Abstract
The central role of the Bcl-2 family in regulating apoptotic cell death was first identified in the 1980s. Since then, significant in-roads have been made in identifying the multiple members of this family, characterizing their form and function and understanding how their interactions determine whether a cell lives or dies. In this review we focus on the recent progress made in characterizing the proapoptotic Bcl-2 family members, Bax and Bak. This progress has resolved longstanding controversies, but has also challenged established theories in the apoptosis field. We will discuss different models of how these two proteins become activated and different ‘modes' by which they are inhibited by other Bcl-2 family members. We will also discuss novel conformation changes leading to Bak and Bax oligomerization and speculate how these oligomers might permeabilize the mitochondrial outer membrane.
Keywords: apoptosis, Bak, Bax, conformation change, oligomerization
Facts
Bax and Bak are activated by BH3-only proteins and inhibited by prosurvival Bcl-2 proteins via direct interactions.
Bax and Bak undergo major conformation changes during transition from inactive monomers to activated oligomers.
Bax and Bak oligomers are responsible for permeabilization of the mitochondrial outer membrane (MOM).
Open Questions
Are both proposed binding sites on Bax essential for its activation by BH3-only proteins?
What molecular features present in Bax and Bak but not in the prosurvival proteins allow conformation change, oligomerization and pore formation?
Does Bax and Bak pore formation involve insertion of their α5/α6 helices as a membrane-spanning hairpin?
What protein:protein interfaces allow Bax and Bak to form high molecular weight oligomeric pores?
The Bcl-2 Family: Guardians at the ‘Mitochondrial Gate'
Mitochondria have a critical role in cell survival through their generation of ATP via oxidative phosphorylation. However, these organelles also have a dark side, as lurking at their surface are the Bcl-2 family of proteins that second mitochondria into the cell death pathway. Two key members of the Bcl-2 family are the proapoptotic proteins Bax and Bak, which convert from harmless monomers into deadly oligomers that form pores in the MOM. These pores are conduits for proapoptotic factors such as cytochrome c to translocate to the cytoplasm. The result is twofold: the loss of cytochrome c from mitochondria disables energy production, and cytosolic cytochrome c instigates a proteolytic cascade that dismantles the cell.1
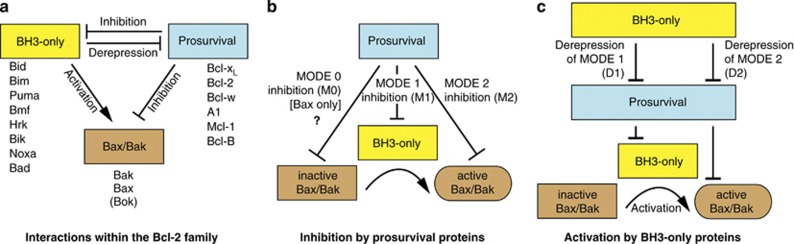
Given their critical role in mediating mitochondrial apoptosis,2, 3 Bax and Bak have to be strictly regulated by other members of the Bcl-2 family (Figure 1a). At least five mammalian ‘prosurvival' proteins have been identified that can inhibit Bax and Bak via three distinct mechanisms: MODE 0, MODE 1 and MODE 2 (Figure 1b). MODE 1 and MODE 2 were recently assigned to describe the indirect and direct inhibition of Bax and Bak, respectively.4 Here we assign MODE 0 to the newly described mechanism in healthy cells by which prosurvival proteins regulate Bax mitochondrial localization. On the other hand, eight or more proapoptotic ‘BH3-only' proteins have been found to initiate apoptosis by triggering Bax and Bak activation (Figure 1c). Historically, BH3-only proteins have been placed into discrete subsets, with those able to directly activate Bax and Bak termed ‘activators' and those that target prosurvival proteins to indirectly activate Bax and Bak called ‘sensitizers'.5 However, recent evidence suggests that this strict categorization may no longer be appropriate.6, 7, 8, 9, 10 We therefore discuss BH3-only proteins only by their ability to either ‘activate' Bax and Bak (Box 1) or ‘derepress' prosurvival proteins.
Figure 1.
Schematic representation of the Bcl-2 family interaction network. (a) The Bcl-2 family can be divided into three classes: the proapoptotic Bax/Bak proteins, the proapoptotic BH3-only proteins and the prosurvival proteins. The prosurvival proteins inhibit the activity of the proapoptotic Bcl-2 family members. The BH3-only proteins activate Bax and Bak either directly (activation) or indirectly (derepression). Despite Bok's amino-acid sequence similarity to Bax and Bak, its apoptotic role is currently unclear.95 (b) Inhibition by prosurvival proteins may occur via three ‘MODES'. During MODE 0 inhibition (M0), prosurvival proteins such as Bcl-xL bind to peripheral Bax at mitochondria and retro-translocate it to the cytosol. During MODE 1 inhibition (M1), prosurvival proteins sequester BH3-only proteins to stop them activating Bax and Bak. During MODE 2 inhibition (M2), prosurvival proteins bind to activated Bax and Bak to prevent their homo-oligomerization. (c) The BH3-only proteins can cause Bax and Bak activation in two ways. While certain BH3-only proteins can directly bind to and activate Bax and Bak, other BH3-only proteins interact with prosurvival proteins to compete off the activating BH3-only proteins (Derepression of MODE 1, D1) or activated Bax/Bak (Derepression of MODE 2, D2)
Box 1. Molecular features of activating BH3-only proteins.
