studies 1 and 2 | 332.3 ± 6.1gδ | 287.1 ± 4.2g* | 356.6 ± 5.6g |
| 1 Studies 5 and 6 | 426.3 ± 7.7gδ | 334.0 ± 9.2g* | 477.5 ± 12.6g |
| Study 4 | 155.2 ± 3.2g | 118.4 ± 5.3gΦ | --- |
| Study 4 | |||
| Body fat (%): | 10.3 ± 0.2 | 8.7 ± 0.3Φ | --- |
| Fat-free mass (g) | 124.6 ± 2.7g | 97.4 ± 4.1gΦ | --- |
| 24-h food intake (g) | 14.4 ± 0.7g | 12.9 ± 0.5gΦ | --- |
| 24-h food intake/FFM: | 0.116 ± 0.01 | 0.135 ± 0.01 | --- |
Study 1 and 2: N = 10 (obesity prone), 8–9 (obesity resistant), and 8 (Sprague-Dawley). Study 4: N = 6/group. Study 5–6: N = 7 (obesity prone), 7–10 (obesity resistant), and 7 (Sprague-Dawley).
P < 0.05 versus obesity prone and/or Sprague-Dawley.
P < 0.05 versus Sprague-Dawley
P < 0.05 versus obesity prone
ANOVA: P < 0.0001
2.1 Study 1. The OX1R antagonist in the rLH reduces OXA-stimulated SPA most effectively in OR rats
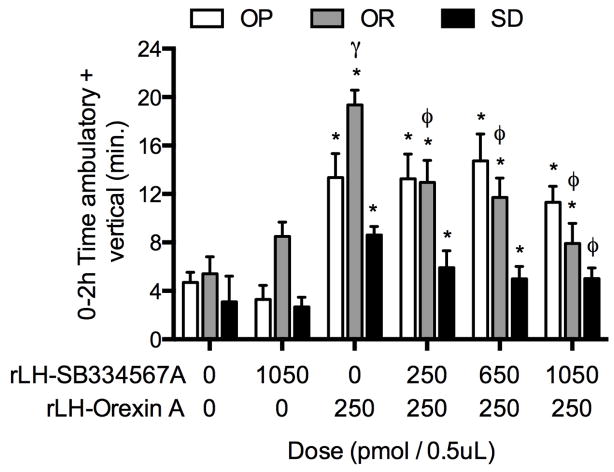
To determine whether the OX1R receptor in the rLH mediated the elevated SPA response to OXA after infusion into the rLH in OR rats, the OX1R antagonist was infused prior to OXA into the rLH. Two-factor repeated measures ANOVA indicated a significant interaction between phenotype and treatment two-hour post-injection (P = 0.0008, Figure 1). When the data were separated by phenotype to compare effects of the OX1R antagonist on OXA-stimulated SPA within each phenotype, the analysis indicated a significant treatment effect on SPA. Orexin A infused into the rLH significantly increased SPA relative to vehicle in all phenotypes (Figure 1). Spontaneous physical activity stimulated by OXA was significantly greater in OR rats compared to both OP and Sprague-Dawley rats in the two-hour post-injection period (P < 0.05 and P < .0005, respectively, Figure 1). Pretreatment with the OX1R antagonist reduced SPA stimulated by OXA in OR and Sprague-Dawley rats but had no effect on OXA-stimulated SPA in OP rats (Figure 1). All three doses of the OX1R antagonist reduced OXA-stimulated SPA in OR rats (250 pmol: P < 0.05, 650 pmol: P < 0.005, 1050 pmol: P < 0.0005) but only the highest dose of the OX1R antagonist reduced OXA-stimulated SPA in Sprague-Dawley rats (P < 0.05).
Figure 1.
(study 1): Orexin A (OXA) infusion into the rostral lateral hypothalamus (rLH) stimulates spontaneous physical activity (SPA) in obesity resistant (OR) rats to a greater extent compared to obesity prone (OP) or Sprague-Dawley (SD) rats. Pre-treatment with the orexin one receptor (OX1R) antagonist (SB-334867-A) in the rLH reduced SPA stimulated by OXA in OR and Sprague-Dawley rats but failed to reduce OXA-stimulated SPA in OP rats (Repeated measures ANOVA: OP: F(5,50) = 10.8, P < 0.0001; OR: F(5,40) = 10.9, P < 0.0001; SD: F(5,35) = 5.4, P = 0.008, respectively. All doses of the OX1R antagonist reduced OXA-stimulated SPA in OR rats but only the highest dose of the antagonist reduced OXA-stimulated SPA in Sprague-Dawley rats. * P < .05 as compared to vehicle/vehicle for each phenotype, φ P < .05 as compared to vehicle/orexin A for each phenotype; γ P < .05 as compared to vehicle/orexin A for OP and Sprague-Dawley rats. Data represent mean ± SEM. N = 10 (obesity prone), 9 (obesity resistant), and 8 (Sprague-Dawley).
