Abstract
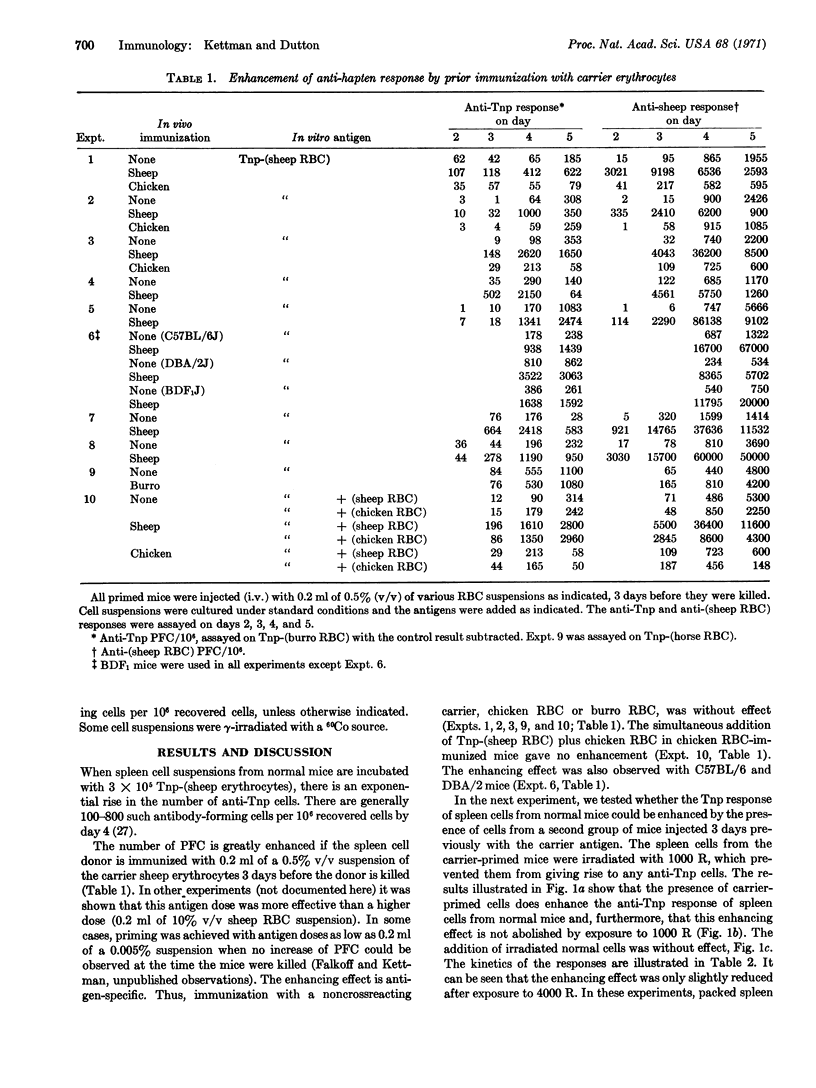
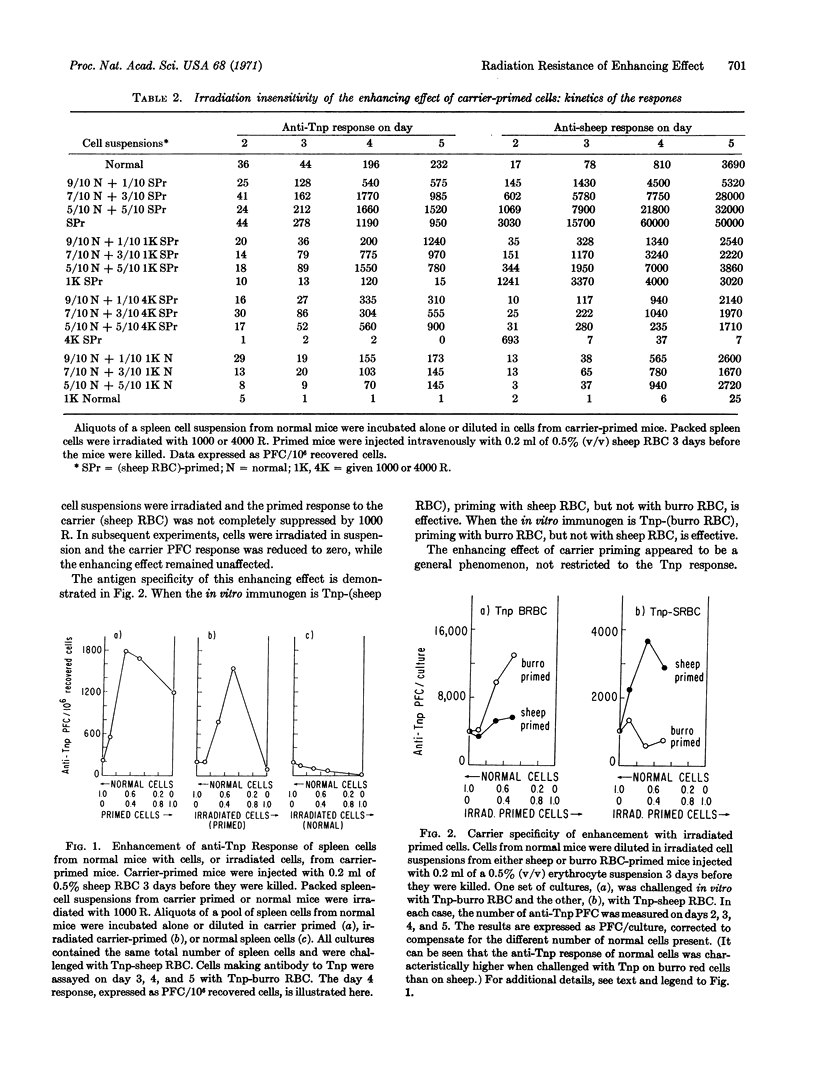
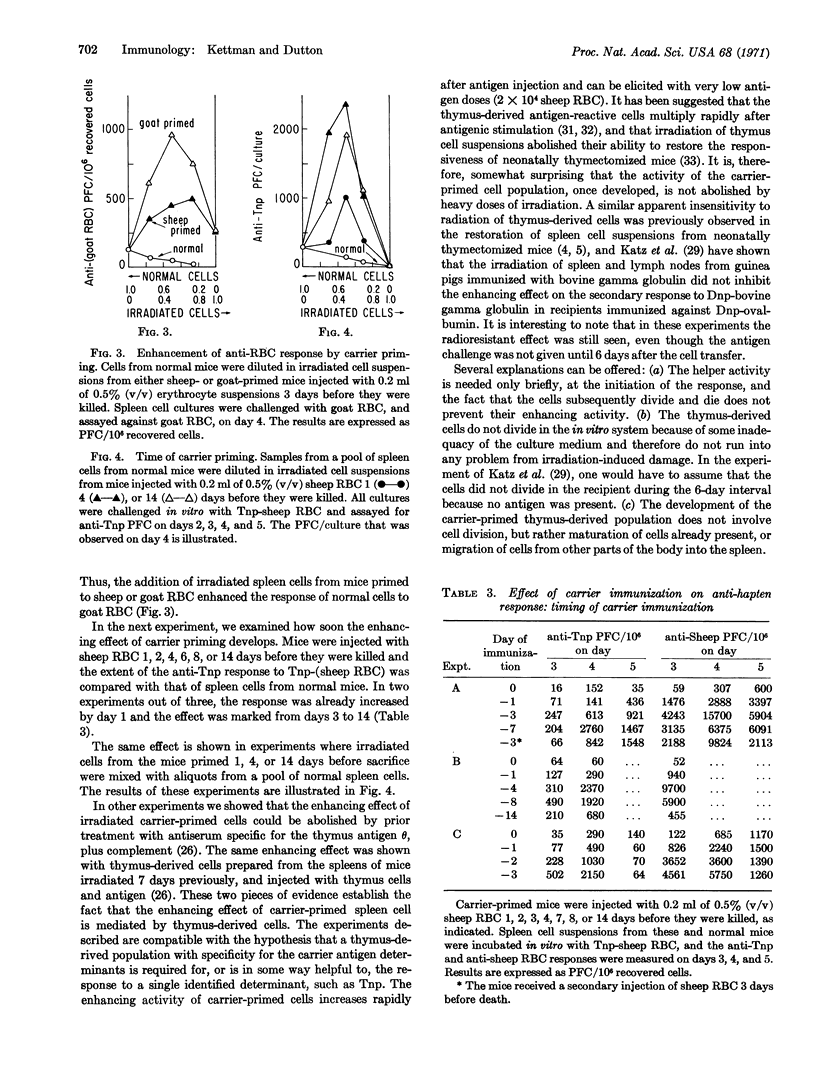
The in vitro primary response of mouse spleen cell suspensions to 2,4,6-trinitrophenyl(Tnp)-erythrocytes has been studied. The number of anti-Tnp plaque-forming cells that arise after antigenic stimulation in vitro is greatly enhanced by prior immunization in vivo with the carrier erythrocyte. The enhancement is antigen specific. The priming for an enhanced response can be elicited with very low antigen doses and is often apparent 24 hr after immunization. It is marked from day 3 to day 14. Spleen cells from carrier-primed mice will enhance the anti-Tnp response of normal cells when mixed cultures of the two cell populations are challenged with Tnp-erythrocytes in vitro. The carrier-primed cells mediating this enhancing effect are thymus derived.
The development of the thymus-derived, carrier-specific cell population has been generally assumed to involve the antigenic stimulation of cell proliferation. It was, therefore, somewhat surprising to find that the enhancing effect of the carrier-primed cells, once they had been generated, is not inhibited by x-irradiation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Claman H. N., Chaperon E. A., Triplett R. F. Immunocompetence of transferred thymus-marrow cell combinations. J Immunol. 1966 Dec;97(6):828–832. [PubMed] [Google Scholar]
- Dutton R. W., McCarthy M. M., Mishell R. I., Raidt D. J. Cell components in the immune response. IV. Relationships and possible interactions. Cell Immunol. 1970 Jul;1(2):196–206. doi: 10.1016/0008-8749(70)90007-9. [DOI] [PubMed] [Google Scholar]
