Abstract
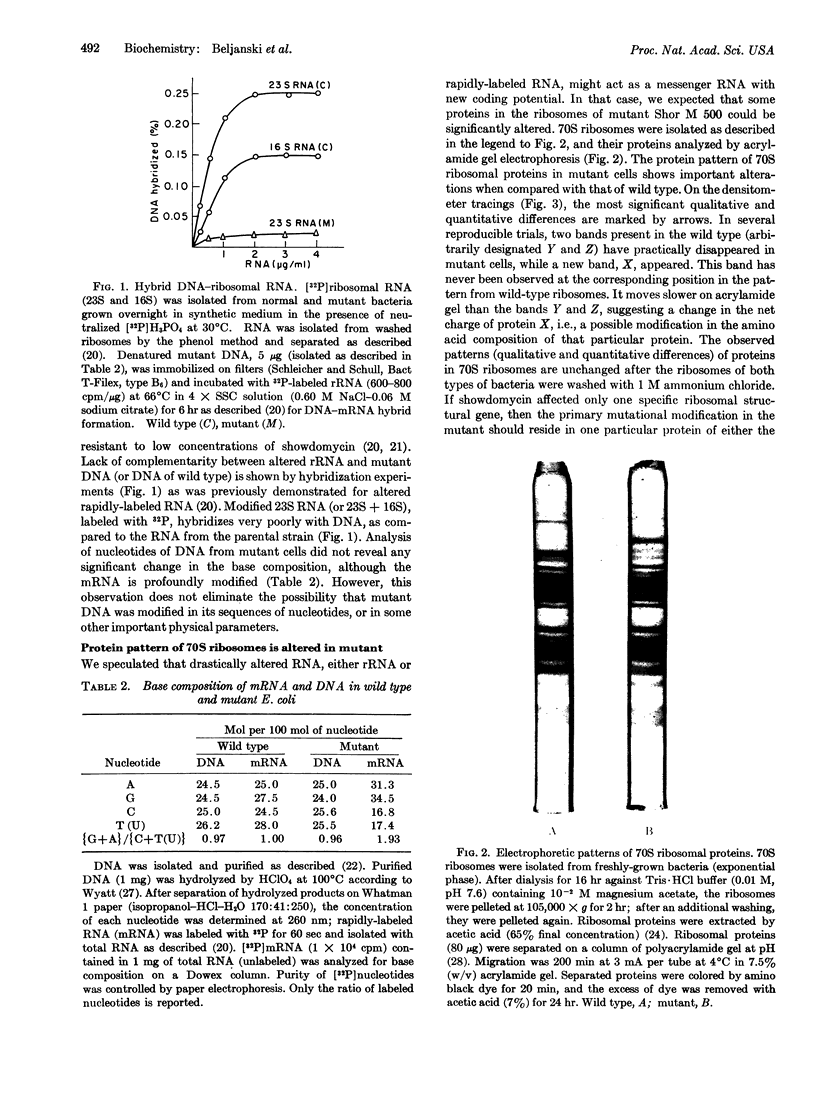
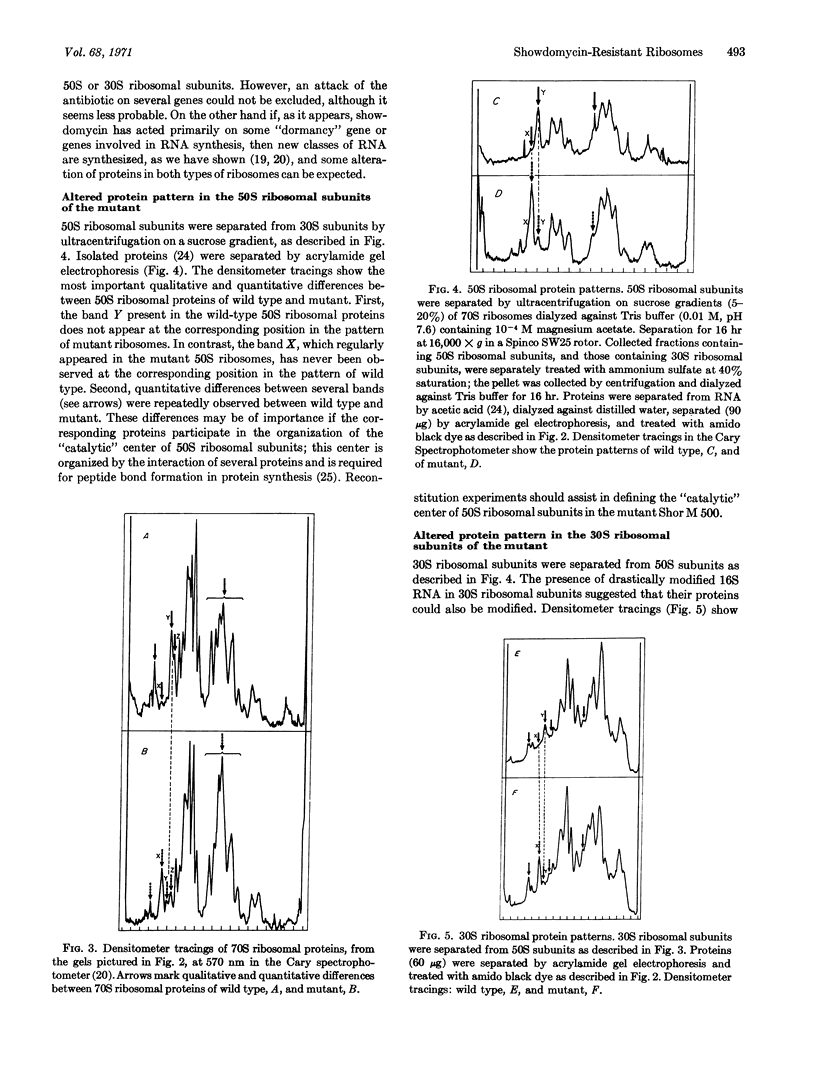
In a mutant of Escherichia coli resistant to showdomycin, both the 50S and 30S ribosomal subunits contain RNA species in which the purine concentration greatly exceeds that of pyrimidines. The same is true for total rapidly-labeled RNA. The modified ribosomal RNA hybridizes poorly with homologous DNA, which is apparently unchanged in base composition. Acrylamide gel electrophoresis of mutant ribosomal proteins shows a highly altered protein pattern for both ribosomal subunits, although the activity of these ribosomes is not decreased.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apirion D. Three genes that affect Escherichia coli ribosomes. J Mol Biol. 1967 Dec 14;30(2):255–275. [PubMed] [Google Scholar]
- Beljanski M., Beljanski M. Synthèse dans Escherichia coli des ARN dont la structure primaire diffère totalement de celle de l'ADN. C R Acad Sci Hebd Seances Acad Sci D. 1968 Sep 16;267(12):1058–1060. [PubMed] [Google Scholar]
- Beljanski M., Bourgarel P., Beljanski M. Showdomycine etiosynthèse d'ARN non complémentaires de l'ADN. I. Ann Inst Pasteur (Paris) 1970 Mar;118(3):253–276. [PubMed] [Google Scholar]
- Beljanski M., Bourgarel P., Chassagne J. Synthèse chez les bactéries d'ARN nouveaux n'étant pas la copie de l'ADN. C R Acad Sci Hebd Seances Acad Sci D. 1969 Jul 16;269(2):240–243. [PubMed] [Google Scholar]
- Bollen A., Davies J., Ozaki M., Mizushima S. Ribosomal Protein Conferring Sensitivity to the Antibiotic Spectinomycin in Escherichia coli. Science. 1969 Jul 4;165(3888):85–86. doi: 10.1126/science.165.3888.85. [DOI] [PubMed] [Google Scholar]
