SUMMARY
Hormone suppression given before or after cytotoxic treatment stimulates recovery of spermatogenesis from endogenous and transplanted spermatogonial stem cells (SSC) and restores fertility in rodents. To test whether the combination of hormone suppression and transplantation could enhance the recovery of spermatogenesis in primates, we irradiated (7 Gy) the testes of 12 adult cynomolgus monkeys and treated 6 of them with GnRH-antagonist (GnRH-ant) for 8 weeks. At the end of this treatment, we transfected cryopreserved testicular cells with GFP-lentivirus and autologously transplanted them back into one of the testes. The only significant effect of GnRH-ant treatment on endogenous spermatogenesis was an increase in the percentage of tubules containing differentiated germ cells (tubule differentiation index; TDI) in the sham-transplanted testes of GnRH-ant-treated monkeys compared to radiation-only monkeys at 24 weeks after irradiation. Although transplantation alone after irradiation did not significantly increase the TDI, detection of lentiviral DNA in the sperm of one radiation-only monkey indicated that some transplanted cells colonized the testis. However, the combination of transplantation and GnRH-ant clearly stimulated spermatogenic recovery as evidenced by several observations in the GnRH-ant-treated monkeys receiving transplantation: (a) significant increases (~20%) in the volume and weight of the testes compared to the contralateral sham-transplanted testes and/or to the transplanted testes of the radiation-only monkeys; (b) increases in TDI compared to the transplanted testes of radiation-only monkeys at 24 weeks (9.6% vs. 2.9%; P=0.05) and 44 weeks (16.5% vs. 6.1%, P=0.055); (c) detection of lentiviral sequences in the sperm or testes of five of the GnRH-ant–treated monkeys; and (d) significantly higher sperm counts than in the radiation-only monkeys. Thus hormone suppression enhances spermatogenic recovery from transplanted SSC in primates and may be a useful tool in conjunction with spermatogonial transplantation to restore fertility in men after cancer treatment.
Keywords: Radiation, spermatogenesis, infertility, transplantation, GnRH-antagonist
INTRODUCTION
As many as 30% of male survivors of cancer in childhood and young adulthood are at risk of sterility due to treatment with high-dose chemotherapy, total-body irradiation, or irradiation with scatter to the genital region (Thomson et al., 2002; Meistrich et al., 2005). Whereas adults have the option of cryopreserving semen before therapy to ensure that they can produce offspring, prepubertal or peripubertal patients cannot supply appropriate semen sample either due to sperm insufficiency or sociological reasons. Thus they do not currently have any fertility preservation choices that have proven effective. Development of new methods of fertility preservation to prevent these effects or restore normal reproductive function after cytotoxic treatment are of great importance to these young male cancer survivors.
If spermatogonial stem cells (SSC) survive after cancer therapy, there is the possibility for endogenous spermatogenic recovery either by spontaneous or stimulated differentiation of these cells. Suppression of gonadotropins and testosterone stimulated endogenous recovery of spermatogenesis from surviving stem cells in rats after exposure to cytotoxic agents, which was surprising since testosterone and follicle-stimulating hormone (FSH) are the hormones responsible for completion of the process of spermatogenesis (Meistrich & Kangasniemi, 1997; Shetty et al., 2000; Shetty et al., 2006). Transient suppression of these hormones after radiation stimulated recovery of spermatogenesis and fertility in both rats and in mice (Meistrich et al., 2001; Wang et al., 2010). Furthermore, hormone suppression in rats during or after exposure to the cancer chemotherapy agents procarbazine or busulfan also stimulated spermatogenic recovery and restored fertility (Velez de la Calle & Jegou, 1990; Meistrich et al., 1999; Udagawa et al., 2001) . Of the several clinical studies attempting to use hormonal suppression to preserve human spermatogenesis after radiation or chemotherapy (reviewed in (Shetty & Meistrich, 2005), only one was successful (Masala et al., 1997). The one study using hormonal suppression after prepubertal radiation or chemotherapy to stimulate recovery (Thomson et al., 2002) was unsuccessful, probably because the high-dose treatment killed all stem cells (Shetty & Meistrich, 2005).
If SSC are completely lost after gonadotoxic therapy, harvesting and cryopreservation of tissue or a cell suspension containing SSC prior to therapy and a method to produce sperm from those cells is the only way to preserve fertility in prepubertal and peripubertal males. Several techniques are being tested for potential future production of sperm, including SSC transplantation, testicular tissue grafting, and in vitro development of sperm (Brinster, 2007; Rodriguez-Sosa & Dobrinski, 2009; Sato et al., 2011). Only SSC transplantation has the potential to restore spermatogenesis from an individual’s own testis in vivo, enabling the recipient male to father his own genetic children, possibly through normal coitus. Hence, autologous transplantation of SSC, such as those collected and cryopreserved before therapy, is an important potential option for fertility preservation (Orwig & Schlatt, 2005; Brinster, 2007). Intratesticular transplantation of cryopreserved testicular cell populations has been well documented to restore fertility in rodent models and some farm animals (Honaramooz & Yang, 2011). However, there are only two reports of modest spermatogenic recovery after transplantation of cryopreserved germ cell suspensions into irradiated monkey testes (Schlatt et al., 2002; Jahnukainen et al., 2011), but the progeny of the donor cells could not be distinguished from endogenous-derived cells. In a recent study, however, spermatogenesis could be restored from either autologously or allogeneically transplanted genetically marked germ cells in rhesus monkeys exposed to busulfan (Hermann et al., 2012).
Experiments in rats showed that spermatogonial differentiation is blocked after radiation because of damage to the somatic compartment but not to the spermatogonia (Zhang et al., 2007) and that the block could be ameliorated by hormone suppression. These findings suggest that hormone suppression should also enhance differentiation and recovery from transplanted germ cells by improving the niche and somatic environment. The enhancement of colonization and differentiation of transplanted spermatogonia via suppression of gonadotropins and intratesticular testosterone has been demonstrated in busulfan-treated and in irradiated recipient rats (Ogawa et al., 1999; Zhang et al., 2007) and mice (Ogawa et al., 1998; Dobrinski et al., 2001; Ohmura et al., 2003), resulting in donor-derived fertility in two of these studies (Zhang et al., 2003; Wang et al., 2010). Comparison of stimulation of recovery of endogenous and donor spermatogenic recovery by hormone suppression in irradiated mice showed a greater stimulation of the recovery from transplanted cells. This result indicates that, besides stimulating proliferation or differentiation of both endogenous and transplanted spermatogonial stem cells, hormone suppression also has a positive effect on homing of transplanted cells (Wang et al., 2010).
To test whether these concepts of stimulation of spermatogenic recovery by hormonal suppression could be applied to primates, we treated irradiated cynomolgus monkeys with a gonadotropin-releasing hormone antagonist (GnRH-ant) in conjunction with spermatogonial stem cell transplantation. Our hypothesis was that GnRH-ant treatment enhances spermatogenic recovery from surviving endogenous and from autologously transplanted SSC in irradiated monkeys.
MATERIALS AND METHODS
Animals
A total of 16 adult (6- to 10-year-old) male cynomolgus monkeys (Macaca fascicularis) were purchased from Charles River Laboratories from their facility in Houston, Texas. The animals were individually housed in steel cages in a facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care at The University of Texas MD Anderson Cancer Center. They were fed Harlan TEKLAD Primate diet #7195 with daily enrichment foods, such as seeds, peanuts, fruits, and vegetables. Their environment was maintained at a constant temperature (75°F–80°F) and humidity (40%–55%) with a 12-hour light/12-hour dark cycle.
For xenotransplantation of monkey testicular cells, adult nude (Swiss nu-nu/Ncr) mice bred at The University of Texas MD Anderson Cancer Center were used as recipients. The animals were maintained on a 12-hour light/12-hour dark cycle and were allowed food and water ad libitum.
All animal care and treatment protocols were approved by the Institutional Animal Care and Use Committee of MD Anderson Cancer Center.
Experimental design
A preliminary experiment was performed involving four monkeys: one untreated control, one receiving GnRH-ant only, one receiving testicular radiation only, and one receiving both radiation and GnRH-ant (Fig. S1). No transplantations were performed.
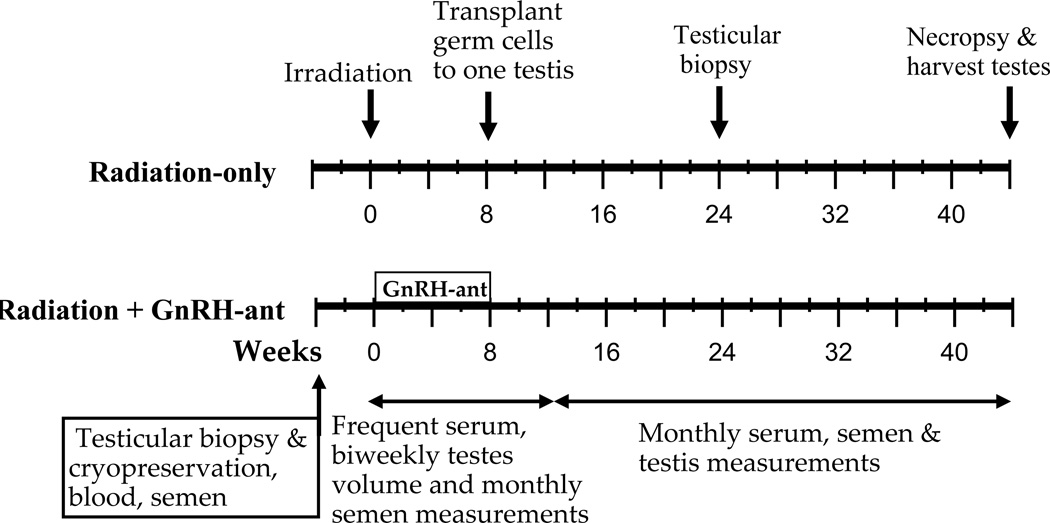
In the main experiment, twelve other monkeys were divided into two treatment groups of six each, such that the age and weight distributions were similar (Table S1). All monkeys underwent irradiation followed by autologous germ cell transplantation into one testis (Fig. 1). One group received GnRH-ant treatment and the other group received no hormone-suppressive treatment.