In early studies, only Bid and Bim were categorized as BH3-only proteins that can activate Bax and Bak.5 This has been challenged with evidence that Puma can activate Bax,6, 96 that Noxa can bind to and induce Bak activation7 and that peptides from all BH3-only members can trigger Bax- and Bak-mediated permeabilization of liposomes if used at high concentrations.10
Recent structural and mutational studies allow a more detailed definition of which residues in the BH3 domain promote binding and activation of Bax53 and Bak.55 In general, BH3-only proteins bind in a similar manner to the grooves of Bax/Bak and the prosurvival proteins. Specifically, as seen in the BidBH3 peptide binding to Bax, four hydrophobic residues (h1–h4) in the BH3 make contact with four hydrophobic pockets in the groove, and a salt bridge forms between a conserved aspartate (BidD95) and a conserved arginine (BaxR109) (see Figure). Notably, a new h0 position in Bid and Bim as well as Noxa and Bad contributes to their activating function.53, 55
Mutagenesis has indicated that both the hydrophobic interactions and the salt bridge are essential for the binding of activating BH3 peptides to the Bax groove.53, 55 A single substitution of the h1 residue in the Noxa BH3 domain (NoxaC25I) was sufficient to turn Noxa into an activating BH3 peptide,53 whereas Bad required at least three substitutions to be able to activate Bax and Bak.53, 55 At the other end of the BH3 domain (beyond h4), there is little contact with Bax, and mutation did not affect binding to Bax.8, 53 However substitution at the h5 of Bad and Noxa seemed to contribute to their gain-of-function activity for Bak.55 In addition, the glycine in the GDE sequence was important, as Bid G94A lost binding to Bax52 and the Bad S118G change contributed to its gain-of-function for Bax and Bak.53, 55 Finally, BidA91W lost its ability to activate Bak.55 Together the data suggest that not one single residue but rather a combination of residues determines whether a BH3-only protein can activate Bax and Bak.
Binding of BH3 peptides to the rear pocket of Bax requires at least some of the above described residues,6, 35, 36, 97 consistent with the rear pocket and hydrophobic groove displaying a similar distribution of charge and hydrophobicity. Further analysis may identify mutations that distinguish between the two potential activation sites in Bax to better understand their role in triggering Bax anchorage into the membrane versus Bax conformation change and oligomerization.

BH3 domain residues involved in binding to the groove of Bax or Bak. (a) Surface representation of a Bax structure that has a BidBH3 peptide bound to the Bax groove (4BD2). Note that the hydrophobic residues (gray side chains) and the aspartate residue (red side chain) in the BidBH3 peptide make contact with the hydrophobic pockets and the arginine (indicated in blue) in the groove of Bax, respectively. (b) Sequence alignment of the indicated BH3 domains, highlighting five conserved hydrophobic residues (gray bars) and the invariant aspartate (red bar). Residues in orange are discussed in the text
Dys-regulated Bcl-2 proteins are associated with a plethora of diseases, making the Bcl-2 family members and their interactions a focus for novel therapeutics.11, 12 BH3 mimetics such as ABT737, its derivative ABT263, and the newly developed ABT199, which bind and inhibit prosurvival Bcl-2 proteins, provide the stepping stones towards customized, selective drugs that activate the apoptotic program in cancer cells13, 14, 15 and have encouraged the search for anticancer drugs that directly activate Bax and Bak.16
Conversely, as excess apoptosis contributes to ischemia reperfusion injury and diseases such as amyotrophic lateral sclerosis17, 18 there is interest in developing inhibitors of Bax and Bak.19 Although treating chronic degenerative conditions may not be tractable, acute inhibition of apoptosis, for example in preventing reperfusion injury following organ transplantation, may be of significant benefit. Thus, defining the regulatory landscape that exquisitely controls Bax and Bak will unlock the clinical potential of these critical effector proteins.
In this review, we focus on recent insights into the structural transitions of Bax and Bak from inactive monomers to active oligomers, and the major checkpoints employed by the cell to regulate this transition. The findings are summarized in a model that incorporates both biochemical and structural data supporting the transitions (Figure 2).
Figure 2.
Bax and Bak transitions are regulated by other Bcl-2 family members. (a) Schematic representation of the Bax/Bak protein sequence indicating the location of the nine α-helices. Helices of interest are colored in red (α2), green (α5), black (α9) or blue (latch helices α6, α7, α8). In addition, the four BH domains and regions that constitute the groove and rear pocket are marked. (b) Schematic representation of a model for Bax and Bak activation and oligomerization. Bax and Bak proteins are represented as cylindrical bundles using the same color scheme as in (a). Bax in healthy cells: peripheral Bax shuttles between the MOM and cytosol mediated by prosurvival Bcl-2 proteins (blue box) (MODE 0 inhibition, M0). Note that in healthy cells a tail-anchored population of Bax has also been observed to retro-translocate independent of prosurvival Bcl-2 proteins. Bak is constitutively inserted in the MOM in healthy cells. Bax/Bak activation: BH3-only proteins (yellow box) activate Bax and Bak and cause α9 exposure and membrane insertion (Bax only), α2/BH3 domain exposure, N-terminal exposure, α1/α2 loop displacement and core/latch dissociation. To prevent Bax and Bak activation, prosurvival proteins sequester the activating BH3-only proteins (MODE 1 inhibition, M1). In turn, certain BH3-only proteins promote apoptosis by binding the prosurvival proteins and thereby compete off the activating BH3-only proteins (Derepression of MODE 1, D1). Bax/Bak dimer/oligomerization: activated Bax and Bak dimerize by interaction of the exposed BH3 domain of one molecule with the groove of a second molecule and vice versa. These symmetric dimers further oligomerize to form a complex of unknown size (n) that permeabilizes the membrane. By directly binding and sequestering activated Bax and Bak, prosurvival Bcl-2 proteins prevent dimerization (MODE 2 inhibition, M2). In turn, BH3-only proteins interfere with the prosurvival inhibition by binding the prosurvival proteins and thereby competing off activated Bax and Bak (Derepression of MODE 2, D2)
Bax and Bak in Healthy Cells: Wolves in Sheep's Clothing
Like the prosurvival proteins, Bax and Bak protein sequences contain all four Bcl-2 homology (BH) domains, including the re-defined BH4 domain20 (Figure 2a). In addition, several structures of inactive Bax and Bak21, 22, 23 reveal that the protein fold of the two proapoptotic proteins is strikingly similar to that of their prosurvival counterparts.24, 25 Thus, based on sequence and structure, what distinguishes Bax and Bak from the prosurvival Bcl-2 proteins is unclear.
