Abstract
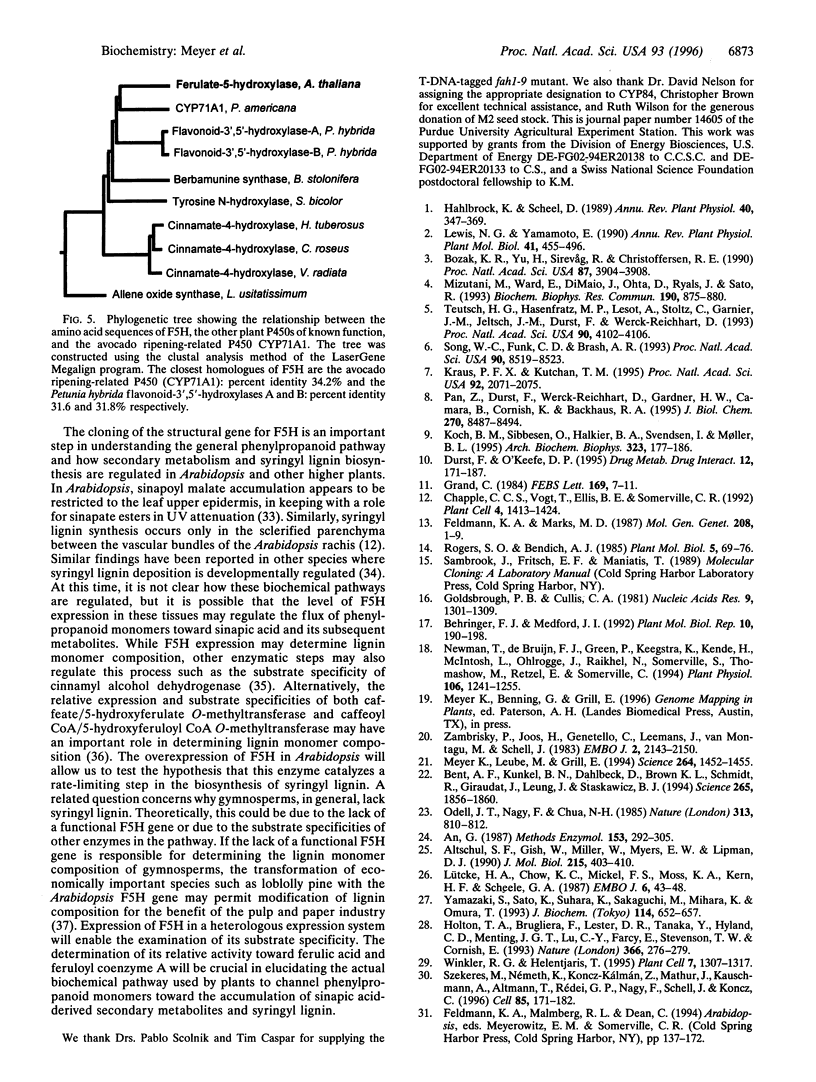
The fah1 mutant of Arabidopsis is defective in the accumulation of sinapic acid-derived metabolites, including the guaiacyl-syringyl lignin typical of angiosperms. Earlier results indicated that the FAH1 locus encodes ferulate-5-hydroxylase (F5H), a cytochrome P450-dependent monooxygenase (P450) of the general phenylpropanoid pathway. We have cloned the gene encoding this P450 by T-DNA tagging and have confirmed the identity of the cloned gene by complementation of the mutant phenotype. F5H shows 34% amino acid sequence identity with the avocado ripening-induced P450 CYP71A1 and 32% identity with the flavonoid-3',5'-hydroxylases of Petunia hybrida. In contrast, it shares much less homology with cinnamate-4-hydroxylase, a P450 that catalyzes the hydroxylation of cinnamic acid three steps earlier in the general phenylpropanoid pathway. Since the highest degree of identity between F5H and previously sequenced P450s is only 34%, F5H identifies a new P450 subfamily that has been designated CYP84.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Bent A. F., Kunkel B. N., Dahlbeck D., Brown K. L., Schmidt R., Giraudat J., Leung J., Staskawicz B. J. RPS2 of Arabidopsis thaliana: a leucine-rich repeat class of plant disease resistance genes. Science. 1994 Sep 23;265(5180):1856–1860. doi: 10.1126/science.8091210. [DOI] [PubMed] [Google Scholar]
- Bozak K. R., Yu H., Sirevåg R., Christoffersen R. E. Sequence analysis of ripening-related cytochrome P-450 cDNAs from avocado fruit. Proc Natl Acad Sci U S A. 1990 May;87(10):3904–3908. doi: 10.1073/pnas.87.10.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapple C. C., Vogt T., Ellis B. E., Somerville C. R. An Arabidopsis mutant defective in the general phenylpropanoid pathway. Plant Cell. 1992 Nov;4(11):1413–1424. doi: 10.1105/tpc.4.11.1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durst F., O'Keefe D. P. Plant cytochromes P450: an overview. Drug Metabol Drug Interact. 1995;12(3-4):171–187. doi: 10.1515/dmdi.1995.12.3-4.171. [DOI] [PubMed] [Google Scholar]
- Goldsbrough P. B., Cullis C. A. Characterisation of the genes for ribosomal RNA in flax. Nucleic Acids Res. 1981 Mar 25;9(6):1301–1309. doi: 10.1093/nar/9.6.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holton T. A., Brugliera F., Lester D. R., Tanaka Y., Hyland C. D., Menting J. G., Lu C. Y., Farcy E., Stevenson T. W., Cornish E. C. Cloning and expression of cytochrome P450 genes controlling flower colour. Nature. 1993 Nov 18;366(6452):276–279. doi: 10.1038/366276a0. [DOI] [PubMed] [Google Scholar]
