Abstract
Candida species exhibit a variety of ploidy states and modes of sexual reproduction. Most species possess the requisite genes for sexual reproduction, recombination, and meiosis, yet only a few have been reported to undergo a complete sexual cycle including mating and sporulation. Candida albicans, the most studied Candida species and a prevalent human fungal pathogen, completes its sexual cycle via a parasexual process of concerted chromosome loss rather than a conventional meiosis. In this study, we examine ploidy changes in Candida tropicalis, a closely related species to C. albicans that was recently revealed to undergo sexual mating. C. tropicalis diploid cells mate to form tetraploid cells, and we show that these can be induced to undergo chromosome loss to regenerate diploid forms by growth on sorbose medium. The diploid products are themselves mating competent, thereby establishing a parasexual cycle in this species for the first time. Extended incubation (>120 generations) of C. tropicalis tetraploid cells under rich culture conditions also resulted in instability of the tetraploid form and a gradual reduction in ploidy back to the diploid state. The fitness levels of C. tropicalis diploid and tetraploid cells were compared, and diploid cells exhibited increased fitness relative to tetraploid cells in vitro, despite diploid and tetraploid cells having similar doubling times. Collectively, these experiments demonstrate distinct pathways by which a parasexual cycle can occur in C. tropicalis and indicate that nonmeiotic mechanisms drive ploidy changes in this prevalent human pathogen.
INTRODUCTION
Many yeast species can reproduce by both sexual and asexual mechanisms. Asexual reproduction is generally utilized when conditions are favorable for growth, while sexual reproduction is favored when nutrients are limiting (1). Sexual reproduction can generate increased levels of genomic diversity that promote adaptation to a changing or hostile environment (2). However, sex is also associated with an increased cost relative to asexual reproduction (3, 4). The ability to switch between sexual and asexual modes of reproduction (facultative sex) allows for reallocation of resources to sexual reproduction during periods of low fitness and also provides a mechanism for coping with deleterious mutations (5, 6).
Saccharomyces cerevisiae, the most widely studied of the ascomycetes, has served as a model organism for the study of mating and meiosis in fungi. It exhibits a mating cycle in which yeast cells alternate between haploid and diploid phases. Mating between haploid MATa and MATα cells generates diploid a/α cells that can subsequently undergo meiosis to form haploid ascospores (7, 8). Similar to S. cerevisiae, several human fungal pathogens have been shown to exhibit extant sexual programs (4, 8, 9). Cryptococcus neoformans, a basidiomycete that is a causative agent of fungal meningoencephalitis, exhibits both homothallic (same-sex) and heterothallic (intersex) mating (10, 11). This has direct implications for pathogenesis as sexual spores of C. neoformans are infectious propagules in animal models of infection (12). Furthermore, phylogenetic analysis of the sibling species Cryptococcus gattii has implicated α-α same-sex mating in the generation of a highly virulent isolate responsible for an ongoing outbreak that initiated on Vancouver Island (13–15). Aspergillus fumigatus, a prevalent airborne, ascomycetous pathogen, was also recently shown to undergo a complete sexual program complete with the formation of recombinant ascospores (16).
Candida species represent an important genus of human pathogens and these ascomycetes vary in their potential to undergo sexual reproduction. Several clinically relevant species reside within the so-called Candida clade, a group of species that translate the CUG codon as serine instead of leucine, as in the universal genetic code (17, 18). Within this clade, Candida lusitaniae, Candida guilliermondii, Debaryomyces hansenii, and Lodderomyces elongisporus have been reported to undergo full sexual cycles that include sporulation (19–23). In the case of C. lusitaniae, studies reveal that although this species lacks several conserved meiosis genes, it completes its sexual cycle through Spo11-dependent recombination and sporulation (17, 20). The meiotic process leads to high levels of aneuploidy, and resulting asci contain a dyad of ascospores rather than the conventional tetrads formed by S. cerevisiae (20).
The most prevalent human fungal pathogen is Candida albicans, a Candida clade member that undergoes both homothallic and heterothallic mating (24–26). Mating in this species is regulated by a phenotypic switch in which cells must transition from the conventional white state to the alternative opaque state to become mating competent (27). C. albicans possesses a number of genes implicated in meiosis (17, 28) but has not been observed to undergo a conventional meiotic program. In its place, ploidy reduction occurs through a process of concerted chromosome loss that results in high levels of chromosomal aneuploidy (29, 30). This parasexual chromosome loss has been proposed to promote adaptation through the generation of a large pool of genetically diverse progeny (31). Recombination during the parasexual process is dependent on the conserved meiosis-specific protein Spo11, indicating parallels with a conventional meiosis (30). Furthermore, C. albicans has recently been shown to form viable haploid cells that can undergo either auto-diploidization or mating to return to the diploid state (32).
Candida tropicalis is also a clinically relevant Candida clade species and is rapidly emerging as a major cause of candidiasis on a global scale (33). Until recently, C. tropicalis strains were believed to be asexual, but it is now apparent that a and α diploid cells can undergo efficient mating to generate tetraploid a/α cells (34, 35). C. tropicalis mating is controlled by a white-opaque transition related to that in C. albicans. However, it is not known if C. tropicalis cells can complete a sexual or parasexual program to return to the diploid state.
In this study, we tested the ability of genetically marked tetraploid strains of C. tropicalis to undergo a conventional meiosis or a parasexual (nonmeiotic) reduction in ploidy. We show that tetraploid cells can be induced to undergo a parasexual process when grown on sorbose medium. The resulting progeny are diploid, and a or α cells are capable of remating to form tetraploid cells, thus establishing a complete parasexual cycle for C. tropicalis. We also observed that C. tropicalis tetraploid cells are unstable during prolonged propagation in rich medium, eventually returning to the diploid state after ∼240 generations. Furthermore, we show that C. tropicalis diploid cells outcompete tetraploid cells during prolonged coculture in vitro. Taken together, our work compares the fitness of diploid and tetraploid states in C. tropicalis and demonstrates that this species can complete a parasexual cycle via distinct ploidy reduction pathways.
MATERIALS AND METHODS
Media.
Standard media were prepared as previously described (36, 37). Yeast extract peptone dextrose (YPD) contained 1% yeast extract, 2% peptone, and 2% glucose. YPD medium was supplemented with 200 μg/ml nourseothricin (YPD-NAT) to select for nourseothricin-resistant (NATR) strains (38). Sporulation medium #1 consisted of 1% potassium acetate, 0.05% dextrose, 0.1% yeast extract, and 0.01% complete amino acid mix. Sporulation medium #2 was Gorodkowa medium and consisted of 1% peptone, 0.5% NaCl, and 0.1% glucose. Potato dextrose agar (PDA) medium was 3.9% Difco potato dextrose agar and 0.07% tartaric acid. Minimal medium was 0.7% yeast nitrogen base (YNB) and 2% glucose. Sorbitol medium contained 18.2% sorbitol, 0.7% YNB, 2% glucose, complete amino acids, and 25 μg/ml uridine. Malt extract medium was 3% malt extract and 0.5% peptone. Sheep's blood agar was Columbia agar with 5% sheep's blood (Becton, Dickinson, and Co). V8 medium contained 5% V8 juice and 0.05% KH2PO4 (pH 7.0). Serum medium was YPD medium supplemented with 10% fetal bovine serum. Low-phosphate medium contained YNB without phosphate (CYN6701; Formedium, Ltd., Hunstanton, England) and KH2PO4 (10 μM) and was adjusted to pH 4.7 with 3 M HCl. Synthetic low-ammonium dextrose (SLAD) medium was YNB (without amino acids or ammonium sulfate) supplemented with 50 μM ammonium sulfate and 2% glucose. Sorbose medium contained 0.7% yeast nitrogen base, 2% sorbose, and 25 μg/ml uridine (with or without amino acids). 2-Deoxygalactose (2-DOG) and presporulation media were prepared as described previously (29). Solid media contained 2% agar.
Strain and plasmid construction.
All strains used in this study are listed in Table 1. Gene deletions in C. tropicalis strains were performed using the SAT1 flipper strategy (38). Strains were transformed with ∼1 μg of DNA using a modified electroporation protocol, as described previously (20, 34). In brief, cells were grown overnight at 30°C in 3 ml of YPD medium and diluted into 50-ml YPD cultures, which were grown overnight at 30°C. Samples were centrifuged and resuspended in 0.1 M lithium acetate, 7.5 mM Tris-HCl (pH 8.0), 1 mM EDTA, and 10 mM dithiothreitol for 1 h at room temperature. After cells were washed twice with water and once with 1 M sorbitol, they were resuspended in the liquid that remained after decantation. For each transformation, ∼50 μl of cells was mixed with 10 to 15 μl of DNA, and the mixture was electroporated at 1.8 kV using a MicroPulsor Electroporator (Bio-Rad). The cells were immediately resuspended in 1 ml of cold 1 M sorbitol, followed by a recovery period of 4 h at 30°C in 1 ml of YPD medium. Finally, cells were plated onto YPD medium containing 200 μg/ml nourseothricin (YPD-NAT) to select for cells that had incorporated the construct into the genome through homologous recombination. PCR was used to confirm proper integration into the correct locus. In order to flip out the SAT1 marker from the genome, cells were grown in liquid yeast extract-peptone (YP) medium supplemented with 2% maltose for 5 days at 30°C and plated on YPD medium containing 20 μg/ml nourseothricin. Small colonies were replica patched onto YPD and YPD-NAT plates to confirm loss of nourseothricin resistance. For double mutants, the transformation process was repeated, and PCR was used to confirm loss of the open reading frame (ORF).
Table 1.