Figure 1.
Overall design of the main study. Monkeys were evaluated before treatment and periodically after exposure to radiation, hormone suppression, and transplantation. Evaluation included sampling of serum and semen, measurements of testis volume and weight, and testis biopsies as indicated. All 12 monkeys were given testicular irradiation; six then underwent GnRH-ant–mediated hormone suppression for 8 weeks, while the other six did not. At the end of the 8-week period, all monkeys received autologous transplantation of GFP-lentivirus–labeled germ cells into one testis.
General surgical and post-surgical procedures
Monkeys undergoing testicular biopsy and spermatogonial transplantation were first sedated with IM injection of telazol (2.2–4.4 mg/kg; Fort Dodge Animal Health, Fort Dodge, IA) and then anesthetized with 1–3% isoflurane (Butler Schein Animal Health, Dublin, OH) in oxygen. Before all surgical manipulations, 2% lidocaine (Hospira, Inc., Lake Forest, IL) (IM) was instilled into the surgical site to provide local anesthesia. All surgical procedures were performed under aseptic conditions. Postsurgically, all animals received, at the discretion of the Clinical Veterinarian, one daily IM injection of Baytril antibiotics (5mg/kg) for a week post-surgery, and an analgesic (buprenorphine; 0.01–0.03 mg/kg; Bedford Laboratories, Bedford, OH) prior to and at the end of the day of surgery, and 2 times per day for up to 3 days as needed by the appearance of the animal under constant monitoring.
Irradiation
The monkeys were anesthetized with telazol and were maintained with isoflurane. Each monkey was irradiated to the testes, using a cobalt-60 gamma-irradiator, based on dosimetry performed in a simulated phantom made from 1.5% agarose. Tissue-equivalent bolus material (5-mm thick) was placed over the scrotum to provide a build-up layer. For dose uniformity, both postero-anterior and antero-posterior positions were used, and half of the dose was given in each position. The monkeys were irradiated at a total calculated dose of 7 Gy at a rate of 73–78 cGy/minute, maintaining a field size of ~10 × 10-cm and a source-to-skin distance of 76.5 cm measured to the bolus.
Mouse recipients underwent irradiation to eliminate endogenous spermatogenesis prior to transplantation of monkey testicular cells. The mice were restrained in a plastic chamber and then placed into a metal shield module with a 3-cm diameter hole, so that only the lower abdominal and scrotal area of the animal was irradiated. Radiation was delivered by a cesium-137 gamma-ray unit (Zhang et al., 2006) as an initial 1.5-Gy dose followed by a second dose of 12 Gy.
GnRH antagonist treatment
The GnRH-ant Acyline was obtained from the Contraceptive Discovery and Development Branch (formerly Contraception and Reproductive Health Branch) of the Eunice Kennedy Shriver National Institute of Child Health and Human Development (Bioqual; Rockville, MD). A stock solution of Acyline (2 mg/ml) in 5% aqueous mannitol was prepared as needed and stored at 4°C for a maximum of 1 week. Various GnRH-ant treatment regimens were used in the preliminary experiment to determine the most effective dose regimen for suppressing serum testosterone (Fig. S2). One unirradiated monkey was initially given daily subcutaneous injections of Acyline at 50 µg/kg/day for 2 weeks, followed by twice-weekly injections, at doses of 200 µg/kg (Monday) and 300 µg/kg (Thursday) during weeks 3 and 4 and 300 and 450 µg/kg during weeks 5 through 8. One irradiated monkey was initially given a bolus injection of 600 µg/kg and then twice-weekly injections at doses of 200 µg/kg and 300 µg/kg from weeks 3 through 8. On the basis of those results, the monkeys in the main experiment were given twice-weekly subcutaneous injections of Acyline on Mondays and Thursdays at doses of 200 µg/kg and 300 µg/kg, respectively. The hormone-suppressive treatment was started immediately after irradiation, since in irradiated rats this efficiently stimulated recovery of spermatogenesis from surviving stem cells (Meistrich & Kangasniemi, 1997). Hormone suppression was continued for 8 weeks and at the end of the eighth week, transplantation was performed.
Semen and blood collection
Semen was obtained from anesthetized monkeys by electro-ejaculation using a rectal probe (Beltron Instruments, Longmont, CO). The probe was inserted gently into the rectum with the electrodes adjacent to the prostate. Stimulation was applied for 1 second every 3–5 seconds, initially at 10 volts and gradually increased to 15 volts until an ejaculate was obtained. The sample was allowed to liquefy at 37°C for an hour before sperm were counted in the exudate using a hemacytometer. Sperm counts were expressed per total ejaculate (volume of exudate plus remaining coagulum). The exudate was stored at −80°C for later polymerase chain reaction (PCR) analysis of lentiviral DNA.
Blood (5–10 ml) was drawn from each monkey by venipuncture of the saphenous vein with the animal under ketamine (Fort Dodge Animal Health, Fort Dodge, IA) sedation. Serum was prepared and stored at −20°C.
Testicular measurements and sampling
Testis volume was determined by measuring the length and width of each testis within the scrotum of anesthetized monkeys with calipers and modeling the testis as a prolate ellipsoid, applying the following formula: testis volume = π × width2 × length/6. Since the pretreatment volume of all testes were measured, testis volumes could be presented as a fraction of the pretreatment volume, providing a correction for interanimal variability.
Testicular biopsy specimens were collected from anesthetized animals by making an incision in the scrotal skin and then in the tunica albuginea to expose the testicle. Biopsy samples of up to1 g, depending on the size of the testis, to obtain cells for transplantation or of 100 mg for histological and hormone studies, were collected from a region midway between the poles avoiding the major blood vessels and the rete testis. At the end of the study, the remaining testes were harvested intact, weighed, and prepared for histology. Absolute testis weights are given since pretreatment testis weights were not known; thus there is more interanimal variability than in testis volume, which is normalized to the pretreatment value.
In 15 of the 16 monkeys studied, we did not observe any adverse effects of multiple testicular biopsies or the transplantation procedure on the testes. No focal or generalized damage to somatic structures or inflammation was observed. Only in one monkey (main experiment, #5, radiation-only) the sham-transplanted testis became almost completely necrotic after the 24-week biopsy and was excluded from the analysis at subsequent time points. Thus, biopsy by itself does not seem to be deleterious to the remaining testicular tissue, and occasional necrosis may be a result of damage to a major blood vessel.
Preparation of testis cells for transplantation
The testis cells were prepared with slight modification of previously published procedures (Hermann et al., 2007). Biopsy samples were digested with collagenase type IV (1 mg/ml; Worthington Biochemical Corporation, Columbus, OH) and DNase I (100 µg/ml; Sigma-Aldrich, , St. Louis, MO) in Hanks’ balanced salt solution (HBSS; Gibco/Life Technologies, Grand Island, NY) for 5–10 minutes at 37°C with vigorous shaking. Dispersed seminiferous tubules were sedimented and washed in HBSS to remove interstitial cells. Isolated seminiferous tubules were further digested with trypsin (2.5 mg/ml; Gibco) containing 1 mM EGTA, 1 mM MgCl2, and DNase I (0.4 mg/ml) in HBSS for 10–15 minutes at 37°C with pipetting. The cell suspension was filtered through a 70-µm nylon mesh, pelleted, and resuspended at 40 × 106 per ml in minimum essential medium α (MEMα; Gibco) containing 10% fetal bovine serum (FBS).
Cells were aliquoted into cryovials, and an equal volume of freezing medium (MEMα + 20% FBS + 20% dimethyl sulfoxide [DMSO]) was added drop-wise. Vials were frozen at −1°C/minute in controlled-rate freezing containers (Nalge Nunc International, Penfield, NY) to −80°C and stored in liquid nitrogen.
Lentiviral Transfection of Testicular Cells
Prior to use, the frozen vials with testicular cells were thawed rapidly at 37°C, excess MEMα + 10% FBS was added to the cell mixture drop-wise, and cells were washed three times. Cells were transfected with a lentiviral vector modified from the FUGW construct (Lois et al., 2002) and containing EF1α (promoter)–EGFP (Hermann et al., 2012) which was obtained from the Transgenic and Molecular Research Core at Magee-Womens Research Institute. Cells were incubated overnight with the lentivirus particles in MEMα containing 10% FBS and polybrene (6 µg/ml; Sigma-Aldrich) at a total multiplicity of infection (MOI) of 60 (three additions at MOI 20, at 3-hour intervals). Lentivirus-treated cells were washed several times with fresh medium to remove excess lentivirus. The labeling of SSC by EGFP-lentivirus by this method was demonstrated previously although the labeling efficiency was apparently low (Hermann et al., 2012).
Autologous transplantation
Each monkey underwent autologous transplantation of cells into one testis 8 weeks after irradiation essentially as described (Hermann et al., 2012). Briefly, cells prepared for transplantation were suspended at approximately 1.3 × 108 cells/ml in MEMα containing 10% FBS, trypan blue (Sigma-Aldrich; 0.4 mg/ml), 20% (v/v) Optison ultrasound contrast agent (GE Healthcare, Waukesha, WI), 1% antibiotic-antimycotic (a combination of penicillin, streptomycin, and amphotericin B; Gibco), and DNase I (0.1 mg/ml) in a total volume of as much as 1 ml, depending on recipient testis size and number of available cells. The cells were transplanted via ultrasound-guided injections into the rete testis. A 13MHz linear superficial probe and a MicroMaxx ultrasound machine (Sonosite, Bothell, WA) were used to visualize the rete testis space and to guide a 25-gauge, 2” spinal needle into the space. Cells were injected under slow constant pressure and chased with saline solution. The average total numbers of viable cells injected into the radiation-only monkeys and the irradiated and GnRH-ant–treated monkeys were 56 × 106 and 81 × 106, respectively (Table S1). The contralateral testes were sham transplanted at the same time by injection of the suspension medium with all constituents except the cells.
