Abstract
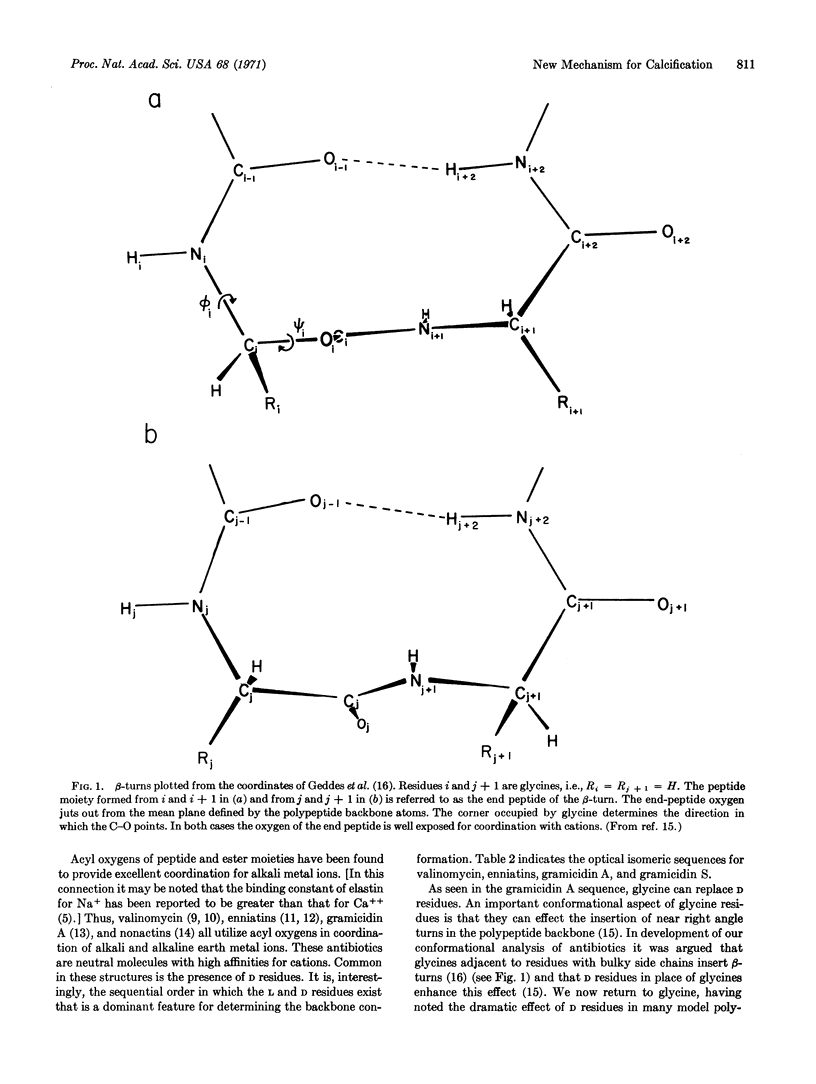
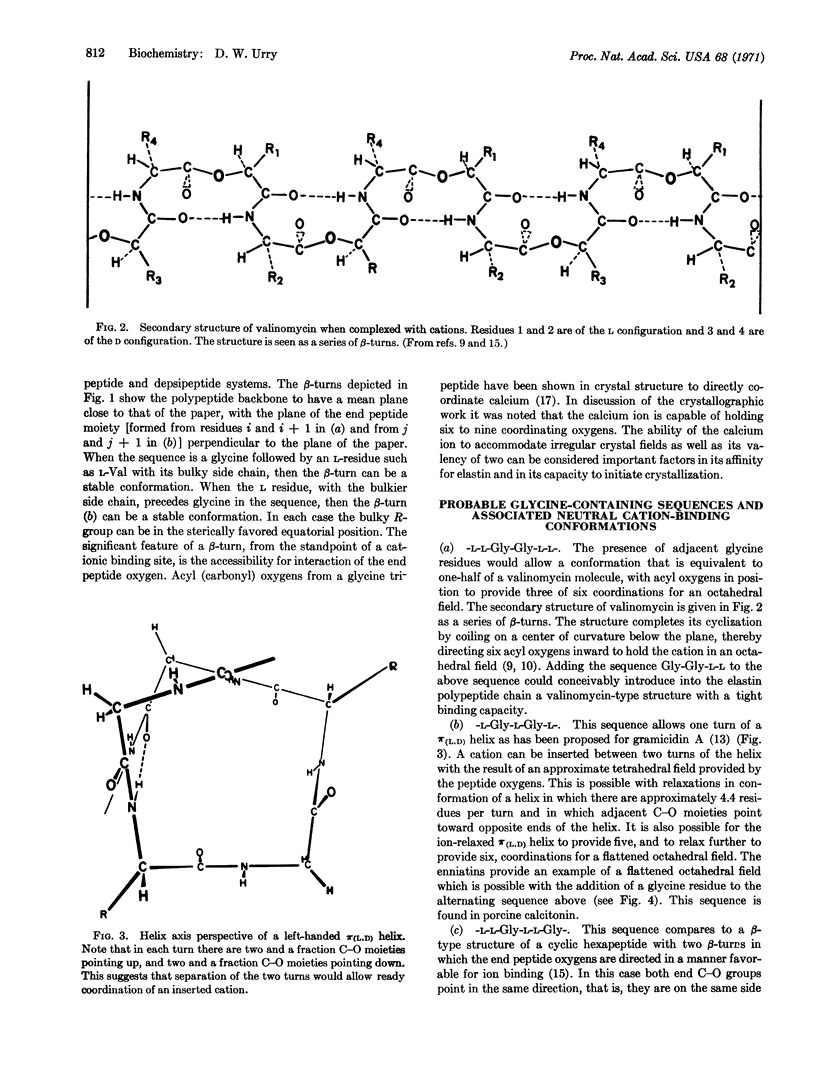
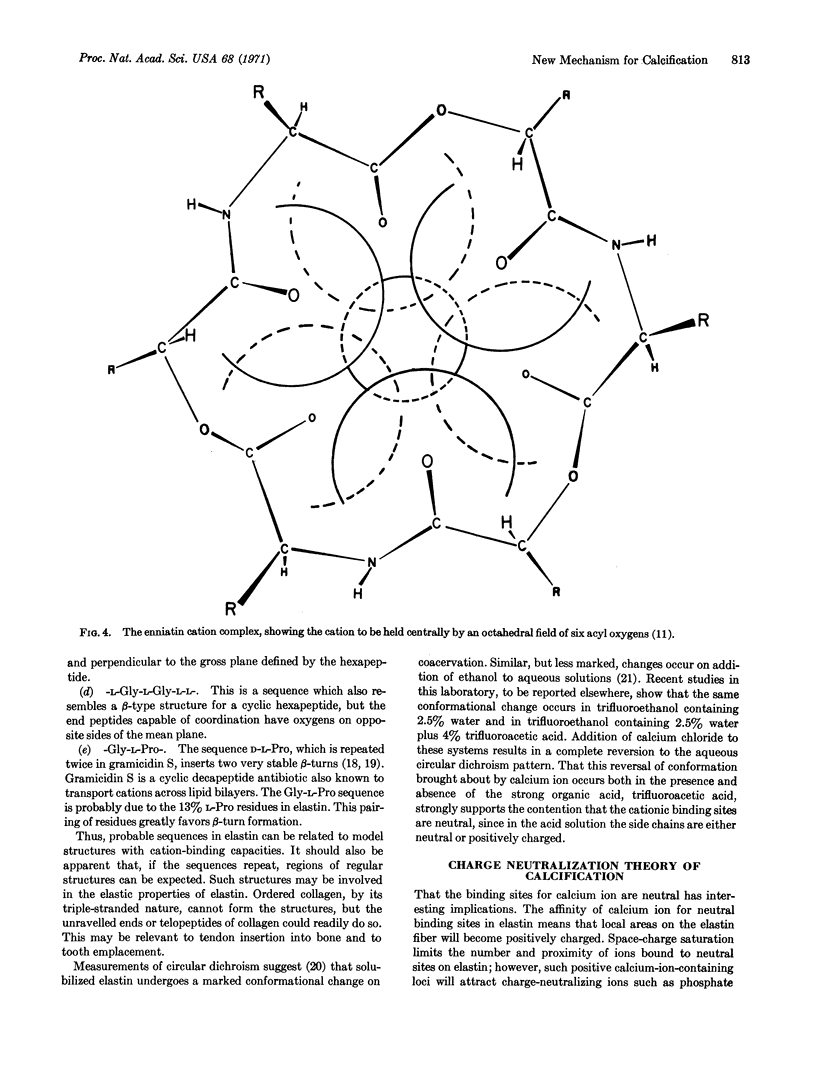
Neutral, uncharged binding sites for calcium ions are proposed for elastin and collagen. The sites utilize, particularly from a conformational viewpoint, the most striking feature of the amino acid composition, that is, the high glycine content. Glycines favor the formation of β-turns and associated conformations that are known, from studies on ion-transporting antibiotics, to interact with cations. By analogy with certain antibiotics, which are uncharged polypeptides and depsipeptides that bind cations by coordination with neutral acyl oxygens, it is proposed that calcium-ion binding also utilizes uncharged coordinating groups, i.e., neutral sites, in the protein matrix. The protein matrix, which becomes positively charged by virtue of the bound calcium ions, attracts neutralizing phosphate and carbonate ions, which then allow further calcium ion binding. The driving force is, therefore, the affinity of calcium ions for the neutral nucleation sites.
