Abstract
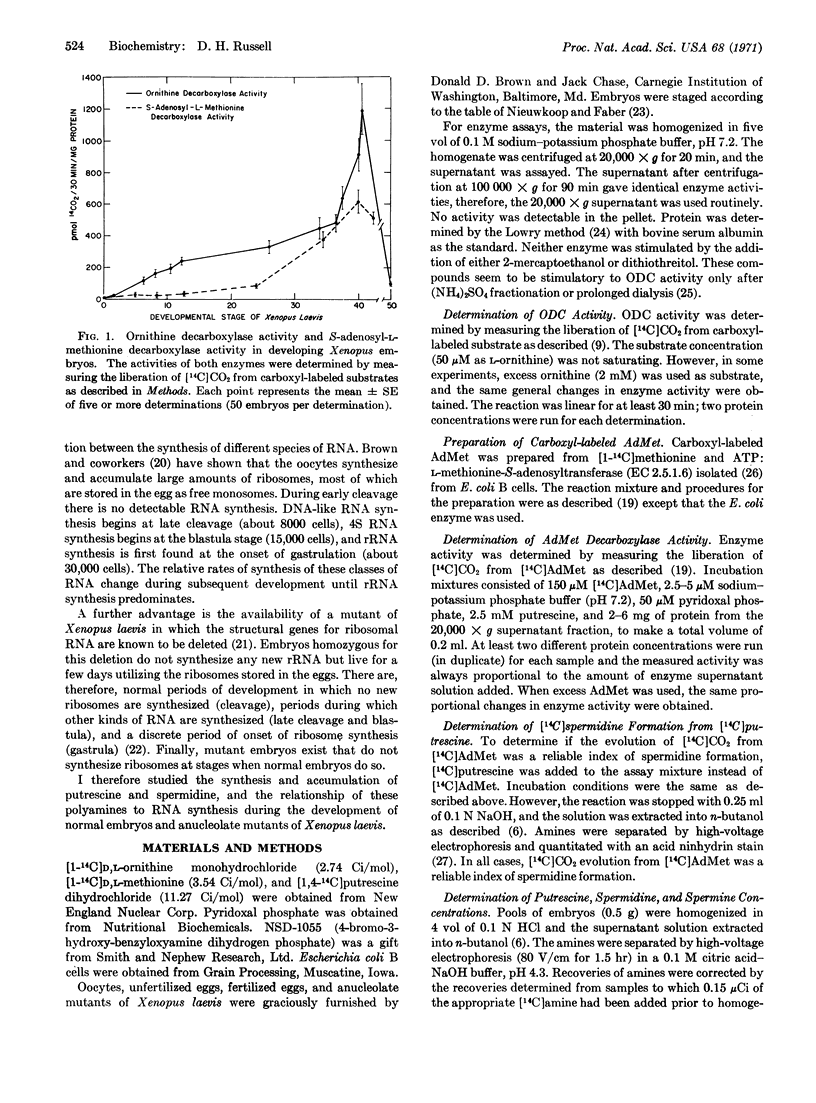
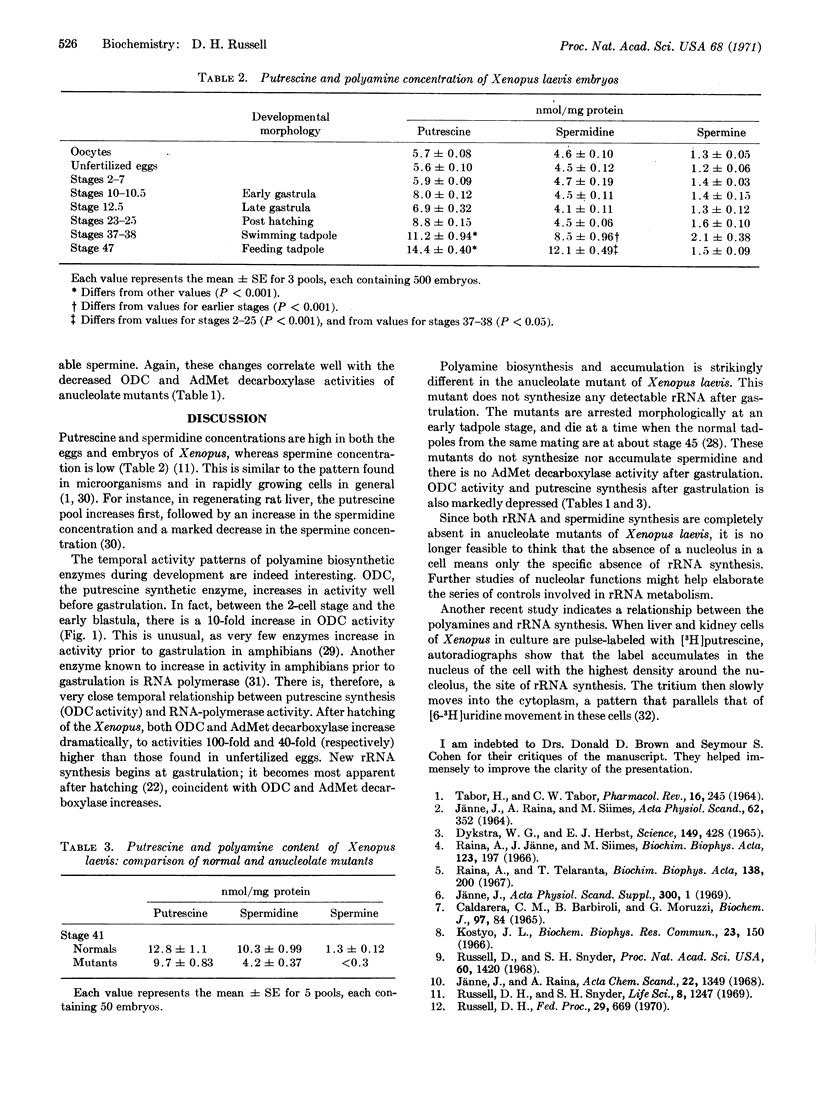
Ornithine decarboxylase (EC 4.1.1.17), the enzyme that catalyzes the synthesis of putrescine from ornithine, increases dramatically in developing Xenopus embryos. Between the 2-cell stage and early blastula stage, activity increases 10-fold, and in swimming tadpoles, the enzyme activity is 100-fold higher than that present in either unfertilized eggs or 2-cell embryos. S-adenosyl-L-methionine decarboxylase, an enzyme that catalyzes spermidine synthesis from putrescine and S-adenosyl-L-methionine, increases 40-fold in activity during the development of Xenopus, but does not increase in activity prior to gastrulation. Concomitant with these enzyme changes, putrescine and spermidine concentrations are elevated during the development of Xenopus embryos. Maximal accumulations are present in the swimming tadpole and correspond to maximal enzyme activities. Anucleolate-mutant embryos of Xenopus, which do not synthesize new ribosomes, have no detectable S-adenosyl-L-methionine decarboxylase activity and do not accumulate spermidine after gastrulation. Ornithine decarboxylase activity is depressed in these mutants and putrescine accumulation is decreased also. The activity of some dehydrogenases that increase in Xenopus embryos after gastrulation show normal increases in the anucleolate mutants. Thus, the synthesis of putrescine and spermidine in embryos correlates with the onset of ribosomal-RNA synthesis and the formation of a viable nucleolus in the embryonic cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abraham K. A. Studies on DNA-dependent RNA polymerase from Escherichia coli. 1. The mechanism of polyamine induced stimulation of enzyme activity. Eur J Biochem. 1968 Jun;5(1):143–146. doi: 10.1111/j.1432-1033.1968.tb00348.x. [DOI] [PubMed] [Google Scholar]
- BROWN D. D., GURDON J. B. ABSENCE OF RIBOSOMAL RNA SYNTHESIS IN THE ANUCLEOLATE MUTANT OF XENOPUS LAEVIS. Proc Natl Acad Sci U S A. 1964 Jan;51:139–146. doi: 10.1073/pnas.51.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarera C. M., Barbiroli B., Moruzzi G. Polyamines and nucleic acids during development of the chick embryo. Biochem J. 1965 Oct;97(1):84–88. doi: 10.1042/bj0970084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldarera C. M., Moruzzi M. S., Barbiroli B., Moruzzi G. Spermine and spermidine of the prostate gland of orchiectomized rats and their effect on RNA polymerase activity. Biochem Biophys Res Commun. 1968 Oct 24;33(2):266–271. doi: 10.1016/0006-291x(68)90779-1. [DOI] [PubMed] [Google Scholar]