Bax and Bak contain nine α-helices, with a hydrophobic α5 at the protein core, surrounded by amphipathic helices (Figures 2 and 3a). The C-terminal α9 helix contains a transmembrane domain (TM) that anchors the proteins in the MOM. The hydrophobic BH3 domain, located in α2, is nicely tucked away in inactive Bax and Bak and becomes exposed during activation to facilitate hetero- and homo-oligomerization.9, 26, 27 In addition, a hydrophobic groove is located on the surface of Bax and Bak involving residues from the C terminus of α2 to the N terminus of α5 and residues in α8. Notably, a similar surface groove in the prosurvival proteins has been characterized as the receptor site for the BH3 domain of proapoptotic Bcl-2 members.28, 29, 30, 31, 32, 33, 34 Given the importance of this structural motif in regulating protein–protein interactions of the prosurvival proteins, one may predict that Bax/Bak regulation could also occur via binding of proapoptotic BH3 domains to the groove of Bax and Bak. However, in inactive Bax, the groove is normally occupied by its own TM.22 Interestingly, on the opposite side of Bax, and masked by the α1/α2 loop, is a shallower groove or ‘rear pocket' involving α1/α6 helices (Figures 2a and 3a). As this rear pocket has a similar distribution of hydrophobicity and charge as the canonical surface groove, it may be a trigger site for Bax activation.35, 36
Figure 3.
Binding of BH3 peptides to the groove and rear pocket of Bax promotes conformation change and oligomerization. Cartoon and surface overlays of Bax structures and models that support the transitions of Bax and Bak are described in Figure 2. Color scheme of the helices as in Figure 2. (a) NMR structure of the full-length Bax monomer (1F16). Note, the locations of the hydrophobic surface groove, rear pocket, BH3 domain and TM are indicated. (b) Model of a BimBH3 peptide (magenta tube) bound to the rear pocket of full-length Bax calculated from NMR data. Note that binding of the BimBH3 peptide displaced the α1/α2 loop (red arrow). BimBH3 binding coincided with the exposure of the N-terminal 6A7 epitope (marked in yellow), α9 helix and the BH3 domain (black arrows). (c) Crystal structure of a BidBH3 peptide (magenta tube) bound to the groove of BaxΔC21 (4BD2). Note that BidBH3 peptide binding induced partial α2 displacement (short red arrow) and dissociation of the C-terminal α6–α8 helices (latch) from the N-terminal α1–α5 helices (core) (long red arrow). (d) Crystal structure of the α2–α5 Bax ‘core' forming a BH3:groove dimer (4BDU). Note that aromatic residues (dark gray) on helices α4 and α5 form a lipophilic surface. Although the lipophilic surface is concave in the homodimer structure, whether this curvature is retained when Bax is associated with the MOM and whether the curvature is important for membrane permeabilization is unknown. (e) Overlay of the Bax:BidBH3 complex (colored:magenta, from c) with one half of the Bax BH3:groove dimer (in gray, from d). Note, to form the BH3:groove dimer, no major rearrangements of the ‘core' helices occur other than the exposure of the BH3 domain (α2, red arrow)
Sequestration of the TM of Bax into its own groove renders the protein mainly cytosolic in healthy cells. However, a small portion of Bax is loosely attached to mitochondria,37, 38, 39 as it can be extracted using sodium carbonate.40 Until very recently, it was thought that after an apoptotic stimulus, Bax actively translocates to mitochondria.41, 42 However, elegant studies using FLIP (fluorescence loss in photobleaching) and FRAP (fluorescence recovery after photobleaching) indicate that Bax localizes to mitochondria in healthy cells, but is actively retro-translocated to the cytosol (discussed below).37, 40, 43 In such a model, inhibition of retro-translocation following apoptotic insults causes Bax to accumulate at mitochondria, thereby sensitizing cells to apoptosis.
In contrast to Bax, Bak is constitutively inserted into the MOM in healthy cells,44, 45 presumably via α9. The Bak α9 is more hydrophobic than that of Bax, and appears to prefer the hydrophobic membrane environment rather than the amphipathic environment of the Bak groove. Consistent with this, when the Bak tail was mutated to make it more hydrophilic (Bax-like), the mutant Bak protein became more cytosolic with binding of the tail to the groove.44, 46
Bax and Bak Activation: the Jekyll to Hyde Metamorphosis
When Bax and Bak become activated, they undergo major conformation changes involving the C terminus (of Bax only), N terminus, the BH3 domain and the α5 and α6 helices, resulting in proteins that have been literally turned inside out (Figure 2b).26, 35, 45, 47, 48, 49 How these changes are triggered, the sequence of events and which changes are actually required for oligomer formation and apoptotic function are still under investigation.50
Direct activation of Bax and Bak by BH3-only proteins
Bax and Bak can be activated by direct binding of certain BH3-only proteins (Figure 1c). While BH3-only family members bind readily to prosurvival Bcl-2 proteins, an interaction of the former with Bax and Bak has been difficult to capture. In the ‘hit and run' model it was proposed that binding of BH3-only proteins to Bax and Bak is only transient,51 presumably because the induced changes in Bax and Bak conformation lead to the disengagement of the activating proteins from Bax and Bak. Some of the first evidence for direct activation were derived from a Bid mutant that was unable to bind to prosurvival proteins but could still bind and activate Bax.52 Since then, several studies have captured or implicated direct binding and activation of Bax and Bak by BH3-only proteins using biochemical, structural, as well as genetic approaches.4, 7, 35, 36, 46, 53, 54, 55, 56 However, Bax and Bak activation may also be caused by other factors such as changes in intracellular environment, post-translational modification and interaction with mitochondrial membrane components and non-Bcl-2 proteins (Box 2). Thus, whether direct activation of Bax and Bak by BH3-only proteins is needed in all circumstances remains to be elucidated.57, 58
Box 2. Non-Bcl-2 factors regulating Bax and Bak.
Several factors have been implicated in regulating Bax and Bak independently of, or in concert with, other Bcl-2 family members.
Stimuli that initiate Bax and Bak activation include mild heat, hydrogen peroxide, and low or high pH,98, 99, 100, 101 as well as non-Bcl-2 proteins such as p53 and Bif-1.102, 103, 104 In addition, post-translational modification such as dephosphorylation of Bax and Bak has been implicated in their activation and oligomerization.105, 106, 107 However, an absolute requirement for this dephosphorylation seems unlikely, given that recombinant Bax and Bak can permeabilize liposomes79, 108, 109 and that Bak proapoptotic function does not rely on dephosphorylation.110 In addition, membrane components such as cardiolipin111 and sphingolipids112 may co-operate with BH3-only proteins to promote Bax and Bak activation.
