Nel is a thrombospondin-1–like extracellular glycoprotein that is predominantly expressed in the vertebrate nervous system. It stimulates the genesis of retinal ganglion cells (RGCs) by promoting their differentiation and survival during development and is essential for production of proper numbers of RGCs.
Abstract
For correct functioning of the nervous system, the appropriate number and complement of neuronal cell types must be produced during development. However, the molecular mechanisms that regulate the production of individual classes of neurons are poorly understood. In this study, we investigate the function of the thrombospondin-1–like glycoprotein, Nel (neural epidermal growth factor [EGF]-like), in the generation of retinal ganglion cells (RGCs) in chicks. During eye development, Nel is strongly expressed in the presumptive retinal pigment epithelium and RGCs. Nel overexpression in the developing retina by in ovo electroporation increases the number of RGCs, whereas the number of displaced amacrine cells decreases. Conversely, knockdown of Nel expression by transposon-mediated introduction of RNA interference constructs results in decrease in RGC number and increase in the number of displaced amacrine cells. Modifications of Nel expression levels do not appear to affect proliferation of retinal progenitor cells, but they significantly alter the progression rate of RGC differentiation from the central retina to the periphery. Furthermore, Nel protects RGCs from apoptosis during retinal development. These results indicate that Nel positively regulates RGC production by promoting their differentiation and survival during development.
INTRODUCTION
The vertebrate CNS is composed of a diverse range of morphologically and functionally distinct types of cells, and the correct functioning of the nervous system is critically dependent on the production of a sufficient and balanced number of each cell type. This cellular diversity arises from multipotent progenitor cells by complex developmental mechanisms, including cell proliferation, fate determination, differentiation, and survival. Identification of the molecules and mechanisms that generate the proper number of neuronal cell types is one of the major goals of developmental biology.
The retina has served as an excellent model system for studying the mechanisms of cell production in the vertebrate CNS. During development, progenitor cells in the presumptive neural retina give rise to six major classes of neurons and one glial cell class in an evolutionally conserved order, which follows a histogenetic sequence in the retina (Price et al., 1987; Holt et al., 1988; Wetts and Fraser, 1988). The first cells that emerge from the retinal progenitor cells are retinal ganglion cells (RGCs), which are the sole output neurons in the retina and locate in the innermost layer (the ganglion cell layer [GCL]) of the mature retina (Prada et al., 1991). Whereas cell-intrinsic mechanisms play crucial roles in retinal cell diversification (Cayouette et al., 2006), several extrinsic signals derived from various sources in the eye influence RGC development (Isenmann et al., 2003; Agathocleous and Harris, 2009), including fibroblast growth factors (FGFs; McCabe et al., 1999, 2006; Martinez-Morales et al., 2005), Sonic hedgehog (Shh; Zhang and Yang, 2001a; Masai et al., 2005), vascular endothelial growth factor (VEGF; Hashimoto et al., 2006), and Delta-Notch signaling (Austin et al., 1995). Considering the complexity of cellular and molecular interactions that mediate retinal development, however, it seems likely that additional extracellular signals are involved in regulation of RGC development.
Nel (neural epidermal growth factor [EGF]-like) is an extracellular glycoprotein that was initially identified in chickens (Matsuhashi et al., 1995, 1996). In mammals, two related genes were subsequently identified and termed Nel-like 1 (Nell1) and 2 (Watanabe et al., 1996; Kuroda et al., 1999). Based on sequence similarities, Nell2 appeared to be the mammalian orthologue of chicken Nel. In this article, we refer to both Nel and Nell2 as Nel. The Nel/Nell proteins have significant structural similarities with thrombospondin-1, which plays important roles in a wide range of physiological and pathological conditions, such as tumor growth and metastasis, angiogenesis, wound healing, inflammation, and synaptogenesis (Adams and Lawler, 2011). The Nel protein contains an N-terminal thrombospondin-1 domain, five chordin-like/von Willebrand factor C domains, and six EGF-like domains (Matsuhashi et al., 1995, 1996). During development, Nel is expressed in many different regions of the developing nervous system, including the retina, cerebral cortex, hippocampus, amygdala, and spinal cord (Matsuhashi et al., 1995; Oyasu et al., 2000; Nelson et al., 2002, 2004). Previous studies showed that Nel stimulates differentiation of neuronal progenitor cells in the spinal cord and mitogenesis in dorsal root ganglia in chicks (Nelson et al., 2004). In addition, we previously demonstrated that Nel inhibits outgrowth of RGC axons and induces growth cone collapse and axon retraction, and suggested that Nel acts as an inhibitory axon guidance molecule in establishment of the layer specificity in the visual projection (Jiang et al., 2009; Nakamura et al., 2012). It was also shown that Nel promotes survival of cortical and hippocampal neurons in vitro (Aihara et al., 2003) and of RGCs after optic nerve injury in vivo (Munemasa et al., 2012). In adults, targeted disruption of the Nel gene results in significant enhancement of long-term potentiation in the dentate gyrus (Matsuyama et al., 2004). Moreover, Nel-deficient mice show impairment of spatial learning, further suggesting its functions in regulation of synaptic plasticity in the hippocampus (Matsuyama et al., 2005). These results indicate that Nel plays crucial and diverse functions in the developing and adult nervous system. The cell surface receptor(s) for Nel remains to be identified.
In the developing retina, Nel is strongly expressed in RGCs (Nelson et al., 2002). However, its functions in retinal development remain elusive. In this study, we investigate the functions of Nel in development of RGCs in the chick retina. Our results indicate that Nel positively regulates RGC production by both stimulating RGC differentiation and inhibiting programmed cell death.
RESULTS
Nel is expressed in retinal pigment epithelium and RGCs in the developing chick eye
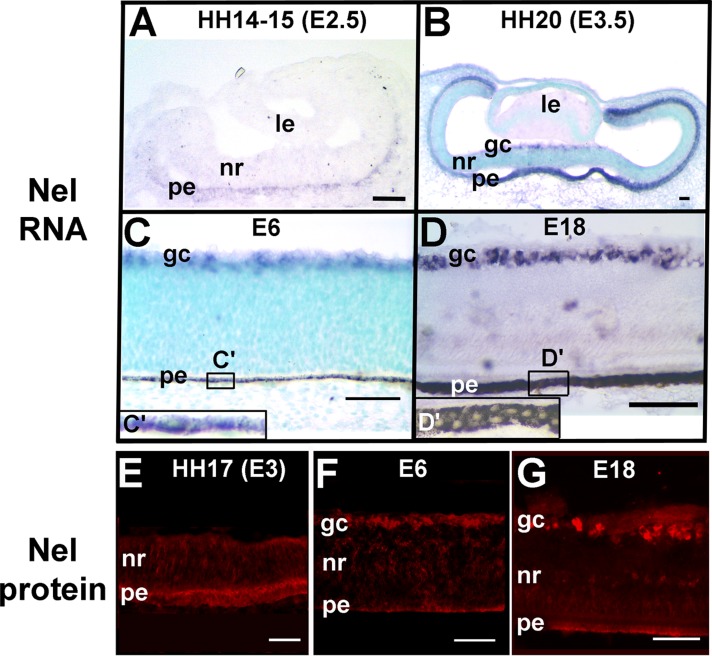
To explore potential functions of Nel in retinal development, we first examined its spatial and temporal expression patterns in the developing chick eye by RNA in situ hybridization. In chicks, invagination of the primary optic vesicle starts at Hamburger Hamilton stage (HH) 14 (embryonic day [E] 2.5), and the double-layered optic cup is formed at HH15 (E2.5). At those stages, Nel RNA expression was detected in the outer layer of the optic cup, the presumptive retinal pigment epithelium (RPE; Figure 1A). At HH20 (E3.5), Nel expression in the RPE increased, and, in addition, significant expression of Nel RNA was detected in cells that occupy the inner surface of the central retina (Figure 1B), where newly differentiated RGCs reside. Nel RNA expression in RGCs increased between E4 and E6 (Figure 1C). Expression of Nel persisted and continued to be restricted to the RPE and RGCs at least until E18 (Figure 1, C′, D, and D′). The observed strong expression of Nel in RGCs is consistent with previous reports (Nelson et al., 2002; Wang et al., 2007).
FIGURE 1:
RNA and protein expression of Nel in the developing chick retina. (A–D) Nel expression detected by RNA in situ hybridization. Retinal sections prepared from different stages of chick embryos were hybridized with an RNA probe for Nel (violet). (A) HH14–15 (E2.5), (B) HH20 (E3.5), (C, C′) E6, and (D, D′) E18. RNA expression of Nel is initially restricted to the presumptive RPE (pe; A) but is also detected in RGCs (gc) at later stages (B–D). Nel RNA expression in the RPE and RGCs persists until at least E18 (D). In C and D, higher-magnification views of the RPE (areas indicated by small rectangles) are shown in insets (C′ and D′, respectively). In B and C, the sections are counterstained with methyl green (light blue). (E–G) Nel protein distribution detected by immunohistochemistry. Sections of HH17 (E3), E6 (F), or E18 (G) retina were treated with anti-Nel antibody (red). The Nel protein is mostly localized in the RPE and RGC layer. le, lens; nr, neural retina. Scale bars, 50 μm.
