Abstract
OBJECTIVE
Anti-oxidative drugs continue to be developed for the treatment of atherosclerosis. Apocynin is an NADPH-oxidase-inhibitor with anti-inflammatory properties. We used contrast enhanced ultrasound (CEU) molecular imaging to assess whether short-term apocynin therapy in atherosclerosis reduces vascular oxidative stress and endothelial activation
APPROACH AND RESULTS
Genetically-modified mice with early atherosclerosis were studied at baseline and after 7 days of therapy with apocynin (4mg/kg/d I.P.) or saline. CEU molecular imaging of the aorta was performed with microbubbles targeted to vascular cell adhesion molecule 1 (VCAM-1; MBV), to platelet GPIbα (MBPl), and control microbubbles (MBCtr). Aortic VCAM-1 was measured using Western Blot. Aortic ROS generation was measured using a lucigenin assay. Hydroethidine (HE) oxidation was used to assess aortic superoxide generation. Baseline signal for MBV (1.3±0.3 A.U.) and MBPl (1.5±0.5 A.U.) was higher than for MBCtr (0.5±0.2 A.U., p<0.01). In saline-treated animals, signal did not significantly change for any microbubble agent whereas short-term apocynin significantly (p<0.05) reduced VCAM-1 and platelet signal (MBV: 0.3±0.1, MBPl: 0.4±0.1 MBCtr: 0.3±0.2 A.U., p=0.6 between agents). Apocynin reduced aortic VCAM-1 expression by 50% (p<0.05). However, apocynin therapy did not reduce either ROS content, superoxide generation, or macrophage content.
CONCLUSIONS
Short-term treatment with apocynin in atherosclerosis reduces endothelial cell adhesion molecule expression. This change in endothelial phenotype can be detected by molecular imaging before any measurable decrease in macrophage content, and is not associated with a detectable change in oxidative burden.
Keywords: Apocynin, Atherosclerosis, Oxidative Stress, Microbubbles, Molecular Imaging
INTRODUCTION
Endothelial activation is a key step both in the initiation of atherosclerotic lesions as well as in their progression to a late stage, where inflammatory cell burden and susceptibility to acute atherothrombotic complications are high. Oxidative stress plays a major role in supporting and amplifying the endothelial activation in atherosclerosis1. The family of NOX NADPH oxidase present in plaque macrophages, and in native endothelial and smooth muscle cells is a major source of reactive oxygen species (ROS) and therefore represents a potential therapeutic target2.
Apocynin is a polyphenolic drug that has been isolated from plant extracts and inhibits assembly of the NOX2 isoform of the NADPH oxidase enzyme complex3. In mice with advanced atherosclerosis, long-term therapy with apocynin has been shown to reduce endothelial adhesion molecule expression, platelet adhesion, and plaque growth; while in hypercholesterolemic rabbits apocynin started at a much earlier stage of disease has been shown to prevent development of atherosclerotic lesions4. It is unknown whether the beneficial effects of apocynin occur early after initiation of therapy. With regards to mechanism, it is unknown whether apocynin’s effects are entirely due to a reduction in oxidative stress since polyphenolic drugs such as apocynin have anti-inflammatory effects independent of their anti-oxidant properties5, 6. Direct anti-inflammatory action independent of anti-oxidant properties has been substantiated by the reduced adhesion molecule expression in cultured endothelial cells exposed to apocynin7, 8.
In this study we addressed many of these knowledge gaps. We performed in vivo ultrasound molecular imaging to test the hypothesis that short-term administration of apocynin in a model of early atherosclerosis reduces endothelial activation and platelet adhesion, two factors that are recognized to play an important role in plaque progression. Ex vivo techniques were used to evaluate whether these effects were associated with a reduction in vessel oxidative stress.
MATERIALS AND METHODS
Materials and Methods are available in the online-only Supplement.
RESULTS
Effect of apocynin on VCAM-1 expression and endothelial platelet adhesion
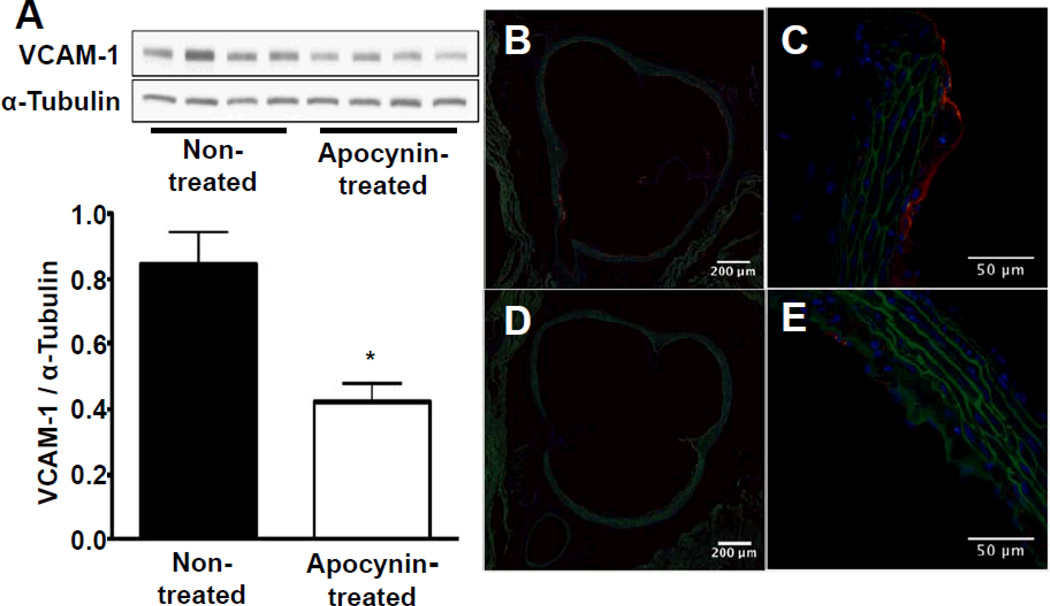
Apocynin treatment for 1 week reduced total aortic wall expression of VCAM-1 on Western blot by 50% (Figure 1A). On immunohistochemistry, small regions of neointimal formation were observed in the aortic root and proximal ascending portion. VCAM-1 staining was present on the endothelial lining and on macrophages in fibrofatty lesions in non-treated animals. In apocynin-treated animals, VCAM-1 expression was reduced both on endothelial cells as well as on macrophages within lesions, while the total amount of macrophages present in plaques remained unchanged (Figure 2). The reduction in endothelial VCAM-1 expression was evident both in regions overlying plaques as well as on endothelium in regions without plaques (Figure 1B–E).