On the other hand, certain interactions can regulate Bax and Bak negatively. For example, in healthy cells, the voltage-dependent-anion-channel2 (VDAC2)113, 114 is proposed to enhance recruitment of Bak to the mitochondria115 and to keep Bak in an inactive state.113, 114, 116 Likewise, Pin1 and the E3 ligase IBRDC2 may function as negative regulators of Bax, presumably by affecting its conformation or targeting it for degradation.117, 118
Finally, the mitochondrial fission and fusion machinery may remodel the membrane environment to regulate Bax and Bak activation and oligomerization.111119, 120
Whether all of the above-mentioned non-Bcl-2 factors are necessary for regulating Bax and Bak requires further investigation. However, it is conceivable that these factors may tweak an apoptotic response in certain cell types under certain conditions.
The MOM may have a significant role in direct activation, as two landmark papers using full-length recombinant Bcl-2 proteins in liposomes in combination with FRET (Förster resonance energy transfer) showed that Bax and truncated Bid (tBid) only interact when a membrane is present.59, 60 After membrane insertion, tBid could then drive the membrane insertion of Bax.59, 60, 61 This led to the ‘embedded together‘ model, which proposed an important role for MOM in all interactions between the Bcl-2 family members.62
Bax and Bak conformation changes triggered by BH3-only proteins
An early conformation change in Bax is eversion of α9 from the Bax groove, to allow the peripherally attached protein to integrate into the MOM. From the structure of inactive Bax it was inferred that BH3-only proteins may not be capable of directly displacing α9 from the groove.22 However, several studies indicate that BH3-only proteins or peptides can bind to the α1/α6 side (rear pocket) of Bax,6, 9, 36, 46, 63 and thus might provoke Bax α9 exposure indirectly. Indeed, an NMR study showed that binding of a stapled BimBH3 peptide to the rear pocket of Bax induced chemical shifts in residues belonging to the α9 helix (Figure 3b).35 As Bak is constitutively localized at mitochondria, presumably with its α9 helix already inserted into the membrane, initial activation at the rear pocket would not be required. In support of this, BH3 peptides from activating BH3-only proteins do not bind to the α1/α6 region of Bak.46
Besides Bax α9 exposure, several other conformation changes were associated with binding of a stapled BimBH3 peptide to the Bax rear pocket (Figure 3b). Displacement of the loop between α1 and α2 coincided with exposure of the 6A7 epitope in α1 and the BH3 domain, both hallmarks of Bax activation.35 While these hallmark conformation changes may have been a direct consequence of Bim binding to the rear pocket they may also have been caused by subsequent binding of Bim to the canonical groove once it is vacated by α9. This sequential binding was first highlighted by Kim et al,9 and is supported by linkage of BH3 peptides to Bax residues in the rear pocket as well as the canonical groove.46 A two-step activation for Bax would also explain why the Bax S184V variant that constitutively inserts α9 in the MOM is inactive until stimulated by proapoptotic factors.43, 49
Supporting the importance of the groove as an activation site, a crystal structure of Bax lacking the α9 helix, and thereby representing Bax with an unoccupied groove analogous to Bax S184V, displayed BH3 peptides of Bid and Bax bound to its groove (Figure 3c).53 The peptides bound to the Bax groove in a similar manner to which they bind to the groove of prosurvival proteins. However, binding to Bax results in increased movement of the α2/α3 side of the Bax groove away from the bound peptide, with a partial displacement of the α2 helix. This opening of the groove might weaken the contact between the BH3 peptide and Bax, providing a structural rationale for transient binding of BH3-only proteins to Bax. The Bax crystal structures also provided evidence for a novel conformation change, termed the ‘core/latch dissociation', in which α5 and α6 unhinge allowing the α6–α8 helices (termed ‘latch') to dissociate from the α1–α5 helices (termed ‘core') (Figure 3c). This conformation change was required for Bax function in mitochondrial assays, as its proapoptotic function was inhibited upon cysteine-tethering of α5 and α6.53 Interestingly, the structural re-organization that occurs during core/latch dissociation exposes the N terminus of α1, providing a structural mechanism for the exposure of the N-terminal epitopes during Bax (and possibly also Bak) activation.45, 49
The Bax α5 and α6 helices have previously been implicated in its pore-forming function, by everting from their inactive localization and inserting as a hairpin into the MOM, analogous to the pore-forming domains of bacterial toxins such as colicin A and Diphtheria toxin.24, 64 Consistent with this hypothesis, peptides based on Bax α5 and/or α6 helices of Bax have pore-forming activity.65, 66, 67 Most compelling were cell studies in which the Bax α5/α6 helices became buried in the MOM prior to oligomerization.47 Our recent structural studies indicate that α5 and α6 of Bax dissociate during activation,53 suggesting that membrane insertion of these helices may not occur as a hairpin (see below).
Several biochemical and structural approaches show that, in contrast to Bax, Bak activation involves BH3-only protein or BH3 peptide binding only to the canonical groove.7, 46, 55 First, by testing different mutants of Bak and BH3-only proteins (including reciprocal size-swap variants) in binding and Bak oligomerization assays, the activation site of BH3-only proteins was mapped to the Bak groove.7 Second, BH3 peptides can link to the groove of Bak but not to its α1/α6 region.46 Third, in a very recent NMR structure, a BidBH3 peptide bound to the groove of Bak55 in a similar manner to its engagement with the Bax groove.53 Binding of BH3 peptides to the Bak groove coincided with the exposure of the Bak BH3 domain and the N terminus,55 both of which are similar conformation changes to those seen in Bax.53 Intriguingly, these Bak conformation changes were blocked by tethering the peptide to the groove, suggesting that Bak can only change conformation when the activating peptide leaves the groove,55 consistent with the ‘hit and run' model. Whether Bak undergoes core/latch dissociation like Bax remains to be determined.