Because Nel is a secreted protein, it could in principle diffuse distances to be distributed in neighboring layers and tissues. We therefore examined the Nel protein distribution in the developing retina by immunohistochemistry using anti-Nel antibody (Jiang et al., 2009). The distribution of the Nel protein showed a very similar pattern to that of Nel RNA expression (Figure 1, E–G), indicating that most of the secreted Nel protein remains in the layers of its origin. This result is consistent with our previous observation that Nel RNA and protein show similar layer-specific distributions in the developing chick optic tectum (Jiang et al., 2009). This limited diffusion of the Nel protein may be due to its heparin-binding activity (Kuroda et al., 1999; Nakamura et al., 2012), and the Nel protein may be trapped by heparin sulfate proteoglycans in situ soon after secretion from Nel-expressing cells.
Nel overexpression increases RGC number in vivo
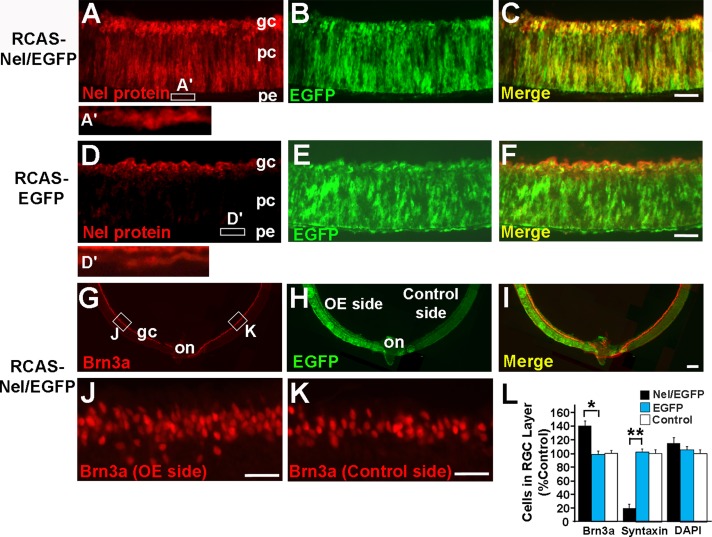
The expression patterns of Nel in early retinal development raised the possibility that Nel may be involved in retinal neurogenesis, particularly in development of RGCs. To test this possibility directly in vivo, we constructed a replication-competent retroviral vector that drives bicistronic expression of Nel and an enhanced green fluorescent protein (EGFP) marker (RCAS-Nel-IRES-EGFP). As a negative control, we used the RCAS-IRES-EGFP vector, which expresses only EGFP. The expression vectors were transfected into the optic vesicle at HH9–11 (E1.5) by in ovo electroporation, and expression of the transgenes was assessed at later stages by fluorescence of EGFP and immunohistochemistry using anti-Nel antibody (Jiang et al., 2009; Figure 2, A–F).
FIGURE 2:
Nel overexpression results in increase in RGC number. (A–F) Overexpression of Nel by using RCAS-Nel-IRES-EGFP. The retrovirus construct was electroporated into the optic vesicle of HH9–11 (E1.5) chick embryos, and retinal sections were prepared at E5. An area of Nel overexpression (A–C) and a corresponding area transfected with the control RCAS-IRES-EGFP (D–F) are shown. Nel expression was detected by anti-Nel antibody (red), and transfection domains are marked by the EGFP reporter (green). (C, F) Merged images of the left two images. (A′, D’) Higher-magnification views of the RPE (areas indicated by small rectangles in A and D, respectively). In Nel/EGFP transfection domains, ectopic expression of Nel in progenitor cells (pc) of the neural retina is detected. In addition, staining for the Nel protein in RGCs (gc) and RPE (pe) is stronger compared to that in control areas. (G–L) Effects of Nel overexpression on RGC production. Retinal sections were prepared at E8 from embryos transfected with RCAS-Nel-IRES-EGFP, and the production of RGCs was evaluated by immunohistochemistry for Brn3a (red). The transgene was introduced into half of the retina (overexpression [OE] side), which is marked by the EGFP reporter (green). (I) Merged image of G and H. (J, K) Higher-magnification views of Nel-overexpressing and corresponding untransfected areas in G (indicated by small rectangles), respectively. The number of Brn3a-positive cells significantly increased in Nel ectopic domains. on, optic nerve head. Scale bars, 50 μm. (L) Quantification of cell numbers in the GCL. The numbers of Brn3a-positive RGCs, syntaxin-positive displaced amacrine cells, and total cells in the GCL (4′,6-diamidino-2-phenylindole) in areas transfected with Nel and EGFP (Nel/EGFP) or EGFP only (EGFP) were compared with those in equivalent areas of the contralateral (untransfected) eye (Control) and indicated as mean ± SEM. *p < 0.001, **p < 0.0005. n = 6 embryos. ANOVA test.
To examine effects of Nel overexpression on RGC development, we incubated embryos transfected with the RCAS-Nel-IRES-EGFP vector until E8, when production of RGCs is mostly complete (Prada et al., 1991). Retinal sections were then prepared and stained for Brn3a, a specific marker for RGCs in the retina (Xiang et al., 1995). Retinal neurogenesis occurs in a central-to-peripheral gradient (Snow and Robson, 1995; McCabe et al., 1999) and also shows slight temporal-to-nasal and dorsal-to-ventral gradients (Prada et al., 1991). We therefore compared the numbers of Brn3a-positive cells between equivalent areas (e.g., central-temporal area) of transfected and untransfected (contralateral) retinas. We found that the number of Brn3a-positive cells increased by approximately 40% in regions transfected with RCAS-Nel-IRES-EGFP (Figure 2, G–L). Transfection of the control vector that expresses only EGFP did not affect the production of Brn3a-positive cells in the retina (Figure 2L).
During vertebrate retinal development, significant numbers of amacrine cells, which are mainly located in the superficial part of the inner nuclear layer (INL), migrate to the GCL. In chicks, those “displaced” amacrine cells are produced between E5.5 and E6.5 and comprise 20–35% of the total number of cells in the GCL (Galvez et al., 1977; Ehrlich and Morgan, 1980; Layer and Vollmer, 1982). Therefore next we examined whether Nel overexpression affects the number of displaced amacrine cells by using the amacrine cell marker syntaxin. We found that in the retinal regions where Nel is overexpressed, the number of displaced amacrine cells in the GCL significantly decreases. The total cell numbers in the GCL showed a slight increase after Nel overexpression, although this difference was not statistically significant (Figure 2L). These results indicate that Nel overexpression promotes the genesis of RGCs and decreases the number of displaced amacrine cells in the GCL.
Decrease of RGC number by RNA interference knockdown of Nel
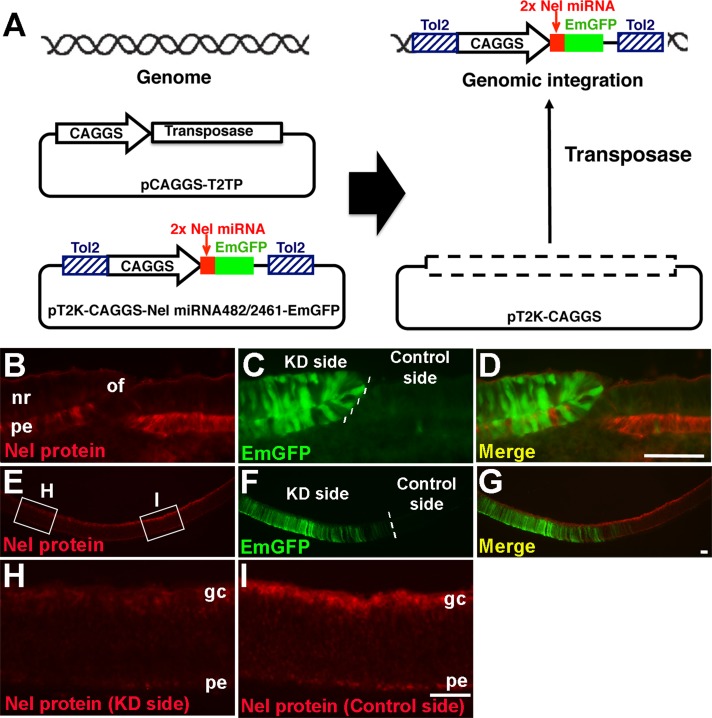
Next we examined whether Nel is essential for the production of the proper number of RGCs in vivo. To test this, we designed artificial microRNAs (miRNAs) to target Nel expression and introduced them into the developing eye by in ovo electroporation. Because RGC production continues over at least 5–6 d, it was necessary that the production of the artificial miRNA be maintained throughout the period of RGC generation without significant reduction in expression. To achieve stable expression of transgenes, we used a transposon-mediated gene transfer system (Sato et al., 2007) that uses the Tol2 transposable element (Koga et al., 1996). We made a Tol2 transposon construct (pT2K-CAGGS-Nel miRNA482/2461-emerald GFP [EmGFP]) that expresses Nel-specific miRNA and an EmGFP marker under the CAGGS promoter. As a negative control, we used a transposon vector carrying nonspecific miRNA sequences with the EmGFP marker gene (pT2K-CAGGS-Control miRNA-EmGFP). When introduced into cells with a transposase activity, the expression cassette would be excised from the plasmid and integrated into the host genome, allowing stable expression of the miRNA and the EmGFP marker (Figure 3A). We electroporated the transposon vector of Nel miRNA with the transposase expression vector pCAGGS-T2TP into the optic vesicle at HH9–11 (E1.5). Electroporation efficiency was monitored by expression of EmGFP, and effects of RNA interference (RNAi) on Nel expression was evaluated by immunohistochemistry using anti-Nel antibody. At E4.5, endogenous expression of Nel was found to be significantly reduced in EmGFP-expressing cells in the RPE (Figure 3, B–D). At E8, reduction of Nel expression was also obvious in RGCs (Figure 3, E–I).