Figure 1.
Assessment of vascular cell adhesion molecule 1 (VCAM-1) expression after 7 days of saline or apocynin treatment. A, VCAM-1 protein expression in the ascending aorta assessed by Western blot in nontreated (lanes 1–4) vs apocynin-treated (lanes 5–8) animals, n=4 per group, *P<0.05 vs nontreated animals. Representative examples of fluorescent immunohistochemistry images of the base of the aorta demonstrating endothelial VCAM-1 expression (red fluorescence) in a nontreated animal at 10-fold magnification (B), in the same animal at 40-fold magnification (C), and reduced VCAM-1 expression in an apocynin-treated animal (D and E). Autofluorescence delineating vessel anatomy is shown in green, 4´,6-diamidino-2-phenylindole staining of the nuclei in blue.
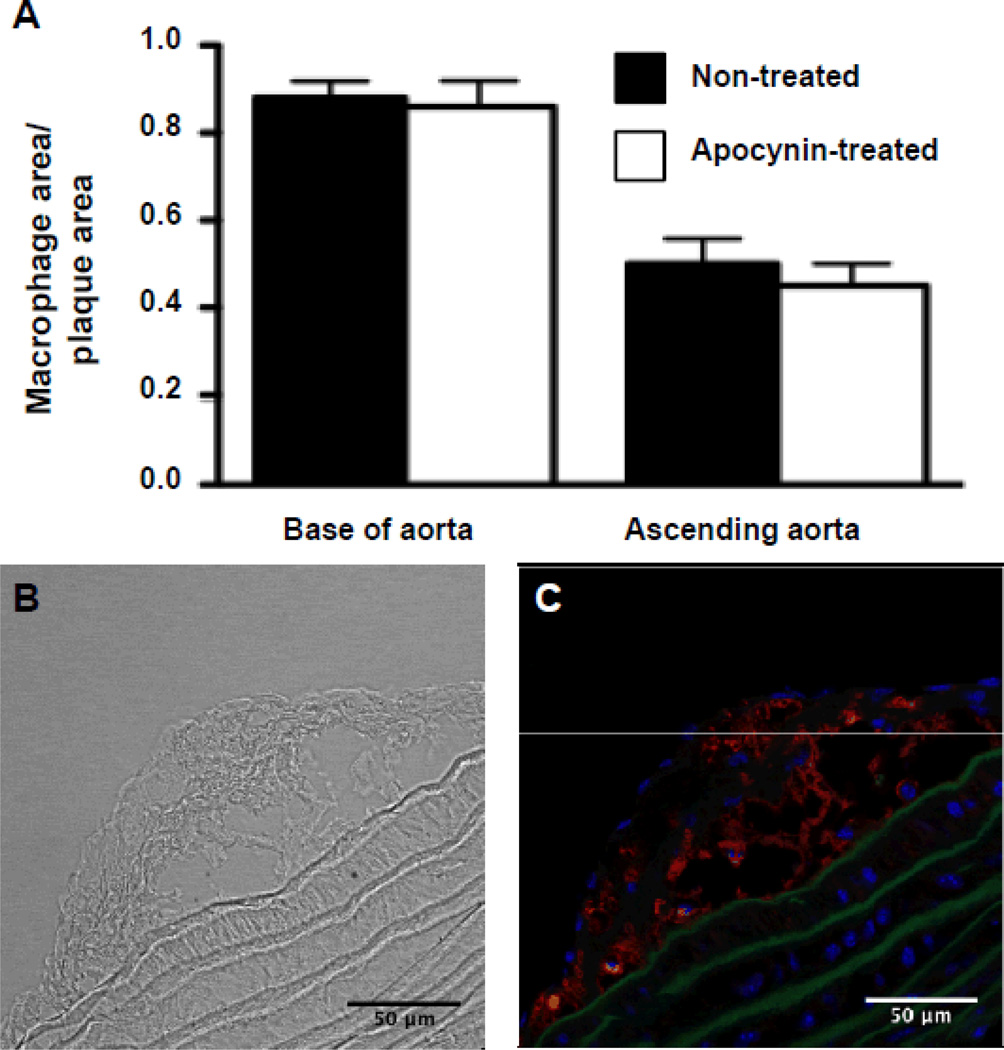
Figure 2.
Assessment of plaque macrophage content after 7 days of saline or apocynin treatment. A, Percentage of the plaque area covered with macrophages (Mac-2 staining) at the base of the aorta and in the ascending aorta (n=8 in each group; P=nonsignificant vs non-treated). B, Example of trans-illumination image used for plaque delineation. C, Example of Mac-2 staining used for quantification of macrophage content in the plaque.
