Abstract
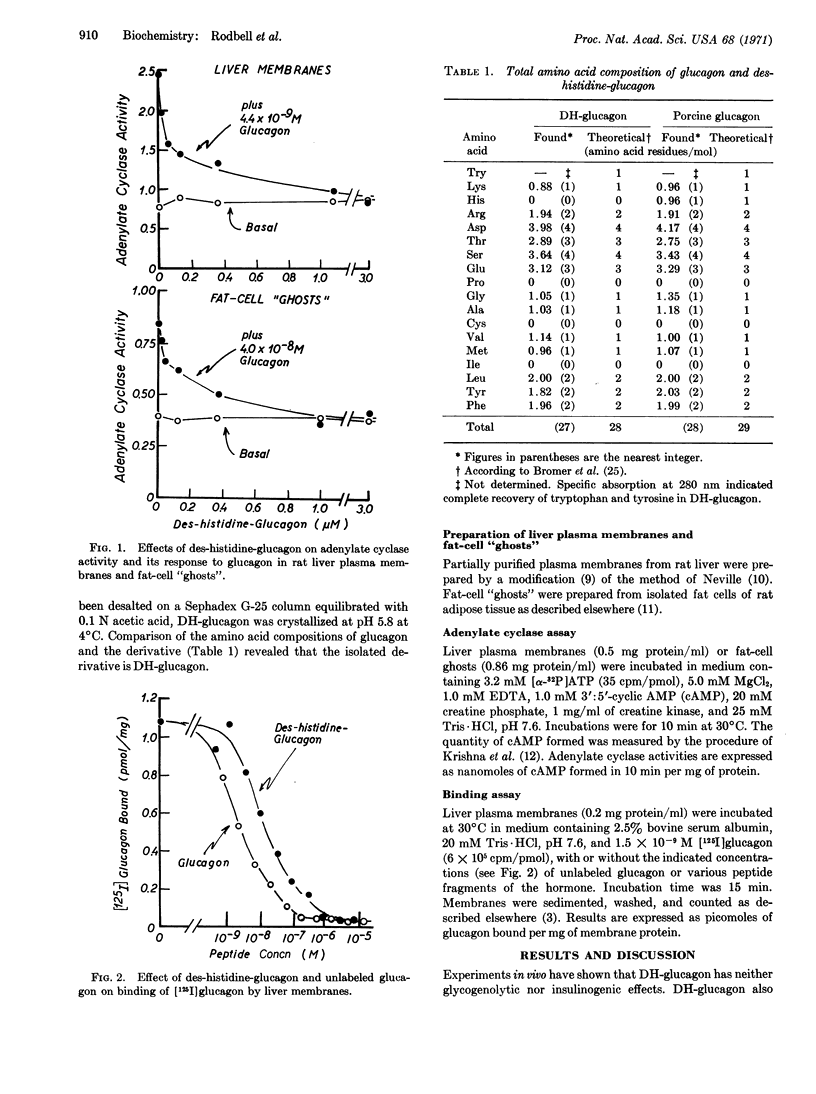
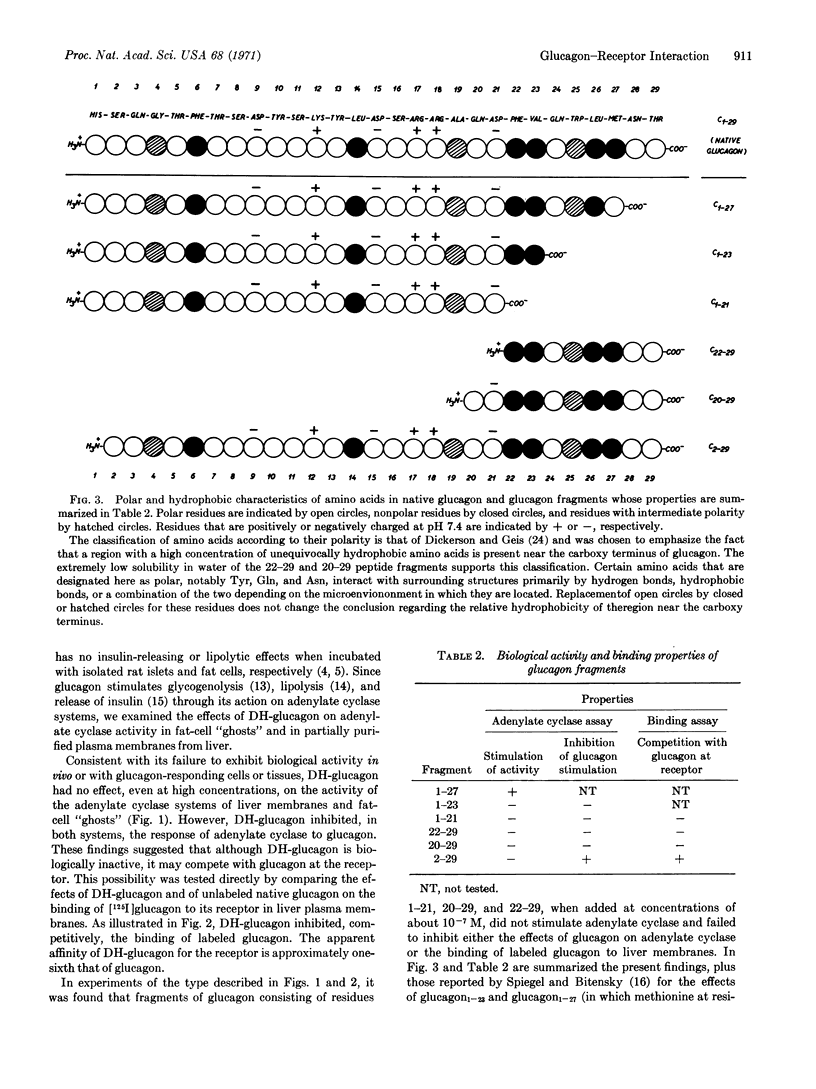
Des-histidine-glucagon (DH-glucagon, glucagon2-29) does not activate the glucagon-sensitive adenylate cyclase system present in either liver plasma membranes or in fat-cell “ghosts”, but inhibits the response of these systems to submaximal concentrations of glucagon. DH-glucagon also inhibits, competitively, the binding of [125I]glucagon to its receptor in liver plasma membranes. Amino-terminal fragments of glucagon (glucagon1-21, glucagon1-23) and carboxy-terminal fragments (glucagon20-29, glucagon22-29) failed to activate adenylate cyclase, to inhibit the response of the enzyme to glucagon, or to compete with labeled glucagon at its receptor.
It is concluded that the amino-terminal histidine residue of glucagon is essential for biological activity and that a hydrophobic near-carboxy-terminal region (residues 22-27) is essential for binding of glucagon to its receptor. Amino-terminal histidine may also contribute to the binding of glucagon, since the apparent affinity of DH-glucagon for the receptor is only about one-sixth that of glucagon. Thus, essentially the entire molecule of glucagon must be considered to be the biologically active species.