2.2 Study 2. Selective stimulation of OX2R in the rLH increases SPA for a longer duration in OP and Sprague-Dawley rats compared to OR rats
We sought to determine whether selective stimulation of OX2R in the rLH would elicit a differential SPA response between phenotypes like stimulation of OX1R by OXA. There was a main effect of phenotype and treatment on SPA one-hour post-injection (F(2,23) = 3.9, P = 0.0349 and F(3,69) = 10.7, P < 0.0001, respectively, Figure 2). Two-hour post-injection, there was a main effect of treatment (F(3,69) = 4.8, P = 0.0043) but the effect of phenotype failed to reach statistical significance (F(2,23) = 3.4 P = 0.0504). There was a treatment effect of the OX2R agonist on SPA in all phenotypes one-hour post-injection. Overall the OR rats had the highest levels of SPA across the dose range. The 650 pmol dose of the OXR agonist significantly stimulated SPA in OR rats (P < 0.05) and SPA stimulated by this dose in OR rats was significantly greater than in Sprague-Dawley rats (P < 0.05). In OP rats the highest dose of the OX2R agonist significantly stimulated SPA in the one hour post-injection period (P < 0.005). In Sprague-Dawley rats the 250 and 1050 pmol doses of the agonist significantly stimulated SPA (P < .05 and P < .005, respectively). The stimulatory effect of the OX2R agonist persisted in OP (Figure 2) but not in OR and Sprague-Dawley rats during the two-hour post-injection period.
Figure 2.
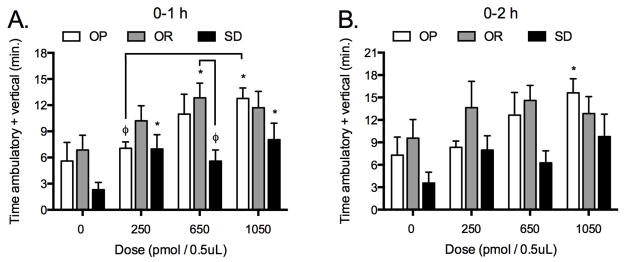
(study 2): Selective stimulation of the orexin two receptor (OX2R) receptor with Ala11, D-Leu15-Orexin B in the rostral lateral hypothalamus (rLH) increases SPA in obesity prone (OP), obesity resistant (OR) and Sprague-Dawley (SD) rats. Data in the one and two-hours post-injection shown in panel A and B, respectively. (Repeated measures ANOVA 1-h and 2-h, respectively: OP: F(3,27) = 5.1, P = 0.0062 and F(3,27) = 4.0, P = 0.0171; OR: F(3,21) = 3.6, P = 0.0299 and F(3,21) = 0.8, P = 0.4841; SD: F(3,21) = 5.0, P = 0.0088 and F(3,21) = 3.0, P = 0.0529. * p < .05 as compared to vehicle. φ p < .05. Data represent mean ± SEM. N = 10 (obesity prone), 8 (obesity resistant), and 8 (Sprague-Dawley). Please note different y-axes.
2.3 Study 3. OXA-stimulated arousal is similar between OR and OP rats
We determined whether OXA-stimulated SPA was secondary to arousal since OXA stimulates both SPA and arousal. Two-factor repeated measures ANOVA indicated a main effect of treatment on AW, SWS and REM sleep but no effect of phenotype during the one-hour post-injection period (Figure 3). Therefore the treatment effect was similar between phenotypes. Orexin A significantly increased AW and reduced SWS and REM sleep, and had no effect on quiet wake during the one-hour post-injection interval (Figure 3).
Figure 3.
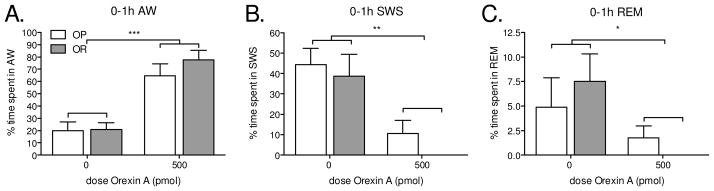
(study 3): Orexin A in the in the rostral lateral hypothalamus stimulates active wake (AW) and reduces slow wave sleep (SWS) and rapid eye movement sleep (REM) equally in obesity prone (OP) and obesity resistant (OR) rats during the one-hour post-injection period. Obesity resistant rats spent zero minutes in slow wave sleep or REM sleep after the orexin A injection. There was no effect of orexin A on quiet wake (data not shown). Two-factor repeated measures ANOVA for the main effects of phenotype and treatment: AW F(1,10) = 0.7, P = 0.4202 and F(1,10) = 39.6, P < 0.0001. QW: F(1,10) = 0.01, P = 0.9344 and F(1,10) = 1.2, P = 0.2964. SWS: F(1,10) = 1.4, P = 0.2586 and F(1,10) = 20.6, P = 0.0011. REM: F(1,10) = 0.5, P = 0.8284 and F(1,10) = 5.2, P = 0.0457. * p < .05, ** p < .005, and ***p < .0001 as compared to vehicle-treated rats. Data represent mean ± SEM. N = 6/group. Please note different y-axes.
2.4 Study 4. OR rats ran more than OP rats and wheel running was directly proportional to 24-h SPA
We hypothesized that OR rats would run more than OP rats and greater wheel running would align with greater 24-h SPA levels. As expected, untrained OR rats ran significantly more on the running wheel compared to OP rats over the 24-h period (Figure 4A). This greater propensity to run was due to more wheel running revolutions during the light cycle (Figure P < 0.0001: OP = 196.0 ± 38.60 and OR = 762.7 ± 27.1 wheel revolutions) since wheel running during the dark cycle was similar between groups (P = .2427: OP = 569.9 ± 115.8 and OR = 732.3 ± 68.3 wheel revolutions). Running wheel revolutions were positively associated with time spent moving in the SPA chambers over the 24-h period (Figure 4B).