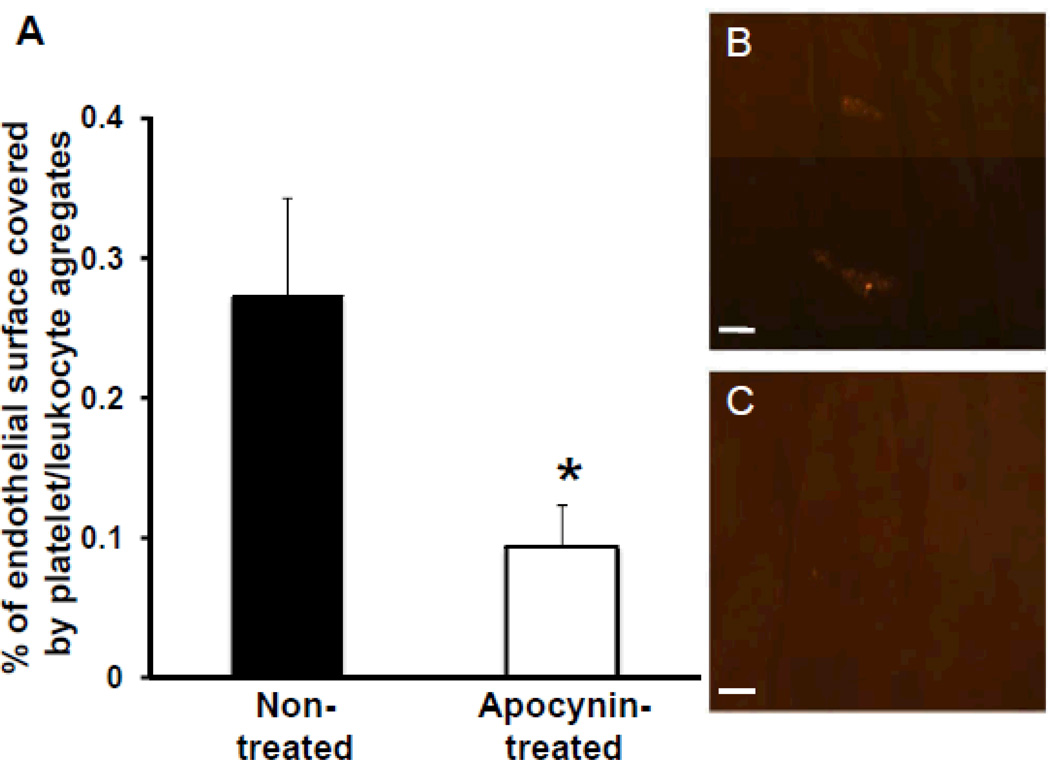
In vivo labeling of platelets with rhodamine-6G allowed visualization of platelet/leukocyte aggregates on the vascular endothelial surface. Platelet/leukocyte aggregates were present in regions with early plaques, but also in regions of the aortic endothelial surface with a normal appearance. Both the number of platelet/leukocyte aggregates per square millimeter (5.0±0.44 in apocynin treated animals vs 9.6±0.52 in non-treated animals, p<0.01) and the percentage of endothelial surface covered by platelet/leukocyte aggregates was significantly reduced in animals that were treated with apocynin (Figure 3).
Figure 3.
Assessment of platelet adhesion on the aortic endothelial surface after 7 days of saline or apocynin treatment. A, Percentage of the endothelial surface of the ascending aorta covered with platelet/leukocyte aggregates (n=5 in each group), *P<0.05 vs nontreated animals. Examples of en face fluorescence microscopy demonstrating 2 platelet/leukocyte aggregates on normal appearing endothelial surface in a nontreated animal (B) and absence of platelet/leukocyte aggregates in an apocynin-treated animal (C). Scale bar, 25 µm.
Effect of apocynin on vascular oxidative stress
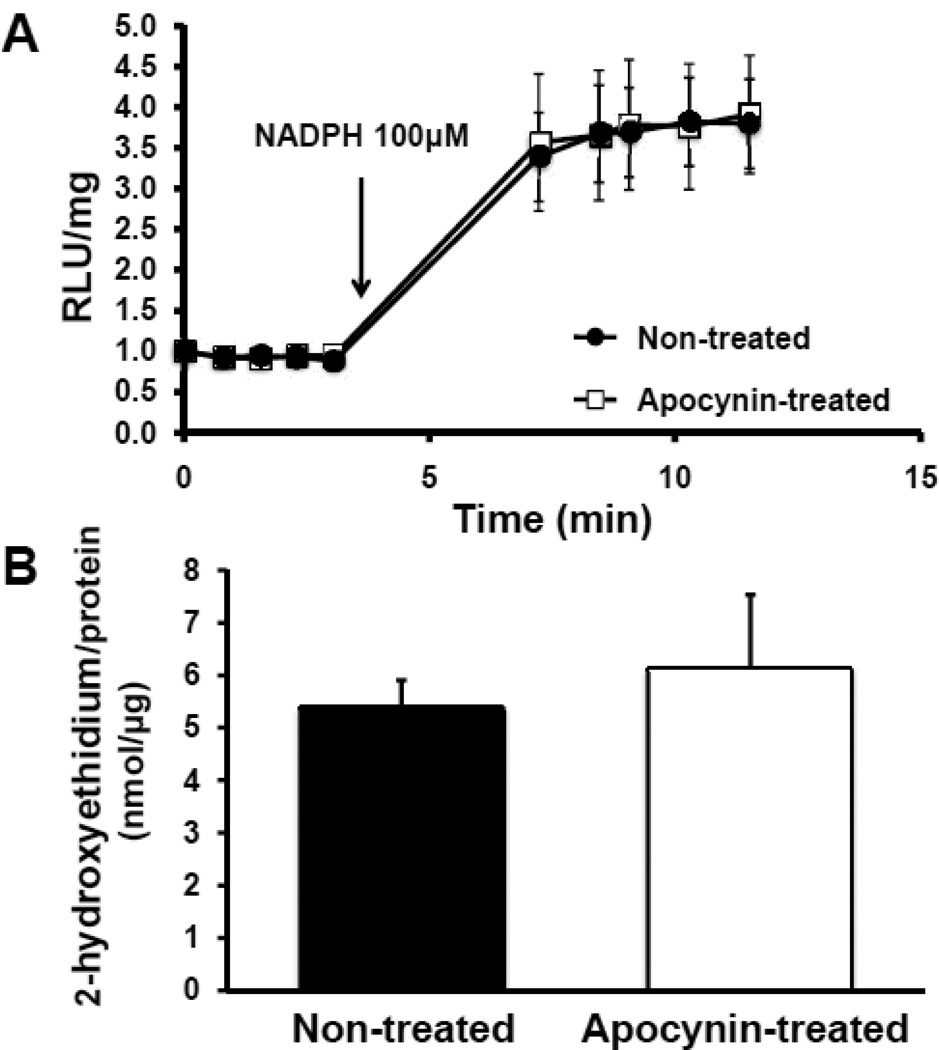
Lucigenin assays showed robust NADPH oxidase activity in whole aortic rings of non-treated mice. In mice treated with apocynin, NADPH-dependent lucigenin chemiluminescence was not different compared to non-treated mice. Given the potential of lucigenin to undergo redox cycling and generate artifactual signals, high pressure liquid chromatography (HPLC) of tissue extracts after exposure of aortic rings to HE was performed to directly assess tissue superoxide content. In accordance with the results of the lucigenin assays, HE oxidation to EOH was not different between the two animal groups (Figure 4).