Restraint of Bax and Bak by prosurvival proteins
The prosurvival Bcl-2 family members have long been recognized as critical guardians of Bax/Bak activity. It has now become clear that they can act via different ‘MODES' to prevent Bax and Bak activation and oligomerization.4
MODE 0
The level of Bax at mitochondria is tightly regulated in healthy cells as Bax is trafficked away from the MOM to the cytosol,37, 40, 43 As Bcl-xL was found to ferry peripherally associated Bax to the cytosol,37, 40 this represents a new way of keeping Bax in check and is defined here as ‘MODE 0' inhibition (Figure 1b). The molecular mechanism underpinning this newly described ‘retro-translocation' remains obscure. Although binding of Bax and Bcl-xL during retro-translocation has not been shown, mutagenesis of the BH3 domain of Bax and the groove of Bcl-xL inhibited retro-translocation implicating a direct interaction.37 This BH3:groove interaction indicates that the peripheral Bax ‘cargo' has undergone at least certain activation steps including exposure of its BH3 domain but has not yet reached the stage of membrane integration or oligomerization. Such conformation changes that are normally associated with activation during apoptosis may have been induced by the association of Bax with membranes.68
Once retro-translocated, the complex of the cargo (Bax) and ferry (Bcl-xL) must dissociate as Bax in the cytosol is monomeric and does not require interactions with other proteins to maintain its inactive conformation.69, 70 Further, as cytosolic Bax does not expose its N terminus or its BH3 domain, Bax must revert to its inactive conformer consistent with the reversible conformation change in Bax induced by interaction with membranes.68
Bax retro-translocation independent of prosurvival Bcl-2 proteins has recently been reported.43 However, retro-translocation in this case involved Bax that was tail-anchored in the MOM rather than peripherally associated, suggesting that different populations of Bax may exist in a healthy cell with distinct mechanisms governing their subcellular localization.
Although the molecular mechanism governing Bax retro-translocation and whether prosurvival Bcl-2 proteins are necessary requires further investigation, controlling Bax subcellular localization clearly represents an important mechanism to regulate apoptotic function. Considering that only 100 molecules of Bax per mitochondria are necessary for pore formation,71 retro-translocation might be critical in finetuning a cell's response to apoptotic stimuli.
MODE 1
In response to apoptotic stimuli prosurvival proteins can sequester BH3-only proteins to prevent them from activating Bax and Bak. This ‘MODE 1' inhibition4 (Figure 1b) is likely facilitated by sequestering the BH3 domain of the BH3-only proteins into the prosurvival groove.28, 29, 34 However, recent evidence suggests that regions other than the BH3 domain might also be involved, as mutation in the BH3 domain of Bim (Bim2A) that was sufficient to abrogate binding of a BimBH3 peptide to Bcl-xL in vitro72 did not abolish interaction of full-length Bim with Bcl-xL in live cells.73
MODE 2
Irrespective of the activating mechanism (see Box 2), once Bax and Bak are activated, prosurvival proteins directly bind to the activated proteins to prevent their homo-oligomerization resulting in ‘MODE 2' inhibition (Figure 1b).4 Again, MODE 2 occurs via sequestration of the exposed BH3 domain of Bax and Bak into the groove of the prosurvival proteins,27, 31, 32 putting the BH3:groove interaction central to the myriad of interactions that govern cell fate.
MODE 1 and MODE 2 may require a conformation change in the prosurvival proteins induced by binding of BH3-only proteins.59 This ‘activation' of prosurvival Bcl-2 proteins may simply anchor α9 in the MOM,74 or involve a more drastic insertion of the α5 and α6 helices,75, 76 similar to the proposed conformation change in Bax.47 That proapoptotic proteins ‘activate' prosurvival proteins seems at first glance to be counter-intuitive. However, BH3-only proteins may bind to and induce conformation change in any of the structurally similar multi-domain Bcl-2 proteins, regardless of whether they are prosurvival or proapoptotic. The prosurvival proteins might thus act as a dominant negative form of Bax, competing with Bax for binding of BH3-only proteins and later on for activated Bax to prevent Bax homo-oligomerization.77
Derepression of MODE 1 and MODE 2: indirect activation of Bax and Bak by BH3-only proteins
MODE 1 and MODE 2 inhibition by prosurvival proteins can be overcome by upregulating BH3-only proteins, which bind and inhibit the prosurvival Bcl-2 family members and thus indirectly promote Bax and Bak activation (Figure 1c). When these upregulated BH3-only proteins interact with prosurvival proteins to compete off activating BH3-only proteins,5, 8, 60, 78, 79 the process is termed ‘MODE 1 derepression'.4 In addition, derepression also takes place when the BH3-only proteins compete off activated Bax and Bak from the prosurvival proteins, termed ‘MODE 2 derepression',4 akin to the ‘indirect activation' model.58, 80, 81 Notably, according to Llambi et al.4 derepression of MODE 2 complexes is more difficult than MODE 1 complexes.
Taken together, the prosurvival proteins, the BH3-only proteins, and Bax/Bak partake in a dynamic triad of competitive interactions (Figure 1a). The relative affinities of these interactions and the cellular concentration of each protein determine whether Bax and Bak (i) remain inactive, (ii) become activated but bind prosurvival proteins (in which case the cell is reprieved) or (iii) become activated and self-associate to form a pore (in which case the cell is generally doomed).
Bax and Bak Oligomerization: Making Doughnuts and Daisy Chains
Once activated, Bax and Bak have exposed hydrophobic regions that need to be buried in a membrane or a protein interface, leading to the formation of membrane-spanning high-molecular weight oligomers.