- Koch B. M., Sibbesen O., Halkier B. A., Svendsen I., Møller B. L. The primary sequence of cytochrome P450tyr, the multifunctional N-hydroxylase catalyzing the conversion of L-tyrosine to p-hydroxyphenylacetaldehyde oxime in the biosynthesis of the cyanogenic glucoside dhurrin in Sorghum bicolor (L.) Moench. Arch Biochem Biophys. 1995 Oct 20;323(1):177–186. doi: 10.1006/abbi.1995.0024. [DOI] [PubMed] [Google Scholar]
- Kraus P. F., Kutchan T. M. Molecular cloning and heterologous expression of a cDNA encoding berbamunine synthase, a C--O phenol-coupling cytochrome P450 from the higher plant Berberis stolonifera. Proc Natl Acad Sci U S A. 1995 Mar 14;92(6):2071–2075. doi: 10.1073/pnas.92.6.2071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landry L. G., Chapple C. C., Last R. L. Arabidopsis mutants lacking phenolic sunscreens exhibit enhanced ultraviolet-B injury and oxidative damage. Plant Physiol. 1995 Dec;109(4):1159–1166. doi: 10.1104/pp.109.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis N. G., Yamamoto E. Lignin: occurrence, biogenesis and biodegradation. Annu Rev Plant Physiol Plant Mol Biol. 1990;41:455–496. doi: 10.1146/annurev.pp.41.060190.002323. [DOI] [PubMed] [Google Scholar]
- Lütcke H. A., Chow K. C., Mickel F. S., Moss K. A., Kern H. F., Scheele G. A. Selection of AUG initiation codons differs in plants and animals. EMBO J. 1987 Jan;6(1):43–48. doi: 10.1002/j.1460-2075.1987.tb04716.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer K., Leube M. P., Grill E. A protein phosphatase 2C involved in ABA signal transduction in Arabidopsis thaliana. Science. 1994 Jun 3;264(5164):1452–1455. doi: 10.1126/science.8197457. [DOI] [PubMed] [Google Scholar]
- Mizutani M., Ward E., DiMaio J., Ohta D., Ryals J., Sato R. Molecular cloning and sequencing of a cDNA encoding mung bean cytochrome P450 (P450C4H) possessing cinnamate 4-hydroxylase activity. Biochem Biophys Res Commun. 1993 Feb 15;190(3):875–880. doi: 10.1006/bbrc.1993.1130. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Newman T., de Bruijn F. J., Green P., Keegstra K., Kende H., McIntosh L., Ohlrogge J., Raikhel N., Somerville S., Thomashow M. Genes galore: a summary of methods for accessing results from large-scale partial sequencing of anonymous Arabidopsis cDNA clones. Plant Physiol. 1994 Dec;106(4):1241–1255. doi: 10.1104/pp.106.4.1241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell J. T., Nagy F., Chua N. H. Identification of DNA sequences required for activity of the cauliflower mosaic virus 35S promoter. 1985 Feb 28-Mar 6Nature. 313(6005):810–812. doi: 10.1038/313810a0. [DOI] [PubMed] [Google Scholar]