Figure 4.
Assessment of reactive oxygen species generation after 7 days of saline or apocynin treatment. A, Superoxide-generating activity of whole aortic rings after the addition of 100 µmol/L NADPH at 4 minutes. Measurements represent relative light units (RLU).P=nonsignificant (ns) between saline-treated and apocynin-treated animals (n=10 in each group). B, High pressure liquid chromatography analysis of 2-hydroxyethidium generated in vascular rings exposed to 50 µmol/L hydroethidine. P=ns between saline-treated and apocynin-treated animals (n=8 in each group).
Molecular Imaging of VCAM-1 expression and platelet adhesion
High frequency ultrasound imaging was not of sufficient quality for evaluation in one animal in each group. In the remaining animals, there were no differences in left ventricular ejection fraction, peak aortic flow velocity or aortic diameter, indicating that apocynin treatment did not lead to hemodynamic differences that could potentially influence targeted microbubble adhesion (table).
Table.
Echocardiographic data (mean ± 1 SD)
| Saline- treated (n=8) |
Apocynin- treated (n=9) |
P- value | |
|---|---|---|---|
| Ejection fraction (%) | 58.4±13.1 | 61.6±8.8 | ns |
| Aortic internal diameter (mm) | 1.54±0.09 | 1.50±0.12 | ns |
| Aortic peak systolic velocity (m/s) | 0.58±0.15 | 0.68±0.03 | ns |
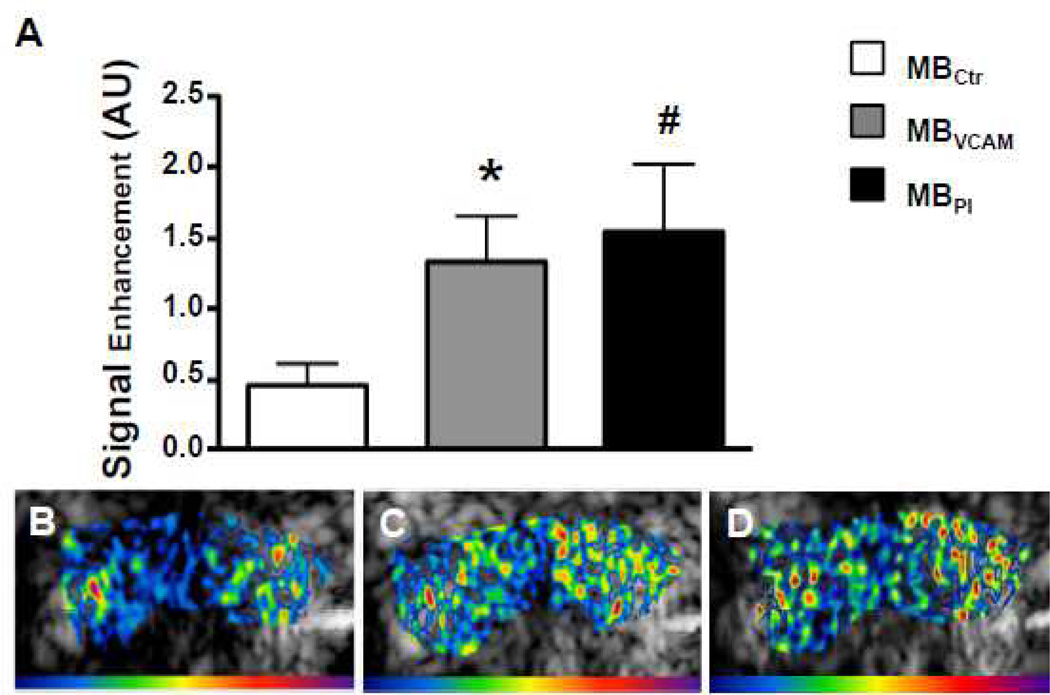
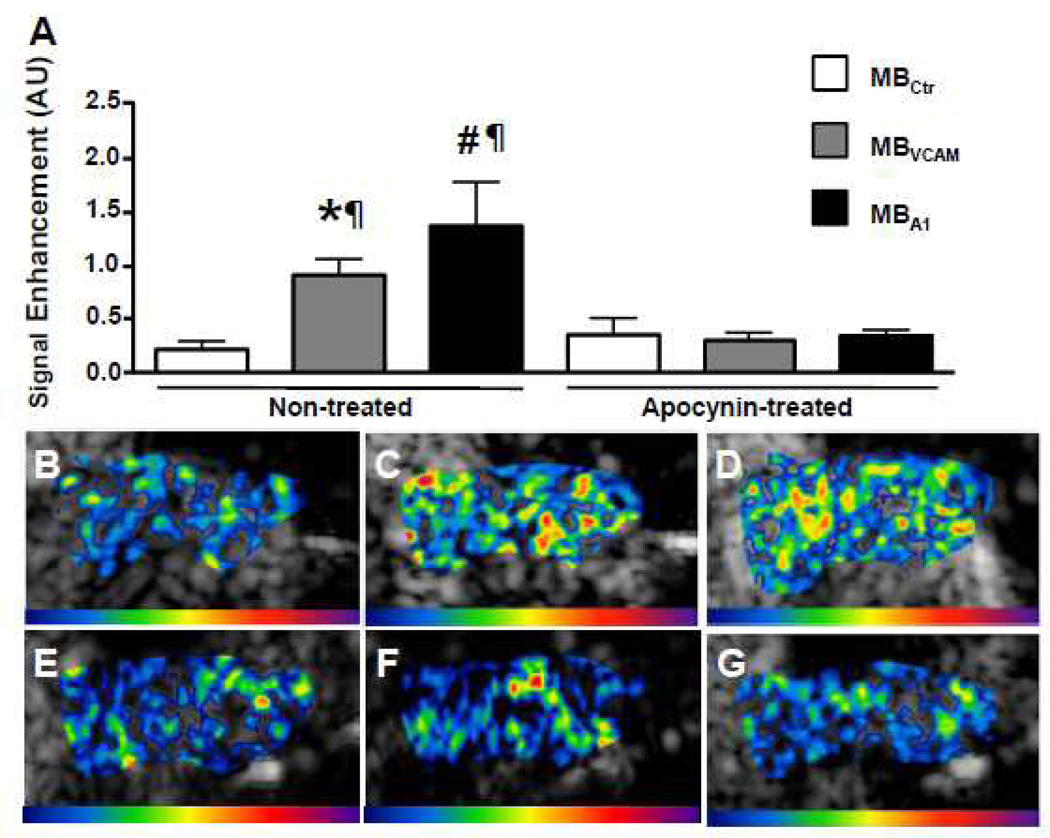
CEU molecular imaging in the ascending aorta at baseline showed greater signal enhancement for VCAM-1-targeted and platelet-targeted microbubbles compared to control microbubbles (Figure 5). After 7 days of treatment with apocynin, signal for VCAM-1 targeted and platelet targeted microbubbles was not different from control microbubble signal (Figure 6). In contrast, in animals treated with saline injections, the signal for VCAM-1 and platelets was elevated significantly