Two main oligomer models have been proposed. An asymmetric, single-interface oligomer model, or ‘daisy-chain' model, was originally suggested for Bax82 and is supported by studies that define a BH3:α1/α6 interface.35 A recent model of a Bak octameric pore was also based on a single-interface mechanism.83
An alternative symmetric or two-interface model was proposed for Bak, based on a cysteine-linkage approach in mammalian cells26 and was later supported for Bax in different linkage studies.84, 85 In this model, the exposed Bak BH3 domain engages the canonical hydrophobic surface groove of a partner Bak molecule in a similar manner to its interaction with the prosurvival groove. However, the BH3:groove interaction in a Bax or Bak homodimer is symmetric, with the BH3 domain of the second Bax/Bak molecule binding into the groove of the first in a reciprocal fashion (Figure 2b). The symmetric BH3:groove dimer model was supported by electron paramagnetic resonance (EPR)-spin labeling of recombinant Bax and Bak in liposomes,86, 87 and more recently by evidence that the basic oligomeric unit of activated Bak is a homodimer under native conditions.88
The symmetric model is also supported by a recent crystal structure of the Bax α2–α5 region.53 The α2–α5 of Bax, which contains the BH3 domain and the hydrophobic surface groove, was shown to be sufficient for oligomerization.89 Intriguingly, when a similar α2–α5 Bax construct was expressed as a GFP fusion protein and crystallized, it spontaneously formed a symmetric BH3:groove dimer (Figure 3d).53 In this dimer structure, the BaxBH3 domain bound to the Bax groove in a similar fashion as activating BH3 peptides (Figure 3e), indicating that similar BH3 domain residues might be crucial for Bax activation as well as Bax dimerization. This also indicates that for a Bax homodimer to form, the activating BH3 domain must first leave the Bax groove, consistent with the ‘hit and run' model.
Interestingly, one half of the BH3:groove homodimer closely resembles the ‘core' of the Bax:BidBH3 complex (Figure 3e), suggesting that no major rearrangements of the Bax core, other than the BaxBH3 exposure, are necessary to form the BH3:groove dimer. Thus, the α5 helix remains adjacent to α3 and α4, and, together with α4, lines the base of the BH3:groove dimer, projecting a high concentration of hydrophobic aromatic residues into the milieu (Figure 3d). One may therefore speculate that core/latch dissociation exposes lipophilic residues of α4, α5 and α6 and that BH3:groove homodimers nucleate the oligomerization of this activated form. The locally concentrated lipophilic residues could then penetrate the membrane bilayer to displace the phospholipid headgroups to provoke positive membrane curvature and eventually membrane rupture. Such a mechanism is potentially analogous to the ‘carpet model' of pore formation described for certain bacterial toxins such as melittin, whereby antimicrobial peptides aggregate in the plane of the membrane leading to membrane rupture.90, 91
This proposed mechanism for permeabilization of the MOM by Bax and Bak by an in-plane interaction with the membrane (Figure 2b) is consistent with the reduced labeling of the Bax α5/α6 helices with a hydrophilic label during activation.47 However, it suggests that Bax and Bak α5/α6 helices may not traverse the MOM as a membrane-spanning hairpin and therefore may not function analogously to colicin A and Diphtheria toxin. That the Bax or Bak α5/α6 helices may not insert as a hairpin is supported by several lines of evidence. First, the α5 is not everted in the structure of dimerized Bax.53 Second, a disulfide tether between α4 and α5 helices of Bax did not inhibit apoptotic function, providing further support that the α5 remains associated with the ‘core' domain.53 Third, α5 and α6 must separate for Bax to mediate cell death.53 And finally, EPR spin labeling suggested that α5 remains associated with the core in activated Bak and that α6 exhibits only shallow insertion into liposomal membranes.87
That the basic oligomeric unit of Bax and Bak is a symmetric homodimer,26, 53, 85, 86, 87, 88 is not consistent with the daisy-chain model of oligomerization. Rather in a symmetric model a second interface is necessary for dimers to multimerize in order to form the higher order oligomeric pore.84 Regions outside of the core α2–5 domain such as the α6 helices have been implicated in this requisite second interface in Bax and Bak.85, 87, 88, 92 Therefore, core-latch dissociation may not only serve to expose a lipophilic surface to engage the MOM, it may also reposition the latch domain, (that includes the α6), to facilitate higher order oligomerization. However, how this second interface enables dimers to multimerize, and consequently the structure of the putative apoptotic pore, is unknown. Whether the apoptotic pore involves dimers assembled in a closed conformation (a ‘doughnut'), a linear assembly, or a disordered aggregate, as well as whether lipids are critical constituents of the pore remains to be determined.
What's Next?
Although recent novel approaches have provided significant insight into how Bax and Bak are activated to kill cells, further research is clearly needed. A structure of a high-molecular weight oligomer of Bax and/or Bak would be a major step forward and would determine whether Bax and Bak form proteinaceous pores.50, 93 The alternative is that they form lipidic pores.67, 94 If so, intercalated lipids will significantly hinder characterization of the pore by conventional structural approaches and so elegant biophysical approaches in the presence of a membrane may be needed.
Exciting times lie ahead. We anticipate that future advances in our understanding of how Bax and Bak are activated and how they function will expose these critical apoptotic proteins as targets for novel therapeutics.
Acknowledgments
We thank Rachel Uren and Peter Czabotar for critical reading of the manuscript, and Colin Hockings for advice. DW is supported by a Deutsche Forschungsgemeinschaft (DFG) postdoctoral fellowship (Germany). GD and RK are supported by Australian Research Council Future Fellowships. The work was supported by operational infrastructure grants through the Victorian State Government Operational Infrastructure Support and the Australian Government NHMRC IRIISS.
Glossary
- BH
Bcl-2 homology
- EPR
electron paramagnetic resonance
- MOM
mitochondrial outer membrane
- tBid
truncated Bid
- TM
transmembrane domain
The authors declare no conflict of interest.
