Abstract
Purified yeast phenylalanyl-tRNA synthetase can aminoacylate (yeast) tRNAPhe, (wheat) tRNAPhe, and (Escherichia coli) tRNA1Val (1, 2). We now report that this synthetase can also aminoacylate (E. coli) tRNAPhe and (E. coli) tRNA1Ala. Highly purified (E. coli) tRNAPhe is heterologously aminoacylated to approximately 90% of the extent achieved with the homologous enzyme (crude E. coli phenylalanyl-tRNA synthetase). Pure (E. coli) tRNA1Ala (the major species) is heterologously aminoacylated to 70% of the extent achieved with the homologous synthetase (crude E. coli alanyl-tRNA synthetase).
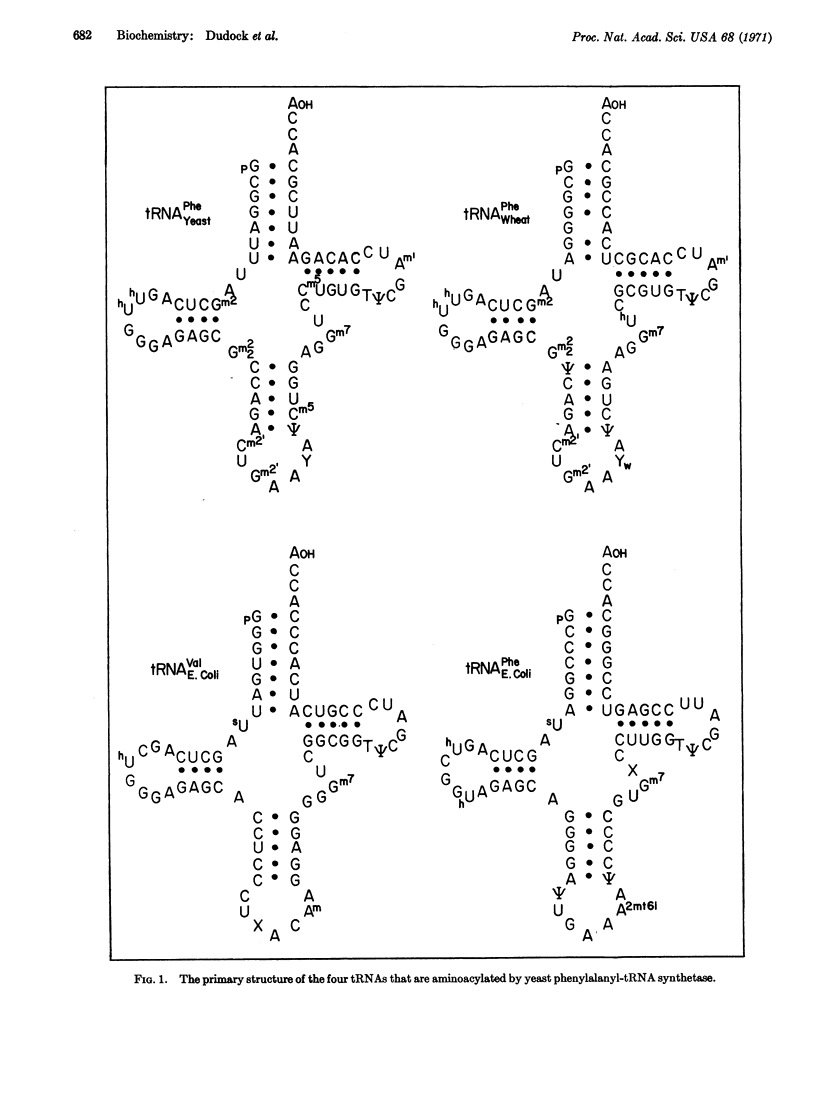
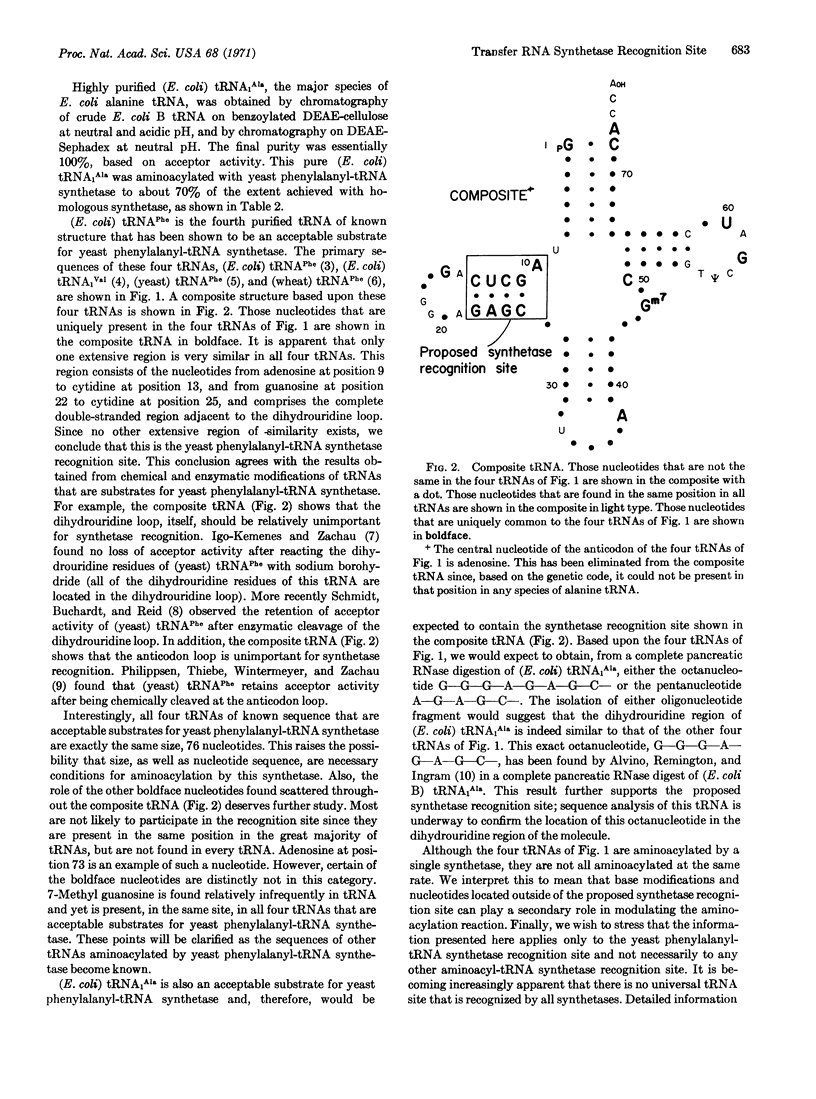
(E. coli) tRNAPhe is the fourth purified transfer RNA of known sequence to be shown to be an acceptable substrate for purified yeast phenylalanyl-tRNA synthetase. A comparison of these sequences shows that only one region is extremely similar in all four tRNAs. This region is located adjacent to the dihydrouridine loop, and consists of the nucleotides [Formula: see text] We conclude that this is the synthetase recognition site for yeast phenylalanyl-tRNA synthetase.
This conclusion is further supported by partial fragment analysis of (E. coli) tRNA1Ala.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvino C. G., Remington L., Ingram V. M. Chemical studies on amino acid acceptor ribonucleic acids. 8. Degradation of purified alanine Escherichia coli B transfer ribonucleic acid by pancreatic ribonuclease. Biochemistry. 1969 Jan;8(1):282–288. doi: 10.1021/bi00829a040. [DOI] [PubMed] [Google Scholar]
- Barrell B. G., Sanger F. The sequence of phenylalanine tRNA from E. coli. FEBS Lett. 1969 Jun;3(4):275–278. doi: 10.1016/0014-5793(69)80157-2. [DOI] [PubMed] [Google Scholar]