Figure 4.
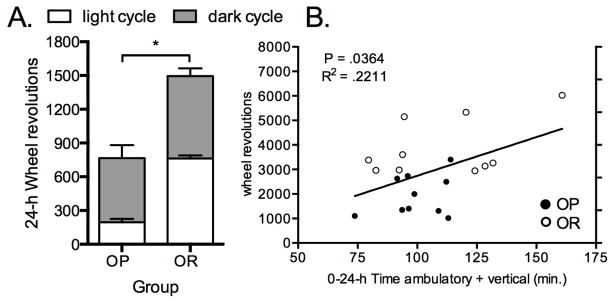
(A) Young untrained six-week old obesity resistant (OR) rats have a greater propensity to run on a running wheel compared to obesity prone (OP) rats. (B) Wheel running revolutions were directly proportional to 24-h time spent moving. *P = 0.0003 for 24-h and P < .0001 for the light cycle. Data represent mean ± SEM. N = 6/group.
2.5 Study 5. Pretreatment with the OX1R antagonist in the SN reduces OXA-stimulated SPA in OR rats only
The OX1R antagonist was infused prior to OXA into the SN to determine the contribution of OX1R to OXA-stimulated SPA and whether this effect was phenotype-dependent. Two-factor repeated measures ANOVA indicated a main effect of phenotype and treatment one and two-hours post-injection (1-h F(2,16) = 19.2, P < 0.0001 and F(3,48) = 13.5, P < 0.0001; 2-h F(2,16) = 19.0, P < 0.0001 and F(3,48) = 10.8, P < 0.0001, Figure 5). When the data were separated by phenotype to determine the effect of the OX1R antagonist on OXA-stimulated SPA within each phenotype, the analysis indicated a significant treatment effect on all phenotypes with one exception. Unlike one-hour post-injection, there was no treatment effect in OP rats during the two-hour post-injection period. Orexin A-stimulated SPA after infusion into the SN was significantly greater in OR rats relative to both OP and Sprague-Dawley rats (Figure 5). Orexin A in the SN significantly increased SPA in all phenotypes relative to vehicle (Figure 5). Pre-treatment with the OX1R antagonist in the SN reduced OXA-stimulated SPA in OR rats (P < 0.05) but failed to reduce OXA-stimulated SPA in OP or Sprague-Dawley rats (P > .05 for all comparisons).
Figure 5.
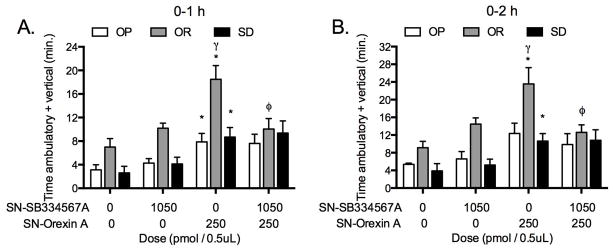
(study 5): The orexin 1 receptor (OX1R) antagonist (SB-334867-A) in the substantia nigra (SN) blocked SPA stimulated by orexin A (OXA) in the SN in obesity resistant (OR) but not in obesity prone (OP) or Sprague-Dawley (SD) rats one and two hours post-injection (Panel A and B, respectively). Repeated measures ANOVA 1-h and 2-h, respectively: OP: F(3,15) = 3.9, P = 0.0300 and F(3,15) = 2.9, P = 0.0690; OR: F(3,18) = 7.2, P = 0.0022 and F(3,18) = 6.3, P = 0.0040; SD: F(3,15) = 6.3 P = 0.0053 and F(3,15) 4.4, P = 0.0198. * p < .05 as compared to vehicle/vehicle for each phenotype, φ p < .05 as compared to vehicle/orexin A for OR rats. γ P < .05 as compared to vehicle/orexin A for OP and Sprague-Dawley rats. Data represent mean ± SEM. N = 6 (obesity prone), 7 (obesity resistant), and 6 (Sprague-Dawley).