The charge neutralization theory of calcification suggests a fundamental role of organic anions, for example sulfated mucopolysaccharides, in regulating bone formation and in retardation of atherosclerosis. The proposed mechanism contains elements that tend to unify several theories on the pathogenesis of atherosclerosis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dobler M., Dunitz J. D., Krajewski J. Structure of the K+ complex with enniatin B, a macrocyclic antibiotic with K+ transport properties. J Mol Biol. 1969 Jun 28;42(3):603–606. doi: 10.1016/0022-2836(69)90249-6. [DOI] [PubMed] [Google Scholar]
- Franzblau C., Lent R. W. Studies on the chemistry of elastin. Brookhaven Symp Biol. 1968 Jun;21(2):358–377. [PubMed] [Google Scholar]
- Geddes A. J., Parker K. D., Atkins E. D., Beighton E. "Cross-beta" conformation in proteins. J Mol Biol. 1968 Mar 14;32(2):343–358. doi: 10.1016/0022-2836(68)90014-4. [DOI] [PubMed] [Google Scholar]
- HALL D. A. The reaction between elastase and elastic tissue. 1. The substrate. Biochem J. 1955 Mar;59(3):459–465. doi: 10.1042/bj0590459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilbourn B. T., Dunitz J. D., Pioda L. A., Simon W. Structure of the K+ complex with nonactin, a macrotetrolide antibiotic possessing highly specific K+ transport properties. J Mol Biol. 1967 Dec 28;30(3):559–563. doi: 10.1016/0022-2836(67)90370-1. [DOI] [PubMed] [Google Scholar]
- Mammi M., Gotte L., Pezzin G. Evidence for order in the structure of alpha-elastin. Nature. 1968 Oct 26;220(5165):371–373. doi: 10.1038/220371b0. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Urry D. W. Solution conformation of valinomycin-potassium ion complex. Science. 1970 May 29;168(3935):1091–1092. doi: 10.1126/science.168.3935.1091. [DOI] [PubMed] [Google Scholar]
- Ohnishi M., Urry D. W. Temperature dependence of amide proton chemical shifts: the secondary structures of gramicidin S and valinomycin. Biochem Biophys Res Commun. 1969 Jul 23;36(2):194–202. doi: 10.1016/0006-291x(69)90314-3. [DOI] [PubMed] [Google Scholar]
- Ovchinnikov Y. A., Ivanov V. T., Evstratov A. V., Bystrov V. F., Abdullaev N. D., Popov E. M., Lipkind G. M., Arkhipova S. F., Efremov E. S., Shemyakin M. M. The physicochemical basis of the functioning of biological membranes: dynamic conformational properties of enniatin B and its K+ complex in solution. Biochem Biophys Res Commun. 1969 Nov 6;37(4):668–676. doi: 10.1016/0006-291x(69)90863-8. [DOI] [PubMed] [Google Scholar]
- Petruska J. A., Sandberg L. B. The amino acid composition of elastin in its soluble and insoluble state. Biochem Biophys Res Commun. 1968 Oct 24;33(2):222–228. doi: 10.1016/0006-291x(68)90772-9. [DOI] [PubMed] [Google Scholar]
- Pinkerton M., Steinrauf L. K., Dawkins P. The molecular structure and some transport properties of valinomycin. Biochem Biophys Res Commun. 1969 May 22;35(4):512–518. doi: 10.1016/0006-291x(69)90376-3. [DOI] [PubMed] [Google Scholar]
- Schiffmann E., Corcoran B. A., Martin G. R. The role of complexed heavy metals in initiating the mineralizing of "elastin" and the precipitation of mineral from solution. Arch Biochem Biophys. 1966 Jul;115(1):87–94. doi: 10.1016/s0003-9861(66)81042-1. [DOI] [PubMed] [Google Scholar]
- Stern A., Gibbons W. A., Craig L. C. A conformational analysis of gramicidin S-A by nuclear magnetic resonance. Proc Natl Acad Sci U S A. 1968 Oct;61(2):734–741. doi: 10.1073/pnas.61.2.734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urry D. W., Starcher B., Partridge S. M. Coacervation of solubilized elastin effects a notable conformational change. Nature. 1969 May 24;222(5195):795–796. doi: 10.1038/222795a0. [DOI] [PubMed] [Google Scholar]
- Urry D. W. The gramicidin A transmembrane channel: a proposed pi(L,D) helix. Proc Natl Acad Sci U S A. 1971 Mar;68(3):672–676. doi: 10.1073/pnas.68.3.672. [DOI] [PMC free article] [PubMed] [Google Scholar]