- Dudock B. S., DiPeri C., Michael M. S. On the nature of the yeast phenylalanine tran- sfer ribonucleic acid synthetase recognition site. J Biol Chem. 1970 May 10;245(9):2465–2468. [PubMed] [Google Scholar]
- Dudock B. S., Katz G., Taylor E. K., Holley R. W. Primary structure of wheat germ phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1969 Mar;62(3):941–945. doi: 10.1073/pnas.62.3.941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igo-Kemenes T., Zachau H. G. On the specificity of the reduction of transfer ribonucleic acids with sodium borohydride. Eur J Biochem. 1969 Oct;10(3):549–556. doi: 10.1111/j.1432-1033.1969.tb00723.x. [DOI] [PubMed] [Google Scholar]
- Philippsen P., Thiebe R., Wintermeyer W., Zachau H. G. Splitting of phenylalanine specific tRNA into half molecules by chemical means. Biochem Biophys Res Commun. 1968 Dec 30;33(6):922–928. doi: 10.1016/0006-291x(68)90400-2. [DOI] [PubMed] [Google Scholar]
- Rajbhandary U. L., Chang S. H., Stuart A., Faulkner R. D., Hoskinson R. M., Khorana H. G. Studies on polynucleotides, lxviii the primary structure of yeast phenylalanine transfer RNA. Proc Natl Acad Sci U S A. 1967 Mar;57(3):751–758. doi: 10.1073/pnas.57.3.751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt J., Buchardt B., Reid B. R. Effect of cleaving the dihydrouridine loop and the ribothymidine loop on the amino acid acceptor activity of yeast phenylalanine transfer ribonucleic acid. J Biol Chem. 1970 Nov 10;245(21):5743–5750. [PubMed] [Google Scholar]
- Taglang R., Waller J. P., Befort N., Fasiolo F. Amino-acylation du tRNA-1-Val de Escherichia coli par la phénylalanyl-tRNA synthétase de levure. Eur J Biochem. 1970 Feb;12(3):550–557. doi: 10.1111/j.1432-1033.1970.tb00886.x. [DOI] [PubMed] [Google Scholar]
- Yaniv M., Barrell B. G. Nucleotide sequence of E. coli B tRNA1-Val. Nature. 1969 Apr 19;222(5190):278–279. doi: 10.1038/222278a0. [DOI] [PubMed] [Google Scholar]