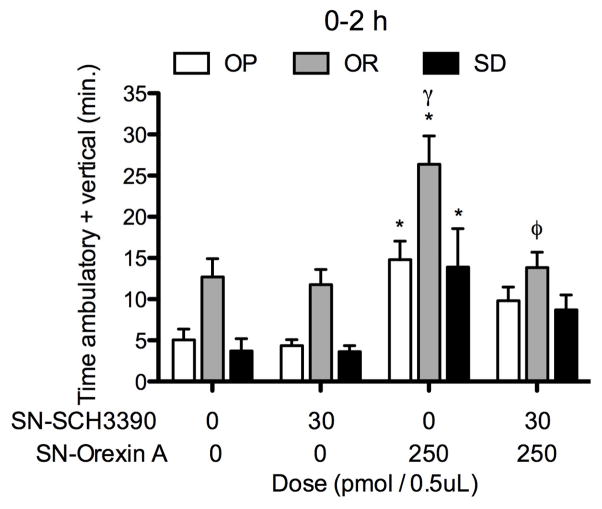
2.5 Study 6. OXA in the SN elicits greater SPA in OR rats and antagonism of DA1R in the SN reduces OXA-stimulated SPA in OR rats only
We tested whether OXA-stimulated SPA after infusion in the SN was phenotype-dependent like OXA-stimulated SPA after rLH infusion. We also determined whether the elevated SPA response to OXA in the SN in OR rats was mediated by DA1R. To do so, the DA1R antagonist was infused prior to OXA into SN. Two-factor repeated measures ANOVA indicated main effects of phenotype and treatment on SPA two-hours post-injection (F(2,21) = 23.9, P < 0.0001 and F(3,21) = 14.2, P < 0.0001, Figure 6). Separating the data by phenotype indicated a significant treatment effect on SPA for all phenotypes (Figure 6). Orexin A significantly stimulated SPA relative to vehicle in all phenotypes and the stimulatory effect of OXA on SPA after SN infusion was significantly greater in OR rats relative both OP and Sprague-Dawley rats (Figure 6). The DA1R antagonist was more effective in blocking OXA-stimulated SPA in OR rats as compared to OP or Sprague-Dawley rats (Figure 6). In contrast to OP rats where there was no effect of the DA1R antagonist on OXA-stimulated SPA, the DA1R antagonist blocked OXA-stimulated SPA in OR rats. In Sprague-Dawley rats, the DA1R antagonist had no effect on OXA-stimulated SPA in the one-hour post-injection period but blocked OXA-stimulated SPA during the two-hour post-injection period (1-h and 2-h: P > 0.05 and P < 0.05, respectively).
Figure 6.
(study 6): Orexin A (OXA) infusion into the substantia nigra (SN) stimulates spontaneous physical activity (SPA) in obesity resistant (OR) rats more than obesity prone (OP) or Sprague-Dawley (SD) rats. Pre-treatment with the selective dopamine one receptor (DA1R) antagonist SCH3390 in the SN reduced SPA stimulated by OXA in OR and Sprague-Dawley rats. Repeated measures ANOVA for the two-hour post-injection period: OP: F(3,18) = 9.1, P = 0.0007; OR: F(3,27) = 6.7, P = 0.0016; SD: F(3,18) = 3.7, P = 0.0314. * p < .05 as compared to vehicle/vehicle for each phenotype, φ p < .05 as compared to vehicle/orexin A for each phenotype; γ p < .05 as compared to vehicle/orexin A for OP and Sprague-Dawley rats. Data represent mean ± SEM. N = 7 (obesity prone), 10 (obesity resistant), and 7 (Sprague-Dawley).
DISCUSSION
Previous studies of SPA in OR rats have demonstrated greater sensitivity to OXA stimulation and greater expression of orexin 1 and 2 receptors in the rLH (Teske et al., 2006). These studies extend those observations by demonstrating that OR rats have greater sensitivity to blockade of orexin receptors in rLH and greater short-term sensitivity to stimulation of the orexin 2 receptor. Unlike OP and Sprague-Dawley rats, all doses of the OX1R antagonist reduced OXA-stimulated SPA in OR rats. We further demonstrate that orexin A increased arousal equally in lean and obese rodents, OR rats ran more and wheel running was directly related to 24-h SPA levels. In addition, the OX1R antagonist, SB-334867-A, and the DA1R antagonist, SCH3390, in SN more effectively reduced SPA stimulated by OXA in OR rats. These data are novel and demonstrate that 1) orexin and dopamine action differs between obesity resistant and prone rats, 2) OXA-stimulated SPA is not merely due to enhanced arousal and 3) OR rats may be intrinsically more motivated to perform physical activity. Together these data highlight critical neural pathways and brain sites of action underlying the heightened basal and OXA-stimulated SPA response in OR rats and supports the hypothesis that increased orexin action at the rLH contributes to increased SPA, which is a crucial factor in obesity resistance (Teske et al., 2006, Kotz et al., 2008, Kotz et al., 2012).
Orexin A stimulates both arousal and SPA. While we have shown that OR rats are more responsive to treatments that stimulate SPA such as caloric restriction (Teske and Kotz, 2009), the elevated SPA response to OXA in OR rats might have been secondary to enhanced arousal. If OXA-stimulated SPA was secondary to enhanced arousal one would expect OR rats to have greater arousal following central OXA infusion relative to OP rats. Instead, OXA-stimulated arousal is similar between lean and obese, indicating that greater SPA in lean rats cannot be due to commensurate arousal (Figure 3). We acknowledge that energy expenditure due to OXA-stimulated SPA and arousal must be determined to distinguish which factor (SPA and arousal) contributes most to adiposity. Elevated SPA response to OXA in OR rats suggests an intrinsic drive to be more physically active since previous work demonstrates central OXA modulates motivation (Thorpe et al., 2005a, Choi et al., 2010, Sharf et al., 2010).
We measured voluntary wheel running and SPA over the 24-h period to investigate whether a greater propensity for physical activity would parallel adiposity status and 24-h SPA. To do so, we tested whether the propensity to run differed between OR and OP rats and whether wheel running was associated with 24-h SPA levels. Six-week old OR rats voluntarily ran significantly more in running wheels (Figure 4A), which was directly associated with 24-h SPA (Figure 4B). Like high SPA levels shown previously in OR rats (Teske et al., 2006, Kotz et al., 2008, Teske et al., 2012), this greater propensity to run appears to be intrinsic and spontaneous in nature and could not be due to differences in learning given that we did not train and rats were not allowed prior access to the running wheels before testing. Our data parallel that in rats selectively-bred for high aerobic capacity, where rats with high capacity for running also displayed high SPA levels (Waters et al., 2008, Novak et al., 2010). Together these data show that greater voluntary exercise correlates with elevated 24-h SPA levels, and shows no indication of compensation for increases in one activity with decreases in another. Nonetheless we acknowledge that other processes likely moderate differences in running wheel activity in our study since only 22% of the variance wheel running was accounted for by SPA and that compensatory changes in SPA after wheel running would be best tested with SPA and wheel running measured simultaneously. Overall, these findings would be expected to contribute to lower adiposity levels in OR rats (Teske et al., 2012).