FIGURE 3:
Knockdown of Nel expression by transposon-mediated transfer of artificial miRNA. (A) A scheme showing transposition of a Tol2-flanked expression cassette for artificial Nel miRNA and EmGFP by transposase. When a Tol2 transposon construct (pT2K-CAGGS-Nel miRNA482/2461-EmGFP) is introduced into cells with a transposase expression construct (pCAGGS-T2TP), the Tol2-flanked cassette is excised from the vector and integrated into the host genome by the transposase activity (modified from Sato et al., 2007). (B–I) RNAi knockdown of Nel expression in the developing eye. A transposon construct containing an expression cassette of Nel miRNA and EmGFP was cotransfected with a transposase expression vector into the optic vesicle by in ovo electroporation at HH9–11 (E1.5). Retinal sections were prepared at E4.5 (B–D) or E8 (E–I), and expression of Nel was examined by immunohistochemistry using anti-Nel antibody (red). Half of the retina was transfected with the transposon constructs and marked by EmGFP (knockdown [KD] side, green). The boundary between transfected and untransfected (Control) sides is indicated by a dotted line in C and F. (D, G) Merged images of the left two images. (B–D) At E4.5, Nel expression is significantly reduced in the RPE (pe) cells that express EmGFP. Note the complementary pattern of Nel immunostaining and EmGFP expression. (E–I) At E8, Nel expression in the RPE and RGCs (gc) is decreased on the knockdown side (left side of the section). (H, I) Higher-magnification views of knockdown and corresponding untransfected areas in E (indicated by small rectangles), respectively. Scale bars, 50 μm. nr, neural retina; of, optic fissure.
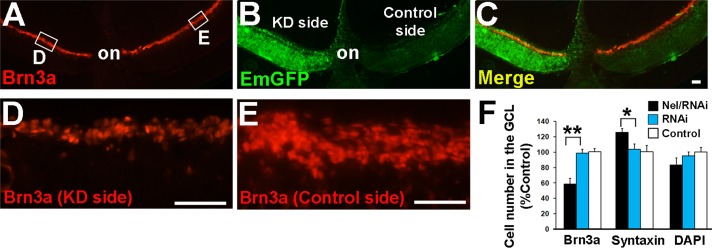
Then we examined whether RNAi knockdown of Nel affects the numbers of RGCs and displaced amacrine cells. Whereas transfection of the control vector did not cause any changes in the RGC or displaced amacrine cell numbers, introduction of the Nel miRNA construct with the transposase expression vector resulted in an approximately 40% reduction in the number of Brn3a-positive cells and to a lesser extent, an increase in the number of displaced amacrine cells (Figure 4). Taken together, these results indicate that Nel regulates the number of RGCs positively and that of displaced amacrine cells negatively and is essential for the generation of the proper numbers of retinal neurons during development.
FIGURE 4:
Reduction of the RGC number by RNAi knockdown of Nel expression. A transposon construct of artificial Nel miRNA and EmGFP (pT2K-CAGGS-Nel miRNA482/2461-EmGFP) was cotransfected with a transposase expression vector (pCAGGS-T2TP) into the optic vesicle by in ovo electroporation at HH9–11 (E1.5). Retinal sections were prepared at E8 and stained for Brn3a (red). (A–C) Representative section through the optic nerve head (on). The transgenes were introduced into half (knockdown [KD] side, green) of the retina. Compared to the control side, the number of Brn3a-positive cells is significantly reduced on the knockdown side. (D, E) Higher-magnification views of RNAi knockdown (D) and corresponding untransfected (E) areas in A (indicated by small rectangles). Scale bars, 50 μm. (F) Quantification of cell numbers in the GCL. The numbers of Brn3a-positive RGCs, syntaxin-positive displaced amacrine cells, and total cells in the GCL (4′,6-diamidino-2-phenylindole) in areas transfected with Nel miRNA or control miRNA were compared with those in equivalent areas in the contralateral (untransfected) eye (Control) and plotted as mean ± SEM. *p < 0.05, **p < 0.0005. n = 6 embryos. ANOVA test.
Effects of Nel on other types of retinal cells
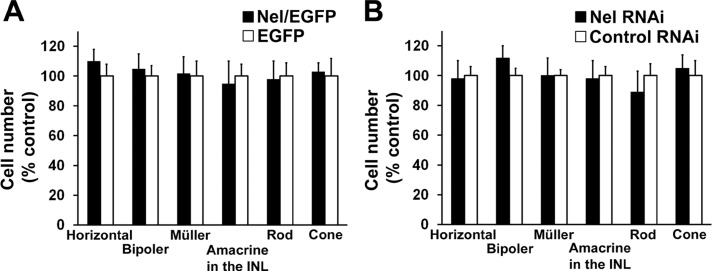
Next we tested whether Nel overexpression or knockdown affects the production of other types of retinal cells. To this end, we examined the numbers of different types of retinal cells at E18 by using AP2α as a bipolar cell marker, Pax6 as a horizontal cell marker, rhodopsin as a marker for rods, visinin as a marker for cones, and vimentin as a Müller glia marker. As shown in Figure 5, neither overexpression nor RNAi knockdown caused significant changes in the numbers of those retinal cells. In addition, the amacrine cell numbers in the INL were not significantly altered by the modulations of Nel expression levels. These results indicate that the effects of Nel expression are confined to the GCL of the retina.
FIGURE 5:
Effects of Nel on production of different retinal cell types. Expression constructs for Nel cDNA (A) or artificial miRNA (B) were transfected into the optic vesicle by in ovo electroporation at HH9–11 (E1.5). The numbers of different types of retinal cells in transfected areas were compared with those in corresponding areas transfected with control vectors (EGFP in A, control RNAi in B) at E18. No significant differences were detected in the numbers of AP2α-positive bipolar cells, Pax6-positive horizontal cells, rhodopsin-positive rods, visinin-positive cones, or vimentin-positive Müller glia. In addition, the amacrine cell numbers in the INL were not significantly altered. n = 6 embryos. ANOVA test.
Nel does not significantly affect proliferation of retinal progenitor cells
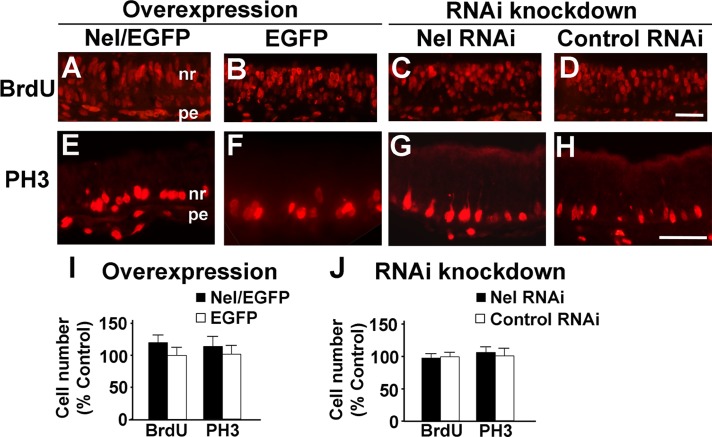
The potential mechanisms by which Nel positively regulates RGC production include stimulation of retinal progenitor proliferation, promotion of RGC differentiation, and inhibition of apoptosis. First, we tested whether Nel can enhance proliferation of progenitor cells in the developing retina. We electroporated RCAS-Nel-IRES-EGFP into the optic vesicle at HH9–11 (E1.5) and counted the number of bromodeoxyuridine (BrdU)-positive cells after 3 h of in vivo labeling at E6. No significant difference in the number of BrdU-positive cells was observed between Nel-overexpressing and control areas (Figure 6, A, B, and I). In addition, RNAi knockdown of Nel expression did not affect the number of BrdU-positive cells (Figure 6, C, D, and J). In a separate set of experiments, retinal sections were examined by immunohistochemistry using anti–phosphohistone H3 (PH3) antibody. Neither overexpression nor RNAi knockdown of Nel caused any significant change in the number of PH3-positive cells (Figure 6, E–J). These results suggest that Nel does not serve as a mitogen during early retinal development.