Strains used in this study
| Strain | Genotype | MTL type | Ploidy |
|---|---|---|---|
| CAY2059 | arg4/arg4 | a/a | Diploid |
| CAY2060 | arg4/arg4 | a/a | Diploid |
| CAY2881 | his1/his1 GAL1/gal1::SAT1 | α/α | Diploid |
| CAY3031 | his1/his1 GAL1/gal1 | α/α | Diploid |
| CAY3371 | his1/his1 gal1/gal1::SAT1 | α/α | Diploid |
| CAY3372 | his1/his1 gal1/gal1::SAT1 | α/α | Diploid |
| CAY3373 | his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | a/a/α/α | Tetraploid |
| CAY3374 | his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | a/a/α/α | Tetraploid |
| CAY3375 | his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | a/a/α/α | Tetraploid |
| CAY4090 | his1/HIS1 arg4/ARG4 gal1::SAT1/GAL1 | a/α | Diploid |
| CAY4276 | his1/HIS1 arg4/ARG4 gal1/GAL1 | a/α | Diploid |
| CAY4277 | his1/HIS1 arg4/ARG4 gal1/GAL1 | a/α | Diploid |
| CAY4281 | his1/HIS1 arg4/ARG4 gal1::SAT1/GAL1 | a/α | Diploid |
| CAY4283 | his1/HIS1 arg4/ARG4 gal1::SAT1/GAL1 | a/α | Diploid |
| CAY4311 | his1/HIS1 arg4/ARG4 gal1/GAL1 | a/α | Diploid |
| CAY4539 | his1/HIS1 arg4/ARG4 gal1/GAL1 ura3::SAT1/URA3 | a/α | Diploid |
| CAY4888 | his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1/GAL1/GAL1 | a/a/α/α | Tetraploid |
| CAY4890 | his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1/GAL1/GAL1 | a/a/α/α | Tetraploid |
In order to delete genes using the SAT1 flipper, the 5′ and 3′ regions of the gene of interest were PCR amplified and cloned into the plasmid pSFS2a (38). The 5′ flank of the target gene was digested with ApaI/XhoI, and the 3′ flank was digested with SacI/SacII and cloned into pSFS2a. The resulting plasmid was digested with ApaI/SacI in order to liberate the deletion cassette. Correct integration of the cassette into the genome was confirmed by PCR. All oligonucleotides used in this study are listed in Table 2.
Table 2.
Oligonucleotides used in this study
| Oligonucleotide no. | Oligonucleotide name | Sequence |
|---|---|---|
| 562 | CtMTLα2 Check 1 | TAA AAC ATT AAG CAT AGA GGA CAA AGA A |
| 563 | CtMTLα2 Check 2 | AAC TTC AAA TGC AAA ATG TAA AAC ATA C |
| 564 | CtMTLa1 Check 1 | GGA TAA AGA GAG CTT AAG TTC AGA AGA G |
| 565 | CtMTLa1 Check 2 | AAG TAT CAT CTG TCT TAT CTG ATT CCT TC |
| 641 | CtHIS1 (ApaI) −900 | GGA GCG GGG CCC ACC TCA AGA GAT GAA TGA CA |
| 642 | CtHIS1 (XhoI) 0 | GCC GGC TCG AGA ACT CTA AAC TAT GAT TGT ATT C |
| 643 | CtHIS1 (SacII) +900 | GGA GCG CCG CGG AGT TCA TCT GTA TAA TAG TAC A |
| 644 | CtHIS1 (SacI) +1,800 | GGC CGC GAG CTC TCC ACA CAT TCA TAA ATG TTC A |
| 645 | CtHIS1 5′ Check −950 | CCA AGG ACA ACA CTC GTG CAG T |
| 646 | CtHIS1 3′ Check + 1,900 | CAA ATT GAT CAC TTC TCT AGT CGA |
| 647 | CtHIS1 5′ ORF +200 | AGG GTA ATT GTG ATT TGG GTA |
| 648 | CtHIS1 3′ ORF +600 | CTT GGA AGA AAC CAA GTG AG |
| 657 | CtARG4 (ApaI) −900 | GGA GCG GGG CCC TGA ATG TGT TGG TGG TGA TG |
| 658 | CtARG4 (XhoI) 0 | GCC GGC CTC GAG GAC ATT GTT GTT AGA ATA AGT T |
| 659 | CtARG4 (NotI) +1,400 | GGA GCG GCG GCC GCA CAT TCA TTC TCC TTC CCT CTC |
| 660 | CtARG4 (SacII) +2,300 | GGC CGC CGC GGG AAT ATT GAT TGT TGA TTG TTG |
| 661 | CtARG4 5′ Check −950 | GAC GTT GTT TAC CCT TGG TAT C |
| 662 | CtARG4 3′ Check + 2400 | TTG TGG AAT GTA ATT TGC ACA C |
| 663 | CtARG4 5′ ORF +200 | TCA GGA TTA GAA GAA ATT CGT G |
| 664 | CtARG4 3′ ORF +600 | TGG ACT TTG ATT AAC TCT GGT T |
| 1046 | CtGAL1 (ApaI) −900 | GGC GCC GGG CCC GAA ACG AAG ACC TAA TTC ACC C |
| 1047 | CtGAL1 (XhoI) +50 | GCC GGC CTC GAG GGT TCA TTA GAA TGA GGA TCA G |
| 1048 | CtGAL1 (SacII) +1,200 | GGA GCG CCG CGG GAA TCA CAG GGC TGA TTT AG |
| 1049 | CtGAL1 (SacI) +2,150 | GGC CGC GAG CTC CGT TAA TTG TCA CCT ATC TAG AC |
| 1050 | CtGAL1 5′ Check −950 | GGG ATC TAG TCA GGG AAG TTC |
| 1051 | CtGAL1 3′ Check + 2200 | CTT CTA ATA CCC CAT AGT AC |
| 1052 | CtGAL1 5′ ORF +250 | CAT CAT AAC CAA CAC AGA TAG C |
| 1053 | CtGAL1 3′ ORF +650 | CGT AAA CTG CTT GTA CCA ATG |
| 1054 | CtADE2 (ApaI) −950 | GGC GCC GGG CCC CCT GCG TAT TCT TAC GTA CA |
| 1055 | CtADE2 (XhoI) 0 | GCC GGC CTC GAG TGA CCA CCT CCT AAA ATA CC |
| 1056 | CtADE2 (SacII) +1,500 | GGA GCG CCG CGG GAG GTA TTC CAG TAG CTA CTG |
| 1057 | CtADE2 (SacI) +2,500 | GGC CGC GAG CTC TCG CAC CAT GCG ATT CCT TG |
| 1058 | CtADE2 5′ check | CGA CCT GCG TAT TCT TAC GT |
| 1059 | CtADE2 3′ check | GAG ACA ATT GCT ACG CTG CG |
| 2295 | CtURA3 (ApaI) −450 | GGC GCC GGG CCC GAA ATC TAT CAA GCT TCA TGT GAC |
| 2296 | CtURA3 (XhoI) 0 | GCC GGC CTC GAG GGA TGT TTA GAT GCT CTT TC |
| 2297 | CtURA3 (SacII) +750 | GGC GCC CCG CGG GAT ATA GAG AAG CCG GTT GG |
| 2298 | CtURA3 (SacI) +1300 | CCG GCG AGC TCG GGA TGA TGA TCA AGT TGA TG |
| 2299 | CtURA3 5′ ORF −800 | GGA GAC TAC TCC ATC ATC GT |
| 2300 | CtURA3 3′ ORF +1,500 | GTG TAT TTG CAG GTT ATG GG |
C. tropicalis mating assay.
Mating in C. tropicalis was carried out using two methods. In the first, strains with deletions at the HIS1 or ARG4 locus were streaked onto YPD medium, and cells were resuspended in liquid Spider medium and grown for 3 to 5 days at 30°C. Following incubation, equal amounts (optical density at 600 nm [OD600] of 0.5 or ∼107 cells) of each strain were mixed together in 100 μl of YPD medium and pipetted onto 0.8-μm-pore-size nitrocellulose filters on the surface of Spider medium plates. Plates were incubated at 30°C for 5 days, after which cells were collected from the filters and streaked onto His−/Arg− plates in order to select for successful mating products. PCR using primers directed at MTLa1 and MTLα2 (Table 2) were used to confirm the presence of both mating types in the mating products. Ploidy was checked using flow cytometry as described below. The second method used the same steps, except that cells from YPD medium were streaked directly onto plates containing Spider medium and grown at room temperature for 5 days.
Flow cytometric analysis.
Cytometric analysis of cells was performed essentially as described previously (25). Cells were grown in 500 μl of YPD medium to an OD600 of ∼0.5 and fixed in 70% ethanol at 4°C overnight. Cells were then washed twice in water and resuspended in 0.5 mM Tris-HCl, pH 8.0, followed by sonication at low power. Following this, cells were resuspended in freshly prepared 2 mg/ml RNase A solution in 0.5 mM Tris-HCl, pH 8.0 (heated to 98°C for 10 min and allowed to slowly cool to room temperature). Samples were incubated at 37°C for 2 h, centrifuged, and resuspended in freshly prepared 5 mg/ml pepsin in 55 mM HCl at 37°C for 45 min. They were then washed with 0.5 mM Tris-HCl, pH 7.5, sonicated at low power, and resuspended in 200 μl of 0.5 mM Tris-HCl, pH 7.5. Fifty microliters of each sample was stained with 500 μl of Sytox Green dye (1 μM) at 4°C for 1 to 48 h. A total of 10,000 cells from each sample were run on a MACSQuant Analyzer 10 (Miltenyi Biotech), and data were analyzed using FlowJo (Tree Star Inc.).
Chromosome loss assay.
Chromosome loss in C. tropicalis was measured using a method similar to that described for C. albicans (29). Tetraploid strains CAY3373, CAY3374, and CAY3375 were grown on YPD medium, streaked onto test medium, and incubated at 30°C or 37°C for 8 to 10 days. Cells were then removed from the plates and resuspended in water. The OD600 was measured and used to plate ∼103 cells on YPD medium and ∼105 cells on 2-DOG plates. The percentage of chromosome loss was calculated as described previously (29).
Batch culture evolution assay.
