Abstract
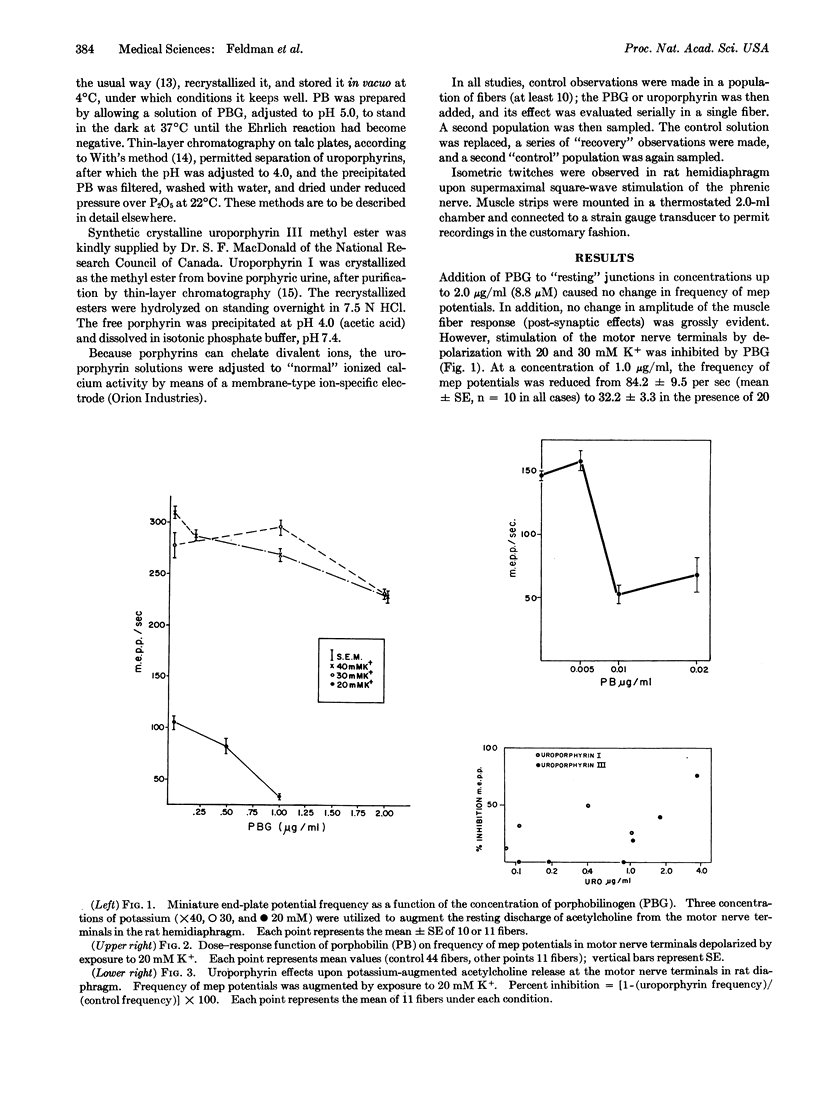
The rat hemidiaphragm was studied in vitro as a test system to evaluate the effects of heme precursors and the uroporphyrins upon neuromuscular excitability. Porphobilinogen and porphobilin had no effect on the resting miniature end-plate potential frequency, but the K+-augmented frequency was significantly reduced. Porphobilin and porphobilinogen gave 50% of their maximal effect at concentrations of 0.008 and 0.6 μg/ml, respectively; the effect increased with concentration. Uroporphyrin I at 0.05-1.0 μg/ml caused a 25% decrease in frequency, but the effect did not increase with concentration. At similar concentrations, uroporphyrin III was without effect.