Our finding that SPA stimulated by OXA into SN was greater in lean rats is consistent with greater SPA response to OXA after infusion into rLH and hypothalamic paraventicular nucleus (Novak et al., 2006, Teske et al., 2006). Orexin one and dopamine one receptor antagonists were more effective at blocking OXA-stimulated SPA in OR rats and reduced SPA levels in OR rats to that observed in obese rats. These findings accord with previous work in Sprague-Dawley rats showing OXA infusion into the LH and SN stimulates SPA (Kotz et al., 2002, Kotz et al., 2006, Teske et al., 2006) and DA1R antagonists block OXA-stimulated SPA (Nakamura et al., 2000, Kotz et al., 2006). We are the first to show that pretreatment of the SN with the OX1R antagonist block OXA-stimulated SPA. This interaction between orexin and dopamine modulation of SPA is congruent with literature suggesting that dopamine modulates OXA neurotransmission (Thorpe et al., 2005a, Aston-Jones et al., 2010, Garland et al., 2011). Orexin-containing neurons project to neurons immunopositive for tyrosine hydroxylase, the precursor required for dopamine synthesis (Nakamura et al., 2000) and to brain areas critically involved in motor control such as the SN, ventral tegmental area and prefrontal cortex (Fadel and Deutch, 2002). Orexin A excites dopamine neurons (Korotkova et al., 2006) and stimulates DA1R-mediated dopamine release (Vittoz and Berridge, 2006). Moreover, that lean and obese rats in our study had differential response to the DA1R antagonist is consistent with mice bred for high and low wheel running (Garland et al., 2011). Given the reciprocal projections between orexin and dopamine containing neurons, orexin modulation of dopamine (Vittoz and Berridge, 2006, Espana et al., 2011, Calipari and Espana, 2012, Patyal et al., 2012) and GABA release (Korotkova et al., 2002) OXR and DAR expression in the SN (Richfield et al., 1987, Hervieu et al., 2001) it is plausible that orexin acts on dopaminergic and GABAergic neurons in the SN to promote SPA by stimulating dopamine release in the SN and movement through the nigrostriatal pathway. Collectively, these studies support the idea that differential orexin and dopamine signaling though both OX1R and DA1R may contribute to the enhanced SPA response to OXA after infusion in the rLH and SN in OR rats, and that orexin and dopamine neural pathways mediate SPA in a phenotype-dependent manner.
In conclusion, we demonstrate that obesity resistant rats are more sensitive to antagonism of OX1R and agonists of OX2R, OXA-stimulated arousal is similar between lean and obese rats, lean rats run more on a wheel than obese rats, running is positively associated with 24-h SPA levels and that OX1R and DA1R antagonists in SN reduced SPA in OR rats to levels observed in obese rats. These data suggest that elevated signaling through OX1R and DA1R may promote a greater response to SPA stimulating agents such as OXA in OR rats. Since SPA-generated energy expenditure contributes significantly to overall energy expenditure, these studies suggest that orexin projections from the lateral hypothalamus stimulates orexin and dopamine signaling in the substantia nigra to promote SPA, which is congruent with orexin and dopamine regulation of other behaviors including arousal and drug-seeking.
Highlights.
OX1R and DA1R antagonists reduced OXA-induced SPA more effectively in lean rats
OXA increased arousal equally in lean and obese rodents.
OR rats ran more and wheel running was directly related to 24-hour SPA levels.
Acknowledgments
Funding for this research and publication was supported by an ACSM Foundation Research Grant from the American College of Sports Medicine Foundation, University of Minnesota Doctoral Dissertation Fellowship, Minnesota Partnership for Biotechnology and Genomics, Department of Veterans Affairs (F7212W to JAT) and the National Institutes of Health (NIDDK R01DK078985 to CMK).
ABBREVIATIONS
- AW
active wake
- DA1R
dopamine one receptor
- rHL
rostral lateral hypothalamus
- OP
obesity prone
- OR
obesity resistant
- OXA
orexin A
- OXR
orexin receptor
- OX1R
orexin one receptor
- OX2R
orexin two receptor
- REM
rapid eye movement
- SPA
spontaneous physical activity
- SN
substantia nigra
- SWS
slow wave sleep
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Charles J. Billington, Email: billi005@umn.edu.
Catherine M. Kotz, Email: kotzx004@umn.edu.