Xenotransplantation to mice
Seminiferous tubules of adult nude mice were injected via the efferent ducts with 7–10 µl of donor testis cell suspension containing about 40 × 106 cells/ml at 3 weeks after testicular irradiation as described previously (Zhang et al., 2006). One to three recipient testes per monkey cell suspension was successfully transplanted for this study. At 10 weeks after transplantation, intact seminiferous tubules were recovered, dispersed, fixed, and stained in whole-mount with an anti-rhesus testis-cell antibody (Hermann et al., 2007). Samples were dehydrated stepwise in methanol and then incubated in MeOH:DMSO:H2O2 (4:1:1) for 2–3 hours. The rhesus testis-cell antibody was used at a 1:800 dilution and detected with goat anti-rabbit IgG conjugated to AlexaFluor 488 (1:300 dilution; Invitrogen, Carlsbad, CA). Samples were mounted with Vectashield medium containing DAPI (Vector Laboratories, Burlingame, CA) on slides with raised coverslips and visualized by fluorescence microscopy. The DAPI staining was used to determine the position of the donor rhesus cells within the seminiferous epithelium. Donor stem cell–derived colonies with at least four cells exhibiting spermatogonial morphology located on the basement membrane of the recipient seminiferous tubule (<100 µm between cells) were counted (Hermann et al., 2009).
Detection of lentiviral vector DNA in sperm and testis
Attempts to detect green fluorescent protein (GFP)–positive sperm or cells using direct fluorescence or immunofluorescent staining of the testicular sections, as had been used with GFP-transfected rat SSC (Ryu et al., 2007), were unsuccessful, in accordance with other studies with monkey testis cells (Hermann et al., 2012). Thus PCR was used to screen for the presence of lentiviral genetic material. DNA was extracted from as many as 1.5 × 107 monkey sperm from each sample (Hermann et al., 2012). To eliminate somatic cells, sperm were suspended in 700 µl phosphate-buffered saline solution (PBS) with 0.2% sodium dodecyl sulfate and pelleted (Zheng et al., 2000). The pellets were resuspended in 300 µl Cell Lysis Solution (Puregene, Cat#158906; Qiagen, Valencia, CA) and then mixed with 33 µl of 100 mM dithiothreitol and 30 µl of proteinase K (20 mg/ml). Samples were then incubated at 55°C overnight. Each sample was supplemented with 100 µl Protein Precipitation Solution (Cat#158910; Qiagen) and vortexed. Samples were subjected to centrifugation, and supernatants were collected. For samples that contained fewer than 1.5 × 107 sperm, 2 µl of glycogen (20 mg/ml) was added to enhance DNA precipitation. Then 1 ml of ice-cold 100% ethanol was added to each sample, mixed thoroughly and subjected to centrifugation. The resulting pellets were washed with 70% ethanol and air-dried.
For monkeys with spermatogenesis in at least 4% of tubules, DNA was extracted from testis slices using Qiagen AllPrep DNA/RNA Mini Kit (Cat #80204). For each PCR reaction, 6–200 ng DNA template and 0.75 U Platinum Taq High Fidelity (Invitrogen) were diluted in a final 15-µl volume containing 0.1 mM deoxy-NTPs, 2.5 mM MgSO4, 0.2 µM of each primer, and buffer. A touch-down PCR protocol was used: 5 minutes at 94°C, then 28 cycles of 30 seconds at 94°C, 30 seconds initially at 70°C with the annealing temperature decreasing by 0.5°C every cycle, and 45 seconds at 72°C, followed by 20 more cycles at the final annealing temperature (56°C) and a final extension step at 72°C for 10 minutes. The amplified DNA was visualized in ethidium bromide–stained agarose gels.
Primers were designed for amplifying the HIV envelope glycoprotein (env) gene and GFP gene in the lentiviral vector and the primate-specific gene BC042682 of rhesus monkeys, which has the same size and sequence in the cynomolgus macaques (Table S2). To confirm that all the sperm and testis DNA samples contained good quality monkey DNA, primer pair BC1 for BC043682 was used; it showed a strong signal in all samples. To detect lentiviral vector DNA sequences, primer pairs for env and GFP, designated env1 and GFP1, respectively, were used initially. Samples were then subjected to another round of nested PCR for more sensitive detection using env2 or GFP2 primer pair. Later, the most sensitive primer pair, env2, was used directly for the remaining sperm and all the testis samples. The nested PCR or the env2 primer pair alone detects positive signals from as low as 0.1 ng of sperm DNA from a monkey (M036) previously shown to have transfected donor-derived sperm in the ejaculate (Hermann et al., 2012).
Hormone assays
Intratesticular testosterone was measured in tissue (20–67 mg) from each biopsy that was frozen immediately in liquid nitrogen, stored at −20°C, and homogenized at the time of radioimmunoassay (RIA) (Boekelheide et al., 2005). Serum testosterone and intratesticular testosterone concentrations were measured using coated-tube RIA kits (TKTT1, Siemens Health Care Diagnostics, Deerfield, IL) according to a method described elsewhere (Shetty et al., 2011). The intraassay and interassay coefficients of variation were 10% and 16%, respectively. The sensitivity of testosterone assay was 0.041 ng/ml.
Circulating concentrations of FSH and luteinizing hormone (LH) were determined by using homologous RIA reagents supplied by the National Hormone and Peptide Program as described previously (Ramaswamy et al., 2003). The sensitivities of the LH and FSH assays were 0.12 ng/ml and 0.06 ng/ml, respectively, using 100-µl samples. The intraassay and interassay coefficients of variation were 6% and 15%, respectively, for FSH, and 3% and 9%, respectively, for LH.
Histological procedures
The monkey testis tissue was fixed in Bouin solution and embedded in paraffin or methacrylate, and sections were stained with periodic acid Schiff reagent and hematoxylin. The stained sections were quantitatively assessed by scoring seminiferous tubule cross-sections at regular intervals across the whole tissue section for the presence or absence of germ cells and the most advanced germ cell type present. In the single biopsy samples taken at interim time points after irradiation, an average of 309 tubules (range: 144–515) were counted per testis in in the main experiment and 138 tubules (79–159) were counted in the preliminary experiment. In whole testes harvested at the end of the studies, the testes were transversely sliced into 5–6 pieces and every alternate slice was used for analysis. Since the slices from the mid region were large, they were halved into two, one of which was used for histological scoring. An average of 3980 tubules (range: 1985–5143) were scored in these testes in the main experiment and 3617 tubules (range 3539–3695) were scored in the preliminary experiment. It should be noted that the tubule count data for the 24 week time point is from the single biopsy samples, and while it may not be the exact representation of the spermatogenesis in the whole testis, it certainly should be indicative. At 44 weeks large portions of the testes were systematically analyzed and hence likely to be more accurate estimations of spermatogenesis. A tubule differentiation index (TDI) that represents the percentage of seminiferous tubule cross sections containing at least one differentiated germ cell type (B spermatogonia or later stages), was computed. In addition, the extent of the progression of germ cell differentiation was assessed by determining the percentages of tubules with germ cells that contained spermatocytes, round spermatids or elongating/elongated spermatids as the latest germ cell type present; no tubules containing only spermatogonia were observed.
Statistical analysis
The testis weights and TDI were represented as arithmetic means ± SEM. For sperm counts, FSH, LH, and testosterone measurements, the averages and SEM were calculated on log-transformed data. The significance of differences between treatment groups was evaluated by the statistical tests indicated in the figure legends. Since the parameters measured were not normally distributed, nonparametric statistics were used. Comparisons between groups of independent samples were done using the Mann-Whitney test. Wherever possible when samples were related (e.g., same monkeys or testes at different time points, contralateral testes from same monkeys), more powerful paired tests, such as the Wilcoxon signed-rank test, was used. A computer-assisted statistics program (PASW statistics 17, SPSS Inc, Chicago, IL) was used. A value of P<0.05 for the asymptotic significance was considered statistically significant.
RESULTS
Preliminary experiment
A preliminary experiment with four monkeys was performed to find an appropriate dose of the GnRH-ant, Acyline, for hormone suppression and to obtain information on the effect of a 7-Gy dose of radiation on spermatogenesis (Fig. S1).
The bolus dose of 600 µg/kg of Acyline (given to the irradiated monkey) transiently suppressed serum testosterone level to 0.6 ng/ml, but levels returned to normal within 7 days. However, daily injections (to the unirradiated monkey), initially at 50 µg/kg/day, for 2 weeks effectively suppressed serum testosterone levels to about 3 ng/ml (Fig. S2A). Twice a week Acyline injections of 200 µg/kg and 300 µg/kg were enough to keep the serum testosterone levels at about 3 ng/ml in the unirradiated monkey during weeks 3 and 4 and reduced them to <1 ng/ml in the irradiated monkey during weeks 3–8. Giving a slight increase in dose of the twice-weekly injections, to 300 and 450 µg/kg, during weeks 5–8 did not further suppress serum testosterone levels in the unirradiated monkey. The testosterone suppression was rapidly reversible, and testosterone level was restored to normal levels within 1 or 2 weeks of the end of treatment. These treatment regimens suppressed intratesticular testosterone levels to between 10% and 20% of the control levels in both monkeys at the end of the 8-week treatment (Fig. S2B).