over control signal to a degree that was similar to baseline. In the subgroup of animals that were imaged before and after treatment, apocynin lead to a significant decrease in VCAM-1 targeted signal (from 1.80±0.51 to 0.30±0.10, p=0.046) and a strong trend for decrease in platelet-targeted signal (2.21±0.80 to 0.35±0.07, p=0.078). In animals imaged before and after treatment with saline injections, signal for VCAM-1 (1.44± 0.55 vs. 1.00± 0.23, p=1.00) and platelet-targeted signal (1.84± 0.88 vs. 1.26± 0.40, p=0.69) did not decrease significantly.
Figure 5.
Molecular imaging of the ascending aorta before the start of treatment.A, Mean±SEM background-subtracted signal intensity for microbubbles targeted to vascular cell adhesion molecule 1 (VCAM-1; MBVCAM), to glycoprotein Ibα on activated thrombocytes (MBPl), and control microbubbles (MBCtr). *P<0.01 vs MBctr, #P<0.05 vs MBctr(n=12). Examples of color-coded contrast-enhanced ultrasound (CEU) images after injection of MBCtr (B), of MBVCAM (C), and of MBPl (D). The color scale for the CEU images is shown at the bottom of each frame.
Figure 6.
Molecular imaging of the ascending aorta after 7 days of saline or apocynin treatment. A, Mean±SEM background-subtracted signal intensity in saline-treated (n=9) and apocynin-treated (n=10) animals for microbubbles targeted to vascular cell adhesion molecule 1 (VCAM-1; MBVCAM), to glycoprotein Ibα on activated thrombocytes (MBPl), and control microbubbles (MBCtr). *P<0.01 vs MBctr, #P<0.01 vs MBctr. ¶P<0.05 vs the same microbubble in apocynin-treated animals. Examples of color-coded contrast-enhanced ultrasound (CEU) images after injection of MBCtr (B), of MBVCAM (C), and of MBPl (D) in saline-treated animals. In the same order, examples of color-coded CEU images after injection of MBCtr (E), of MBVCAM (F), and of MBPl (G) in apocynin-treated animals are shown.