C. tropicalis diploid and tetraploid strains were maintained in a manner similar to that used for S. cerevisiae (39). Frozen stocks were streaked onto YPD medium and subsequently grown overnight in liquid YPD medium. The overnight culture (100 μl) was diluted into 10 ml of YPD medium and grown at 30°C. Three replicates of each cell line were maintained. Cells were diluted 1:100 (100 μl of culture into a fresh tube of 10 ml of YPD medium) daily (24 h ± 1 h) for 42 days. Such dilution allows for approximately 6.64 (log2 100) mitotic divisions before the population returns to stationary phase (39). A 500-μl sample was taken from each line every 3 days (72 h ± 1 h) and frozen in 500 μl of 1:1 YPD medium–50% glycerol at −80°C for subsequent cytometric analysis. For analysis of ploidy change, control diploid and tetraploid cells were used to define gates for regions where diploid G1 and tetraploid G2 peaks were present. The percentage of total cells present in the peaks was calculated by dividing the number of cells in the gated region by the total cell count.
In vitro competition assay.
Diploid and tetraploid strains were compared for fitness in coculture experiments. The frozen stock of each strain was grown on YPD plates overnight at 30°C and subsequently grown in 3 to 5 ml of YPD cultures overnight at 30°C. A hemocytometer was used to determine the concentration of cells present. Competing strains were then mixed together in equal amounts of ∼107 CFU in 10 ml of YPD medium. Two independent cultures were used for each assay in each independent experiment. Cells were diluted 1:100 (100 μl of culture into a fresh tube of 10 ml of YPD medium) daily (24 h ± 1 h) for 7 days. This allowed for a maximum of ∼6.64 divisions each day. After 1 week, an equal number of cells were plated onto YPD and YPD-NAT plates, and the percentage of NATR cells was calculated.
In vivo competition assay.
Female BALB/c mice (18 to 20g; Charles River Laboratories) were made neutropenic by intraperitoneal injection of 200 μg of anti-Gr1 monoclonal antibody (clone: RB6-8C5; BioXCell) 24 h prior to infection. C. tropicalis strains were grown overnight in liquid YPD medium at 30°C. On the day of infection, C. tropicalis cells were diluted to an OD600 of 0.2 into 10 ml of fresh YPD medium and grown at 30°C for 5 h. Log phase cells were collected and washed three times in sterile phosphate-buffered saline (PBS). Cell concentrations were measured with a hemocytometer. Mice were infected with a mixture of two C. tropicalis strains at a ∼50:50 ratio, with a total inoculum of ∼1.0 × 106 CFU by injection into the lateral tail vein. Each C. tropicalis competition mixture included one isolate resistant to nourseothricin (NATR) and one isolate sensitive to nourseothricin (NATS). Dilutions of the inoculum were plated onto YPD agar and YPD-NAT agar to confirm initial CFU counts. Mice were euthanized at 48 h postinfection, and the liver, spleen, brain, and kidneys were isolated from each mouse. The organs were homogenized using a gentleMACS Dissociator. Dilutions of each tissue homogenate were plated onto YPD medium and incubated at 30°C for 24 h to calculate the total fungal burden from each organ. Colonies were then replica plated from YPD medium onto YPD-NAT medium to calculate the percentage of the recovered C. tropicalis population that included NAT resistance. Four to eight mice were used for each combination of C. tropicalis strains. Neutropenia was monitored by collecting blood daily through retro-orbital bleeding of mice. Blood samples were stained with fluorescein isothiocyanate (FITC)-labeled anti-Gr1-antibody, and neutrophil populations were identified by FITC staining, cell size, and cell complexity using flow cytometry. Neutropenia in mice treated with the anti-Gr1 antibody was confirmed 4 days following injection with the antibody (data not shown). Animal protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) of Brown University.
Measurement of doubling times.
Strains used in the competition assay were streaked onto YPD plates and grown overnight at 30°C and subsequently resuspended in liquid YPD medium. Cells were diluted to an OD600 of 0.2 in 1 ml of YPD medium, and four 150-μl samples of each strain were transferred into a 96-well plate. The plate was incubated at 30°C in a Synergy HT multimode plate reader (BioTek), and the OD600 was measured every 30 min. To calculate the doubling time of each strain, readings were averaged and plotted against time as a semilogarithmic graph.
Statistical analysis.
Statistical analysis was completed using the PAST (Paleontological Statistics Software for Education and Data Analysis) software package. Overall, parametric tests were used whenever data sets were found to be normally distributed via the Anderson-Darling method; otherwise, nonparametric tests were used. A P value of 0.05 was used throughout as a measure of significant difference. For the chromosome loss assay, a Bonferroni-corrected Mann-Whitney U test was used. For the batch culture evolution experiments, Tukey's test was used for comparisons between diploid and tetraploid samples on the same given day. The in vitro competition data were compared using an independent-samples t test. The in vivo competition data were compared using one-way analysis of variance (ANOVA).
RESULTS
A screen for ploidy reduction in C. tropicalis tetraploids.
Previous studies showed that C. tropicalis a and α diploid cells are able to mate to form stable a/α tetraploid cells (34, 35). In order to test for conditions that result in a reduction of ploidy in C. tropicalis tetraploid cells, we constructed a genetically marked tetraploid strain as outlined in Fig. 1. Diploid α/α strains were constructed that were homozygous mutants for HIS1 and GAL1 genes (MTLα/α Δgal1/Δgal1 Δhis1/Δhis1) and mated to diploid a/a cells in which both copies of the ARG4 gene had been deleted (MTLa/a Δarg4/Δarg4). The resulting tetraploids were therefore heterozygous at the MTL, GAL1, HIS1, and ARG4 loci (Fig. 1). We note that the chromosomal locations of these loci have not been determined for C. tropicalis although each locus resides on a different supercontig (17). Three tetraploid strains (CAY3373, CAY3374, and CAY3375) resulting from independent mating experiments (see Fig. S1 in the supplemental material) were used to screen for chromosome loss. S. cerevisiae and C. albicans strains expressing GAL1 are unable to grow on medium containing 2-deoxygalactose (2-DOG) as the carbon source (40, 41), and we similarly used 2-DOG in positive selection for C. tropicalis cells that had lost both copies of the wild-type GAL1 allele.
Fig 1.
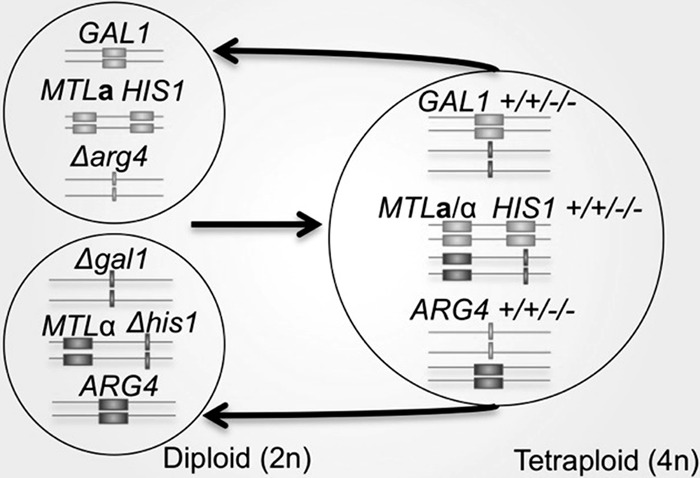
A genetic selection to monitor ploidy reduction in C. tropicalis. Diploid a/a (CAY2059 and CAY2060) and α/α (CAY3371 and CAY3372) C. tropicalis cells were mated to generate tetraploid strains (CAY3373, CAY3374, and CAY3375) that were heterozygous at the GAL1, MTL, HIS1, and ARG4 loci. Loss of GAL1 function was screened for by selection on 2-deoxygalactose (2-DOG) medium, which selects for Δgal1 cells. Chromosomal positions of genes are implicated based on chromosome positioning in the related species C. albicans. Linkage of MTL and HIS1 loci in C. tropicalis was also demonstrated in the present studies.
The genetically marked tetraploid strains were grown under a series of environmental conditions to identify those that could induce either parasexual chromosome loss or meiosis in C. tropicalis. As a proxy for ploidy reduction, we initially monitored loss of GAL1 alleles via selection on 2-DOG medium. A total of 14 different laboratory media were tested, including rich (YPD), S. cerevisiae presporulation, sorbose, potato dextrose agar (PDA), malt extract (malt), sorbitol, YPD-serum, low-phosphate, low-nitrogen (SLAD), and blood agar media. Cultures were grown at 30°C and 37°C for 8 to 10 days and subsequently plated onto YPD medium to determine the total number of viable cells and onto 2-DOG medium to determine the number of cells that had lost GAL1.
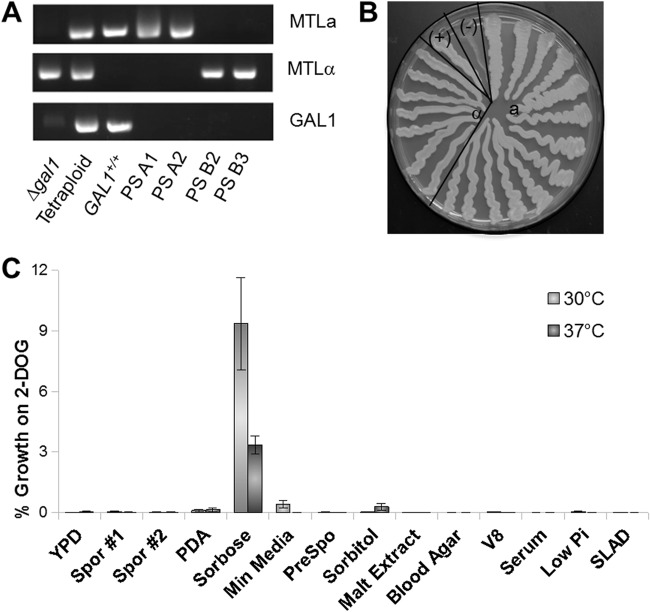
Of all the media tested, sorbose medium induced the greatest formation of Δgal1/Δgal1 cells from C. tropicalis tetraploid cells (Fig. 2C). For cells grown at 30°C on sorbose medium, 9.4% of viable cells were resistant to 2-DOG (2-DOGR) compared to 0.0028% of those from YPD medium (P = 2.3E−6). Similarly, at 37°C, 3.3% of viable cells taken from sorbose medium were found to be 2-DOGR, compared to 0.039% of cells from YPD medium (P = 1.9E−4). Analysis of cells grown on other types of laboratory media generally revealed low rates of formation of 2-DOGR colonies, similar to those of cells grown on YPD medium. For example, testing on S. cerevisiae sporulation medium at 30°C and 37°C resulted in 0.058% and 0.019% of cells becoming 2-DOGR, respectively. These data indicate that growth on sorbose medium leads to increased rates of gal1− colony formation in C. tropicalis tetraploid strains relative to other culture conditions. Interestingly, growth on presporulation medium led to negligible loss of GAL1, which was not significantly different from growth on YPD medium at 30°C (P = 1.0) and 37°C (P = 1.0). This finding contrasts with results in C. albicans, where incubation on presporulation medium at 37°C led to high rates of GAL1 loss from tetraploid cells (29, 30).