References
- Arch JR, Hislop D, Wang SJ, Speakman JR. Some mathematical and technical issues in the measurement and interpretation of open-circuit indirect calorimetry in small animals. Int J Obes (Lond) 2006;30:1322–1331. doi: 10.1038/sj.ijo.0803280. [DOI] [PubMed] [Google Scholar]
- Asahi S, Egashira S, Matsuda M, Iwaasa H, Kanatani A, Ohkubo M, Ihara M, Morishima H. Development of an orexin-2 receptor selective agonist, [Ala(11), D-Leu(15)]orexin-B. Bioorg Med Chem Lett. 2003;13:111–113. doi: 10.1016/s0960-894x(02)00851-x. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, Richardson KA. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Res. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman JM, Roecker AJ, Mercer SP, Bednar RA, Reiss DR, Ransom RW, Meacham Harrell C, Pettibone DJ, Lemaire W, Murphy KL, Li C, Prueksaritanont T, Winrow CJ, Renger JJ, Koblan KS, Hartman GD, Coleman PJ. Proline bis-amides as potent dual orexin receptor antagonists. Bioorg Med Chem Lett. 2008;18:1425–1430. doi: 10.1016/j.bmcl.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Brisbare-Roch C, Dingemanse J, Koberstein R, Hoever P, Aissaoui H, Flores S, Mueller C, Nayler O, van Gerven J, de Haas SL, Hess P, Qiu C, Buchmann S, Scherz M, Weller T, Fischli W, Clozel M, Jenck F. Promotion of sleep by targeting the orexin system in rats, dogs and humans. Nat Med. 2007;13:150–155. doi: 10.1038/nm1544. [DOI] [PubMed] [Google Scholar]
- Butler AA, Kozak LP. A recurring problem with the analysis of energy expenditure in genetic models expressing lean and obese phenotypes. Diabetes. 2010;59:323–329. doi: 10.2337/db09-1471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calipari ES, Espana RA. Hypocretin/orexin regulation of dopamine signaling: implications for reward and reinforcement mechanisms. Frontiers in behavioral neuroscience. 2012;6:54. doi: 10.3389/fnbeh.2012.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DL, Davis JF, Fitzgerald ME, Benoit SC. The role of orexin-A in food motivation, reward-based feeding behavior and food-induced neuronal activation in rats. Neuroscience. 2010;167:11–20. doi: 10.1016/j.neuroscience.2010.02.002. [DOI] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci U S A. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duxon MS, Stretton J, Starr K, Jones DN, Holland V, Riley G, Jerman J, Brough S, Smart D, Johns A, Chan W, Porter RA, Upton N. Evidence that orexin-A-evoked grooming in the rat is mediated by orexin-1 (OX1) receptors, with downstream 5-HT2C receptor involvement. Psychopharmacology (Berl) 2001;153:203–209. doi: 10.1007/s002130000550. [DOI] [PubMed] [Google Scholar]
- Espana RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Even PC, Nadkarni NA. Indirect calorimetry in laboratory mice and rats: principles, practical considerations, interpretation and perspectives. Am J Physiol Regul Integr Comp Physiol. 2012;303:R459–476. doi: 10.1152/ajpregu.00137.2012. [DOI] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Fetissov SO, Meguid MM, Sato T, Zhang LH. Expression of dopaminergic receptors in the hypothalamus of lean and obese Zucker rats and food intake. Am J Physiol Regul Integr Comp Physiol. 2002;283:R905–910. doi: 10.1152/ajpregu.00092.2002. [DOI] [PubMed] [Google Scholar]
- Garland T, Jr, Schutz H, Chappell MA, Keeney BK, Meek TH, Copes LE, Acosta W, Drenowatz C, Maciel RC, van Dijk G, Kotz CM, Eisenmann JC. The biological control of voluntary exercise, spontaneous physical activity and daily energy expenditure in relation to obesity: human and rodent perspectives. J Exp Biol. 2011;214:206–229. doi: 10.1242/jeb.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger BM, Behr GG, Frank LE, Caldera-Siu AD, Beinfeld MC, Kokkotou EG, Pothos EN. Evidence for defective mesolimbic dopamine exocytosis in obesity-prone rats. FASEB J. 2008;22:2740–2746. doi: 10.1096/fj.08-110759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara J, Beuckmann CT, Nambu T, Willie JT, Chemelli RM, Sinton CM, Sugiyama F, Yagami K, Goto K, Yanagisawa M, Sakurai T. Genetic ablation of orexin neurons in mice results in narcolepsy, hypophagia, and obesity. Neuron. 2001;30:345–354. doi: 10.1016/s0896-6273(01)00293-8. [DOI] [PubMed] [Google Scholar]
- Hervieu GJ, Cluderay JE, Harrison DC, Roberts JC, Leslie RA. Gene expression and protein distribution of the orexin-1 receptor in the rat brain and spinal cord. Neuroscience. 2001;103:777–797. doi: 10.1016/s0306-4522(01)00033-1. [DOI] [PubMed] [Google Scholar]