The GnRH-ant treatment was biologically effective in suppressing spermatogenesis, as indicated by the reduction in testicular volume and the shrinkage of tubules with sloughed germ cells in the unirradiated monkey at the end the 8-week treatment (Fig. S3). Both effects were reversible: testis volume had recovered with normal histology at the next biopsy 12 weeks later.
In the monkey treated with radiation alone, 0.6%, 0%, and 0.7% of the tubule cross-sections contained germ cells at 8, 20, and 44 weeks, respectively, after irradiation. Although the irradiated monkey treated with GnRH-ant showed no germ cells in the biopsy sampled at the 8-week time point, germ cells were observed in 1.5% and 6.2% of tubule cross-sections at 20 and 44 weeks, respectively.
Main experiment
We used the experimental design shown in Figure 1 to determine the benefits of hormone suppression alone, spermatogonial transplantation alone and the two approaches combined on the recovery of spermatogenesis after radiation. Pre-irradiation testicular biopsies from both testes, amounting to ~5% of the testis and an average of 2.2 g tissue, were collected from each monkey (Table S1). Histologic analysis showed normal spermatogenesis in all testes (data not shown). Cell suspensions prepared from this tissue yielded an average of 277 million cells per monkey with 80% viability (131± 19 cells/g tissue); there was no significant difference in this yield and viability between the monkeys who went on to receive GnRH-ant treatment and the radiation-only group. All suspensions were cryopreserved.
Response to irradiation
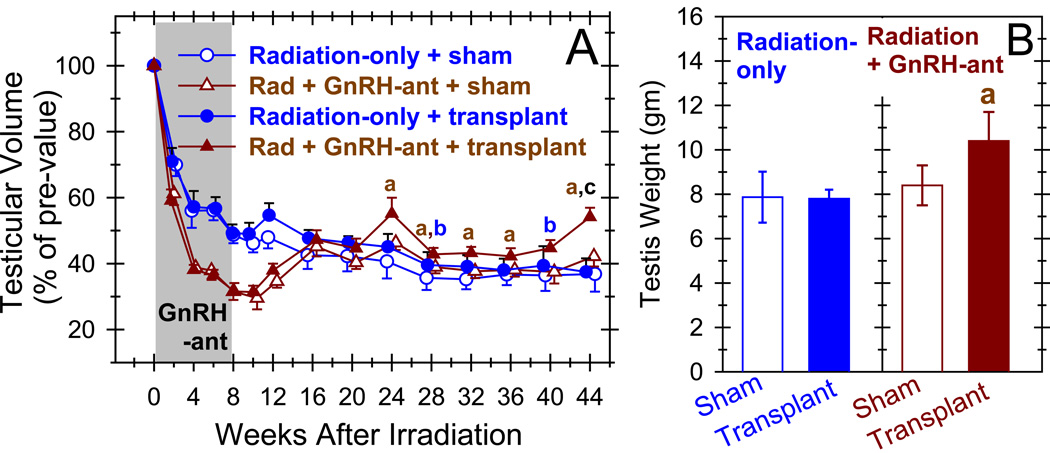
The radiation appropriately depleted endogenous spermatogenesis; testis size in the radiation-only monkeys declined to 49% of that of pretreatment controls by 8 weeks (Fig. 2A). At 24 and 44 weeks after irradiation, only 3% and 7%, respectively, of tubule cross-sections in the sham-transplanted testis contained germ cells, (Figs. 3B, 4A & Fig. S4). This increase in TDI with time was statistically significant (P=0.043). All tubules with germ cells contained cells at the spermatocyte stage or later; no tubule cross-sections containing only spermatogonia were observed. At 24 and 44 weeks, respectively 22% and 67% of the tubules containing germ cells had late spermatids.
Figure 2.
Increases in testis volume and weight suggest that combined hormone suppression and germ cell transplantation promote spermatogenic recovery. Testicular volume (expressed as a percentage of the pretreatment volume) (A) and testis weight at the end of the study, at 44 weeks after irradiation (B) are shown in radiation-only monkeys and monkeys that received GnRH-ant, for both the testes that did or did not receive transplantation at 8-weeks after irradiation. The shaded area represents the period of hormone suppression. Recovery of testis volume during the period from week 24 to week 44 and the testis weight at week 44 was compared between various groups, and significant (P<0.05) differences are shown by different letters, as follows: a: between the transplanted and sham-transplanted testes of GnRH-ant–treated monkeys (Wilcoxon test); b: between the transplanted and sham-transplanted testes of radiation-only monkeys (Wilcoxon test); c: between the transplanted testes of GnRH-ant–treated and radiation-only monkeys (Mann-Whitney test). The differences between the sham-transplanted testes of GnRH-ant–treated and radiation-only monkeys were not significant.
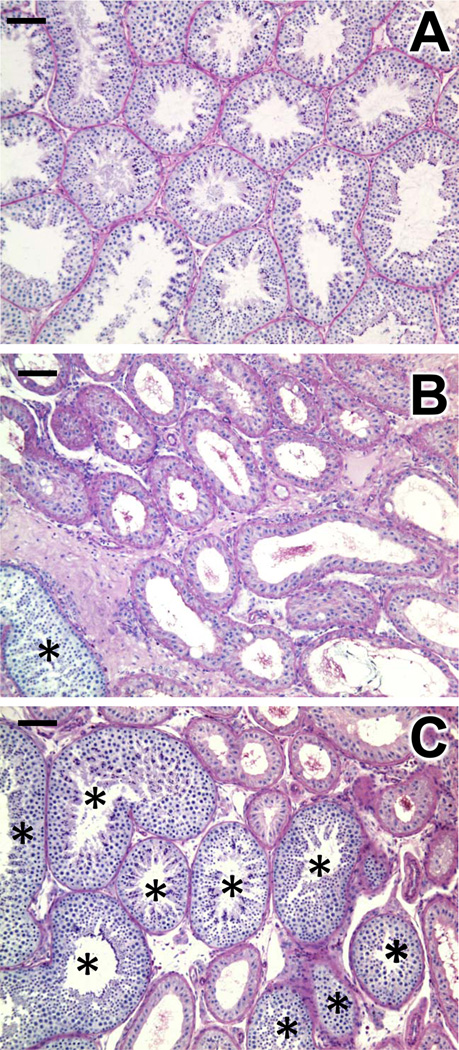
Figure 3.
Combined hormone suppression and germ cell transplantation results in spermatogenic recovery. The three panels show histology of representative monkey testes. (A) Testis from the unirradiated untreated monkey used in the preliminary experiment. (B) Testis of a monkey that received radiation 44 weeks previously and neither GnRH-ant treatment nor transplantation. Very few of the tubules contain germ cells (marked with *) in this radiation-only monkey. (C) Testis of a monkey that received radiation 44 weeks previously and underwent both GnRH-ant treatment and germ cell transplantation. Note the large cluster of tubules containing germ cells. Bar represents 100 µm.
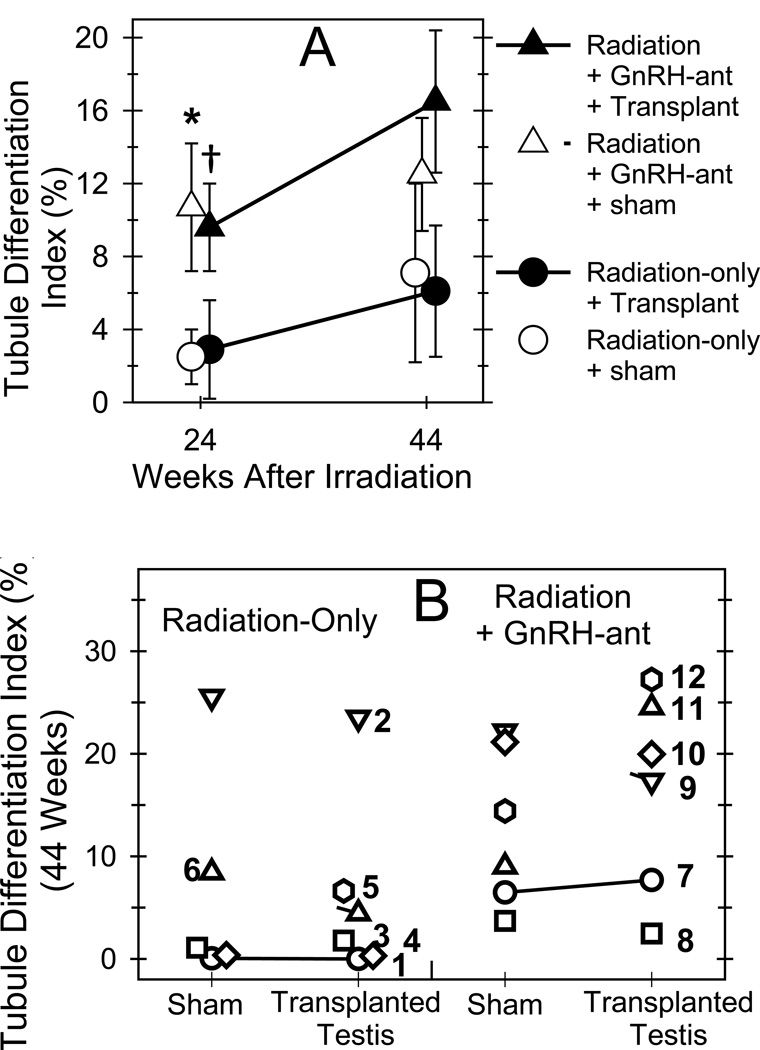
Figure 4.