Fig 2.
Chromosome loss in C. tropicalis tetraploid strains. (A) A Δgal1 MTLα strain (CAY3371) was mated with a GAL1+/+ MTLa strain (CAY2059) to generate the starting tetraploid strain (CAY3373) that was heterozygous at the GAL1 and MTL loci. Cells grown on sorbose medium for 8 to 10 days and selected on 2-DOG medium are shown as parasexual (PS) products. Parasexual colonies were screened for cells containing only MTLa (PS A1 and PS A2) or MTLα (PS B2 and PS B3) loci. (B) A 2-DOG plate showing selection of Δgal1 cells homozygous at the MTL locus after growth on sorbose for 8 to 10 days. CAY3371 (Δgal1 MTLα) and CAY3373 (the parental tetraploid) are shown as positive and negative controls, respectively. (C) Graph showing tetraploid stability under various medium conditions at 30°C and 37°C. The y axis represents a linear scale of percentage growth on 2-DOG medium. Bars represent means ± standard errors (n = 2 for minimal medium, sorbitol, malt extract, blood agar, V8, serum, low Pi, and SLAD; n = 3 for sporulation medium 1; n = 4 for sporulation medium 2, PDA, and presporulation medium; n = 5 for YPD medium; n = 7 for sorbose).
Tetraploid cells recovered from sorbose medium have reduced ploidy.
Growth on 2-DOG medium is indicative of loss of the wild-type GAL1 alleles but is not necessarily demonstrative of chromosome loss. In order to characterize the chromosome content in progeny cells selected on 2-DOG medium, we first analyzed the genotype of 2-DOGR cells at the MTL locus. PCR was used to identify cells that had lost both copies of MTLα (two such progeny are shown in Fig. 2A). Since we observed that MTL and HIS1 loci are genetically linked (these loci are also linked in C. albicans, where they both reside on chromosome 5), α progeny were identified by an inability to grow on medium lacking histidine. In total, we observed that 13 out of 198 2-DOGR colonies (6.6%) were MTLa, with the rest of the colonies being heterozygous at the MTL locus, while 7 out of 460 colonies (1.5%) were MTLα. Cells were also directly picked from colonies on sorbose plates and restreaked on YPD medium, and 8 colonies out of 84 (9.52%) were MTLa by PCR screening. We identified a single MTLα isolate directly from sorbose plates out of 376 tested (0.27%). These experiments establish that MTL alleles were concomitantly lost during growth on sorbose medium and that chromosome loss was not completely random as MTLa cells were generated at a higher frequency than MTLα cells. The reason for the bias in MTL loss is not known; however, it is possible that His− cells are selected against during growth on sorbose medium (even though this medium was supplemented with amino acids), thereby promoting the recovery of MTLa cells over MTLα cells.
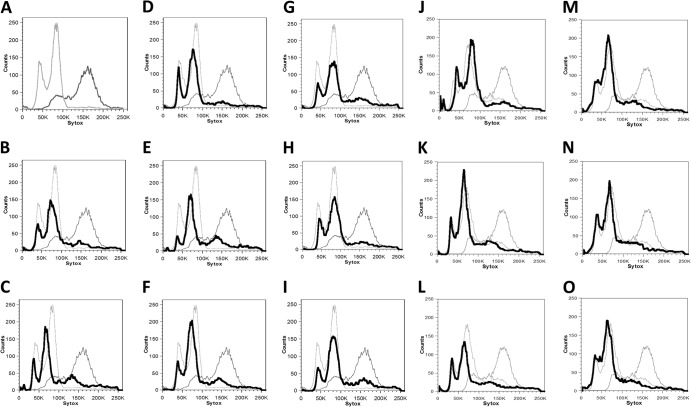
We next used flow cytometry to determine the overall ploidy of selected parasexual progeny. Eight 2-DOGR progeny colonies that were homozygous at the MTL locus, consisting of four that were MTLa (Fig. 3B to E) and four that were MTLα (Fig. 3F to I), were chosen for cytometric analysis. While small variations in ploidy states were observed between the parasexual progeny, all of the progeny were similar in DNA content to control diploid strains. We also examined the ploidy of colonies selected directly from 2-DOG plates with unknown MTL status. Again, all samples analyzed showed peaks that were similar to those in the control diploid strain (Fig. 3J to O), which has a ploidy profile similar to that of other parental diploid strains (see Fig. S1 in the supplemental material). Overall, this analysis establishes that growth on sorbose medium leads to increased rates of ploidy reduction in C. tropicalis, resulting in progeny cells that are diploid, or close to diploid, in DNA content.
Fig 3.
Cell cytometric analysis of parasexual cells. The x axis (Sytox) represents a linear scale of fluorescence, and the y axis represents a linear scale of cell number. (A) Comparison of the initial Δgal1 diploid strain (CAY3371; light gray) with the starting tetraploid (CAY3373; dark gray). These strains are also included for reference in panels B to O. Eight independent colonies were isolated from 2-DOG selection after growth on sorbose for 8 to 10 days and identified as MTLa (B to E) or MTLα (F to I) isolates and catalogued as parasexual strains A1 to A4 and B2 to B5, respectively (Table 3). (J to O) Cell cytometric analysis of tetraploid cells exposed to sorbose and selected for loss of gal1 only, taken from plates grown at 30°C (parasexual strains F1, F2, and F7 in panels J to L, respectively) and 37°C (parasexual strains F9, F11, and G1 in panels M to O, respectively). Samples in panels A to I and J to O were analyzed on separate days, and thus, control diploid and tetraploid plots show minor differences.
Parasexual progeny cells are mating competent.
Given that the progeny cells arising from sorbose medium include a and α diploid cell types, we tested whether these cells could undergo additional rounds of mating to reform tetraploid cells. MTLα progeny that were Δhis1/Δhis1 were mated with a control MTLa strain that is auxotrophic for arginine (CAY2060). Within 3 to 5 days, cocultured cells began to exhibit a mating response (Fig. 4A) and formed zygotes (Fig. 4B), indicative of productive mating. Mating cells were also plated to His−/Arg− medium, and the resulting colonies were identified as MTLa/α cells (Fig. 4C). The ploidy states of these mating products were examined using flow cytometry and showed peaks close to those of a tetraploid control (Fig. 4D to G). Remated tetraploids could also be induced to undergo chromosome loss when grown on sorbose medium at 30°C or 37°C, again as indicated by the generation of 2-DOG-resistant colonies (data not shown). Together, these experiments demonstrate that a parasexual cycle can be established for C. tropicalis, in which cells transition between diploid and tetraploid states.
Fig 4.
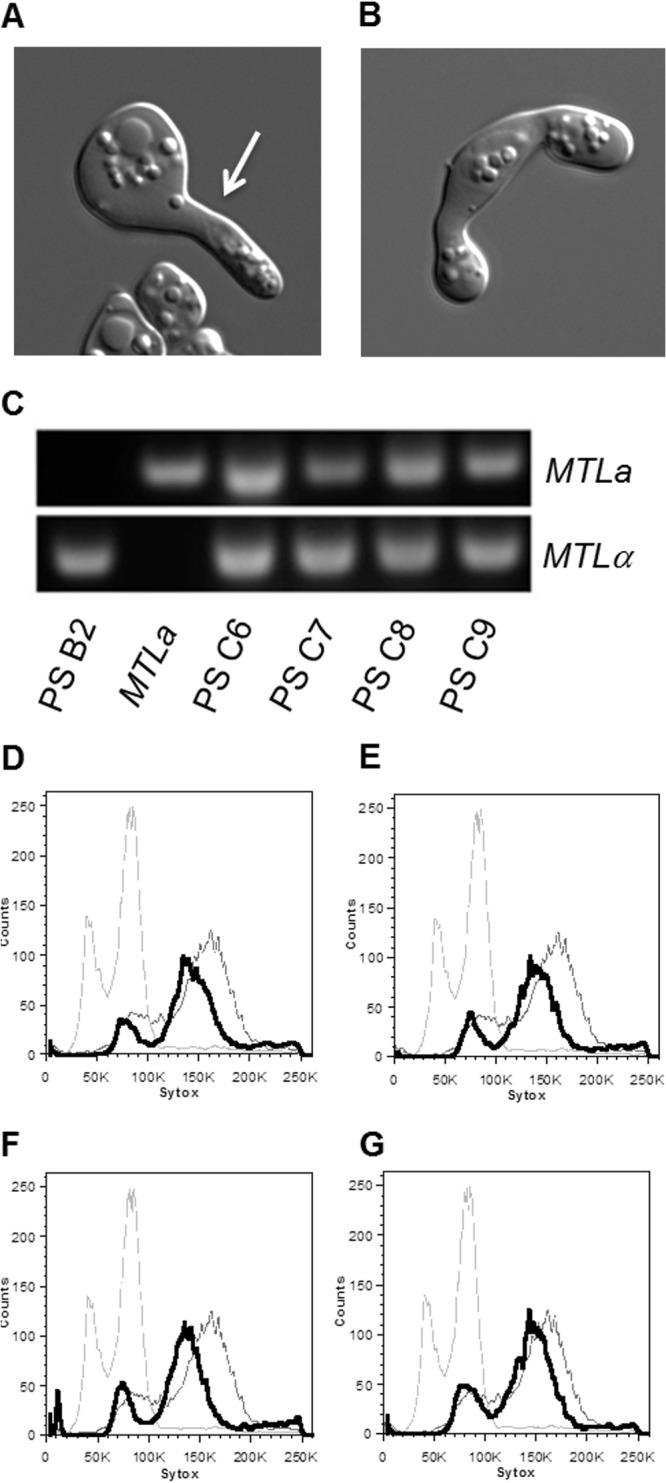
C. tropicalis parasexual progeny are mating competent. (A and B) Representative images of mating projections (A) and zygotes (B) seen during mating of parasexual progeny with wild-type strains. (C) Characterization of MTL loci from four parasexual remating products (parasexual strains [PS] C6 to C9), compared with the parasexual parent (B2) and MTLa parental strain (CAY2060). (D to G) Cell cytometric data showing ploidy of parasexual remating products PS C6 (D), PS C7 (E), PS C8 (F), and PS C9 (G). The x axis (Sytox) represents a linear scale of fluorescence, and the y axis (counts) represents a linear scale of cell number. Diploid (light gray) and tetraploid (dark gray) controls are shown in the background as described in the legend of Fig. 3.