- Jones DN, Gartlon J, Parker F, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Hatcher JP, Johns A, Porter RA, Hagan JJ, Hunter AJ, Upton N. Effects of centrally administered orexin-B and orexin-A: a role for orexin-1 receptors in orexin-B-induced hyperactivity. Psychopharmacology (Berl) 2001;153:210–218. doi: 10.1007/s002130000551. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. Am J Physiol Endocrinol Metab. 2004;286:E551–559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Brown RE, Sergeeva OA, Ponomarenko AA, Haas HL. Effects of arousal- and feeding-related neuropeptides on dopaminergic and GABAergic neurons in the ventral tegmental area of the rat. Eur J Neurosci. 2006;23:2677–2685. doi: 10.1111/j.1460-9568.2006.04792.x. [DOI] [PubMed] [Google Scholar]
- Korotkova TM, Eriksson KS, Haas HL, Brown RE. Selective excitation of GABAergic neurons in the substantia nigra of the rat by orexin/hypocretin in vitro. Regul Pept. 2002;104:83–89. doi: 10.1016/s0167-0115(01)00323-8. [DOI] [PubMed] [Google Scholar]
- Kotz C, Nixon J, Butterick T, Perez-Leighton C, Teske J, Billington C. Brain orexin promotes obesity resistance. Annals of the New York Academy of Sciences. 2012;1264:72–86. doi: 10.1111/j.1749-6632.2012.06585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Billington CJ. Neuroregulation of nonexercise activity thermogenesis and obesity resistance. Am J Physiol Regul Integr Comp Physiol. 2008;294:R699–710. doi: 10.1152/ajpregu.00095.2007. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Teske JA, Levine JA, Wang C. Feeding and activity induced by orexin A in the lateral hypothalamus in rats. Regul Pept. 2002;104:27–32. doi: 10.1016/s0167-0115(01)00346-9. [DOI] [PubMed] [Google Scholar]
- Kotz CM, Wang C, Teske JA, Thorpe AJ, Novak CM, Kiwaki K, Levine JA. Orexin A mediation of time spent moving in rats: neural mechanisms. Neuroscience. 2006;142:29–36. doi: 10.1016/j.neuroscience.2006.05.028. [DOI] [PubMed] [Google Scholar]
- Levin BE, Triscari J, Sullivan AC. The effect of diet and chronic obesity on brain catecholamine turnover in the rat. Pharmacol Biochem Behav. 1986;24:299–304. doi: 10.1016/0091-3057(86)90354-0. [DOI] [PubMed] [Google Scholar]
- Li A, Nattie E. Antagonism of rat orexin receptors by almorexant attenuates central chemoreception in wakefulness in the active period of the diurnal cycle. J Physiol. 2010;588:2935–2944. doi: 10.1113/jphysiol.2010.191288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki I, Sakurai T, Kunii K, Nakamura T, Yanagisawa M, Goto K. Involvement of the serotonergic system in orexin-induced behavioral alterations in rats. Regul Pept. 2002;104:119–123. doi: 10.1016/s0167-0115(01)00355-x. [DOI] [PubMed] [Google Scholar]
- Mavanji V, Teske JA, Billington CJ, Kotz CM. Elevated sleep quality and orexin receptor mRNA in obesity-resistant rats. Int J Obes (Lond) 2010;34:1576–1588. doi: 10.1038/ijo.2010.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T, Uramura K, Nambu T, Yada T, Goto K, Yanagisawa M, Sakurai T. Orexin-induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000;873:181–187. doi: 10.1016/s0006-8993(00)02555-5. [DOI] [PubMed] [Google Scholar]
- Nixon JP, Zhang M, Wang C, Kuskowski MA, Novak CM, Levine JA, Billington CJ, Kotz CM. Evaluation of a quantitative magnetic resonance imaging system for whole body composition analysis in rodents. Obesity (Silver Spring) 2010;18:1652–1659. doi: 10.1038/oby.2009.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Escande C, Burghardt PR, Zhang M, Barbosa MT, Chini EN, Britton SL, Koch LG, Akil H, Levine JA. Spontaneous activity, economy of activity, and resistance to diet-induced obesity in rats bred for high intrinsic aerobic capacity. Horm Behav. 2010;58:355–367. doi: 10.1016/j.yhbeh.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Kotz CM, Levine JA. Central orexin sensitivity, physical activity, and obesity in diet-induced obese and diet-resistant rats. Am J Physiol Endocrinol Metab. 2006;290:E396–403. doi: 10.1152/ajpendo.00293.2005. [DOI] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity (Silver Spring) 2009;17:1493–1498. doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patyal R, Woo EY, Borgland SL. Local hypocretin-1 modulates terminal dopamine concentration in the nucleus accumbens shell. Frontiers in behavioral neuroscience. 2012;6:82. doi: 10.3389/fnbeh.2012.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C, Pennisi M, Topple A. Bregma, lambda and the interaural midpoint in stereotaxic surgery with rats of different sex, strain and weight. J Neurosci Methods. 1985;13:139–143. doi: 10.1016/0165-0270(85)90026-3. [DOI] [PubMed] [Google Scholar]
- Picker MJ, Allen RM, Morgan D, Levine AS, O’Hare E, Cleary JP. Effects of neuropeptide Y on the discriminative stimulus and antinociceptive properties of morphine. Pharmacol Biochem Behav. 1999;64:161–164. doi: 10.1016/s0091-3057(99)00110-0. [DOI] [PubMed] [Google Scholar]
- Rada P, Bocarsly ME, Barson JR, Hoebel BG, Leibowitz SF. Reduced accumbens dopamine in Sprague-Dawley rats prone to overeating a fat-rich diet. Physiol Behav. 2010;101:394–400. doi: 10.1016/j.physbeh.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richfield EK, Young AB, Penney JB. Comparative distribution of dopamine D-1 and D-2 receptors in the basal ganglia of turtles, pigeons, rats, cats, and monkeys. J Comp Neurol. 1987;262:446–463. doi: 10.1002/cne.902620308. [DOI] [PubMed] [Google Scholar]