- Hartmann K., Dutton R. W., McCarthy M. M., Mishell R. I. Cell components in the immune response. II. Cell attachment separation of immune cells. Cell Immunol. 1970 Jul;1(2):182–189. doi: 10.1016/0008-8749(70)90005-5. [DOI] [PubMed] [Google Scholar]
- Haskill J. S. Density distribution analysis of antigen sensitive cells in the rat. Nature. 1967 Dec 23;216(5121):1229–1231. doi: 10.1038/2161229a0. [DOI] [PubMed] [Google Scholar]
- Hirst J. A., Dutton R. W. Cell components in the immune response. 3. Neonatal thymectomy: restoration in culture. Cell Immunol. 1970 Jul;1(2):190–195. doi: 10.1016/0008-8749(70)90006-7. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Carrier function in anti-hapten immune responses. I. Enhancement of primary and secondary anti-hapten antibody responses by carrier preimmunization. J Exp Med. 1970 Aug 1;132(2):261–282. doi: 10.1084/jem.132.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz D. H., Paul W. E., Goidl E. A., Benacerraf B. Radioresistance of cooperative function of carrier-specific lymphocytes in antihapten antibody responses. Science. 1970 Oct 23;170(3956):462–464. doi: 10.1126/science.170.3956.462. [DOI] [PubMed] [Google Scholar]
- Kettman J., Dutton R. W. An in vitro primary immune response to 2,4,6-trinitrophenyl substituted erythrocytes: response against carrier and hapten. J Immunol. 1970 Jun;104(6):1558–1561. [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller J. F., Mitchell G. F. Cell to cell interaction in the immune response. I. Hemolysin-forming cells in neonatally thymectomized mice reconstituted with thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):801–820. doi: 10.1084/jem.128.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W. Immunization of dissociated spleen cell cultures from normal mice. J Exp Med. 1967 Sep 1;126(3):423–442. doi: 10.1084/jem.126.3.423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishell R. I., Dutton R. W., Raidt D. J. Cell components in the immune response. I. Gradient separation of immune cells. Cell Immunol. 