DISCUSSION
Endothelial activation plays a crucial role in the initiation and progression of atherosclerotic plaque formation. In this study, short term treatment with the polyphenol apocynin in a murine model of early mild atherosclerosis lead to a reduction in endothelial inflammatory phenotype and platelet adhesion. These relatively acute changes were not associated with a measurable reduction in vascular NADPH oxidase activity or superoxide content.
Endothelial adhesion molecule expression is an early and important step in the pathogenesis of atherosclerosis. Deposition of oxidized LDL in the vascular wall leads to endothelial expression of pro-inflammatory cytokines such as IL-1-β and tumor necrosis factor-α. Locally increased cytokine levels result in an upregulation of cell adhesion molecules such as VCAM-1, mediated by the transcriptional factors activated protein-1 (AP-1) and nuclear factor κB (NF-κB), this in turn promotes the recruitment of leukocytes to the vessel wall.
ROS generated by NADPH oxidases both in endothelial cells as well as in leukocytes in nascent plaques are thought to amplify vascular inflammation throughout the pathogenesis of atherosclerosis. Accordingly, mouse models with knockouts of the NADPH oxidase isoforms NOX-1 and NOX-2 or the cytosolic NADPH oxidase subunit p47phox have shown a reduction in atherosclerotic plaque formation9–11. In humans, functional deficiency of the GP91phox subunit is associated with smaller carotid intima-media thickness (CIMT)12. These findings have generated interest in using inhibitors of NADPH oxidases for the treatment of atherosclerosis. Apocynin inhibits NADPH oxidase activity in leukocytes allegedly by impeding the assembly of the cytosolic subunits at the cell membrane13. In cell culture experiments it has been noted that the inhibitory action of apocynin occurs with a delay, suggesting that it has to undergo activation before inhibiting ROS generation. In the presence of H2O2 and myeloperoxidase, apocynin is converted to an apocynin radical and subsequently forms apocynin dimers, which are thought to be the active compounds that result in inhibition of NADPH oxidase activity14. Thus, the effect of apocynin on NADPH oxidase inhibition during the later stages of atherosclerosis may depend on its activation in vascular tissue and require a sufficient inflammatory cell and oxidative stress burden. Notably, however, apocynin also exerts anti-inflammatory effects that are independent of NADPH oxidase inhibition6. Such effects may be of importance during the early stages of atherosclerosis, when inflammatory cell load and oxidative burden are low.
We examined the effects of treatment with apocynin in a murine model of early atherosclerosis in mice with fibrofatty lesions. Assessing treatment effects of potential drug candidates during the early stages of atherosclerosis is of clinical significance, since interventions that are started early during the pathogenesis of atherosclerosis are thought to afford a larger risk reduction for cardiovascular events than interventions that are initiated when clinical atherosclerotic disease is established15. Our data indicate that treatment with apocynin results in a rapid decrease in endothelial expression of VCAM-1. These results are in line with observations in cell culture showing a decrease in VCAM-1 expression in response to apocynin8, and extend observations made in advanced atherosclerosis to earlier stages of plaque development4. The decrease in signal from VCAM-1 targeted microbubbles was more pronounced than differences in VCAM-1 expression determined with Western blot. We believe this to be due to the capability of ultrasound molecular imaging to specifically assess endothelial inflammatory phenotype16 and thus to detect a more pronounced decrease in endothelial VCAM-1 compared to Western blot which assesses expression in the whole vascular wall. In addition, the observation that the decrease in vascular inflammation was not associated with a reduction of vascular NADPH oxidase activity or tissue superoxide content indicates that the antiinflammatory effect of apocynin observed in our study was probably mediated through a ROS-independent mechanism in the very early stages of atherosclerotic plaque development, possibly mediated instead by its effects on cytochrome P450 pathways17.
In addition to endothelial cell inflammatory activation, platelet-endothelial interactions play a role in vascular inflammation and the pathogenesis of atherosclerosis. Platelet-endothelial interactions mediated by P-Selectin and von Willebrand factor- GPIbα ligation in the absence of plaque rupture accelerate plaque formation in murine atherosclerosis18–20. The interaction of activated platelets with the endothelial surface results in the secretion of proinflammatory cytokines CD40L and IL-1β, as well as of chemokines like RANTES (regulated on activation, normal T cell expressed and secreted) and platelet factor 4 (PF4) to the vascular wall, all of which facilitate increased monocyte recruitment21–23. Platelets from patients with functional deficiency of GP91phox subunit of NADPH oxidase or control subject platelets treated with apocynin have reduced in vitro platelet recruitment and aggregation, indicating a direct role of NADPH oxidase in platelet reactivity24. Our molecular imaging results indicate a decrease in platelet-endothelial interactions after treatment with apocynin. While we did not specifically investigate the pathways responsible for the action of apocynin in platelets, previous data indicate that apocynin influences platelet aggregation by mechanisms that are dependent on NADPH oxidase activity25 but also on changes in arachidonic acid metabolism with a decrease in thromboxane A2 formation5.
Several limitations of our study deserve attention. First, as the aim of our study was to assess the acute effects of apocynin treatment on the endothelial inflammatory phenotype, we did not expect an influence of treatment on plaque size and thus did not perform histological analysis. However, long-term treatment with apocynin has been shown to reduce plaque formation in our mouse model5. Also, the dose used in our study was in the low range of doses used in published animal studies, however, there is no established optimal dose, and our data demonstrate an effect of the treatment on both endothelial inflammatory phenotype and platelet adhesion. Furthermore, while we applied well-established techniques to measure NADPH oxidase activity and superoxide content without finding an effect of apocynin therapy, we cannot exclude that locally restricted (e.g. endothelial) and/or changes in other ROS species contributed to the observed effect. Finally, our methods for evaluating platelet adhesion did not allow differentiation of direct endothelial attachment and platelet-monocyte complexes.