C. tropicalis tetraploid cells become diploid during prolonged culture in rich medium.
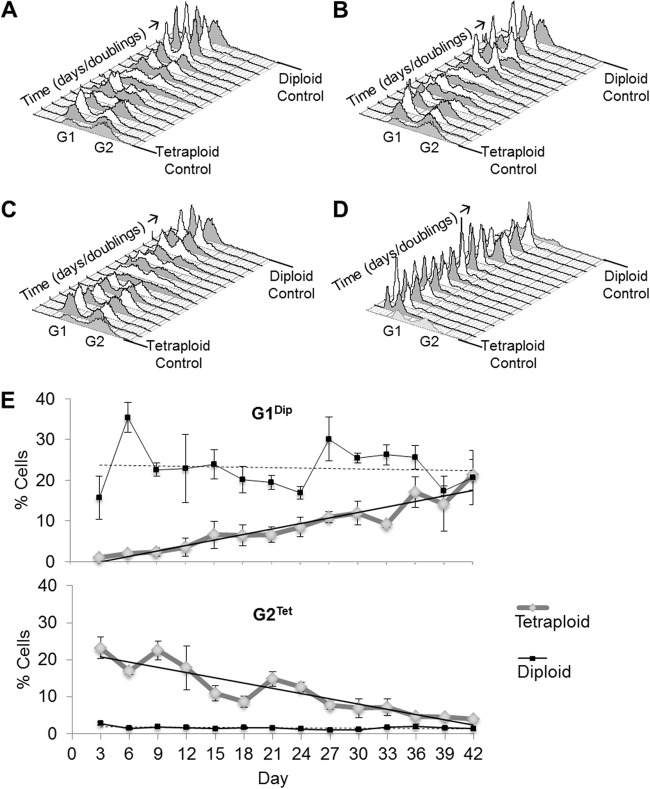
As shown above, chromosome loss in C. tropicalis tetraploid cells is facilitated by growth on sorbose medium over a period of 8 to 10 days. Studies in S. cerevisiae have shown that tetraploid, triploid, and haploid strains trend toward the diploid state when they are cultured over multiple generations, and that the process of chromosome loss from tetraploid to diploid is concerted across the genome (39, 42). We therefore addressed whether ploidy reduction in C. tropicalis tetraploid cells might also occur during extended culture in YPD medium. A genetically marked tetraploid strain (CAY3373) and a control MTLa/α diploid strain (CAY4311) were grown separately in YPD medium in three replicate lines for 42 days at 30°C. Cells were diluted back 1:100 (100 μl into 10 ml of YPD medium) every 24 h allowing for ∼6.6 generations each day, and samples were frozen down every 72 h for subsequent analysis. Flow cytometry showed that all three C. tropicalis tetraploid cell cultures lost ploidy over the course of the experiment and returned to the diploid state (Fig. 5A to C). Interestingly, the temporal profiles of the independent tetraploid lineages showed very similar patterns of ploidy reduction. G1 and G2 peaks were distinct up to day 18 (120 generations), and then multiple peaks were observed between generations 120 and 140. Distinct peaks started to reappear around day 24 (160 generations) and eventually reached a defined diploid state by 36 days (240 generations). Three independent diploid cultures were also propagated in YPD medium for multiple generations, and two of the three showed a steady diploid state across the period of the experiment (Fig. 5D). One diploid lineage unexpectedly showed a trend toward increased ploidy (data not shown).
Fig 5.
Ploidy reduction in C. tropicalis tetraploid strains. (A to C) Cytometric data showing gradual ploidy change from the tetraploid (4n) state to the diploid (2n) state, as evidenced by the leftward shift of G1 and G2 peaks in three independent lines. (D) Cytometric data showing stability of a diploid strain under the same conditions. The x axes represent a linear scale of fluorescence, the y axes represent a linear scale of cell number, and the z axes represent a linear scale of time in days. Control tetraploid (front) and diploid (back) strains are shown on either end for reference. (E) Changes in the G1Dip peak and G2Tet peak during passage of tetraploid strains. Values for control diploid strains are shown in black. The y axis represents the proportion of total cells contained within the given peak with reference to a control strain. The solid and dashed black lines represent the best-fit lines for tetraploids and diploids, respectively. Values represent means ± standard deviations (n = 3 for tetraploids).
To quantify changes in ploidy, we measured the percentage of cells within the diploid G1 peak (G1Dip) and tetraploid G2 peak (G2Tet) relative to control strains. As predicted for tetraploid strains, G1Dip showed an increasing trend over time, while G2Tet decreased to a basal level by 36 days/240 generations (Fig. 5E). Diploid strains showed static trends for both of these parameters, as expected for a stable diploid population, while tetraploid strains converged toward the diploid state by the 42-day time point, or 279 generations (Fig. 5E). The difference in G1Dip between the diploid and tetraploid lines was significant until approximately day 30/200 generations (average P of 0.012), and this significance was lost over the final 12-day period. The G2Tet difference remained statistically significant until day 33/220 generations (average P of 0.0046). Our analysis shows that C. tropicalis tetraploid cells, while generally stable, begin to lose chromosomes during prolonged culture in rich medium, becoming aneuploid and eventually returning to the diploid state in a manner similar to that described in S. cerevisiae.
C. tropicalis diploid cells outcompete tetraploid cells during coculture in vitro.
To investigate the conversion of tetraploid populations to diploid populations, we determined if there was a fitness advantage to propagating in the diploid state. To compare the fitness of tetraploid and diploid cells, we maintained cultures containing cells of both ploidy states in YPD medium for 7 days at 30°C, diluting them 1:100 every 24 h. A nourseothricin-resistant (NATR) diploid strain was grown in competition with a nourseothricin-sensitive (NATS) tetraploid strain, or, conversely, a NATR tetraploid strain was grown in competition with a NATS diploid strain. Nourseothricin resistance served as a selectable marker for determining the fraction of the population that derived from the diploid or tetraploid strain (Fig. 6A). In both cases, the diploid strain used was a prototrophic precursor to the MTLα strain used to construct the tetraploid strain.
Fig 6.
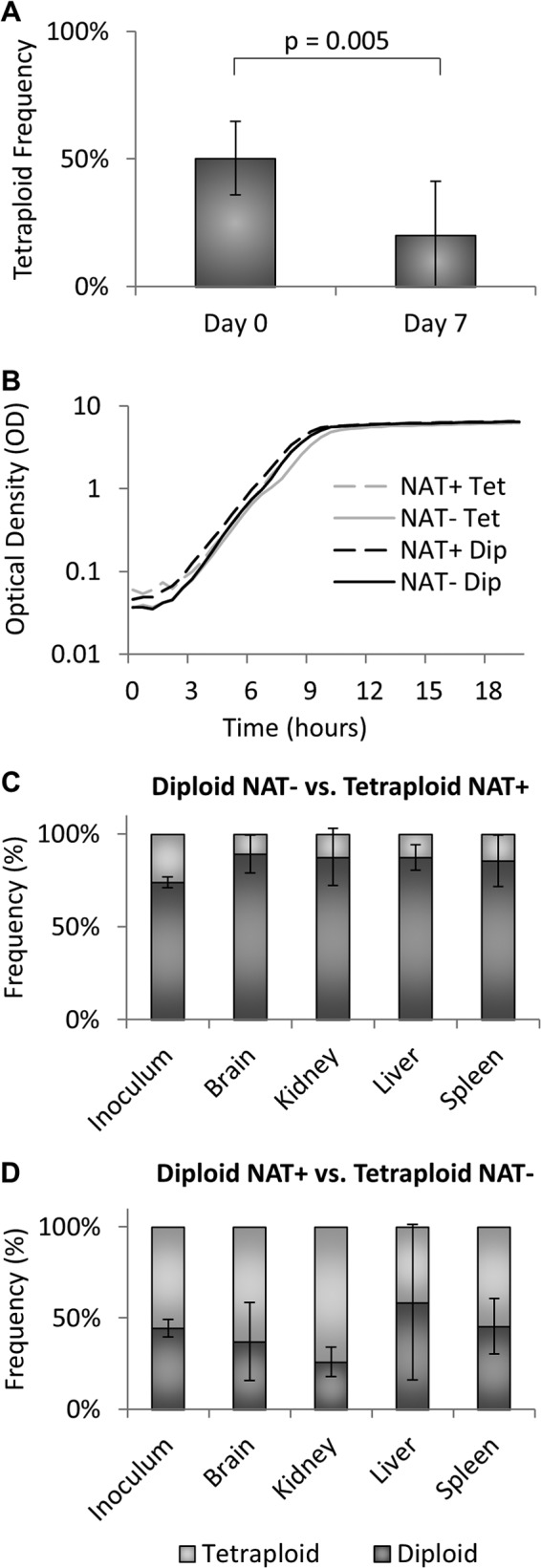
C. tropicalis ploidy competition assays. (A) Coculture of NATR diploid with NATS tetraploid strains and NATR tetraploid with NATS diploid strains. The y axis represents the percentage of tetraploid cells present at the beginning (day 0) and end (day 7) of the experiment. Bars represent means ± standard deviations. NATR diploid strains (CAY4090, CAY4281, and CAY4282) were competed with NATS tetraploid strains (CAY4888 and CAY4890), while NATR tetraploid strains (CAY3373 and CAY3374) were competed with NATS diploid strains (CAY3031, CAY4276, and CAY4277) (P = 0.005; n = 8). (B) Growth curve for strains used in the assay. The x axis represents a linear scale of time in hours, and the y axis represents a logarithmic scale of optical density (OD) as a measure of cell concentration. The experiment was carried out twice, with one representative figure shown here. (C and D) In vivo competition assay showing population frequencies of NATR diploids versus NATS tetraploids (C) and NATR tetraploids versus NATS diploids (D) in murine brain, kidney, liver, and spleen tissue after 48 h, compared to the initial inoculum. Bars represent means ± standard deviations for 4 to 8 mice in independent experiments.