The concentrations of porphobilin and porphobilinogen effective in inhibiting the K+ stimulation, which are several orders of magnitude lower than the effective concentrations of simple amino acids, are those which might reasonably be expected in the sera of patients with acute intermittent porphyria.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COOKSON G. H., RIMINGTON C. Porphobilinogen. Biochem J. 1954 Jul;57(3):476–484. doi: 10.1042/bj0570476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal R. A., Bossenmaier I., Petryka Z. J., Johnson L., Watson C. J. Isolation of porphyrins from porphyria urine by preparative thin-layer chromatography. J Chromatogr. 1968 Nov 5;38(1):100–105. doi: 10.1016/0021-9673(68)85012-5. [DOI] [PubMed] [Google Scholar]
- DE MATTEIS F., RIMINGTON C. The biochemical disturbance in acute intermittent and experimental porphyria. Lancet. 1962 Jun 23;1(7243):1332–1334. doi: 10.1016/s0140-6736(62)92427-3. [DOI] [PubMed] [Google Scholar]
- Davidoff R. A., Shank R. P., Graham L. T., Jr, Aprison M. H., Werman R. Association of glycine with spinal interneurones. Nature. 1967 May 13;214(5089):680–681. doi: 10.1038/214680a0. [DOI] [PubMed] [Google Scholar]
- ELMQVIST D., QUASTEL D. M. PRESYNAPTIC ACTION OF HEMICHOLINIUM AT THE NEUROMUSCULAR JUNCTION. J Physiol. 1965 Apr;177:463–482. doi: 10.1113/jphysiol.1965.sp007605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmqvist D., Feldman D. S. Effects of sodium pump inhibitors on spontaneous acetylcholine release at the neuromuscular junction. J Physiol. 1965 Dec;181(3):498–505. doi: 10.1113/jphysiol.1965.sp007778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FATT P., KATZ B. An analysis of the end-plate potential recorded with an intracellular electrode. J Physiol. 1951 Nov 28;115(3):320–370. doi: 10.1113/jphysiol.1951.sp004675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman D. S., Levere R. D., Lieberman J. S. Presynaptic neuromuscular inhibition by delta-aminolevulinic acid, a porphyrin precursor. Trans Am Neurol Assoc. 1968;93:206–208. [PubMed] [Google Scholar]
- GOLDBERG A., PATON W. D., THOMPSON J. W. Pharmacology of the porphyrins and porphobilinogen. Br J Pharmacol Chemother. 1954 Mar;9(1):91–94. doi: 10.1111/j.1476-5381.1954.tb00822.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GRANICK S., LEVERE R. D. HEME SYNTHESIS IN ERYTHROID CELLS. Prog Hematol. 1964;4:1–47. [PubMed] [Google Scholar]
- Gage P. W., Hubbard J. I. Evidence for a Poisson distribution of miniature end-plate potentials and some implications. Nature. 1965 Oct 23;208(5008):395–396. doi: 10.1038/208395a0. [DOI] [PubMed] [Google Scholar]
- Heilmeyer L., Clotten R. Zur biochemischen Pathogenese der Pophyria acuta intermittens. Klin Wochenschr. 1969 Jan 15;47(2):71–74. doi: 10.1007/BF01745768. [DOI] [PubMed] [Google Scholar]
- KATZ B., MILEDI R. THE EFFECT OF CALCIUM ON ACETYLCHOLINE RELEASE FROM MOTOR NERVE TERMINALS. Proc R Soc Lond B Biol Sci. 1965 Feb 16;161:496–503. doi: 10.1098/rspb.1965.0017. [DOI] [PubMed] [Google Scholar]
- Kosower N. S., Rock R. A. Seizures in experimental porphyria. Nature. 1968 Feb 10;217(5128):565–567. doi: 10.1038/217565a0. [DOI] [PubMed] [Google Scholar]
- LILEY A. W. The quantal components of the mammalian end-plate potential. J Physiol. 1956 Sep 27;133(3):571–587. doi: 10.1113/jphysiol.1956.sp005610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MAUZERALL D., GRANICK S. The occurrence and determination of delta-amino-levulinic acid and porphobilinogen in urine. J Biol Chem. 1956 Mar;219(1):435–446. [PubMed] [Google Scholar]
- Strand L. J., Felsher B. F., Redeker A. G., Marver H. S. Heme biosynthesis in intermittent acute prophyria: decreased hepatic conversion of porphobilinogen to porphyrins and increased delta aminolevulinic acid synthetase activity. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1315–1320. doi: 10.1073/pnas.67.3.1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taddeini L., Watson C. J. The clinical porphyrias. Semin Hematol. 1968 Oct;5(4):335–369. [PubMed] [Google Scholar]
- WATSON C. J., RUNGE W., TADDEINI L., BOSSENMAIER I., CARDINAL R. A SUGGESTED CONTROL GENE MECHANISM FOR THE EXCESSIVE PRODUCTION OF TYPES I AND 3 PORPHYRINS IN CONGENITAL ERYTHROPOIETIC PORPHYRIA. Proc Natl Acad Sci U S A. 1964 Aug;52:478–485. doi: 10.1073/pnas.52.2.478. [DOI] [PMC free article] [PubMed] [Google Scholar]