In summary, we show that in a murine model of early atherosclerosis, treatment with apocynin leads to a rapid decrease in endothelial inflammation and platelet adhesion, which are detectable using ultrasound molecular imaging. Our data indicate that these effects of apocynin are not associated with a measurable decrease in ROS generation.
Supplementary Material
SIGNIFICANCE.
Anti-oxidative drugs continue to be developed for the treatment of atherosclerosis. In this study, we assessed whether short-term therapy with the NADPH oxidase inhibitor apocynin reduces vascular oxidative stress, endothelial inflammatory activation and platelet adhesion in a murine model of early atherosclerosis, and whether molecular imaging is capable of detecting these changes. Assessing the treatment effects of potential drug candidates during the early stages of atherosclerosis is of clinical significance, since interventions that are started early during the pathogenesis of atherosclerosis are thought to afford a larger risk reduction for cardiovascular events than interventions that are initiated late. We show that ultrasound molecular imaging is capable of detecting reductions in endothelial inflammatory activation and in platelet adhesion at a stage before any measurable, treatment associated decrease in macrophage content occurs. These phenotypic changes are not associated with measurable changes in oxidative stress.
ACKNOWLEDGMENTS
Sources of funding: This study was supported by SCORE grants from the Swiss National Science Foundation to Dr. Kaufmann (SNSF 32323B_123819 and 3232B0-141603) and to Dr. Kuster (SNSF 144208), as well as by grants from the U.S. National Institutes of Health (R01-DK-063508, R01-HL-078610, RC1-HL-100659 to Dr. Lindner, and R01 HL42846 to Dr. Ruggeri). Dr. Khanicheh is supported by an MD-PhD start up grant from the University Hospital Basel.
ABBREVIATIONS
- A.U.
arbitrary units
- CEU
contrast enhanced ultrasound
- GPIbα
glycoprotein Ibα
- HE
hydroethidine
- LDL
Low density lipoprotein
- NADPH oxidase
nicotinamide adenine dinucleotide phosphate-oxidase
- RANTES
regulated on activation, normal T cell expressed and secreted
- PF4
platelet factor 4
- ROS
reactive oxygen species
- VCAM-1
vascular cell adhesion molecule 1
- VWF
Von Willebrand Factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures: None.
REFERENCES
- 1.Singh U, Jialal I. Oxidative stress and atherosclerosis. Pathophysiology. 2006;13:129–142. doi: 10.1016/j.pathophys.2006.05.002. [DOI] [PubMed] [Google Scholar]
- 2.Lassegue B, San Martin A, Griendling KK. Biochemistry, physiology, and pathophysiology of nadph oxidases in the cardiovascular system. Circ Res. 2012;110:1364–1390. doi: 10.1161/CIRCRESAHA.111.243972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stolk J, Hiltermann TJ, Dijkman JH, Verhoeven AJ. Characteristics of the inhibition of nadph oxidase activation in neutrophils by apocynin, a methoxy-substituted catechol. Am J Respir Cell Mol Biol. 1994;11:95–102. doi: 10.1165/ajrcmb.11.1.8018341. [DOI] [PubMed] [Google Scholar]
- 4.Liu Y, Davidson BP, Yue Q, Belcik T, Xie A, Inaba Y, McCarty OJ, Tormoen GW, Zhao Y, Ruggeri ZM, Kaufmann BA, Lindner JR. Molecular imaging of inflammation and platelet adhesion in advanced atherosclerosis effects of antioxidant therapy with nadph oxidase inhibition. Circ Cardiovasc Imaging. 2013;6:74–82. doi: 10.1161/CIRCIMAGING.112.975193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Engels F, Renirie BF, Hart BA, Labadie RP, Nijkamp FP. Effects of apocynin, a drug isolated from the roots of picrorhiza kurroa, on arachidonic acid metabolism. FEBS Lett. 1992;305:254–256. doi: 10.1016/0014-5793(92)80680-f. [DOI] [PubMed] [Google Scholar]
- 6.Houser KR, Johnson DK, Ishmael FT. Anti-inflammatory effects of methoxyphenolic compounds on human airway cells. J Inflamm (Lond) 2012;9:6. doi: 10.1186/1476-9255-9-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yu J, Weiwer M, Linhardt RJ, Dordick JS. The role of the methoxyphenol apocynin, a vascular nadph oxidase inhibitor, as a chemopreventative agent in the potential treatment of cardiovascular diseases. Curr Vasc Pharmacol. 2008;6:204–217. doi: 10.2174/157016108784911984. [DOI] [PubMed] [Google Scholar]
- 8.Suzuki Y, Wang W, Vu TH, Raffin TA. Effect of nadph oxidase inhibition on endothelial cell elam-1 mrna expression. Biochem Biophys Res Commun. 1992;184:1339–1343. doi: 10.1016/s0006-291x(05)80029-4. [DOI] [PubMed] [Google Scholar]