FIGURE 6:
Effects of Nel on proliferation of retinal progenitor cells. Expression constructs for Nel cDNA (A, E) or artificial miRNA (C, G) were transfected into the optic vesicle by in ovo electroporation at HH9–11 (E1.5), and effects on cell proliferation were examined by comparing with corresponding areas transfected with control vectors (EGFP in B, F, I; control RNAi in D, H, J). Areas in the central retina are shown. (A–D) Embryos were labeled with BrdU in ovo for 3 h at E6, and retinal sections were prepared and stained with anti-BrdU antibody. (E–H) Retinal sections of E4.5 chicks were stained for PH3. Scale bars, 50 μm. nr, neural retina; pe, pigment epithelium. (I, J) Quantifications of BrdU- and PH3-positive cells. The numbers of stained cells are shown as percentage control in mean ± SEM. No significant differences were detected between Nel overexpression, RNAi knockdown, and their controls. n = 6 embryos. ANOVA test.
Nel accelerates the progression of RGC differentiation wave
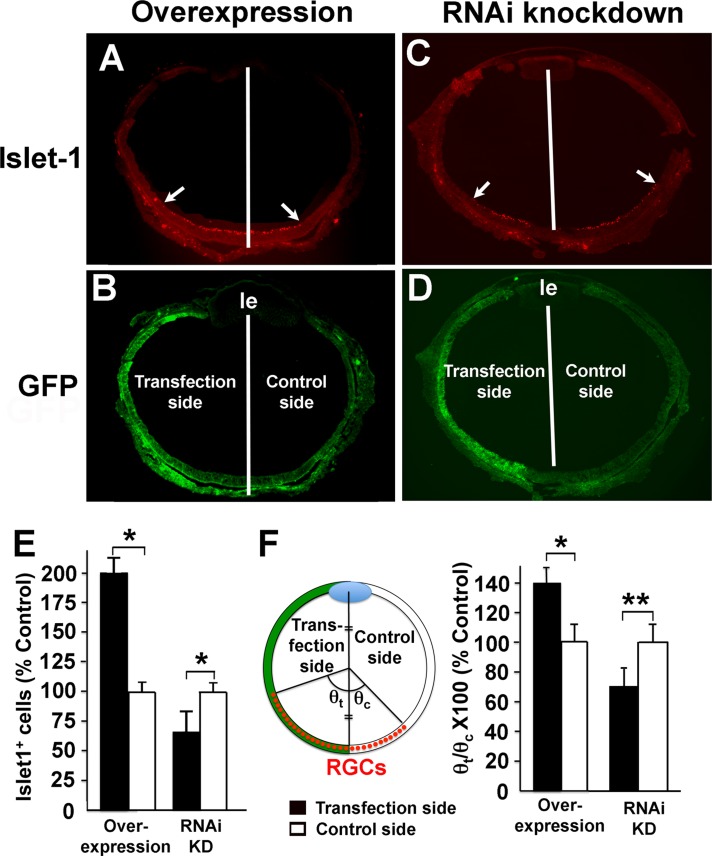
Next we examined the effects of Nel on RGC differentiation. During development, RGC differentiation occurs in a central-to-peripheral gradient, such that RGCs are initially produced in the central retina and then the wave of differentiation proceeds from the central to peripheral retina, much like an expanding circle (Snow and Robson, 1995; McCabe et al., 1999). In addition, it was reported that neurogenesis in the chick retina shows slight temporal-to-nasal and dorsal-to-ventral gradients (Prada et al., 1991). To test whether Nel affects the progression of the RGC differentiation wave, we overexpressed Nel in half (nasal or temporal) of the retina at HH9–11 (E1.5), and examined the progression of RGC differentiation by immunohistochemistry for the RGC marker Islet-1 (Austin et al., 1995) at E4.5, when RGCs are still actively produced. Equal numbers of nasally and temporally transfected retinas were examined. We found that the overexpressing side of the retina contained significantly larger numbers of Islet-1–positive cells than the control side (Figure 7, A, B, and E). Furthermore, compared to the control side, the peripheral edge or front of RGC differentiation reached farther from the central toward peripheral retina (Figure 7, A, B, and F), indicating that Nel overexpression accelerates the movement of the RGC differentiation wave. Conversely, when expression of Nel was knocked down by RNAi in half of the retina, the knockdown side of the retina not only contained fewer Islet-1–positive cells, but also showed slower progression of the RGC differentiation wave (Figure 7, C–F). These results indicate that Nel enhances the rate of RGC differentiation and its progression from the central to peripheral retina.
FIGURE 7:
Enhancement of the progression of RGC differentiation wave by Nel. (A–D) Nel was either overexpressed (A, B) or knocked down (C, D) in half (left side) of the developing retina by in ovo electroporation at HH9–11 (E1.5). Retinal sections were prepared at E4.5, and RGCs were detected by immunohistochemistry using anti–Islet-1 antibody (red). The areas of transgene expression are marked by expression of a GFP reporter (transfection side, green). Compared to the control side, Nel overexpression increased the number of RGCs and accelerated the progression of RGC differentiation wave. Conversely, RNAi knockdown of Nel decreased the RGC number and slowed down the rate of their differentiation. The white lines indicate the midline of the retina, and the arrows show the peripheral edge of RGC differentiation wave. le, lens. (E) Quantification of RGC numbers. The number of Islet-1–positive cells is shown as percentage control in mean ± SEM. (F) Quantification of progression of the RGC differentiation wave. The angles subtended by the arcs of RGC cells were measured on the transfection (θt) and control sides (θc), as indicated. Effects on wave progression were quantified as θt/θc × 100 and plotted as mean ± SEM. Equal numbers of nasally and temporally transfected retinas were examined. *p < 0.005, **p < 0.001. n = 6 embryos each for temporal and nasal transfection. Paired Student's t test.
Nel protects retinal cells from developmental cell death
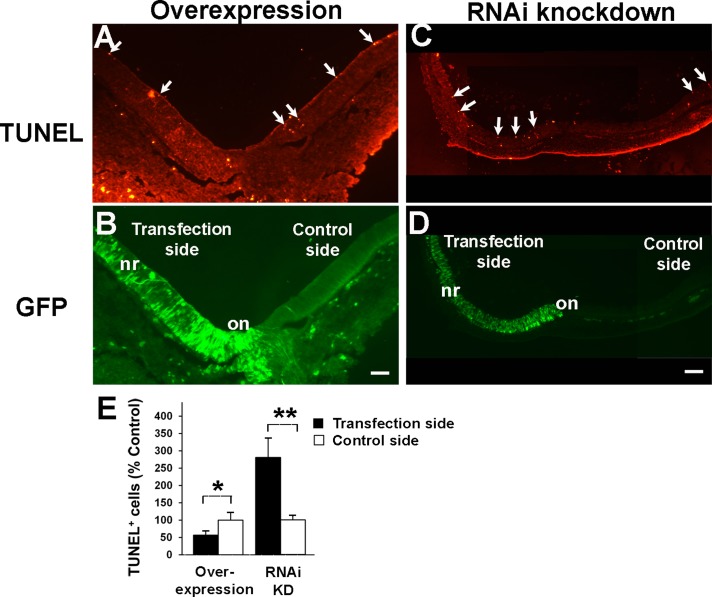
In retinal development, the period of RGC production partly overlaps with the earlier peak phase of developmental cell death (Frade et al., 1997). Therefore next we examined whether manipulations of Nel expression levels affect programmed cell death during retinal development. Consistent with previous reports (Zhang and Yang, 2001a, b), only a few apoptotic cells were detected by terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assay in central regions of the normal E6 retina. Overexpression of Nel resulted in a decrease in the number of TUNEL-positive cells in transfected areas (Figure 8, A, B, and E). Conversely, when Nel expression was knocked down by RNAi, a marked increase in the number of TUNEL-positive cells was observed (Figure 8, C–E). Transfection of control vectors did not change the number of TUNEL-positive cells (Supplemental Figure S1). These results indicate that Nel acts to protect RGCs from programmed cell death during retinal development.
FIGURE 8:
Nel promotes survival of retinal cells during development. (A–D) Nel was either overexpressed (A, B) or knocked down (C, D) in half (left side) of the developing retina by in ovo electroporation at HH9–11 (E1.5). Retinal sections were prepared at E6, and apoptotic cells were detected by TUNEL assay (red). The areas of transgene expression are marked by expression of a GFP reporter (transfection side, green). A subset of TUNEL-positive cells is indicated by arrows. Compared to the control side, Nel overexpression decreased the number of TUNEL-positive cells, whereas RNAi knockdown of Nel drastically increased it. nr, neural retina; on, optic nerve. Scale bars, 100 μm. (E) Quantification of apoptotic cells. The number of TUNEL-positive cells on the transfected side was compared with that on the control side and plotted as mean ± SEM. *p < 0.05, **p < 0.0005. n = 6 embryos. ANOVA test.