Because, as shown elsewhere, the binding of glucagon to its receptor shows characteristics of hydrophobic bonding, and because certain detergents induce conformational changes in the carboxy-terminal binding region of glucagon, the binding is probably of a lipophilic type.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Birnbaumer L., Pohl S. L., Rodbell M. Adenyl cyclase in fat cells. 1. Properties and the effects of adrenocorticotropin and fluoride. J Biol Chem. 1969 Jul 10;244(13):3468–3476. [PubMed] [Google Scholar]
- Birnbaumer L., Rodbell M. Adenyl cyclase in fat cells. II. Hormone receptors. J Biol Chem. 1969 Jul 10;244(13):3477–3482. [PubMed] [Google Scholar]
- Edelhoch H., Lippoldt R. E. Structural studies on polypeptide hormones. I. Fluorescence. J Biol Chem. 1969 Jul 25;244(14):3876–3883. [PubMed] [Google Scholar]
- Gratzer W. B., Beaven G. H., Rattle H. W., Bradbury E. M. A conformational study of glucagon. Eur J Biochem. 1968 Jan;3(3):276–283. doi: 10.1111/j.1432-1033.1968.tb19527.x. [DOI] [PubMed] [Google Scholar]
- Hofmann K., Wingender W., Finn F. M. Correlation of adrenocorticotropic activity of ACTH analogs with degree of binding to an adrenal cortical particulate preparation. Proc Natl Acad Sci U S A. 1970 Oct;67(2):829–836. doi: 10.1073/pnas.67.2.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna G., Weiss B., Brodie B. B. A simple, sensitive method for the assay of adenyl cyclase. J Pharmacol Exp Ther. 1968 Oct;163(2):379–385. [PubMed] [Google Scholar]
- Mutt V., Jorpes J. E. Contemporary developments in the biochemistry of the gastrointestinal hormones. Recent Prog Horm Res. 1967;23:483–503. doi: 10.1016/b978-1-4831-9826-2.50014-3. [DOI] [PubMed] [Google Scholar]
- Neville D. M., Jr Isolation of an organ specific protein antigen from cell-surface membrane of rat liver. Biochim Biophys Acta. 1968 Apr 9;154(3):540–552. doi: 10.1016/0005-2795(68)90014-7. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. Glucagon-sensitive adenyl cylase in plasma membrane of hepatic parenchymal cells. Science. 1969 May 2;164(3879):566–567. doi: 10.1126/science.164.3879.566. [DOI] [PubMed] [Google Scholar]
- Pohl S. L., Birnbaumer L., Rodbell M. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. I. Properties. J Biol Chem. 1971 Mar 25;246(6):1849–1856. [PubMed] [Google Scholar]
- RALL T. W., SUTHERLAND E. W., WOSILAIT W. D. The relationship of epinephrine and glucagon to liver phosphorylase. III. Reactivation of liver phosphorylase in slices and in extracts. J Biol Chem. 1956 Jan;218(1):483–495. [PubMed] [Google Scholar]
- Rodbell M., Birnbaumer L., Pohl S. L. Adenyl cyclase in fat cells. 3. Stimulation by secretin and the effects of trypsin on the receptors for lipolytic hormones. J Biol Chem. 1970 Feb 25;245(4):718–722. [PubMed] [Google Scholar]
- Rodbell M., Krans H. M., Pohl S. L., Birnbaumer L. The glucagon-sensitive adenyl cyclase system in plasma membranes of rat liver. 3. Binding of glucagon: method of assay and specificity. J Biol Chem. 1971 Mar 25;246(6):1861–1871. [PubMed] [Google Scholar]
- Rodbell M. Metabolism of isolated fat cells. V. Preparation of "ghosts" and their properties; adenyl cyclase and other enzymes. J Biol Chem. 1967 Dec 25;242(24):5744–5750. [PubMed] [Google Scholar]
- SCHERAGA H. A., NEMETHY G., STEINBERG I. Z. The contribution of hydrophobic bonds to the thermal stability of protein conformations. J Biol Chem. 1962 Aug;237:2506–2508. [PubMed] [Google Scholar]
- Samols E., Marri G., Marks V. Interrelationship of glucagon, insulin and glucose. The insulinogenic effect of glucagon. Diabetes. 1966 Dec;15(12):855–866. doi: 10.2337/diab.15.12.855. [DOI] [PubMed] [Google Scholar]
- Spiegel A. M., Bitensky M. W. Effects of chemical and enzymatic modifications of glucagon on its activation of hepatic adenyl cyclase. Endocrinology. 1969 Oct;85(4):638–643. doi: 10.1210/endo-85-4-638. [DOI] [PubMed] [Google Scholar]