- 9.Sheehan AL, Carrell S, Johnson B, Stanic B, Banfi B, Miller FJ., Jr Role for nox1 nadph oxidase in atherosclerosis. Atherosclerosis. 2011;216:321–326. doi: 10.1016/j.atherosclerosis.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Judkins CP, Diep H, Broughton BR, Mast AE, Hooker EU, Miller AA, Selemidis S, Dusting GJ, Sobey CG, Drummond GR. Direct evidence of a role for nox2 in superoxide production, reduced nitric oxide bioavailability, and early atherosclerotic plaque formation in apoe−/− mice. Am J Physiol Heart Circ Physiol. 2010;298:H24–H32. doi: 10.1152/ajpheart.00799.2009. [DOI] [PubMed] [Google Scholar]
- 11.Vendrov AE, Hakim ZS, Madamanchi NR, Rojas M, Madamanchi C, Runge MS. Atherosclerosis is attenuated by limiting superoxide generation in both macrophages and vessel wall cells. Arterioscler Thromb Vasc Biol. 2007;27:2714–2721. doi: 10.1161/ATVBAHA.107.152629. [DOI] [PubMed] [Google Scholar]
- 12.Violi F, Pignatelli P, Pignata C, Plebani A, Rossi P, Sanguigni V, Carnevale R, Soresina A, Finocchi A, Cirillo E, Catasca E, Angelico F, Loffredo L. Reduced atherosclerotic burden in subjects with genetically determined low oxidative stress. Arterioscler Thromb Vasc Biol. 2013;33:406–412. doi: 10.1161/ATVBAHA.112.300438. [DOI] [PubMed] [Google Scholar]
- 13.Barbieri SS, Cavalca V, Eligini S, Brambilla M, Caiani A, Tremoli E, Colli S. Apocynin prevents cyclooxygenase 2 expression in human monocytes through nadph oxidase and glutathione redox-dependent mechanisms. Free Radic Biol Med. 2004;37:156–165. doi: 10.1016/j.freeradbiomed.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 14.Johnson DK, Schillinger KJ, Kwait DM, Hughes CV, McNamara EJ, Ishmael F, O'Donnell RW, Chang MM, Hogg MG, Dordick JS, Santhanam L, Ziegler LM, Holland JA. Inhibition of nadph oxidase activation in endothelial cells by ortho-methoxy-substituted catechols. Endothelium. 2002;9:191–203. doi: 10.1080/10623320213638. [DOI] [PubMed] [Google Scholar]
- 15.Ference BA, Yoo W, Alesh I, Mahajan N, Mirowska KK, Mewada A, Kahn J, Afonso L, Williams KA, Sr, Flack JM. Effect of long-term exposure to lower low-density lipoprotein cholesterol beginning early in life on the risk of coronary heart disease: A mendelian randomization analysis. J Am Coll Cardiol. 2012;60:2631–2639. doi: 10.1016/j.jacc.2012.09.017. [DOI] [PubMed] [Google Scholar]
- 16.Kaufmann BA, Sanders JM, Davis C, Xie A, Aldred P, Sarembock IJ, Lindner JR. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116:276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 17.Pietersma A, de Jong N, de Wit LE, Kraak-Slee RG, Koster JF, Sluiter W. Evidence against the involvement of multiple radical generating sites in the expression of the vascular cell adhesion molecule-1. Free Radic Res. 1998;28:137–150. doi: 10.3109/10715769809065800. [DOI] [PubMed] [Google Scholar]
- 18.Burger PC, Wagner DD. Platelet p-selectin facilitates atherosclerotic lesion development. Blood. 2003;101:2661–2666. doi: 10.1182/blood-2002-07-2209. [DOI] [PubMed] [Google Scholar]
- 19.Huo Y, Schober A, Forlow SB, Smith DF, Hyman MC, Jung S, Littman DR, Weber C, Ley K. Circulating activated platelets exacerbate atherosclerosis in mice deficient in apolipoprotein e. Nat Med. 2003;9:61–67. doi: 10.1038/nm810. [DOI] [PubMed] [Google Scholar]
- 20.Massberg S, Brand K, Gruner S, Page S, Muller E, Muller I, Bergmeier W, Richter T, Lorenz M, Konrad I, Nieswandt B, Gawaz M. A critical role of platelet adhesion in the initiation of atherosclerotic lesion formation. J Exp Med. 2002;196:887–896. doi: 10.1084/jem.20012044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Henn V, Slupsky JR, Grafe M, Anagnostopoulos I, Forster R, Muller-Berghaus G, Kroczek RA. Cd40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature. 1998;391:591–594. doi: 10.1038/35393. [DOI] [PubMed] [Google Scholar]
- 22.Hawrylowicz CM, Howells GL, Feldmann M. Platelet-derived interleukin 1 induces human endothelial adhesion molecule expression and cytokine production. J Exp Med. 1991;174:785–790. doi: 10.1084/jem.174.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.von Hundelshausen P, Weber KS, Huo Y, Proudfoot AE, Nelson PJ, Ley K, Weber C. Rantes deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 24.Pignatelli P, Carnevale R, Di Santo S, Bartimoccia S, Sanguigni V, Lenti L, Finocchi A, Mendolicchio L, Soresina AR, Plebani A, Violi F. Inherited human gp91phox deficiency is associated with impaired isoprostane formation and platelet dysfunction. Arterioscler Thromb Vasc Biol. 2011;31:423–434. doi: 10.1161/ATVBAHA.110.217885. [DOI] [PubMed] [Google Scholar]
- 25.Begonja AJ, Gambaryan S, Geiger J, Aktas B, Pozgajova M, Nieswandt B, Walter U. Platelet nad(p)h-oxidase-generated ros production regulates alphaiibbeta3-integrin activation independent of the no/cgmp pathway. Blood. 2005;106:2757–2760. doi: 10.1182/blood-2005-03-1047. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.