DISCUSSION
Functions of Nel in RGC development
Several lines of evidence obtained in this study indicate that Nel positively regulates the production of RGCs during development. First, Nel is strongly expressed in RGCs (Nelson et al., 2002; Wang et al., 2007) and the RPE in the developing eye. Second, gain-of-function studies show that retroviral overexpression of Nel in the developing retina increases RGC number. Third, loss-of-function experiments using RNAi knockdown demonstrate that reduction of Nel expression levels results in decreased number of RGCs. We also showed that alterations of Nel expression levels do not exert obvious effects on cell proliferation but significantly affect the rate of RGC differentiation and developmental cell death, suggesting that Nel enhances the production of RGCs by promoting their differentiation and survival.
During retinal development, RGC differentiation initially occurs in the central retina, and the wave of differentiation proceeds toward the periphery of retina. Our results show that overexpression of Nel accelerates the progression of the RGC differentiation wave, whereas RNAi knockdown of Nel expression retards the movement of the wave front. This effect of Nel appears similar to that previously reported for FGF signaling. During the initial stage of chick retinogenesis, FGF1 is expressed at high levels in the peripheral part of the developing retina (de Iongh and McAvoy, 1992). In a retinal explant culture system, FGF1 promotes the progression of RGC differentiation wave, and inhibition of the FGF receptor signaling inhibits the movement of the wave front (McCabe et al., 1999). In Xenopus embryos, overexpression of FGF2 in retinal progenitor cells results in a 35% increase of RGCs without affecting retinal cell proliferation (Patel and McFarlane, 2000). Whereas both Nel and FGF1 accelerate the progression of RGC differentiation wave, the central-to-peripheral gradient of RGC differentiation is preserved in the presence of excess Nel or FGF1. Given that the cells at the wave front are more mature than those in the peripheral retina, these results may suggest that Nel and FGF1 act to stimulate further differentiation of nascent RGCs in final stages of RGC development (Jiang et al., 2013).
Programmed cell death plays crucial roles in regulation of cell numbers during neural development, and a significant portion of newly generated RGCs undergo programmed cell death during development. The present study shows that whereas Nel overexpression decreases number of apoptotic cells in the developing retina, RNAi knockdown of Nel dramatically increases it. These results suggest that in addition to acceleration of RGC differentiation, suppression of developmental cell death contributes to promotion of RGC production by Nel. Importance of developmental cell death in regulation of RGC numbers has also been shown by studies on brain-derived neurotrophic factor (BDNF). Application of BDNF to chick embryos in ovo significantly inhibits the earlier peak phase of developmental cell death in the retina and results in a 60–90% increase in RGC number (Frade et al., 1997). Nel was shown to promote survival of cortical and hippocampal neurons in primary culture (Aihara et al., 2003). More recently, it was reported that overexpression of Nel in the retina promotes RGC survival in vivo after optic nerve transection (Munemasa et al., 2012). These results indicate that Nel can act as a survival factor for multiple types of neurons.
We found that the number of displaced amacrine cells in the GCL is negatively affected by Nel expression. Given that amacrine cells are among the early-born neurons in the retina and are generated at approximately the same time as RGCs (Cepko et al., 1996), Nel may act to cause a cell fate shift from amacrine cells to RGCs, although the change in amacrine cell number is restricted in the GCL, and no significant effects were observed on amacrine cell number in the INL by Nel overexpression or RNAi knockdown. Alternatively, the displacement of amacrine cells in the GCL may be negatively controlled by the number of RGCs in the layer, and thus the observed effects on the number of displaced amacrine cells may represent secondary effects due to the change of RGC number caused by modulation of Nel expression.
Nel in molecular interaction network during retinal development
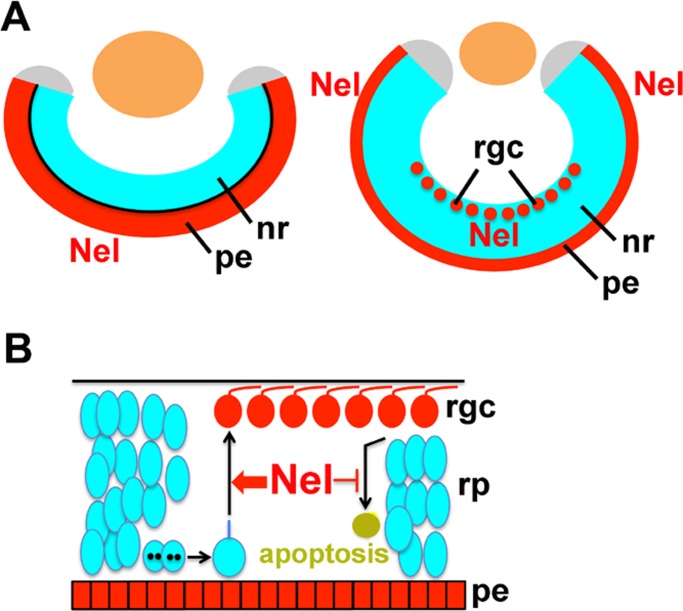
During retinal development, Nel is produced by two different sources in the eye (Figure 9). First, at early stages of retinal development before the first RGCs emerge in the GCL, the presumptive RPE acts as the sole source of Nel in the developing eye. Throughout eye development, the neural retina and the RPE are kept in close apposition, and development of the two tissues occurs in concert (Strauss, 2005). Ablation of the RPE severely disrupts development of the neural retina (Stiemke et al., 1994; Raymond and Jackson, 1995). Interestingly, compared to pigmented wild-type mice, more RGCs are produced during the initial period of retinal neurogenesis in albino mice, which lack pigmentation in the RPE. Furthermore, pharmacological inhibition of pigment production increases the number of RGCs in eyecups isolated from pigmented mice (Rachel et al., 2002). A variety of growth factors are secreted from the RPE, including VEGF, FGF, and pigment epithelium–derived factor (PEDF; Strauss, 2005). In Xenopus, PEDF supports normal development of photoreceptor neurons and Müller cells after removal of the RPE, demonstrating that RPE-derived PEDF plays important roles in the final stages of retinal development (Jablonski et al., 2000, 2001). Although the Nel protein appears to localize mostly in the presumptive RPE at this stage, our results raise the possibility that RPE-derived Nel functions as a part of the molecular network between the RPE and neural retina and acts to increase, directly or indirectly, RGC production in early stages of retinal neurogenesis.
FIGURE 9:
Expression and potential modes of actions of Nel in retinal neurogenesis. (A) During retinal development, Nel is secreted from two different types of cells. Left, at early stages of retinal neurogenesis before RGCs are produced, Nel is expressed exclusively in the presumptive retinal pigment epithelium. Right, at later stages, newly differentiated RGCs also secrete Nel, whereas Nel expression in the RPE is maintained. (B) Nel accelerates the rate of differentiation of retinal progenitor cells into RGCs and inhibits apoptosis of retinal cells. Sources of Nel are indicated in red. nr, neural retina; pe, retinal pigment epithelium; rgc, retinal ganglion cells; rp, retinal progenitor cells.
Second, strong Nel expression was detected in RGCs that had migrated into the GCL. Previous studies showed that differentiated RGCs secrete several factors that affect RGC production. For example, Shh is produced by newly differentiated RGCs and required for the propagation of the neurogenic wave toward the peripheral retina (Masai et al., 2005). Shh also suppresses the generation of additional RGCs behind the neurogenic wave front (Zhang and Yang, 2001a). In addition, VEGF produced by differentiated RGCs increases proliferation of retinal progenitors and decreases production of RGCs (Hashimoto et al., 2006). The present study suggests that Nel is a novel RGC-derived factor that positively regulates RGC production.
The relative contributions and respective functions of RPE- and RGC-derived Nel in RGC production remain elusive. In addition, it is not clear whether RPE-derived Nel affects expression of RGC-derived Nel, or expressions of RPE- and RGC-derived Nel are independently regulated. We plan to address those issues in future studies by creating and analyzing conditional (RPE- and RGC-specific) knockout mice of Nel.
Gene silencing in chick embryos by transposon-mediated integration of artificial miRNA transgenes
Although chick embryos have long been used as a favorite model organism in the field of developmental biology, robust and efficient loss-of-function approaches had not been available for the chick until recently. In the past decade, RNAi has been successfully used in chick embryos by electroporating small interfering RNAs (siRNAs; Hu et al., 2002) or expression vectors for short hairpin RNA (Katahira and Nakamura, 2003) in ovo. More recently, an RCAS retrovirus-based siRNA delivery system was shown to efficiently knock down gene expression in chick embryos (Harpavat and Cepko, 2006).