Following culture for 7 days in YPD medium, cells were plated onto YPD plates with or without nourseothricin to distinguish NATR and NATS colonies. The proportion of tetraploid cells showed a statistically significant decrease from day 0 to day 7 (P = 0.005), comprising only 20% of the population recovered on day 7 despite representing 50% of the initial inoculum (Fig. 6A). Furthermore, occasional NATR diploid cells were recovered despite entering the experiment as tetraploid, while the opposite was not true (data not shown). We conclude that C. tropicalis diploid cells hold a significant fitness advantage over tetraploid cells in vitro and that this is independent of the NAT marker used in these studies, given that tetraploid populations decreased in both NATR and NATS configurations.
To determine if these results were due to differential growth rates between diploid and tetraploid strains, we analyzed the doubling times for each of the strains used in the competition assay. No significant differences in growth rates were observed between diploid and tetraploid strains, as both doubled every 60 min during exponential growth. We also determined that diploid and tetraploid strains were able to grow to the same maximum optical densities in saturated cultures (Fig. 6B and data not shown). These results indicate that the increased fitness of diploid strains is not simply the consequence of faster growth under these culture conditions.
To determine if a fitness advantage of diploid cells was also observed in vivo, we performed a competition experiment using an established murine model for systemic infection (43). Mice were infected via the tail vein with mixed populations of diploid and tetraploid cells, and cells were subsequently recovered from the brain, kidney, liver, and spleen. After 2 days of infection, we saw no change in the relative proportions of diploid and tetraploid strains between the inoculum and the recovered cells (Fig. 6C and D). Thus, the initial NATR diploid inoculum (44.4%) showed similar frequencies in the brain (37.0%), kidney (25.9%), liver (58.5%), and spleen (45.5%) following infection (Fig. 6D). Similarly, the initial frequency of NATR tetraploid strains (26.2%) was not significantly different when they were recovered from any of the infected organs (brain, 10.8%; kidney, 12.5%; liver, 12.7%; spleen, 14.5%) (Fig. 6C). Longer time points during in vivo infection were not possible as C. tropicalis strains led to murine morbidity, with the result that mice had to be euthanized.
DISCUSSION
C. tropicalis was recently discovered to have an extant mating program in which diploid cells fuse to form tetraploid products (34, 35). In this study, we used a genetic selection to identify conditions that induce C. tropicalis tetraploid cells to return to the diploid state. Populations of C. tropicalis tetraploid cells were stable for multiple generations when grown on diverse laboratory media but exhibited chromosome instability when propagated for extended periods in rich (YPD) medium. In addition, tetraploid cells could also be induced to return to the diploid state by selective growth on sorbose medium. The diploid products of chromosome reduction were mating competent, thereby establishing that C. tropicalis can undergo a parasexual program.
The efficiency of chromosome loss in C. tropicalis tetraploid cells was highest when cells were grown on sorbose medium; 3 to 9% of C. tropicalis tetraploid cells became 2-DOGR when incubated on this medium. Assuming a random segregation of chromosomes (and no loss of GAL1 due to recombination), this indicates that 18 to 54% of sorbose-selected colonies became disomic for this chromosome. In contrast, only 0.003 to 0.04% of C. tropicalis cells grown on YPD medium lost GAL1, a difference of 2 to 3 orders of magnitude. Flow cytometric analysis of Δgal1 progeny confirmed that most chromosomes had become disomic during growth on sorbose medium, and thus parasexual progeny exhibited a ploidy similar to that of control diploid cells.
Sorbose medium has also been shown to induce concerted chromosome loss in tetraploid cells of the related species C. albicans (29, 30). In C. albicans, growth on sorbose medium is stressful as most cells in the population are unable to survive on this medium (29, 44). We saw a similar stress response in C. tropicalis, with reduced cell viability on sorbose medium. The survival rate was even lower when cells were grown on sorbose without amino acids (1 to 6%) although there was no difference in loss of the GAL1 marker between the two conditions. In both Candida species, colonies that survive stressful growth on sorbose medium have frequently undergone parasexual chromosome loss. C. albicans tetraploid cells were also previously shown to exhibit chromosome instability when incubated on S. cerevisiae presporulation medium at 37°C (29). In contrast, growth on presporulation medium did not result in high rates of chromosome loss in C. tropicalis. Furthermore, whereas presporulation medium induced high rates of cell death in both C. albicans and C. tropicalis tetraploid cells at 2 days, only C. tropicalis cells recovered viability after 4 days of growth on this medium (data not shown). We surmise that chromosome instability is associated with cell stress, and induction of the parasexual program may be used to survive hostile environmental conditions, as further discussed below.
Notably, none of the conditions tested in this study were found to induce sporulation in C. tropicalis tetraploid cells, and we thus found no evidence for a meiotic program in this species. Identification of a conventional meiosis in C. albicans has been a long-standing goal as it would allow the use of classical genetics in this important human pathogen (45, 46). Similarly, we now report that C. tropicalis appears to undergo a parasexual cycle rather than a true sexual cycle culminating in meiosis. Growth of C. tropicalis tetraploid cells on sorbose medium induced an efficient reduction in ploidy, but the surviving colonies on this medium did not produce any spore-like cells. It therefore remains to be seen if a cryptic meiotic program exists for either C. albicans or C. tropicalis.
C. tropicalis tetraploid cells were also found to undergo a reduction in ploidy to the diploid state during prolonged culture in YPD medium. Strikingly, each of three independent tetraploid lines showed a similar trend toward diploidy via intermediate aneuploid states. This ploidy change began around day 18 in YPD culture following approximately 120 generations of propagation. Since cells were cultured under rich growth conditions, it therefore appears that the parasexual process in Candida species does not take place exclusively under conditions of stress (30, 31), an important factor in considering where chromosome loss may occur in host environments.
Studies of genomic states in S. cerevisiae have established that this organism is also capable of undergoing asexual changes in ploidy. S. cerevisiae cells preferentially propagate as diploid cells, and prolonged culture of haploid, triploid, or tetraploid cells results in their convergence toward the diploid state (39, 42, 47). This genomic convergence is independent of mating or meiosis and may occur by chromosome nondisjunction during mitosis (e.g., to drive a ploidy reduction) or by endoreduplication (e.g., to drive a ploidy increase). Aspergillus nidulans, a primarily haploid ascomycete, also undergoes a reduction in ploidy (from diploid to haploid) via a parasexual mechanism when grown continuously in standard medium (48). It is therefore apparent that extended culture can induce ploidy changes in diverse fungal species although the precise molecular mechanisms driving parasexual ploidy changes remain to be elucidated.
The fitness of C. tropicalis diploid and tetraploid cells was also directly compared in this study. When cocultured in YPD medium for 7 days, diploid cells showed enhanced fitness over tetraploid cells. This is in spite of the fact that diploid and tetraploid cells showed indistinguishable growth rates in vitro. However, coculture in a murine model of infection failed to demonstrate an enhanced fitness of diploids, which may be due to the limited time period (2 days) used for in vivo experiments. It therefore appears that diploid cells exhibit increased fitness over tetraploid cells but that tetraploid cells can stably survive and propagate in in vivo niches, at least for a defined period of time. In S. cerevisiae, haploid cell populations were also shown to be overtaken by diploid cells even though the investigators failed to pinpoint any overt fitness advantage of diploids over haploids (47). Other studies have also failed to identify specific fitness advantages of diploid strains over haploid strains of S. cerevisiae (49, 50). We conclude that C. tropicalis diploid cells hold a cryptic fitness advantage over tetraploid cells but that the nature of the fitness advantage is currently unknown.
Recent studies have revealed that C. albicans diploid cells can also undergo a ploidy reduction to the haploid state in vitro and in vivo (32). This discovery was surprising as C. albicans was thought to be an obligate diploid due to the presence of multiple recessive lethal alleles on different chromosome homologs (51, 52). The formation of C. albicans haploids presumably occurs via a nonmeiotic mechanism as very few chromosomal crossovers were detected in haploid cells (32). To test if C. tropicalis can also exist in the haploid state, we screened for C. tropicalis haploid cells using a diploid strain heterozygous for GAL1, HIS1, ARG4, and URA3. We observed loss of several genetic markers in a subset of the population (Table 3) but have so far been unable to obtain C. tropicalis haploid cells under a variety of in vitro culture conditions (data not shown).
Table 3.