1970 Jul;1(2):175–181. doi: 10.1016/0008-8749(70)90004-3. [DOI] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. II. The source of hemolysin-forming cells in irradiated mice given bone marrow and thymus or thoracic duct lymphocytes. J Exp Med. 1968 Oct 1;128(4):821–837. doi: 10.1084/jem.128.4.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell G. F., Miller J. F. Immunological activity of thymus and thoracic-duct lymphocytes. Proc Natl Acad Sci U S A. 1968 Jan;59(1):296–303. doi: 10.1073/pnas.59.1.296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nossal G. J., Cunningham A., Mitchell G. F., Miller J. F. Cell to cell interaction in the immune response. 3. Chromosomal marker analysis of single antibody-forming cells in reconstituted, irradiated, or thymectomized mice. J Exp Med. 1968 Oct 1;128(4):839–853. doi: 10.1084/jem.128.4.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul W. E., Katz D. H., Goidl E. A., Benacerraf B. Carrier function in anti-hapten immune responses. II. Specific properties of carrier cells capable of enhancing anti-hapten antibody responses. J Exp Med. 1970 Aug 1;132(2):283–299. doi: 10.1084/jem.132.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raff M. C. Role of thymus-derived lymphocytes in the secondary humoral immune response in mice. Nature. 1970 Jun 27;226(5252):1257–1258. doi: 10.1038/2261257a0. [DOI] [PubMed] [Google Scholar]