Footnotes
Edited by G Melino
References
- Liu X, Kim CN, Yang J, Jemmerson R, Wang X. Induction of apoptotic program in cell-free extracts: requirement for dATP and cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/s0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- Wei MC, Zong WX, Cheng EH, Lindsten T, Panoutsakopoulou V, Ross AJ, et al. Proapoptotic BAX and BAK: a requisite gateway to mitochondrial dysfunction and death. Science. 2001;292:727–730. doi: 10.1126/science.1059108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsten T, Ross AJ, King A, Zong W, Rathmell JC, Shiels HA, et al. The combined functions of proapoptotic Bcl-2 family members Bak and Bax are essential for normal development of multiple tissues. Mol Cell. 2000;6:1389–1399. doi: 10.1016/s1097-2765(00)00136-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llambi F, Moldoveanu T, Tait SW, Bouchier-Hayes L, Temirov J, McCormick LL, et al. A unified model of mammalian BCL-2 protein family interactions at the mitochondria. Mol Cell. 2011;44:517–531. doi: 10.1016/j.molcel.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A, Bassik MC, Walensky LD, Sorcinelli MD, Weiler S, Korsmeyer SJ. Distinct BH3 domains either sensitize or activate mitochondrial apoptosis, serving as prototype cancer therapeutics. Cancer Cell. 2002;2:183–192. doi: 10.1016/s1535-6108(02)00127-7. [DOI] [PubMed] [Google Scholar]
- Cartron PF, Gallenne T, Bougras G, Gautier F, Manero F, Vusio P, et al. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol Cell. 2004;16:807–818. doi: 10.1016/j.molcel.2004.10.028. [DOI] [PubMed] [Google Scholar]
- Dai H, Smith A, Meng XW, Schneider PA, Pang YP, Kaufmann SH. Transient binding of an activator BH3 domain to the Bak BH3-binding groove initiates Bak oligomerization. J Cell Biol. 2011;194:39–48. doi: 10.1083/jcb.201102027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Rafiuddin-Shah M, Tu HC, Jeffers JR, Zambetti GP, Hsieh JJ, et al. Hierarchical regulation of mitochondrion-dependent apoptosis by BCL-2 subfamilies. Nat Cell Biol. 2006;8:1348–1358. doi: 10.1038/ncb1499. [DOI] [PubMed] [Google Scholar]
- Kim H, Tu HC, Ren D, Takeuchi O, Jeffers JR, Zambetti GP, et al. Stepwise activation of BAX and BAK by tBID, BIM, and PUMA initiates mitochondrial apoptosis. Mol Cell. 2009;36:487–499. doi: 10.1016/j.molcel.2009.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du H, Wolf J, Schafer B, Moldoveanu T, Chipuk JE, Kuwana T. BH3 domains other than Bim and Bid can directly activate Bax/Bak. J Biol Chem. 2011;286:491–501. doi: 10.1074/jbc.M110.167148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasser A, Cory S, Adams JM. Deciphering the rules of programmed cell death to improve therapy of cancer and other diseases. EMBO J. 2011;30:3667–3683. doi: 10.1038/emboj.2011.307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DR, Kroemer G. Pharmacological manipulation of cell death: clinical applications in sight. J Clin Invest. 2005;115:2610–2617. doi: 10.1172/JCI26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Bellairs JA, Leshchiner ES, Walensky LD. Direct and selective small-molecule activation of proapoptotic BAX. Nat Chem Biol. 2012;8:639–645. doi: 10.1038/nchembio.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochhauser E, Cheporko Y, Yasovich N, Pinchas L, Offen D, Barhum Y, et al. Bax deficiency reduces infarct size and improves long-term function after myocardial infarction. Cell Biochem Biophys. 2007;47:11–20. doi: 10.1385/cbb:47:1:11. [DOI] [PubMed] [Google Scholar]
- Reyes NA, Fisher JK, Austgen K, VandenBerg S, Huang EJ, Oakes SA. Blocking the mitochondrial apoptotic pathway preserves motor neuron viability and function in a mouse model of amyotrophic lateral sclerosis. J Clin Invest. 2010;120:3673–3679. doi: 10.1172/JCI42986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo W, Lee HP, Smith MA, Zhu X, Matsuyama S, Lee HG. Inhibition of Bax protects neuronal cells from oligomeric Abeta neurotoxicity. Cell death & disease. 2012;3:e309. doi: 10.1038/cddis.2012.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvansakul M, Yang H, Fairlie WD, Czabotar PE, Fischer SF, Perugini MA, et al. Vaccinia virus anti-apoptotic F1L is a novel Bcl-2-like domain-swapped dimer that binds a highly selective subset of BH3-containing death ligands. Cell Death Differ. 2008;15:1564–1571. doi: 10.1038/cdd.2008.83. [DOI] [PubMed] [Google Scholar]
- Moldoveanu T, Liu Q, Tocilj A, Watson MH, Shore G, Gehring K. The x-ray structure of a BAK homodimer reveals an inhibitory zinc binding site. Mol Cell. 2006;24:677–688. doi: 10.1016/j.molcel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Suzuki M, Youle RJ, Tjandra N. Structure of Bax: coregulation of dimer formation and intracellular localization. Cell. 2000;103:645–654. doi: 10.1016/s0092-8674(00)00167-7. [DOI] [PubMed] [Google Scholar]
- Wang H, Takemoto C, Akasaka R, Uchikubo-Kamo T, Kishishita S, Murayama K, et al. Novel dimerization mode of the human Bcl-2 family protein Bak, a mitochondrial apoptosis regulator. J Struct Biol. 2009;166:32–37. doi: 10.1016/j.jsb.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Muchmore SW, Sattler M, Liang H, Meadows RP, Harlan JE, Yoon HS, et al. X-ray and NMR structure of human Bcl-xL, an inhibitor of programmed cell death. Nature. 1996;381:335–341. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- Petros AM, Medek A, Nettesheim DG, Kim DH, Yoon HS, Swift K, et al. Solution structure of the antiapoptotic protein bcl-2. Proc Natl Acad Sci USA. 2001;98:3012–3017. doi: 10.1073/pnas.041619798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Sim HW, Puthalakath H, Adams JM, Colman PM, et al. To trigger apoptosis Bak exposes its BH3 domain and homo-dimerizes via BH3:grooove interactions. Mol Cell. 2008;30:369–380. doi: 10.1016/j.molcel.2008.04.005. [DOI] [PubMed] [Google Scholar]