Combined hormone suppression and germ cell transplantation induced greater development of germ cells in monkey testes than either approach alone. The average (A) and individual (B) percentages of tubules showing differentiated germ cells (tubule differentiation indices) in radiation-only and irradiated GnRH-ant–treated monkeys that received autologous transplantation of testicular cells to one of the testes. Tubule differentiation indices were compared between various groups at week 24 and at week 44, and significant (P<0.05) differences are shown as follows: *: between the transplanted testes of GnRH-ant–treated and radiation-only monkeys (Mann-Whitney test); †: between the sham-transplanted testes of GnRH-ant–treated and radiation-only monkeys (Mann-Whitney test). (B) The tubule differentiation indices for individual monkeys, with the individual animal numbers indicated, were obtained at 44 weeks after irradiation. Only the transplanted testis of monkey #5 is shown, as the sham-transplanted testis became necrotic after week 24.
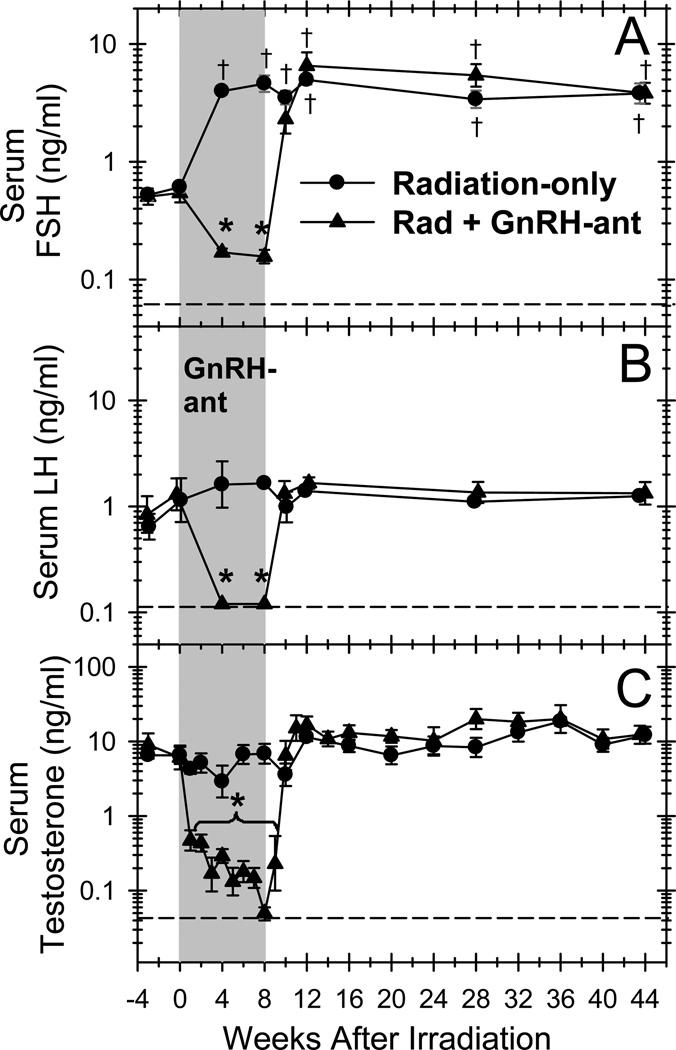
Radiation did not induce any changes in serum testosterone or LH levels (Fig. 5). However, as expected, the loss of germ cells after radiation caused large increases in serum FSH levels in the radiation only monkeys within 4 weeks after irradiation and in the irradiated, GnRH-ant–treated monkeys after the hormone suppression was stopped.
Figure 5.
Serum hormone levels are suppressed by treatment with GnRH-ant and return to normal levels when the treatment is stopped. Serum FSH (A), LH (B), and testosterone (C) levels in radiation-only and irradiated monkeys treated with GnRH-ant for 8 weeks are shown. The shaded area represents the period of hormone suppression. Dashed lines indicate minimum levels of detection. The serum hormone levels after irradiation with or without GnRH-ant treatment were compared to the respective values before irradiation, and significant (P<0.001) differences are shown for the values lower ( *) and higher (†) than the pre-irradiation values (Dunnet test).
Effect of hormone suppression alone
Treatment of the irradiated monkeys with GnRH-ant markedly suppressed serum FSH, LH, and testosterone levels during the treatment period (Fig. 5) and resulted in a more extreme decline in testis volume (Fig. 2A). However, after the treatment period the hormone levels and testis volumes returned within 2 to 8 weeks to the levels observed in radiation-only monkeys
The effect of hormone suppression on endogenous spermatogenic recovery was assessed by comparing the volume, weight, and histology of the sham-transplanted testes of GnRH-ant–treated monkeys with those of the radiation-only monkeys at times ≥24 weeks after irradiation. Testis volumes were slightly but not significantly greater in the GnRH-ant–treated monkeys than in the radiation-only monkeys at all time points from 24 to 44 weeks (Fig. 2A). No differences were observed in the average testis weights when removed (Fig. 2B).
Histologic analysis at 24 weeks after irradiation showed that, whereas only 2.5% of the tubule cross-sections in the sham-transplanted testes of the radiation-only monkeys contained differentiated germ cells, 10.7% of the tubules in the sham-transplanted testes of the GnRH-ant–treated monkeys were recovering spermatogenesis (P=0.037) (Fig. 4A, and Fig. S5). At 44 weeks after irradiation, these TDI values increased to 7.1% (radiation-only) and 12.8% (GnRH-ant), but the difference between the treatment groups was not significant at this time point. Similar to the radiation-only monkeys, 22% and 60% of tubules containing germ cells in the GnRH-ant-treated monkeys had late spermatids at 24 and 44 weeks after irradiation, respectively.
Effect of transplantation alone
At the end of the hormone suppression period, the cryopreserved germ cells from all monkeys were thawed and cultured with EGFP-lentivirus overnight. After incubation, about 113 million cells with 54% viability remained per monkey in the radiation-only group (Table S1). The germ-cell suspensions were injected back into the rete of one of the testes of the monkey from which they were obtained. To validate the presence of stem cells in these preparations, aliquots of the cell suspensions from each monkey were also transplanted to germ cell–depleted nude mice, and donor colonies of monkey spermatogonia were identified by immunostaining (Fig. S6). The cells from the monkeys in the radiation-only group yielded an average of 8.0 ± 2.6 colonies/105 viable cells (Table S3). Based on the xenotransplant assay and the numbers of viable cells autologously transplanted into these monkeys (average of supplementary data, Table S1), we calculated that the radiation-only monkeys received 4600 ± 1500 cells with stem cell potential.
The effect of transplantation alone on spermatogenic recovery was assessed by differences in testis volume, weight, and histology between the transplanted and sham-transplanted testes of radiation-only monkeys and by the presence of lentiviral DNA in their sperm or germ cells. The volumes of the transplanted testes were slightly larger than those of the sham-transplanted testis between 24 and 44 weeks after irradiation, and the difference was significant at two time points (Fig. 2A). However, there was no difference in average testis weight at the end of the study (Fig. 2B). In addition, the average percentage of tubules with differentiated germ cells was not significantly changed by transplantation at either time point (Fig. 4A), and no individual radiation-only monkey showed notably higher percentages of tubules with germ cells in the transplanted testis than in the sham-transplanted testis at the end of the experiment (Fig. 4B).
PCR analysis for lentiviral DNA could only be performed effectively on sperm from three of these monkeys (the other three were azoospermic, Table 1) and on testis tissue from the one monkey that showed germ cells in about 25% of tubules (Fig. 4B) but was azoospermic. Lentiviral genetic material was detected in the sperm of one monkey at several time points after transplantation, indicating that some transplanted SSC did indeed colonize the testis.
Table 1.
Presence or absence of lentiviral vector and/or green fluorescent protein (GFP) DNA in the periodic semen samples and the testes samples collected at the end of the study.
| Treatment | Monkey Number |
Sperm from Semen | Testes |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sham | Trans- planted |
||||||||||||
| Pre- Irrad |
Time after Irradiation (weeks)a |
||||||||||||
| 16 | 20 | 24 | 28 | 32 | 36 | 40 | 44 | 44 | |||||
| Irradiated | 1 | − | 0 | 0 | 0 | n | 0 | n | − | n | nd | nd | |
| 2 | nd | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | − | − | ||
| 3 | nd | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | nd | nd | ||
| 4 | − | 0 | 0 | nd | n | 0 | 0 | 0 | 0 | nd | nd | ||
| 5 | nd | 0 | nd | nd | 0 | nd | − | − | 0 | nd | nd | ||
| 6 | − | + | − | − | − | − | + | − | + | −b | −b | ||
| Irradiated + GnRH-ant |
7 | − | 0 | − | − | − | − | + | − | + | −b | −b | |
| 8 | − | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | nd | nd | ||
| 9 | − | 0 | + | − | − | + | + | − | ne | − | − | ||
| 10 | − | 0 | 0 | − | − | − | + | − | + | − | − | ||
| 11 | − | 0 | − | nd | n | − | n | ne | n | − | + | ||
| 12 | − | 0 | ne | n | + | nd | + | n | − | − | − | ||
Abbreviations: n = no ejaculate; ne = not enough ejaculate for analysis; 0 = azoospermic; + = presence of lentiviral vector and/or GFP DNA in the semen sample; − =absence of lentiviral vector and/or GFP DNA in the semen sample; nd = not done.
Note transplantation was done 8 weeks after irradiation
Low numbers of germ cells in testes (see Fig. 4B).
Effects of combined hormone suppression and transplantation
For transplantation into the GnRH-ant treated monkeys an average of 134 million cells with 64% viability (Table S1) were used, a small portion of each was also used for xenotransplantation. The xenotransplantation assay indicated that these cells yielded 7.6 ± 2.8 colonies/105 viable cells (Table S3). From these numbers, we calculated that the GnRH-ant-treated monkeys received 6900 ± 2800 cells with stem cell potential. Although the radiation-only monkeys received only 4600 ± 1500 potential stem cells, the numbers were not significantly different between the groups.