In loss-of-function analysis of this study, we introduced artificial miRNA transgenes into the developing chick retina by using a transposon-mediated gene transfer system (Sato et al., 2007). This method offers several distinct advantages. First, it supports stable expression of the transgene and thus persistent production of miRNA. Electroporation of conventional expression vectors permits only transient expression of the transgene, usually lasting only for several days in embryonic cells, because of the inability of the electroporated plasmid to be integrated into chromosomes. In contrast, the present method involves chromosomal integration of the expression cassette by activity of coelectroporated transposase and therefore can achieve stable expression of the transgenes. Second, the cells that express the transgene can be easily traced because each of the transfected cells is clearly marked by the EmGFP marker. Third, because expression of miRNA and EmGFP is driven by the same CAGGS promoter, the risk of promoter interference is avoided, which is one of the common problems with retroviral vectors containing an additional promoter to attain dual gene expression (e.g., Yee et al., 1987)). Finally, the areas of transfection can be controlled by properly choosing the type and positions of electrodes. Because this method does not use replication-competent retroviral vectors, there is no transmission of the transgene to neighboring cells after the electroporation. Within the transfected retinas, areas that do not express the transgene serve as a good internal control. Therefore this system provides a stable and efficient loss-of-function option and can contribute to the analysis of gene functions during chick development.
Diverse functions of Nel in visual system development
In the chick visual system, Nel is expressed not only in RGCs (projecting neurons), but also in the optic tectum (the main target of RGC axons in nonmammalian vertebrates). Nel expression in the chick tectum is detected as early as E5. Expression levels of Nel significantly increase between E8 and E12, and the high expression is maintained at least until E18. First RGC axons reach the tectum by E6, and RGC axons project to the specific layers (“retinorecipient laminae”) in the superficial part of the tectum between E12 and E18. During this period, Nel expression in the tectum is confined to deeper layers of the tectum, which RGC axons do not normally innervate (“nonretinorecipient laminae”). Because Nel inhibits outgrowth of RGC axons and induces growth cone collapse and axon retraction in vitro, these results suggest that Nel may act as an inhibitory axon guidance cue that prevents RGC axons from invading inappropriate layers in the deeper part of the tectum and thus contribute to the establishment of the layer specificity in the visual projection (Jiang et al., 2009; Nakamura et al., 2012). In addition, in view of the functions of Nel in RGC production revealed in this study, it seems plausible that Nel could also have roles in differentiation and survival of tectal neurons in nonretinorecipient laminae.
It is intriguing that Nel exerts both positive (promotion of differentiation and survival) and negative (inhibitory effects on axons) effects on RGCs. Several other axon guidance molecules were also reported to exert both positive and negative effects on neurons. Such examples include ephrins, Wnt (Flanagan, 2006), and netrins (Moore et al., 2007). Because the Nel protein consists of different domains, it seems likely that different domains interact with distinct receptors and exert different functions, as is the case for thrombospondin-1 (Adams and Lawler, 2011). Interestingly, Nel exerts neuroprotective effects on cultured neurons at concentrations lower than those required for inhibitory effects on RGC axons (Aihara et al., 2003). This may suggest that Nel exerts inhibitory axon guidance functions and neuroprotective effects through distinct receptors that have different affinities for the ligand. Alternatively, the same receptor(s) may mediate both positive and negative signals in response to different concentrations of the ligand, as shown for Eph receptor–ephrin interactions (Hansen et al., 2004; Matsuoka et al., 2005). Further studies, including identification of its receptors, would be required to fully understand the molecular mechanisms of Nel functions. Nonetheless, it has become increasingly apparent that Nel is a multifunctional molecule that plays crucial roles in development of the vertebrate nervous system and its functions.
MATERIALS AND METHODS
Chick embryos
Fertilized White Leghorn chicken eggs were purchased from Henry Stewart (Louth, United Kingdom) and incubated at 38°C until use.
In situ RNA hybridization
In situ RNA hybridization was performed using digoxigenin-labeled probes for Nel as previously described (Jiang et al., 2009), using 12- to 30-μm frozen sections of the embryonic retina.
Plasmid constructs
For construction of the RCAS-IRES-EGFP plasmid, cDNA sequences containing IRES-EGFP (nucleotide numbers 666–1973) of the pIRES2-EGFP vector (Clontech, Mountain View, CA) were amplified by PCR. Both the 5′ and 3′ PCR primers contain an artificial ClaI site, and the 3′ ClaI site was protected by a 5′ guanine, thus preventing ClaI cleavage when methylated, as previously described (McLaughlin et al., 2003). The PCR product was cloned into the ClaI site of the replication-competent avian retroviral vector RCAS (Fekete and Cepko, 1993). The RCAS-Nel-IRES-EGFP vector was constructed by inserting the protein-coding region of Nel cDNA (GenBank accession number NM_001030740.1, nucleotide numbers 118–2565) into the RCAS-IRES-EGFP vector upstream of the IRES at the functional ClaI site.
For RNAi knockdown, we designed pairs of single-stranded DNA oligonucleotides (one encoding target pre-miRNA and the other corresponding its complement) for 10 potential target sequences by using RNAi Designer (Invitrogen, Carlsbad, CA; https://rnaidesigner.invitrogen.com/rnaiexpress/). After annealing, the double-stranded oligonucleotides were individually cloned into the pcDNA6.2-GW/EmGFP-miRNA vector (Invitrogen). Knockdown effects of each construct were evaluated by cotransfection with a Nel-AP expression vector into HEK293T cells, followed by measurement of AP activity in the culture media. The two most potent target sequences were selected (corresponding to nucleotide numbers 482–502 and 2461–2481, respectively) and tandemly cloned into the pcDNA6.2-GW/EmGFP-miRNA vector (pcDNA6.2-GW–Nel miRNA482/2461-EmGFP) according to the manufacturer's instructions. To construct the pT2K-CAGGS-Nel miRNA482/2461-EmGFP vector, cDNA sequences encoding the two pre-miRNA and EmGFP of the pcDNA6.2-GW/Nel miRNA 482/2461-EmGFP vector were amplified by PCR with an artificial EcoRI site on both ends (5′-GGGAATTCTCTGGCTAACTAGAGAAC-3′ and 5′-CCGAATTCCCTCTAGATCAACCACT-3′) and cloned into the pT2K-CAGGS vector. The control pT2K-CAGGS-Control miRNA-EmGFP vector was constructed by cloning the corresponding region of the pcDNA6.2-GW/EmGFP-miRNA negative control plasmid (Invitrogen) into the pT2K-CAGGS vector using the same set of PCR primers and restriction enzyme sites as mentioned. The pT2K-CAGGS and pCAGGS-T2TP vectors were kindly provided by Yoshiko Takahashi (Kyoto University, Kyoto, Japan) and Koichi Kawakami (National Institute of Genetics, Mishima, Japan), respectively.
In ovo electroporation
In ovo electroporation was performed as described previously (Harada et al., 2008). Briefly, a small amount of plasmid DNA solution (5 μg/μl each) mixed with fast green (dye tracer) was injected into the lumen of the optic vesicle of HH9–11 (E1.5) chick embryos in windowed eggs. Parallel platinum-coated electrodes spaced at 3 mm were placed perpendicular to the long axis of the embryo, one electrode being on the anterior (nasal) side and the other electrode on the posterior (temporal) side of the optic vesicle. Five square pulses (25 V, 50 ms/s) were applied by a CUY21 electroporator (Nepa Gene, Ichikawa, Japan). The levels of ectopic expression varied in different embryos. Only the embryos that showed significant levels of expression were used for analysis. Equal numbers of nasally and temporally transfected retinas were analyzed.
Immunohistochemistry and TUNEL assay
For immunohistochemistry, embryonic retinas were fixed with 4% paraformaldehyde, cryoprotected with 30% sucrose in phosphate-buffered saline (PBS), embedded in OCT compound, frozen, and sectioned at 12–30 μm. Immunohistochemistry for Nel protein was performed as described previously (Jiang et al., 2009), except that Alexa Fluor 594–conjugated anti-rabbit immunoglobulin G (IgG; 1:1000; Invitrogen) was used as secondary antibody. For immunostaining of Brn3a, Islet-1, PH3, Pax6, and AP2α, sections were processed for antigen retrieval in a citrate buffer solution (10 mM trisodium citrate, 0.05% Tween-20) at 95°C for 3 min, blocked in a blocking solution (10% donkey serum, 0.3% Triton X-100 in PBS) for 1 h at room temperature, and treated with primary and secondary antibodies in an antibody dilution solution (0.3% Triton X-100, 1% bovine serum albumin in PBS). The following antibodies were used: anti-Brn3a (1:100, clone 5A3.2; Millipore, Billerica, MA), anti–Islet-1 (1:50, clone 39.4D5; Developmental Studies Hybridoma Bank [DSHB], University of Iowa, Iowa City, IA), anti-PH3 (1:100; Millipore), anti-syntaxin (1:2000; Sigma-Aldrich, St. Louis, MO), anti-Pax6 (1:200; DSHB), anti-PKCα (1:2000; Sigma-Aldrich), anti-vimentin (1:2000; DSHB), anti-rhodopsin (1:200; Sigma-Aldrich), anti-visinin (1:100; DSHB); anti–AP2-α (1:200; DSHB), Alexa Fluor 594–conjugated anti-mouse IgG (1:1,000; Invitrogen), and Alexa Fluor 488–conjugated anti-rabbit IgG (1:400; Invitrogen). The TUNEL assay was performed by using the In Situ Cell Death Detection Kit, TMR red (Roche Applied Science, Indianapolis, IN), according to the manufacturer's instructions. For quantification, the number of positive cells in transfected retinal regions was scored in a high-power optic field and compared with that in equivalent regions of the contralateral (untransfected) retinas or corresponding control areas. At least three fields were examined for each retina, and at least six retinas were analyzed for each type of treatment. Equal numbers of nasally and temporally transfected retinas were analyzed.