- Rodgers RJ, Halford JC, Nunes de Souza RL, Canto de Souza AL, Piper DC, Arch JR, Upton N, Porter RA, Johns A, Blundell JE. SB-334867, a selective orexin-1 receptor antagonist, enhances behavioural satiety and blocks the hyperphagic effect of orexin-A in rats. Eur J Neurosci. 2001;13:1444–1452. doi: 10.1046/j.0953-816x.2001.01518.x. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richardson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Sharf R, Sarhan M, Brayton CE, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin signaling via the orexin 1 receptor mediates operant responding for food reinforcement. Biol Psychiatry. 2010;67:753–760. doi: 10.1016/j.biopsych.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet DC, Levine AS, Billington CJ, Kotz CM. Feeding response to central orexins. Brain Res. 1999;821:535–538. doi: 10.1016/s0006-8993(99)01136-1. [DOI] [PubMed] [Google Scholar]
- Teske JA, Billington CJ, Kuskowski MA, Kotz CM. Spontaneous physical activity protects against fat mass gain. Int J Obes (Lond) 2012;36:603–613. doi: 10.1038/ijo.2011.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske JA, Kotz CM. Effect of acute and chronic caloric restriction and metabolic glucoprivation on spontaneous physical activity in obesity-prone and obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2009;297:R176–184. doi: 10.1152/ajpregu.90866.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teske JA, Levine AS, Kuskowski M, Levine JA, Kotz CM. Elevated hypothalamic orexin signaling, sensitivity to orexin A, and spontaneous physical activity in obesity-resistant rats. Am J Physiol Regul Integr Comp Physiol. 2006;291:R889–899. doi: 10.1152/ajpregu.00536.2005. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Cleary JP, Levine AS, Kotz CM. Centrally administered orexin A increases motivation for sweet pellets in rats. Psychopharmacology (Berl) 2005a;182:75–83. doi: 10.1007/s00213-005-0040-5. [DOI] [PubMed] [Google Scholar]
- Thorpe AJ, Teske JA, Kotz CM. Orexin A-induced feeding is augmented by caloric challenge. Am J Physiol Regul Integr Comp Physiol. 2005b;289:R367–R372. doi: 10.1152/ajpregu.00737.2004. [DOI] [PubMed] [Google Scholar]
- Tschop MH, Speakman JR, Arch JR, Auwerx J, Bruning JC, Chan L, Eckel RH, Farese RV, Jr, Galgani JE, Hambly C, Herman MA, Horvath TL, Kahn BB, Kozma SC, Maratos-Flier E, Muller TD, Munzberg H, Pfluger PT, Plum L, Reitman ML, Rahmouni K, Shulman GI, Thomas G, Kahn CR, Ravussin E. A guide to analysis of mouse energy metabolism. Nature methods. 2012;9:57–63. doi: 10.1038/nmeth.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vittoz NM, Berridge CW. Hypocretin/orexin selectively increases dopamine efflux within the prefrontal cortex: involvement of the ventral tegmental area. Neuropsychopharmacology: official publication of the American College of Neuropsychopharmacology. 2006;31:384–395. doi: 10.1038/sj.npp.1300807. [DOI] [PubMed] [Google Scholar]
- Waters RP, Renner KJ, Pringle RB, Summers CH, Britton SL, Koch LG, Swallow JG. Selection for aerobic capacity affects corticosterone, monoamines and wheel-running activity. Physiology & behavior. 2008;93:1044–1054. doi: 10.1016/j.physbeh.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitman DB, Cox CD, Breslin MJ, Brashear KM, Schreier JD, Bogusky MJ, Bednar RA, Lemaire W, Bruno JG, Hartman GD, Reiss DR, Harrell CM, Kraus RL, Li Y, Garson SL, Doran SM, Prueksaritanont T, Li C, Winrow CJ, Koblan KS, Renger JJ, Coleman PJ. Discovery of a potent, CNS-penetrant orexin receptor antagonist based on an n,n-disubstituted-1,4-diazepane scaffold that promotes sleep in rats. Chem Med Chem. 2009;4:1069–1074. doi: 10.1002/cmdc.200900069. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Gotter AL, Cox CD, Doran SM, Tannenbaum PL, Breslin MJ, Garson SL, Fox SV, Harrell CM, Stevens J, Reiss DR, Cui D, Coleman PJ, Renger JJ. Promotion of sleep by suvorexant-a novel dual orexin receptor antagonist. Journal of neurogenetics. 2011;25:52–61. doi: 10.3109/01677063.2011.566953. [DOI] [PubMed] [Google Scholar]
- Winrow CJ, Tanis KQ, Reiss DR, Rigby AM, Uslaner JM, Uebele VN, Doran SM, Fox SV, Garson SL, Gotter AL, Levine DM, Roecker AJ, Coleman PJ, Koblan KS, Renger JJ. Orexin receptor antagonism prevents transcriptional and behavioral plasticity resulting from stimulant exposure. Neuropharmacology. 2010;58:185–194. doi: 10.1016/j.neuropharm.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Yang ZJ, Meguid MM. LHA dopaminergic activity in obese and lean Zucker rats. Neuroreport. 1995;6:1191–1194. doi: 10.1097/00001756-199505300-00029. [DOI] [PubMed] [Google Scholar]