Parasexual strains in this study
| PS group and strain no.a | Cell type or genotype | Isolate no. | Original strain(s) (growth condition) |
|---|---|---|---|
| Selected progeny tested for MTL type | |||
| MTLa/a | |||
| A1 | MTLa/a Δgal1/Δgal1::SAT1 | 1 | CA3373 (sorbose at 30°C) |
| A2 | MTLa/a Δgal1/Δgal1::SAT1 | 2 | CA3373 (sorbose at 30°C) |
| A3 | MTLa/a Δgal1/Δgal1::SAT1 | 3 | CA3373 (sorbose at 30°C) |
| A4 | MTLa/a Δgal1/Δgal1::SAT1 | 4 | CA3373 (sorbose at 30°C) |
| A5 | MTLa/a Δgal1/Δgal1::SAT1 | 5 | CA3373 (sorbose at 30°C) |
| A6 | MTLa/a Δgal1/Δgal1::SAT1 | 6 | CA3373 (sorbose at 30°C) |
| A7 | MTLa/a Δgal1/Δgal1::SAT1 | 7 | CA3373 (sorbose at 37°C) |
| A8 | MTLa/a Δgal1/Δgal1::SAT1 | 8 | CA3373 (sorbose at 37°C) |
| A9 | MTLa/a Δgal1/Δgal1::SAT1 | 9 | CA3373 (sorbose at 37°C) |
| A10 | MTLa/a Δgal1/Δgal1::SAT1 | 10 | CA3373 (sorbose at 37°C) |
| A11 | MTLa/a Δgal1/Δgal1::SAT1 | 11 | CA3374 (sorbose at 30°C) |
| A12 | MTLa/a Δgal1/Δgal1::SAT1 | 12 | CA3374 (sorbose at 37°C) |
| B1 | MTLa/a Δgal1/Δgal1::SAT1 | 13 | CA3375 (sorbose at 30°C) |
| MTLα/α | |||
| B2 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 23 | CA3373 (sorbose at 30°C) |
| B3 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 25 | CA3373 (sorbose at 30°C) |
| B4 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 242 | CA3373 (sorbose at 30°C) |
| B5 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 283 | CA3373 (sorbose at 30°C) |
| B6 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 302 | CA3374 (sorbose at 30°C) |
| B7 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 320 | CA3374 (sorbose at 30°C) |
| B8 | MTLα/α Δgal1/Δgal1::SAT1 Δhis1/Δhis1 | 336 | CA3374 (sorbose at 30°C) |
| Progeny from sorbose tested for MTL type | |||
| B9 | MTLa/a Δgal1/Δgal1::SAT1 | 1 | |
| B10 | MTLa/a | 2 | CA3373 (sorbose at 37°C) |
| B11 | MTLa/a | 3 | CA3373 (sorbose at 37°C) |
| B12 | MTLa/a | 4 | CA3373 (sorbose at 37°C) |
| C1 | MTLa/a | 7 | CA3373 (sorbose at 37°C) |
| C2 | MTLa/a | 9 | CA3374 (sorbose at 37°C) |
| C3 | MTLa/a | 10 | CA3375 (sorbose at 37°C) |
| C4 | MTLa/a | 11 | CA3375 (sorbose at 37°C) |
| Randomly selected progeny from 2-DOG | |||
| F1 | Δgal1/Δgal1::SAT1 | 1 | CA3373 (sorbose at 30°C) |
| F2 | Δgal1/Δgal1::SAT1 | 2 | CA3373 (sorbose at 30°C) |
| F3 | Δgal1/Δgal1::SAT1 | 3 | CA3373 (sorbose at 30°C) |
| F4 | Δgal1/Δgal1::SAT1 | 4 | CA3373 (sorbose at 30°C) |
| F5 | Δgal1/Δgal1::SAT1 | 5 | CA3373 (sorbose at 30°C) |
| F6 | Δgal1/Δgal1::SAT1 | 6 | CA3373 (sorbose at 30°C) |
| F7 | Δgal1/Δgal1::SAT1 | 7 | CA3373 (sorbose at 30°C) |
| F8 | Δgal1/Δgal1::SAT1 | 8 | CA3373 (sorbose at 30°C) |
| F9 | Δgal1/Δgal1::SAT1 | 1 | CA3373 (sorbose at 37°C) |
| F10 | Δgal1/Δgal1::SAT1 | 2 | CA3373 (sorbose at 37°C) |
| F11 | Δgal1/Δgal1::SAT1 | 3 | CA3373 (sorbose at 37°C) |
| F12 | Δgal1/Δgal1::SAT1 | 4 | CA3373 (sorbose at 37°C) |
| G1 | Δgal1/Δgal1::SAT1 | 5 | CA3373 (sorbose at 37°C) |
| G2 | Δgal1/Δgal1::SAT1 | 7 | CA3373 (sorbose at 37°C) |
| G3 | Δgal1/Δgal1::SAT1 | 8 | CA3373 (sorbose at 37°C) |
| G4 | Δgal1/Δgal1::SAT1 | 9 | CA3373 (sorbose at 37°C) |
| Mating products | |||
| C5 | MTLa/a/α/α his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | 5 | B2 × CAY2060; mating product G |
| C6 | MTLa/a/α/α his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | 6 | B2 × CAY2060; mating product G |
| C7 | MTLa/a/α/α his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | 7 | B2 × CAY2060; mating product G |
| C8 | MTLa/a/α/α his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | 19 | B3 × CAY2060; mating product H |
| C9 | MTLa/a/α/α his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | 20 | B3 × CAY2060; mating product H |
| C10 | MTLa/a/α/α his1/his1/HIS1/HIS1 arg4/arg4/ARG4/ARG4 gal1/gal1::SAT1/GAL1/GAL1 | 21 | B3 × CAY2060; mating product H |
| Haploid screen | |||
| D1 | Gal1− | 1 | CAY4539 (2μM H2O2) |
| D2 | Gal1− Ura3− | 2 | CAY4539 (H2O2) |
| D3 | Gal1− | 3 | CAY4539 (H2O2) |
| D4 | Gal1− | 4 | CAY4539 (H2O2) |
| D5 | Gal1− Arg4− | 5 | CAY4539 (H2O2) |
| D6 | Gal1− Ura3− | 6 | CAY4539 (H2O2) |
| D7 | Gal1− | 7 | CAY4539 (H2O2) |
| D8 | Gal1− | 8 | CAY4539 (H2O2) |
| D9 | Gal1− | 9 | CAY4539 (H2O2) |
| D10 | Gal1− | 11 | CAY4539 (H2O2) |
| D11 | Gal1− Ura3− Arg4− | 12 | CAY4539 (H2O2) |
| D12 | Gal1− Arg4− | 13 | CAY4539 (H2O2) |
| E1 | Gal1− Ura3− | 15 | CAY4539 (42°C) |
| E2 | Gal1− | 16 | CAY4539 (42°C) |
| E3 | Gal1− Arg4− | 17 | CAY4539 (42°C) |
| E4 | Gal1− | 19 | CAY4539 (42°C) |
| E5 | Gal1− Ura3− | 20 | CAY4539 (42°C) |
| E6 | Gal1− | 21 | CAY4539 (42°C) |
| E7 | Gal1− | 22 | CAY4539 (42°C) |
PS, parasexual.
What are the consequences of parasexuality for the lifestyles of Candida species? Berman and Hadany (31) have proposed that diverse progeny resulting from parasexuality would have the potential to better survive and adapt to stressful environments. In support of this model, the investigators note that many of the steps in the C. albicans parasexual cycle are induced by stress, and thus parasexuality can generate recombinant forms precisely when needed for adaptation to a hostile environment. Preliminary experiments in C. albicans support this hypothesis as they show that parasexual progeny can better resist stressful conditions in vitro than parental diploid cells (M. P. Hirakawa and R. J. Bennett, unpublished observations). We note that both stressful culture conditions (growth on sorbose) and nonstressful conditions (prolonged culture in YPD medium) induce parasexual chromosome loss in C. tropicalis tetraploid cells. It therefore remains an intriguing question as to the precise conditions that induce the parasexual cycle in nature and the role of this cycle in generating recombinant forms that can promote adaptation to environmental niches.
Supplementary Material
ACKNOWLEDGMENTS
S.K.J. and A.M.P. were supported by predoctoral fellowships F31DE022701 and F31DE022703, respectively. M.P.H. was supported by a Graduate Assistance in Areas of National Need Training Grant P200A100100. This work was supported by National Institutes of Health grant AI081704 (to R.J.B.) and by National Science Foundation Grant MCB1021120 (to R.J.B). R.J.B. also holds an Investigator in the Pathogenesis of Infectious Disease Award from the Burroughs Wellcome Fund.
Footnotes
Published ahead of print 11 October 2013
Supplemental material for this article may be found at http://dx.doi.org/10.1128/EC.00128-13.