Statistical analyses
Statistical significance of the data was determined using analysis of variance (ANOVA) or Student's t test.
Supplementary Material
Acknowledgments
We thank John Flanagan and Lynda Erskine for discussions and critical reading of the manuscript; Lynda Erskine, Neil Vargesson, Bing Lang, and Freyja Bruce for help and advice on immunostaining and the TUNEL assay; and Yvonne Turnbull for technical assistance in the purchase and maintenance of chicken eggs. The pCAGGS-T2TP and pT2K-CAGGS vectors were generous gifts from Koichi Kawakami and Yoshiko Takahashi, respectively. The anti-Pax6 antibody (developed by A. Kawakami), anti–Islet-1 (39.4D5; by T. M. Jessell and S. Brenner-Morton), anti-vimentin (H5; by J. R. Sanes), anti-visinin (7G4; by C. Cepko), and anti–AP-2 alpha (3B5; by T. Williams) were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the National Institute of Child Health and Human Development and maintained by the Department of Biology, University of Iowa, Iowa City, IA. This work was supported by grants from the Royal Society and Biotechnology and Biological Sciences Research Council to M.N.
Abbreviations used:
- BDNF
brain-derived neurotrophic factor
- EGFP
enhanced green fluorescent protein
- EmGFP
emerald green fluorescent protein
- FGF
fibroblast growth factor
- GCL
ganglion cell layer
- HH
Hamburger Hamilton stage
- INL
inner nuclear layer
- miRNA
microRNA
- PEDF
pigment epithelium–derived factor
- PH3
phosphohistone H3
- RGC
retinal ganglion cell
- RNAi
RNA interference
- RPE
retinal pigment epithelium
- Shh
sonic hedgehog
- VEGF
vascular endothelial growth factor
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E13-08-0453) on November 20, 2013.
REFERENCES
- Adams JC, Lawler J. The thrombospondins. Cold Spring Harb Perspect Biol. 2011;3:a009712. doi: 10.1101/cshperspect.a009712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Agathocleous M, Harris WA. From progenitors to differentiated cells in the vertebrate retina. Annu Rev Cell Dev Biol. 2009;25:45–69. doi: 10.1146/annurev.cellbio.042308.113259. [DOI] [PubMed] [Google Scholar]
- Aihara K, Kuroda S, Kanayama N, Matsuyama S, Tanizawa K, Horie M. A neuron-specific EGF family protein, NELL2, promotes survival of neurons through mitogen-activated protein kinases. Brain Res Mol Brain Res. 2003;116:86–93. doi: 10.1016/s0169-328x(03)00256-0. [DOI] [PubMed] [Google Scholar]
- Austin CP, Feldman DE, Ida JA, Jr, Cepko CL. Vertebrate retinal ganglion cells are selected from competent progenitors by the action of Notch. Development. 1995;121:3637–3650. doi: 10.1242/dev.121.11.3637. [DOI] [PubMed] [Google Scholar]
- Cayouette M, Poggi L, Harris WA. Lineage in the vertebrate retina. Trends Neurosci. 2006;29:563–570. doi: 10.1016/j.tins.2006.08.003. [DOI] [PubMed] [Google Scholar]
- Cepko CL, Austin CP, Yang X, Alexiades M, Ezzeddine D. Cell fate determination in the vertebrate retina. Proc Natl Acad Sci USA. 1996;93:589–595. doi: 10.1073/pnas.93.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Iongh R, McAvoy JW. Distribution of acidic and basic fibroblast growth factors (FGF) in the foetal rat eye: implications for lens development. Growth Factors. 1992;6:159–177. [PubMed] [Google Scholar]
- Ehrlich D, Morgan IG. Kainic acid destroys displaced amacrine cells in post-hatch chicken retina. Neurosci Lett. 1980;17:43–48. doi: 10.1016/0304-3940(80)90059-2. [DOI] [PubMed] [Google Scholar]
- Fekete DM, Cepko CL. Replication-competent retroviral vectors encoding alkaline phosphatase reveal spatial restriction of viral gene expression/transduction in the chick embryo. Mol Cell Biol. 1993;13:2604–2613. doi: 10.1128/mcb.13.4.2604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan JG. Neural map specification by gradients. Curr Opin Neurobiol. 2006;16:59–66. doi: 10.1016/j.conb.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Frade JM, Bovolenta P, Martinez-Morales JR, Arribas A, Barbas JA, Rodriguez-Tebar A. Control of early cell death by BDNF in the chick retina. Development. 1997;124:3313–3320. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- Galvez JM, Puelles L, Prada C. Inverted (displaced) retinal amacrine cells and their embryonic development in the chick. Exp Neurol. 1977;56:151–157. doi: 10.1016/0014-4886(77)90145-5. [DOI] [PubMed] [Google Scholar]
- Hansen MJ, Dallal GE, Flanagan JG. Retinal axon response to ephrin-as shows a graded, concentration-dependent transition from growth promotion to inhibition. Neuron. 2004;42:717–730. doi: 10.1016/j.neuron.2004.05.009. [DOI] [PubMed] [Google Scholar]
- Harada H, Takahashi Y, Kawakami K, Ogura T, Nakamura H. Tracing retinal fiber trajectory with a method of transposon-mediated genomic integration in chick embryo. Dev Growth Differ. 2008;50:697–702. doi: 10.1111/j.1440-169X.2008.01067.x. [DOI] [PubMed] [Google Scholar]
- Harpavat S, Cepko CL. RCAS-RNAi: a loss-of-function method for the developing chick retina. BMC Dev Biol. 2006;6:2. doi: 10.1186/1471-213X-6-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Zhang XM, Chen BY, Yang XJ. VEGF activates divergent intracellular signaling components to regulate retinal progenitor cell proliferation and neuronal differentiation. Development. 2006;133:2201–2210. doi: 10.1242/dev.02385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt CE, Bertsch TW, Ellis HM, Harris WA. Cellular determination in the Xenopus retina is independent of lineage and birth date. Neuron. 1988;1:15–26. doi: 10.1016/0896-6273(88)90205-x. [DOI] [PubMed] [Google Scholar]
- Hu WY, Myers CP, Kilzer JM, Pfaff SL, Bushman FD. Inhibition of retroviral pathogenesis by RNA interference. Curr Biol. 2002;12:1301–1311. doi: 10.1016/s0960-9822(02)00975-2. [DOI] [PubMed] [Google Scholar]
- Isenmann S, Kretz A, Cellerino A. Molecular determinants of retinal ganglion cell development, survival, and regeneration. Prog Retin Eye Res. 2003;22:483–543. doi: 10.1016/s1350-9462(03)00027-2. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal Muller cell development and glutamine synthetase expression after removal of the retinal pigment epithelium. Glia. 2001;35:14–25. doi: 10.1002/glia.1066. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Ding Q, Xie X, Libby RT, Lefebvre V, Gan L. Transcription factors SOX4 and SOX11 function redundantly to regulate the development of mouse retinal ganglion cells. J Biol Chem. 2013;288:18429–18438. doi: 10.1074/jbc.M113.478503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Obama H, Kuan SL, Nakamura R, Nakamoto C, Ouyang Z, Nakamoto M. In vitro guidance of retinal axons by a tectal lamina-specific glycoprotein Nel. Mol Cell Neurosci. 2009;41:113–119. doi: 10.1016/j.mcn.2009.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katahira T, Nakamura H. Gene silencing in chick embryos with a vector-based small interfering RNA system. Dev Growth Differ. 2003;45:361–367. doi: 10.1046/j.1440-169x.2003.00705.x. [DOI] [PubMed] [Google Scholar]
- Koga A, Suzuki M, Inagaki H, Bessho Y, Hori H. Transposable element in fish. Nature. 1996;383:30. doi: 10.1038/383030a0. [DOI] [PubMed] [Google Scholar]
- Kuroda S, Oyasu M, Kawakami M, Kanayama N, Tanizawa K, Saito N, Abe T, Matsuhashi S, Ting K. Biochemical characterization and expression analysis of neural thrombospondin-1-like proteins NELL1 and NELL2. Biochem Biophys Res Commun. 1999;265:79–86. doi: 10.1006/bbrc.1999.1638. [DOI] [PubMed] [Google Scholar]
- Layer PG, Vollmer G. Lucifer yellow stains displaced amacrine cells of the chicken retina during embryonic development. Neurosci Lett. 1982;31:99–104. doi: 10.1016/0304-3940(82)90099-4. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Del Bene F, Nica G, Hammerschmidt M, Bovolenta P, Wittbrodt J. Differentiation of the vertebrate retina is coordinated by an FGF signaling center. Dev Cell. 2005;8:565–574. doi: 10.1016/j.devcel.2005.01.022. [DOI] [PubMed] [Google Scholar]