- Dykstra W. G., Jr, Herbst E. J. Spermidine in Regenerating Liver: Relation to Rapid Synthesis of Ribonucleic Acid. Science. 1965 Jul 23;149(3682):428–429. doi: 10.1126/science.149.3682.428. [DOI] [PubMed] [Google Scholar]
- ELSDALE T. R., FISCHBERG M., SMITH S. A mutation that reduces nucleolar number in Xenopus laevis. Exp Cell Res. 1958 Jun;14(3):642–643. doi: 10.1016/0014-4827(58)90175-7. [DOI] [PubMed] [Google Scholar]
- HEILMANN J., BARROLLIER J., WATZKE E. Beitrag zur Aminosäurebestimmung auf Papierchromatogrammen. Hoppe Seylers Z Physiol Chem. 1957;309(4-6):219–220. [PubMed] [Google Scholar]
- JAENNE J., RAINA A., SIIMES M. SPERMIDINE AND SPERMINE IN RAT TISSUES AT DIFFERENT AGES. Acta Physiol Scand. 1964 Dec;62:352–358. doi: 10.1111/j.1748-1716.1964.tb10433.x. [DOI] [PubMed] [Google Scholar]
- Jänne J., Raina A. Stimulation of spermidine synthesis in the regenerating rat liver: relation to increased ornithine decarboxylase activity. Acta Chem Scand. 1968;22(4):1349–1351. doi: 10.3891/acta.chem.scand.22-1349. [DOI] [PubMed] [Google Scholar]
- Jänne J. Studies on the biosynthetic pathway of polyamines in rat liver. Acta Physiol Scand Suppl. 1967;300:1–71. [PubMed] [Google Scholar]
- Jänne J., Williams-Ashman H. G. Mammalian ornithine decarboxylase: activation and alteration of physical behaviour by thiol compounds. Biochem J. 1970 Sep;119(3):595–597. doi: 10.1042/bj1190595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostyo J. L. Changes in polyamine content of rat liver following hypophysectomy and treatment with growth hormone. Biochem Biophys Res Commun. 1966 Apr 19;23(2):150–155. doi: 10.1016/0006-291x(66)90520-1. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lievens A., Brachet J. RNA polymerase activity during development of normal and hybrid amphibian embryos. Biochim Biophys Acta. 1970 Mar 19;204(1):263–266. doi: 10.1016/0005-2787(70)90513-7. [DOI] [PubMed] [Google Scholar]