In contrast to the minimal effects of hormone suppression or transplantation alone on spermatogenic recovery, enhanced spermatogenic recovery from the transplanted cells was clearer in the hormone-suppressed monkeys. The volumes of the transplanted testes in the GnRH-ant–treated monkeys were greater than those of the other groups at all time points starting at 24 weeks after irradiation (Fig. 2A), the difference being significant at nearly all points. The volumes of the GnRH-ant–treated transplanted testes averaged 20% larger than those of the radiation-only transplanted testes. The volumes of the GnRH-ant–treated transplanted testes averaged 17% more than the contralateral sham-transplanted testes, and there also was a significant difference (P=0.043) in testis weights at the end of the study (Fig. 2B).
The transplanted testes of the GnRH-ant–treated monkeys showed the highest percentage of tubules with differentiated germ cells (Figs 3C, 4A & Fig. S7). The TDI value of 9.6% at week 24 was significantly higher than the TDI of 2.9% of the transplanted testes of radiation-only monkeys (P=0.05) and at week 44, the TDI was increased to 16.5%, which was close to being significantly different from the value of 6.1% in the radiation-only monkeys (P=0.055) (Fig. 4A). Although the difference in the average percentages of tubules with germ cells between the transplanted and control testes of the GnRH-ant–treated monkeys was not statistically significant, two of the six monkeys (#s 11 and 12) treated with GnRH-ant showed marked increases (~2-fold) in the percentage of tubules with germ cells in the transplanted vs. the sham-transplanted testis (Fig. 4B). As in the other treatment groups, in the transplanted testes of the GnRH-treated monkeys, there were no tubules with spermatogenesis arrested at the spermatogonial stage and 33% and 66% the tubules showing differentiated germ cells contained late spermatids at 24 and 44 weeks, respectively.
PCR analysis of lentiviral DNA could be performed on sperm and testes of five GnRH-ant–treated monkeys; the sixth monkey (# 8) was azoospermic and had very few tubules with germ cells. Four of the five GnRH-ant–treated monkeys with sperm showed lentivirus DNA in the sperm by PCR analysis (Table 1). The fifth monkey, which produced very scant or sometimes no ejaculate, had lentiviral sequences in DNA extracted from the transplanted testis but not in the DNA from the sham-transplanted testis.
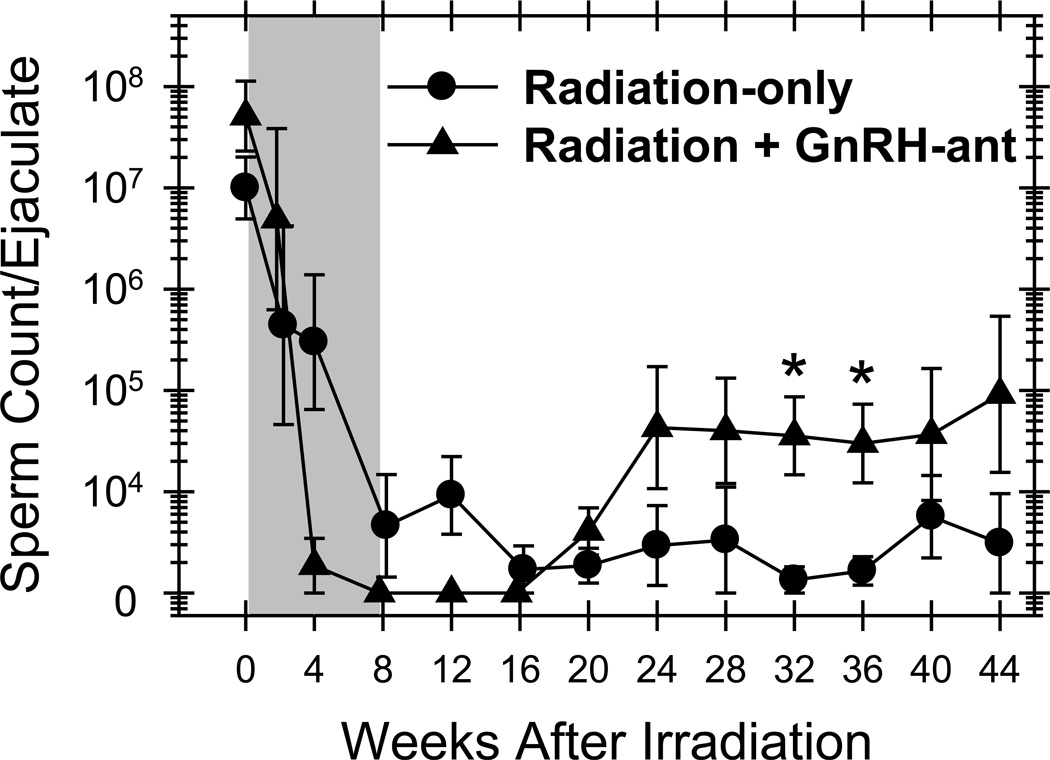
Sperm counts in all the monkeys were assessed. The GnRH-ant–treated monkeys showed higher sperm counts than the radiation-only monkeys at all time points starting 24 weeks after irradiation; the differences at several of the time points were statistically significant (Fig. 6). When data from all these time points were pooled, the statistical significance of the difference between the groups was P<0.001. Furthermore, five of the six GnRH-ant–treated monkeys repeatedly showed >104 sperm per ejaculate (three monkeys ≥1 million), compared to only one of the six radiation-only monkeys (maximum count, 0.26 million).
Figure 6.
Treatment with GnRH-ant stimulates recovery of sperm counts in monkeys transplanted with germ cells. Sperm counts in radiation-only and GnRH-ant–treated irradiated monkeys that received autologous transplantation of testicular cells to one of the testes are shown. The shaded area represents the period of hormone suppression. For the purpose of averaging log-transformed value, azoospermic counts were set at 1,000/ml which is the lower limit of detection of sperm with hemocytometer counting. Recovery of sperm counts was compared between the two groups during the period from week 24 to week 44, and significant (P<0.05) differences are shown by the symbol:* (Mann-Whitney test).
DISCUSSION
In the current study we investigated the ability to enhance recovery of spermatogenesis in irradiated monkeys by hormone suppression, spermatogonial transplantation, and the two strategies combined. Hormone suppression alone appears to accelerate recovery of endogenous spermatogenesis. Transplantation alone did not have any effect on overall spermatogenic recovery, although sperm production from transplanted cells could be demonstrated. However, hormone suppression clearly enhanced spermatogenic recovery from transplanted spermatogonia in this nonhuman primate model.
Hormone suppression alone induced a significant increase in the TDI from 2.7% to 10.7% at 24 weeks after 7-Gy. Although the TDI values in the irradiated GnRH-ant-treated monkeys increased to 12.8% at 44 weeks, the difference between the two treatment groups at that time point was not significant. Thus we suggest that GnRH-ant-treatment may accelerate the initiation of endogenous spermatogenic recovery but may not produce a sustained enhancement. Two previous studies also failed to show any protection or stimulation of recovery in irradiated cynomolgus (Kamischke et al., 2003) or stump-tailed (Boekelheide et al., 2005) macaques.
Acyline suppressed serum testosterone to 2% of control values while, in the previous studies, Cetrorelix suppressed testosterone levels only to 21% (Kamischke et al., 2003) and 10% (Boekelheide et al., 2005) of controls. Moreover, the previous studies employed much higher doses of GnRH-ant (450 µg Cetrorelix/kg/day, compared with 500 µg Acyline/kg/week used here), which was likely the cause of the prolonged suppression of testosterone levels for about 15 weeks after the end of treatment, compared with only 1 to 2 weeks in the present study. Furthermore, in one of those studies (Boekelheide et al., 2005), the volume of the testes of the unirradiated monkeys recovered to only 40% of the pre-treatment values after cessation of hormone suppression, and the volumes of the testes of the irradiated, hormone-suppressed monkeys remained permanently below those of the radiation-only monkeys. In the present study, the biological effect of the GnRH-ant was indeed transient, as evidenced by full recovery of testicular volume to that of non–hormone-suppressed controls within 8 weeks after the end of Acyline treatment.
The absence of substantial recovery with transplantation alone was disappointing in view of earlier reports. Although lentivirus signal in sperm indicated that we achieved transplantation, the enhancement of recovery of spermatogenesis (Schlatt et al., 2002; Jahnukainen et al., 2011) and the incidence of donor marker sequences in sperm (Hermann et al., 2012) were lower than reported in previous studies. Two of these studies used unilateral autologous transplantation of testicular cells in adult cynomolgus monkeys after 2 Gy radiation (Schlatt et al., 2002) or in prepubertal/pubertal rhesus monkeys after 10 Gy (Jahnukainen et al., 2011). In two of five adult monkeys and in one of five immature monkeys (a prepubertal monkey) in those studies, recovery of spermatogenesis was enhanced in the transplanted testis as compared to the sham-transplanted testis. In one of these cases, however, there could have been selective damage to the sham-transplanted testis by a previous unilateral biopsy (Jahnukainen et al., 2011). Following transplantation of SSC in busulfan-treated rhesus monkeys using lentivirus-transfected autologous and allogeneic testicular cells (Hermann et al., 2012), ejaculated sperm from donor cells were detected by PCR in nine of twelve recipients of autologous cells (marked by lentivirus) and two of six recipients of allogeneic cells (microsatellite markers). In one of the allogeneic transplanted recipients, about 10% of the sperm were of donor genotype. In our study we are unaware of any technical problems that might have caused reduced colonization, as cell preparation, cryopreservation, and lentiviral transduction were done according to the same procedures and transplantation was performed by the same individuals as in the previous study (Hermann et al., 2012). Possible factors include the use of a rather high dose of radiation in adult monkeys and the culturing of cells, which was not done in other irradiation studies. Whatever the cause, the low level of colonization with transplantation alone made the system very sensitive to detection of the increase resulting from hormone suppression.