REFERENCES
- 1.Nielsen K, Heitman J. 2007. Sex and virulence of human pathogenic fungi. Adv. Genet. 57:143–173 [DOI] [PubMed] [Google Scholar]
- 2.Goddard MR, Godfray HC, Burt A. 2005. Sex increases the efficacy of natural selection in experimental yeast populations. Nature 434:636–640 [DOI] [PubMed] [Google Scholar]
- 3.Heitman J. 2006. Sexual reproduction and the evolution of microbial pathogens. Curr. Biol. 16:R711–725 [DOI] [PubMed] [Google Scholar]
- 4.Heitman J. 2010. Evolution of eukaryotic microbial pathogens via covert sexual reproduction. Cell Host Microbe 8:86–99 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hadany L, Otto SP. 2007. The evolution of condition-dependent sex in the face of high costs. Genetics 176:1713–1727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hadany L, Otto SP. 2009. Condition-dependent sex and the rate of adaptation. Am. Nat. 174(Suppl 1):S71–S78 [DOI] [PubMed] [Google Scholar]
- 7.Neiman AM. 2005. Ascospore formation in the yeast Saccharomyces cerevisiae. Microbiol. Mol. Biol. Rev. 69:565–584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ni M, Feretzaki M, Sun S, Wang X, Heitman J. 2011. Sex in fungi. Annu. Rev. Genet. 45:405–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler G. 2010. Fungal sex and pathogenesis. Clin. Microbiol. Rev. 23:140–159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon-Chung KJ. 1976. Morphogenesis of Filobasidiella neoformans, the sexual state of Cryptococcus neoformans. Mycologia 68:821–833 [PubMed] [Google Scholar]
- 11.Lin X, Hull CM, Heitman J. 2005. Sexual reproduction between partners of the same mating type in Cryptococcus neoformans. Nature 434:1017–1021 [DOI] [PubMed] [Google Scholar]
- 12.Giles SS, Dagenais TR, Botts MR, Keller NP, Hull CM. 2009. Elucidating the pathogenesis of spores from the human fungal pathogen Cryptococcus neoformans. Infect. Immun. 77:3491–3500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fraser JA, Giles SS, Wenink EC, Geunes-Boyer SG, Wright JR, Diezmann S, Allen A, Stajich JE, Dietrich FS, Perfect JR, Heitman J. 2005. Same-sex mating and the origin of the Vancouver Island Cryptococcus gattii outbreak. Nature 437:1360–1364 [DOI] [PubMed] [Google Scholar]
- 14.Ma H, Hagen F, Stekel DJ, Johnston SA, Sionov E, Falk R, Polacheck I, Boekhout T, May RC. 2009. The fatal fungal outbreak on Vancouver Island is characterized by enhanced intracellular parasitism driven by mitochondrial regulation. Proc. Natl. Acad. Sci. U. S. A. 106:12980–12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Byrnes EJ, III, Li W, Lewit Y, Ma H, Voelz K, Ren P, Carter DA, Chaturvedi V, Bildfell RJ, May RC, Heitman J. 2010. Emergence and pathogenicity of highly virulent Cryptococcus gattii genotypes in the northwest United States. PLoS Pathog. 6:e1000850. 10.1371/journal.ppat.1000850 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.O'Gorman CM, Fuller HT, Dyer PS. 2009. Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus. Nature 457:471–474 [DOI] [PubMed] [Google Scholar]
- 17.Butler G, Rasmussen MD, Lin MF, Santos MA, Sakthikumar S, Munro CA, Rheinbay E, Grabherr M, Forche A, Reedy JL, Agrafioti I, Arnaud MB, Bates S, Brown AJ, Brunke S, Costanzo MC, Fitzpatrick DA, de Groot PW, Harris D, Hoyer LL, Hube B, Klis FM, Kodira C, Lennard N, Logue ME, Martin R, Neiman AM, Nikolaou E, Quail MA, Quinn J, Santos MC, Schmitzberger FF, Sherlock G, Shah P, Silverstein KA, Skrzypek MS, Soll D, Staggs R, Stansfield I, Stumpf MP, Sudbery PE, Srikantha T, Zeng Q, Berman J, Berriman M, Heitman J, Gow NA, Lorenz MC, Birren BW, Kellis M, Cuomo CA. 2009. Evolution of pathogenicity and sexual reproduction in eight Candida genomes. Nature 459:657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miranda I, Silva R, Santos MA. 2006. Evolution of the genetic code in yeasts. Yeast 23:203–213 [DOI] [PubMed] [Google Scholar]
- 19.van der Walt JP. 1966. Lodderomyces, a new genus of the Saccharomycetaceae. Antonie Van Leewenhoek 32:1–5 [DOI] [PubMed] [Google Scholar]
- 20.Reedy JL, Floyd AM, Heitman J. 2009. Mechanistic plasticity of sexual reproduction and meiosis in the Candida pathogenic species complex. Curr. Biol. 19:891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Walt JP, Taylor MB, Liebenberg NV. 1977. Ploidy, ascus formation and recombination in Torulaspora (Debaryomyces) hansenii. Antonie Van Leeuwenhoek 43:205–218 [DOI] [PubMed] [Google Scholar]
- 22.Wickerham LJ, Burton KA. 1954. A clarification of the relationship of Candida guilliermondii to other yeasts by a study of their mating types. J. Bacteriol. 68:594–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gargeya IB, Pruitt WR, Simmons RB, Meyer SA, Ahearn DG. 1990. Occurrence of Clavispora lusitaniae, the teleomorph of Candida lusitaniae, among clinical isolates. J. Clin. Microbiol. 28:2224–2227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Alby K, Schaefer D, Bennett RJ. 2009. Homothallic and heterothallic mating in the opportunistic pathogen Candida albicans. Nature 460:890–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hull CM, Raisner RM, Johnson AD. 2000. Evidence for mating of the “asexual” yeast Candida albicans in a mammalian host. Science 289:307–310 [DOI] [PubMed] [Google Scholar]
- 26.Magee BB, Magee PT. 2000. Induction of mating in Candida albicans by construction of MTLa and MTLα strains. Science 289:310–313 [DOI] [PubMed] [Google Scholar]
- 27.Miller MG, Johnson AD. 2002. White-opaque switching in Candida albicans is controlled by mating-type locus homeodomain proteins and allows efficient mating. Cell 110:293–302 [DOI] [PubMed] [Google Scholar]
- 28.Tzung KW, Williams RM, Scherer S, Federspiel N, Jones T, Hansen N, Bivolarevic V, Huizar L, Komp C, Surzycki R, Tamse R, Davis RW, Agabian N. 2001. Genomic evidence for a complete sexual cycle in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 98:3249–3253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bennett RJ, Johnson AD. 2003. Completion of a parasexual cycle in Candida albicans by induced chromosome loss in tetraploid strains. EMBO J. 22:2505–2515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Forche A, Alby K, Schaefer D, Johnson AD, Berman J, Bennett RJ. 2008. The parasexual cycle in Candida albicans provides an alternative pathway to meiosis for the formation of recombinant strains. PLoS Biol. 6:e110. 10.1371/journal.pbio.0060110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Berman J, Hadany L. 2012. Does stress induce (para)sex? Implications for Candida albicans evolution. Trends Genet. 28:197–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hickman MA, Zeng G, Forche A, Hirakawa MP, Abbey D, Harrison BD, Wang YM, Su CH, Bennett RJ, Wang Y, Berman J. 2013. The “obligate diploid” Candida albicans forms mating-competent haploids. Nature 494:55–59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kothavade RJ, Kura MM, Valand AG, Panthaki MH. 2010. Candida tropicalis: its prevalence, pathogenicity and increasing resistance to fluconazole. J. Med. Microbiol. 59:873–880 [DOI] [PubMed] [Google Scholar]
- 34.Porman AM, Alby K, Hirakawa MP, Bennett RJ. 2011. Discovery of a phenotypic switch regulating sexual mating in the opportunistic fungal pathogen Candida tropicalis. Proc. Natl. Acad. Sci. U. S. A. 108:21158–21163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xie J, Du H, Guan G, Tong Y, Kourkoumpetis TK, Zhang L, Bai FY, Huang G. 2012. N-Acetylglucosamine induces white-to-opaque switching and mating in Candida tropicalis, providing new insights into adaptation and fungal sexual evolution. Eukaryot. Cell 11:773–782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Guthrie C, Fink GR. 1991. Guide to yeast genetics and molecular biology. Academic Press, San Diego, CA [Google Scholar]
- 37.Liu H, Kohler J, Fink GR. 1994. Suppression of hyphal formation in Candida albicans by mutation of a STE12 homolog. Science 266:1723–1726 [DOI] [PubMed] [Google Scholar]
- 38.Reuss O, Vik A, Kolter R, Morschhauser J. 2004. The SAT1 flipper, an optimized tool for gene disruption in Candida albicans. Gene 341:119–127 [DOI] [PubMed] [Google Scholar]
- 39.Gerstein AC, Chun HJ, Grant A, Otto SP. 2006. Genomic convergence toward diploidy in Saccharomyces cerevisiae. PLoS Genet. 2:e145. 10.1371/journal.pgen.0020145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Platt T. 1984. Toxicity of 2-deoxygalactose to Saccharomyces cerevisiae cells constitutively synthesizing galactose-metabolizing enzymes. Mol. Cell. Biol. 4:994–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorman JA, Gorman JW, Koltin Y. 1992. Direct selection of galactokinase-negative mutants of Candida albicans using 2-deoxy-galactose. Curr. Genet. 21:203–206 [DOI] [PubMed] [Google Scholar]
- 42.Gerstein AC, McBride RM, Otto SP. 2008. Ploidy reduction in Saccharomyces cerevisiae. Biol. Lett. 4:91–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noble SM, French S, Kohn LA, Chen V, Johnson AD. 2010. Systematic screens of a Candida albicans homozygous deletion library decouple morphogenetic switching and pathogenicity. Nat. Genet. 42:590–598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Janbon G, Sherman F, Rustchenko E. 1998. Monosomy of a specific chromosome determines l-sorbose utilization: a novel regulatory mechanism in Candida albicans. Proc. Natl. Acad. Sci. U. S. A. 95:5150–5155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Alby K, Bennett RJ. 2010. Sexual reproduction in the Candida clade: cryptic cycles, diverse mechanisms, and alternative functions. Cell. Mol. Life Sci. 67:3275–3285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bennett RJ, Johnson AD. 2005. Mating in Candida albicans and the search for a sexual cycle. Annu. Rev. Microbiol. 59:233–255 [DOI] [PubMed] [Google Scholar]
- 47.Gerstein AC, Otto SP. 2011. Cryptic fitness advantage: diploids invade haploid populations despite lacking any apparent advantage as measured by standard fitness assays. PLoS One 6:e26599. 10.1371/journal.pone.0026599 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schoustra SE, Debets AJ, Slakhorst M, Hoekstra RF. 2007. Mitotic recombination accelerates adaptation in the fungus Aspergillus nidulans. PLoS Genet. 3:e68. 10.1371/journal.pgen.0030068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mable BK. 2001. Ploidy evolution in the yeast Saccharomyces cerevisiae: a test of the nutrient limitation hypothesis. J. Evol. Biol. 14:157–170 [DOI] [PubMed] [Google Scholar]
- 50.Dickinson WJ. 2008. Synergistic fitness interactions and a high frequency of beneficial changes among mutations accumulated under relaxed selection in Saccharomyces cerevisiae. Genetics 178:1571–1578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sarachek A, Weber DA. 1986. Segregant-defective heterokaryons of Candida albicans. Curr. Genet. 10:685–693 [DOI] [PubMed] [Google Scholar]
- 52.Whelan WL, Soll DR. 1982. Mitotic recombination in Candida albicans: recessive lethal alleles linked to a gene required for methionine biosynthesis. Mol. Gen. Genet. 187:477–485 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.