- Pegg A. E., Williams-Ashman H. G. On the role of S-adenosyl-L-methionine in the biosynthesis of spermidine by rat prostate. J Biol Chem. 1969 Feb 25;244(4):682–693. [PubMed] [Google Scholar]
- Raina A., Jänne J., Siimes M. Stimulation of polyamine synthesis in relation to nucleic acids in regenerating rat liver. Biochim Biophys Acta. 1966 Jul 20;123(1):197–201. doi: 10.1016/0005-2787(66)90173-0. [DOI] [PubMed] [Google Scholar]
- Raina A., Telaranta T. Association of polyamines and RNA in isolated subcellular particles from rat liver. Biochim Biophys Acta. 1967 Mar 29;138(1):200–203. doi: 10.1016/0005-2787(67)90604-1. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Medina V. J., Snyder S. H. The dynamics of synthesis and degradation of polyamines in normal and regenerating rat liver and brain. J Biol Chem. 1970 Dec 25;245(24):6732–6738. [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: effect of hypophysectomy and growth hormone on ornithine decarboxylase. Endocrinology. 1969 Feb;84(2):223–228. doi: 10.1210/endo-84-2-223. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H. Amine synthesis in regenerating rat liver: extremely rapid turnover of ornithine decarboxylase. Mol Pharmacol. 1969 May;5(3):253–262. [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H., Medina V. J. Growth hormone induction of ornithine decarboxylase in rat liver. Endocrinology. 1970 Jun;86(6):1414–1419. doi: 10.1210/endo-86-6-1414. [DOI] [PubMed] [Google Scholar]
- Russell D. H., Snyder S. H., Medina V. J. Presence and biosynthesis of putrescine and polyamines in amphibians. Life Sci. 1969 Dec 15;8(24):1247–1254. doi: 10.1016/0024-3205(69)90181-7. [DOI] [PubMed] [Google Scholar]
- Russell D., Snyder S. H. Amine synthesis in rapidly growing tissues: ornithine decarboxylase activity in regenerating rat liver, chick embryo, and various tumors. Proc Natl Acad Sci U S A. 1968 Aug;60(4):1420–1427. doi: 10.1073/pnas.60.4.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TABOR H., TABOR C. W. SPERMIDINE, SPERMINE, AND RELATED AMINES. Pharmacol Rev. 1964 Sep;16:245–300. [PubMed] [Google Scholar]
- Wallace H., Birnstiel M. L. Ribosomal cistrons and the nucleolar organizer. Biochim Biophys Acta. 1966 Feb 21;114(2):296–310. doi: 10.1016/0005-2787(66)90311-x. [DOI] [PubMed] [Google Scholar]