Most importantly, our results, clearly show augmentation of spermatogenic recovery in the transplanted testes of GnRH-ant–treated monkeys by multiple criteria. These testes: (1) had greater weights than the testes of other treatment groups; (2) had increased percentages of tubule cross-sections showing spermatogenesis, including two monkeys with greatly increased spermatogenesis in the transplanted vs. the sham-transplanted testis; (3) had detectable lentivirus-transfected germ cells or sperm in five of six cases; and (4) produced higher sperm counts than those from monkeys not treated with GnRH-ant. Although the quantitative contribution of endogenous vs. transplanted stem cells to this sperm production could not be determined, the presence of lentiviral DNA in most of the samples from hormone suppressed monkeys demonstrates that the increased sperm production must have been derived in part from transplanted cells. Since the stimulation of spermatogenic recovery from donor cells was greater than that from endogenous cells, we conclude that the hormone suppression primarily enhances the homing, colonization, and survival of donor SSC.
It is not clear why the positive signals for the lentiviral DNA in sperm were discontinuous over time. The same phenomenon was also observed after autologous transplantation study of lentivirus-transfected cells to busulfan-treated rhesus monkeys (Hermann et al., 2012). This may have been due to the low labeling efficiency and cyclical release of the sperm originating from a limited number of stem spermatogonia transduced by lentivirus as they self-renewed and differentiated in the tubules.
As indicated in the Results, the GnRH-ant treated monkeys received 48% more potential viable stem cells during the transplantation than did the irradiated-only monkeys, although the numbers varied between animals and were not significantly different between the groups. The recovery of spermatogenesis in transplanted testes of the GnRH-ant treated monkeys compared to the radiation-only ones, indicated by multiple endpoints, appeared to be greater than that which could be contributed by a modest increase in cells transplanted, but we cannot rule out some enhancement due to the greater numbers of functional cells transplanted.
It is useful to analyze the hormone suppression regimen selected in this study; although it was effective in enhancing recovery of spermatogenesis from the transplanted stem cells, changes might produce even greater recovery. Acyline suppressed serum testosterone to 2% of control values while, in the previous studies, Cetrorelix suppressed testosterone levels only to 21% (Kamischke et al., 2003) and 10% (Boekelheide et al., 2005) of controls. Moreover, the previous studies employed much higher doses of GnRH-ant which caused prolonged suppression of testosterone levels after the end of treatment and incomplete recovery of spermatogenesis in an unirradiated monkey (Boekelheide et al., 2005). The initiation of the hormone suppression 8 weeks before transplantation as based on a study in mice indicated that only hormone suppression prior to transplantation induced enhancement of donor-generated spermatogenesis in mice (Dobrinski et al., 2001). However, others found that extending the treatment after transplantation gave slightly greater enhancement (Wang et al., 2010) or that treatment after transplantation was as effective as treatment before transplantation (Ohmura et al., 2003). However, because differentiation of spermatogonia to the B spermatogonial stage in normal monkeys is inhibited when both testosterone and FSH are suppressed by GnRH-ant (Marshall et al., 2005), whereas in rodents hormonal suppression has little effect on premeiotic development, we limited the hormone suppression to the period before transplantation.
Suppression of both testosterone and FSH by using GnRH-ant was employed since that was used in most rodent studies. Since the hormone suppression in this study primarily stimulated recovery from transplanted spermatogonia, efficient homing of these cells to the stem cell niche in the basal region of the epithelium, which involves passage though the tight junctions at the Sertoli cell ("blood-testis") barrier (Kanatsu-Shinohara et al., 2008), may be a critical step. Because androgen suppression increases the permeability of the Sertoli cell barrier (Meng et al., 2005), we suggest that it is androgen suppression that leads to enhanced homing efficiency. However, spermatogenic recovery from transplanted cells involves the processes of stem cell survival, stem-cell proliferation, self-renewal, and differentiation, and it is possible that FSH might actually have a stimulatory role.
The dose of radiation used in the current study is relevant to the human exposures during the radiation therapy. The testicular dose is about 8 Gy is when single-dose total body radiation is given as part of a bone-marrow transplant conditioning regimen for leukemia or Hodgkin’s disease (Anserini et al., 2002; Jacob et al., 1998). The spermatogenic response of the human testis seems similar to that of the monkey as about 10% of these patients, who also received a temporarily sterilizing dose of cyclophosphamide, eventually recovered their sperm count.
Although the present study demonstrates that hormone suppression significantly enhances spermatogenic recovery from transplanted stem spermatogonia in primates, the efficiency of the process is low and must be improved if it is to be clinically effective. Elucidation of the relative roles and mechanisms of testosterone and FSH in the inhibition or stimulation of spermatogenic recovery from donor stem cells after cytotoxic treatment in monkeys will help to develop a better clinical protocol for spermatogenic recovery, perhaps by suppressing only one of the hormones, optimizing suppression time, and/or directly targeting a downstream effector of the hormone action. Further development of the spermatogonial stem cell preparation and technology for transplantation in a clinically relevant nonhuman primate system, along with optimizing hormone suppression, will facilitate addressing issues of safety and feasibility for human applications in the restoration of male fertility after cancer treatment.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by grants HD 061301 from NIH/NICHD to GS, Cancer Center Support Grant CA 16672 from the NIH to MD Anderson Cancer Center, Grant K99/R00 HD062687 from NIH to BPH, and the Florence M. Thomas Professorship in Cancer Research to MLM. We acknowledge the outstanding work of Dana Toomey, who assisted in managing the treatment of the monkeys, sample collections, and animal health issues. We are thankful to Mario Rodriguez for his expert assistance in spermatogonial transplantation in nonhuman primates. We sincerely thank Drs. R.P. Blye, Hyun K. Kim, June Lee, and Min S. Lee of the National Institute for Child Health and Human Development for providing the Acyline. We also thank Kathryn L. Hale for the scientific edition of the manuscript.
Footnotes
DISCLOSURES
The authors have no conflicting financial interests.
AUTHOR CONTRIBUTIONS
GS, KEO & MLM, conception and design; GS, collection of data; RKU, study design, treatment and surgeries; WZ, PCR analysis of semen and testicular samples; SHS, cell preparation and immunostaining; CCW, xenotransplantation and hormone assays; RCT, dosimetry for the testicular radiation of the monkeys. BPH, study design, transplantation of testicular cells into the monkey testes; KEO, transplantation of testicular cells into the monkey testes; MLM & GS analysis of results and preparation of the manuscript.
REFERENCES
- Anserini P, Chiodi S, Spinelli S, Costa M, Conte N, Copello F, Bacigalupo A. Semen analysis following allogeneic bone marrow transplantation. Additional data for evidence-based counselling. Bone Marrow Transplant. 2002;30:447–451. doi: 10.1038/sj.bmt.1703651. [DOI] [PubMed] [Google Scholar]
- Boekelheide K, Schoenfeld H, Hall SJ, Weng CCY, Shetty G, Leith J, Harper J, Sigman M, Hess DL, Meistrich ML. Gonadotropin-releasing hormone antagonist (cetrorelix) therapy fails to protect non-human primates (macaca arctoides) from radiation-induced spermatogenic failure. J Androl. 2005;26:222–234. doi: 10.1002/j.1939-4640.2005.tb01089.x. [DOI] [PubMed] [Google Scholar]
- Brinster RL. Male germline stem cells: from mice to men. Science. 2007;316:404–405. doi: 10.1126/science.1137741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrinski I, Ogawa T, Avarbock MR, Brinster RL. Effect of the GnRH-agonist leuprolide on colonization of recipient testes by donor spermatogonial stem cells after transplantation in mice. Tissue Cell. 2001;33:200–207. doi: 10.1054/tice.2001.0177. [DOI] [PubMed] [Google Scholar]
- Herbst KL. Gonadotropin-releasing hormone antagonists. Curr Opin Pharmacol. 2003;3:660–666. doi: 10.1016/j.coph.2003.06.009. [DOI] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Lin CC, Sheng Y, Tomko J, Rodriguez M, Shuttleworth JJ, McFarland D, Hobbs RM, Pandolfi PP, et al. Characterization, cryopreservation, and ablation of spermatogonial stem cells in adult rhesus macaques. Stem Cells. 2007;25:2330–2338. doi: 10.1634/stemcells.2007-0143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Simorangkir DR, Chu T, Plant TM, Orwig KE. Molecular dissection of the male germ cell lineage identifies putative spermatogonial stem cells in Rhesus macaques. Hum Reprod. 2009;24:1704–1716. doi: 10.1093/humrep/dep073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hermann BP, Sukhwani M, Winkler F, Pascarella JN, Peters KA, Sheng Y, Valli H, Rodriguez M, Ezzelarab M, Dargo G, et al. Spermatogonial stem cell transplantation into rhesus testes regenerates spermatogenesis producing functional sperm. Cell Stem Cell. 2012;11:715–726. doi: 10.1016/j.stem.2012.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honaramooz A, Yang Y. Recent advances in application of male germ cell transplantation in farm animals. Vet Med Int. 2011;Vol. 2011 doi: 10.4061/2011/657860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob A, Barker H, Goodman A, Holmes J. Recovery of spermatogenesis following bone marrow transplantation. Bone Marrow Transplant. 1998;22:277–279. doi: 10.1038/sj.bmt.1701332. [DOI] [PubMed] [Google Scholar]