- Rajewsky K., Schirrmacher V., Nase S., Jerne N. K. The requirement of more than one antigenic determinant for immunogenicity. J Exp Med. 1969 Jun 1;129(6):1131–1143. doi: 10.1084/jem.129.6.1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rittenberg M. B., Pratt K. L. Antitrinitrophenyl (TNP) plaque assay. Primary response of Balb/c mice to soluble and particulate immunogen. Proc Soc Exp Biol Med. 1969 Nov;132(2):575–581. doi: 10.3181/00379727-132-34264. [DOI] [PubMed] [Google Scholar]
- Roseman J. X-ray resistant cell required for the induction of in vitro antibody formation. Science. 1969 Sep 12;165(3898):1125–1127. doi: 10.1126/science.165.3898.1125. [DOI] [PubMed] [Google Scholar]
- Shearer G. M., Cudkowicz G. Distinct events in the immune response elicited by transferred marrow and thymus cells. I. Antigen requirements and priferation of thymic antigen-reactive cells. J Exp Med. 1969 Dec 1;130(6):1243–1261. doi: 10.1084/jem.130.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shortman K., Diener E., Russell P., Armstrong W. D. The role of nonlymphoid accessory cells in the immune response to different antigens. J Exp Med. 1970 Mar 1;131(3):461–482. doi: 10.1084/jem.131.3.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talmage D. W., Hemmingsen H. Cell interaction in antibody synthesis. J Allergy. 1969 Jun;43(6):323–335. doi: 10.1016/0021-8707(69)90077-x. [DOI] [PubMed] [Google Scholar]
- Theis G. A., Thorbecke G. J. The proliferative and anamnestic antibody response of rabbit lymphoid cells in vitro. II. Requirement for adherent and nonadherent cells of the responses to particulate antigens in spleen cell cultures. J Exp Med. 1970 May 1;131(5):970–980. doi: 10.1084/jem.131.5.970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S., Lennox E. S., Porter R. R. Induction in vitro of hapten specific plaque forming cells. Nature. 1970 Dec 12;228(5276):1087–1089. doi: 10.1038/2281087a0. [DOI] [PubMed] [Google Scholar]