- Darnall K. R., Townsend L. B., Robins R. K. The structure of showdomycin, a novel carbon-linked nucleoside antibiotic related to uridine. Proc Natl Acad Sci U S A. 1967 Mar;57(3):548–553. doi: 10.1073/pnas.57.3.548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOROWITZ J., LOMBARD A., CHARGAFF E. Aspects of the stability of a bacterial ribonucleic acid. J Biol Chem. 1958 Dec;233(6):1517–1522. [PubMed] [Google Scholar]
- Krembel J., Apirion D. Changes in ribosomal proteins associated with mutants in a locus that affects Escherichia coli ribosomes. J Mol Biol. 1968 Apr 28;33(2):363–368. doi: 10.1016/0022-2836(68)90194-0. [DOI] [PubMed] [Google Scholar]
- LEBOY P. S., COX E. C., FLAKS J. G. THE CHROMOSOMAL SITE SPECIFYING A RIBOSOMAL PROTEIN IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1964 Dec;52:1367–1374. doi: 10.1073/pnas.52.6.1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Lowry C. V. PHAGE f2 RNA-DIRECTED BINDING OF FORMYLMETHIONYL-TRNA TO RIBOSOMES AND THE ROLE OF 30S RIBOSOMAL SUBUNITS IN INITIATION OF PROTEIN SYNTHESIS. Proc Natl Acad Sci U S A. 1967 Sep;58(3):946–953. doi: 10.1073/pnas.58.3.946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Mizushima S., Ozaki M., Traub P., Lowry C. V. Structure and function of ribosomes and their molecular components. Cold Spring Harb Symp Quant Biol. 1969;34:49–61. doi: 10.1101/sqb.1969.034.01.009. [DOI] [PubMed] [Google Scholar]
- Ozaki M., Mizushima S., Nomura M. Identification and functional characterization of the protein controlled by the streptomycin-resistant locus in E. coli. Nature. 1969 Apr 26;222(5191):333–339. doi: 10.1038/222333a0. [DOI] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SPOTTS C. R., STANIER R. Y. Mechanism of streptomycin action on bacteria: a unitary hypothesis. Nature. 