- Masai I, Yamaguchi M, Tonou-Fujimori N, Komori A, Okamoto H. The hedgehog-PKA pathway regulates two distinct steps of the differentiation of retinal ganglion cells: the cell-cycle exit of retinoblasts and their neuronal maturation. Development. 2005;132:1539–1553. doi: 10.1242/dev.01714. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K. New gene, nel, encoding a M(r) 93 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. 1995;203:212–222. doi: 10.1002/aja.1002030209. [DOI] [PubMed] [Google Scholar]
- Matsuhashi S, Noji S, Koyama E, Myokai F, Ohuchi H, Taniguchi S, Hori K. New gene, nel, encoding a Mr 91 K protein with EGF-like repeats is strongly expressed in neural tissues of early stage chick embryos. Dev Dyn. 1996;207:233–234. doi: 10.1002/aja.1012070202. [DOI] [PubMed] [Google Scholar]
- Matsuoka H, Obama H, Kelly ML, Matsui T, Nakamoto M. Biphasic functions of the kinase-defective Ephb6 receptor in cell adhesion and migration. J Biol Chem. 2005;280:29355x29363. doi: 10.1074/jbc.M500010200. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Aihara K, Nishino N, Takeda S, Tanizawa K, Kuroda S, Horie M. Enhanced long-term potentiation in vivo in dentate gyrus of NELL2-deficient mice. Neuroreport. 2004;15:417–420. doi: 10.1097/00001756-200403010-00007. [DOI] [PubMed] [Google Scholar]
- Matsuyama S, Doe N, Kurihara N, Tanizawa K, Kuroda S, Iso H, Horie M. Spatial learning of mice lacking a neuron-specific epidermal growth factor family protein, NELL2. J Pharmacol Sci. 2005;98:239–243. doi: 10.1254/jphs.fp0050211. [DOI] [PubMed] [Google Scholar]
- McCabe KL, Gunther EC, Reh TA. The development of the pattern of retinal ganglion cells in the chick retina: mechanisms that control differentiation. Development. 1999;126:5713–5724. doi: 10.1242/dev.126.24.5713. [DOI] [PubMed] [Google Scholar]
- McCabe KL, McGuire C, Reh TA. Pea3 expression is regulated by FGF signaling in developing retina. Dev Dyn. 2006;235:327–335. doi: 10.1002/dvdy.20631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin T, Hindges R, Yates PA, O'Leary DD. Bifunctional action of ephrin-B1 as a repellent and attractant to control bidirectional branch extension in dorsal-ventral retinotopic mapping. Development. 2003;130:2407–2418. doi: 10.1242/dev.00467. [DOI] [PubMed] [Google Scholar]
- Moore SW, Tessier-Lavigne M, Kennedy TE. Netrins and their receptors. Adv Exp Med Biol. 2007;621:17–31. doi: 10.1007/978-0-387-76715-4_2. [DOI] [PubMed] [Google Scholar]
- Munemasa Y, Chang CS, Kwong JM, Kyung H, Kitaoka Y, Caprioli J, Piri N. The neuronal EGF-related gene Nell2 interacts with Macf1 and supports survival of retinal ganglion cells after optic nerve injury. PLoS One. 2012;7:e34810. doi: 10.1371/journal.pone.0034810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura R, Nakamoto C, Obama H, Durward E, Nakamoto M. Structure-function analysis of Nel, a thrombospondin-1-like glycoprotein involved in neural development and functions. J Biol Chem. 2012;287:3282–3291. doi: 10.1074/jbc.M111.281485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BR, Claes K, Todd V, Chaverra M, Lefcort F. NELL2 promotes motor and sensory neuron differentiation and stimulates mitogenesis in DRG in vivo. Dev Biol. 2004;270:322–335. doi: 10.1016/j.ydbio.2004.03.004. [DOI] [PubMed] [Google Scholar]
- Nelson BR, Matsuhashi S, Lefcort F. Restricted neural epidermal growth factor-like like 2 (NELL2) expression during muscle and neuronal differentiation. Mech Dev. 2002;119(Suppl 1):S11–S19. doi: 10.1016/s0925-4773(03)00084-4. [DOI] [PubMed] [Google Scholar]
- Oyasu M, Kuroda S, Nakashita M, Fujimiya M, Kikkawa U, Saito N. Immunocytochemical localization of a neuron-specific thrombospondin-1-like protein, NELL2: light and electron microscopic studies in the rat brain. Brain Res Mol Brain Res. 2000;76:151–160. doi: 10.1016/s0169-328x(99)00342-3. [DOI] [PubMed] [Google Scholar]
- Patel A, McFarlane S. Overexpression of FGF-2 alters cell fate specification in the developing retina of Xenopus laevis. Dev Biol. 2000;222:170–180. doi: 10.1006/dbio.2000.9695. [DOI] [PubMed] [Google Scholar]
- Prada C, Puga J, Perez-Mendez L, Lopez R, Ramirez G. Spatial and temporal patterns of neurogenesis in the chick retina. Eur J Neurosci. 1991;3:559–569. doi: 10.1111/j.1460-9568.1991.tb00843.x. [DOI] [PubMed] [Google Scholar]
- Price J, Turner D, Cepko C. Lineage analysis in the vertebrate nervous system by retrovirus-mediated gene transfer. Proc Natl Acad Sci USA. 1987;84:156–160. doi: 10.1073/pnas.84.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachel RA, Dolen G, Hayes NL, Lu A, Erskine L, Nowakowski RS, Mason CA. Spatiotemporal features of early neuronogenesis differ in wild-type and albino mouse retina. J Neurosci. 2002;22:4249–4263. doi: 10.1523/JNEUROSCI.22-11-04249.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- Sato Y, Kasai T, Nakagawa S, Tanabe K, Watanabe T, Kawakami K, Takahashi Y. Stable integration and conditional expression of electroporated transgenes in chicken embryos. Dev Biol. 2007;305:616–624. doi: 10.1016/j.ydbio.2007.01.043. [DOI] [PubMed] [Google Scholar]
- Snow RL, Robson JA. Migration and differentiation of neurons in the retina and optic tectum of the chick. Exp Neurol. 1995;134:13–24. doi: 10.1006/exnr.1995.1032. [DOI] [PubMed] [Google Scholar]
- Stiemke MM, Landers RA, al-Ubaidi MR, Rayborn ME, Hollyfield JG. Photoreceptor outer segment development in Xenopus laevis: influence of the pigment epithelium. Dev Biol. 1994;162:169–180. doi: 10.1006/dbio.1994.1076. [DOI] [PubMed] [Google Scholar]
- Strauss O. The retinal pigment epithelium in visual function. Physiol Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- Wang JT, Kunzevitzky NJ, Dugas JC, Cameron M, Barres BA, Goldberg JL. Disease gene candidates revealed by expression profiling of retinal ganglion cell development. J Neurosci. 2007;27:8593–8603. doi: 10.1523/JNEUROSCI.4488-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe TK, Katagiri T, Suzuki M, Shimizu F, Fujiwara T, Kanemoto N, Nakamura Y, Hirai Y, Maekawa H, Takahashi E. Cloning and characterization of two novel human cDNAs (NELL1 and NELL2) encoding proteins with six EGF-like repeats. Genomics. 1996;38:273–276. doi: 10.1006/geno.1996.0628. [DOI] [PubMed] [Google Scholar]
- Wetts R, Fraser SE. Multipotent precursors can give rise to all major cell types of the frog retina. Science. 1988;239:1142–1145. doi: 10.1126/science.2449732. [DOI] [PubMed] [Google Scholar]
- Xiang M, Zhou L, Macke JP, Yoshioka T, Hendry SH, Eddy RL, Shows TB, Nathans J. The Brn-3 family of POU-domain factors: primary structure, binding specificity, and expression in subsets of retinal ganglion cells and somatosensory neurons. J Neurosci. 1995;15:4762–4785. doi: 10.1523/JNEUROSCI.15-07-04762.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee JK, Moores JC, Jolly DJ, Wolff JA, Respess JG, Friedmann T. Gene expression from transcriptionally disabled retroviral vectors. Proc Natl Acad Sci USA. 1987;84:5197–5201. doi: 10.1073/pnas.84.15.5197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Regulation of retinal ganglion cell production by Sonic hedgehog. Development. 2001a;128:943–957. doi: 10.1242/dev.128.6.943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XM, Yang XJ. Temporal and spatial effects of Sonic hedgehog signaling in chick eye morphogenesis. Dev Biol. 2001b;233:271–290. doi: 10.1006/dbio.2000.0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.