- Pan Z., Durst F., Werck-Reichhart D., Gardner H. W., Camara B., Cornish K., Backhaus R. A. The major protein of guayule rubber particles is a cytochrome P450. Characterization based on cDNA cloning and spectroscopic analysis of the solubilized enzyme and its reaction products. J Biol Chem. 1995 Apr 14;270(15):8487–8494. doi: 10.1074/jbc.270.15.8487. [DOI] [PubMed] [Google Scholar]
- Song W. C., Funk C. D., Brash A. R. Molecular cloning of an allene oxide synthase: a cytochrome P450 specialized for the metabolism of fatty acid hydroperoxides. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8519–8523. doi: 10.1073/pnas.90.18.8519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szekeres M., Németh K., Koncz-Kálmán Z., Mathur J., Kauschmann A., Altmann T., Rédei G. P., Nagy F., Schell J., Koncz C. Brassinosteroids rescue the deficiency of CYP90, a cytochrome P450, controlling cell elongation and de-etiolation in Arabidopsis. Cell. 1996 Apr 19;85(2):171–182. doi: 10.1016/s0092-8674(00)81094-6. [DOI] [PubMed] [Google Scholar]
- Teutsch H. G., Hasenfratz M. P., Lesot A., Stoltz C., Garnier J. M., Jeltsch J. M., Durst F., Werck-Reichhart D. Isolation and sequence of a cDNA encoding the Jerusalem artichoke cinnamate 4-hydroxylase, a major plant cytochrome P450 involved in the general phenylpropanoid pathway. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4102–4106. doi: 10.1073/pnas.90.9.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetten R., Sederoff R. Lignin Biosynthesis. Plant Cell. 1995 Jul;7(7):1001–1013. doi: 10.1105/tpc.7.7.1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkler R. G., Helentjaris T. The maize Dwarf3 gene encodes a cytochrome P450-mediated early step in Gibberellin biosynthesis. Plant Cell. 1995 Aug;7(8):1307–1317. doi: 10.1105/tpc.7.8.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S., Sato K., Suhara K., Sakaguchi M., Mihara K., Omura T. Importance of the proline-rich region following signal-anchor sequence in the formation of correct conformation of microsomal cytochrome P-450s. J Biochem. 1993 Nov;114(5):652–657. doi: 10.1093/oxfordjournals.jbchem.a124232. [DOI] [PubMed] [Google Scholar]
- Ye Z. H., Kneusel R. E., Matern U., Varner J. E. An alternative methylation pathway in lignin biosynthesis in Zinnia. Plant Cell. 1994 Oct;6(10):1427–1439. doi: 10.1105/tpc.6.10.1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zambryski P., Joos H., Genetello C., Leemans J., Montagu M. V., Schell J. Ti plasmid vector for the introduction of DNA into plant cells without alteration of their normal regeneration capacity. EMBO J. 1983;2(12):2143–2150. doi: 10.1002/j.1460-2075.1983.tb01715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]