1961 Nov 18;192:633–637. doi: 10.1038/192633a0. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Maglott D. M., Monro R. E. On the catalytic center of peptidyl transfer: a part of the 50 S ribosome structure. Cold Spring Harb Symp Quant Biol. 1969;34:39–48. doi: 10.1101/sqb.1969.034.01.008. [DOI] [PubMed] [Google Scholar]
- Staehelin T., Meselson M. In vitro recovery o ribosomes and of synthetic activity from synthetically inactive ribosomal subunits. J Mol Biol. 1966 Mar;16(1):245–249. doi: 10.1016/s0022-2836(66)80277-2. [DOI] [PubMed] [Google Scholar]
- Sypherd P. S., O'Neil D. M., Taylor M. M. The chemical and genetic structure of bacterial ribosomes. Cold Spring Harb Symp Quant Biol. 1969;34:77–84. doi: 10.1101/sqb.1969.034.01.012. [DOI] [PubMed] [Google Scholar]
- Tanaka K., Teraoka H., Tamaki M., Otaka E., Osawa S. Erythromycin-resistant mutant of Escherichia coli with altered ribosomal protein component. Science. 1968 Nov 1;162(3853):576–578. doi: 10.1126/science.162.3853.576. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Streptomycin resistance mutation in Escherichia coli: altered ribosomal protein. Science. 1968 Apr 12;160(3824):198–199. doi: 10.1126/science.160.3824.198. [DOI] [PubMed] [Google Scholar]
- Traub P., Nomura M. Structure and function of E. coli ribosomes. V. Reconstitution of functionally active 30S ribosomal particles from RNA and proteins. Proc Natl Acad Sci U S A. 1968 Mar;59(3):777–784. doi: 10.1073/pnas.59.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WALLER J. P., HARRIS J. I. Studies on the composition of the protein from Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1961 Jan 15;47:18–23. doi: 10.1073/pnas.47.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]