- Jahnukainen K, Ehmcke J, Quader MA, Saiful Huq M, Epperly MW, Hergenrother S, Nurmio M, Schlatt S. Testicular recovery after irradiation differs in prepubertal and pubertal non-human primates, and can be enhanced by autologous germ cell transplantation. Hum Reprod. 2011;26:1945–1954. doi: 10.1093/humrep/der160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamischke A, Kuhlmann M, Weinbauer GF, Luetjens M, Yeung C-H, Kronholz HL, Nieschlag E. Gonadal protection from radiation by GnRH antagonist or recombinant human FSH: a controlled trial in a male nonhuman primate (Macaca fascicularis) J Endocrinol. 2003;179:183–194. doi: 10.1677/joe.0.1790183. [DOI] [PubMed] [Google Scholar]
- Kanatsu-Shinohara M, Takehashi M, Takashima S, Lee J, Morimoto H, Chuma S, Raducanu A, Nakatsuji N, Fassler R, Shinohara T. Homing of mouse spermatogonial stem cells to germline niche depends on beta1-integrin. Cell Stem Cell. 2008;3:533–542. doi: 10.1016/j.stem.2008.08.002. [DOI] [PubMed] [Google Scholar]
- Kangasniemi M, Huhtaniemi I, Meistrich ML. Failure of spermatogenesis to recover despite the presence of A spermatogonia in the irradiated LBNF1 rat. Biol Reprod. 1996;54:1200–1208. doi: 10.1095/biolreprod54.6.1200. [DOI] [PubMed] [Google Scholar]
- Lois C, Hong EJ, Pease S, Brown EJ, Baltimore D. Germline transmission and tissue-specific expression of transgenes delivered by lentiviral vectors. Science. 2002;295:868–872. doi: 10.1126/science.1067081. [DOI] [PubMed] [Google Scholar]
- Marshall GR, Ramaswamy S, Plant TM. Gonadotropin-independent proliferation of the pale type A spermatogonia in the adult rhesus monkey (Macaca mulatta) Biol Reprod. 2005;73:222–229. doi: 10.1095/biolreprod.104.038968. [DOI] [PubMed] [Google Scholar]
- Masala A, Faedda R, Alagna S, Satta A, Chiarelli G, Rovasio PP, Ivaldi R, Taras MS, Lai E, Bartoli E. Use of testosterone to prevent cyclophosphamide-induced azoospermia. Ann Intern Med. 1997;126:292–295. doi: 10.7326/0003-4819-126-4-199702150-00005. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Kangasniemi M. Hormone treatment after irradiation stimulates recovery of rat spermatogenesis from surviving spermatogonia. J Androl. 1997;18:80–87. [PubMed] [Google Scholar]
- Meistrich ML, Shetty G. Suppression of testosterone stimulates recovery of spermatogenesis after cancer treatment. Int J Androl. 2003;26:141–146. doi: 10.1046/j.1365-2605.2003.00400.x. [DOI] [PubMed] [Google Scholar]
- Meistrich ML, Vassilopoulou-Sellin R, Lipshultz LI. Gonadal dysfunction. In: DeVita VT, Hellman S, Rosenberg SA, editors. Cancer: Principles and Practice of Oncology. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 2560–2574. [Google Scholar]
- Meistrich ML, Wilson G, Huhtaniemi I. Hormonal treatment after cytotoxic therapy stimulates recovery of spermatogenesis. Cancer Res. 1999;59:3557–3560. [PubMed] [Google Scholar]
- Meistrich ML, Wilson G, Shuttlesworth G, Huhtaniemi I, Reissmann T. GnRH agonists and antagonists stimulate recovery of fertility in irradiated LBNF1 rats. J Androl. 2001;22:809–817. [PubMed] [Google Scholar]
- Meng J, Holdcraft RW, Shima JE, Griswold MD, Braun RE. Androgens regulate the permeability of the blood-testis barrier. Proc Natl Acad Sci U S A. 2005;102:16696–16700. doi: 10.1073/pnas.0506084102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Avarbock MR, Brinster RL. Leuprolide, a gonadotropin-releasing hormone agonist, enhances colonization after spermatogonial transplantation into mouse testes. Tissue Cell. 1998;30:583–588. doi: 10.1016/s0040-8166(98)80039-6. [DOI] [PubMed] [Google Scholar]
- Ogawa T, Dobrinski I, Brinster RL. Recipient preparation is critical for spermatogonial transplantation in the rat. Tissue Cell. 1999;31:461–472. doi: 10.1054/tice.1999.0060. [DOI] [PubMed] [Google Scholar]
- Ohmura M, Ogawa T, Ono M, Dezawa M, Hosaka M, Kubota Y, Sawada H. Increment of murine spermatogonial cell number by gonadotropin-releasing hormone analogue is independent of stem cell factor c-kit signal. Biol Reprod. 2003;68:2304–2313. doi: 10.1095/biolreprod.102.013276. [DOI] [PubMed] [Google Scholar]
- Orwig KE, Schlatt S. Cryopreservation and transplantation of spermatogonia and testicular tissue for preservation of male fertility. J Natl Cancer Inst Monogr. 2005;34:51–56. doi: 10.1093/jncimonographs/lgi029. [DOI] [PubMed] [Google Scholar]
- Ramaswamy S, Marshall GR, Pohl CR, Friedman RL, Plant TM. Inhibitory and stimulatory regulation of testicular inhibin B secretion by luteinizing hormone and follicle-stimulating hormone, respectively, in the rhesus monkey (Macaca mulatta) Endocrinology. 2003;144:1175–1185. doi: 10.1210/en.2002-221078. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Sosa JR, Dobrinski I. Recent developments in testis tissue xenografting. Reproduction. 2009;138:187–194. doi: 10.1530/REP-09-0012. [DOI] [PubMed] [Google Scholar]
- Ryu BY, Orwig KE, Oatley JM, Lin CC, Chang LJ, Avarbock MR, Brinster RL. Efficient generation of transgenic rats through the male germline using lentiviral transduction and transplantation of spermatogonial stem cells. J Androl. 2007;28:353–360. doi: 10.2164/jandrol.106.001511. [DOI] [PubMed] [Google Scholar]
- Sato T, Katagiri K, Gohbara A, Inoue K, Ogonuki N, Ogura A, Kubota Y, Ogawa T. In vitro production of functional sperm in cultured neonatal mouse testes. Nature. 2011;471:504–507. doi: 10.1038/nature09850. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Foppiani L, Rolf C, Weinbauer GF, Nieschlag E. Germ cell transplantation into X-irradiated monkey testes. Hum Reprod. 2002;17:55–62. doi: 10.1093/humrep/17.1.55. [DOI] [PubMed] [Google Scholar]
- Shetty G, Meistrich ML. Hormonal approaches to preservation and restoration of male fertility after cancer treatment. J Natl Cancer Inst Monogr. 2005;34:36–39. doi: 10.1093/jncimonographs/lgi002. [DOI] [PubMed] [Google Scholar]
- Shetty G, Porter KL, Zhou W, Shao SH, Weng CC, Meistrich ML. Androgen suppression-induced stimulation of spermatogonial differentiation in juvenile spermatogonial depletion mice acts by elevating the testicular temperature. Endocrinology. 2011;152:3504–3514. doi: 10.1210/en.2011-0251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty G, Weng CC, Meachem SJ, Bolden-Tiller OU, Zhang Z, Pakarinen P, Huhtaniemi I, Meistrich ML. Both testosterone and FSH independently inhibit spermatogonial differentiation in irradiated rats. Endocrinology. 2006;147:472–482. doi: 10.1210/en.2005-0984. [DOI] [PubMed] [Google Scholar]
- Shetty G, Wilson G, Huhtaniemi I, Shuttlesworth GA, Reissmann T, Meistrich ML. Gonadotropin-releasing hormone analogs stimulate and testosterone inhibits the recovery of spermatogenesis in irradiated rats. Endocrinology. 2000;141:1735–1745. doi: 10.1210/endo.141.5.7446. [DOI] [PubMed] [Google Scholar]
- Thomson AB, Critchley HO, Kelnar CJ, Wallace WH. Late reproductive sequelae following treatment of childhood cancer and options for fertility preservation. Best Pract Res Clin Endocrinol Metab. 2002;16:311–334. doi: 10.1053/beem.2002.0200. [DOI] [PubMed] [Google Scholar]
- Udagawa K, Ogawa T, Watanabe T, Yumura Y, Takeda M, Hosaka M. GnRH analog, leuprorelin acetate, promotes regeneration of rat spermatogenesis after severe chemical damage. Int J Urol. 2001;8:615–622. doi: 10.1046/j.1442-2042.2001.00382.x. [DOI] [PubMed] [Google Scholar]
- Velez de la Calle JF, Jegou B. Protection by steroid contraceptives against procarbazine-induced sterility and genotoxicity in male rats. Cancer Res. 1990;50:1308–1315. [PubMed] [Google Scholar]
- Wang G, Shao SH, Weng CC, Wei C, Meistrich ML. Hormonal suppression restores fertility in irradiated mice from both endogenous and donor-derived stem spermatogonia. Toxicol Sci. 2010;117:225–237. doi: 10.1093/toxsci/kfq191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Renfree MB, Short RV. Successful intra- and interspecific male germ cell transplantation in the rat. Biol Reprod. 2003;68:961–967. doi: 10.1095/biolreprod.102.009480. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Meistrich M. The radiation-induced block in spermatogonial differentiation is due to damage to the somatic environment, not the germ cells. J Cell Physiol. 2007;211:149–158. doi: 10.1002/jcp.20910. [DOI] [PubMed] [Google Scholar]
- Zhang Z, Shao S, Meistrich ML. Irradiated mouse testes efficiently support spermatogenesis derived from donor germ cells of mice and rats. J Androl. 2006;27:365–375. doi: 10.2164/jandrol.05179. [DOI] [PubMed] [Google Scholar]
- Zheng N, Monckton DG, Wilson G, Hagemeister F, Chakraborty R, Connor TH, Siciliano MJ, Meistrich ML. Frequency of minisatellite repeat number changes at the MS205 locus in human sperm before and after cancer chemotherapy. Environ Mol Mutagen. 2000;36:134–145. doi: 10.1002/1098-2280(2000)36:2<134::aid-em8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.