- Zha H, Aime-Sempe C, Sato T, Reed JC. Proapoptotic protein Bax heterodimerizes with Bcl-2 and homodimerizes with Bax via a novel domain (BH3) distinct from BH1 and BH2. J Biol Chem. 1996;271:7440–7444. doi: 10.1074/jbc.271.13.7440. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, et al. Structural insights into the degradation of Mcl-1 induced by BH3 domains. Proc Natl Acad Sci USA. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Dai S, Zhu Y, Marrack P, Kappler JW. The structure of a Bcl-xL/Bim fragment complex: Implications for Bim function. Immunity. 2003;19:341–352. doi: 10.1016/s1074-7613(03)00234-6. [DOI] [PubMed] [Google Scholar]
- Petros AM, Nettseheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, et al. Rationale for Bcl-xL/Bad peptide complex formation from structure, mutagenesis, and biophysical studies. Protein Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, et al. Structure of Bcl-xL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Lee EF, Thompson GV, Wardak AZ, Fairlie WD, Colman PM. Mutation to Bax beyond the BH3 domain disrupts interactions with pro-survival proteins and promotes apoptosis. J Biol Chem. 2011;286:7123–7131. doi: 10.1074/jbc.M110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. Structure of the BH3 domains from the p53-inducible BH3-only proteins Noxa and Puma in complex with Mcl-1. J Mol Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]
- Smits C, Czabotar PE, Hinds MG, Day CL. Structural plasticity underpins promiscuous binding of the prosurvival protein A1. Structure. 2008;16:818–829. doi: 10.1016/j.str.2008.02.009. [DOI] [PubMed] [Google Scholar]
- Gavathiotis E, Reyna DE, Davis ML, Bird GH, Walensky LD. BH3-triggered structural reorganization drives the activation of proapoptotic BAX. Mol Cell. 2010;40:481–492. doi: 10.1016/j.molcel.2010.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavathiotis E, Suzuki M, Davis ML, Pitter K, Bird GH, Katz SG, et al. BAX activation is initiated at a novel interaction site. Nature. 2008;455:1076–1081. doi: 10.1038/nature07396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlich F, Banerjee S, Suzuki M, Cleland MM, Arnoult D, Wang C, et al. Bcl-x(L) retrotranslocates Bax from the mitochondria into the cytosol. Cell. 2011;145:104–116. doi: 10.1016/j.cell.2011.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilmore AP, Metcalfe AD, Romer LH, Streuli CH. Integrin-mediated survival signals regulate the apoptotic function of Bax through its conformation and subcellular localization. J Cell Biol. 2000;149:431–446. doi: 10.1083/jcb.149.2.431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentijn AJ, Upton JP, Gilmore AP. Analysis of endogenous Bax complexes during apoptosis using blue native PAGE: implications for Bax activation and oligomerization. Biochem J. 2008;412:347–357. doi: 10.1042/BJ20071548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt F, Cakir Z, Reichenbach F, Youle RJ, Edlich F. The C-terminal helix of Bcl-x(L) mediates Bax retrotranslocation from the mitochondria. Cell Death Differ. 2013;20:333–342. doi: 10.1038/cdd.2012.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Wolter KG, Youle RJ. Cytosol-to-membrane redistribution of Bax and Bcl-X(L) during apoptosis. Proc Natl Acad Sci USA. 1997;94:3668–3672. doi: 10.1073/pnas.94.8.3668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolter KG, Hsu YT, Smith CL, Nechushtan A, Xi XG, Youle RJ. Movement of Bax from the cytosol to mitochondria during apoptosis. J Cell Biol. 1997;139:1281–1292. doi: 10.1083/jcb.139.5.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenberg B, Wang P, Keeble JA, Rodriguez-Enriquez R, Walker S, Owens TW, et al. Bax exists in a dynamic equilibrium between the cytosol and mitochondria to control apoptotic priming. Mol Cell. 2013;49:959–971. doi: 10.1016/j.molcel.2012.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer PE, Frederick P, Gulbis JM, Dewson G, Kluck RM. Translocation of a Bak C-terminus mutant from cytosol to mitochondria to mediate cytochrome C release: implications for Bak and Bax apoptotic function. PLoS One. 2012;7:e31510. doi: 10.1371/journal.pone.0031510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths GJ, Dubrez L, Morgan CP, Jones NA, Whitehouse J, Corfe BM, et al. Cell damage-induced conformational changes of the pro-apoptotic protein Bak in vivo precede the onset of apoptosis. J Cell Biol. 1999;144:903–914. doi: 10.1083/jcb.144.5.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshchiner ES, Braun CR, Bird GH, Walensky LD. Direct activation of full-length proapoptotic BAK. Proc Natl Acad Sci USA. 2013;110:E986–E995. doi: 10.1073/pnas.1214313110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annis MG, Soucie EL, Dlugosz PJ, Cruz-Aguado JA, Penn LZ, Leber B, et al. Bax forms multispanning monomers that oligomerize to permeabilize membranes during apoptosis. EMBO J. 2005;24:2096–2103. doi: 10.1038/sj.emboj.7600675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu YT, Youle RJ. Nonionic detergents induce dimerization among members of the Bcl-2 family. J Biol Chem. 1997;272:13829–13834. doi: 10.1074/jbc.272.21.13829. [DOI] [PubMed] [Google Scholar]
- Nechushtan A, Smith CL, Hsu YT, Youle RJ. Conformation of the Bax C-terminus regulates subcellular location and cell death. EMBO J. 1999;18:2330–2341. doi: 10.1093/emboj/18.9.2330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westphal D, Dewson G, Czabotar PE, Kluck RM. Molecular biology of Bax and Bak activation and action. Biochim Biophys Acta. 2011;1813:521–531. doi: 10.1016/j.bbamcr.2010.12.019. [DOI] [PubMed] [Google Scholar]
- Wei MC, Lindsten T, Mootha VK, Weiler S, Gross A, Ashiya M, et al. tBID, a membrane-targeted death ligand, oligomerizes BAK to release cytochrome c. Genes Dev. 2000;14:2060–2071. [PMC free article] [PubMed] [Google Scholar]
- Wang K, Yin XM, Chao DT, Milliman CL, Korsmeyer SJ. BID: a novel BH3 domain-only death agonist. Genes Dev. 1996;10:2859–2869. doi: 10.1101/gad.10.22.2859. [DOI] [PubMed] [Google Scholar]
- Czabotar PE, Westphal D, Dewson G, Ma S, Hockings C, Fairlie WD, et al. Bax crystal structures reveal how BH3 domains activate Bax and nucleate its oligomerization to induce apoptosis. Cell. 2013;152:519–531. doi: 10.1016/j.cell.2012.12.031. [DOI] [PubMed] [Google Scholar]
- Merino D, Giam M, Hughes PD, Siggs OM, Heger K, O'Reilly LA, et al. The role of BH3-only protein Bim extends beyond inhibiting Bcl-2-like prosurvival proteins. J Cell Biol. 2009;186:355–362. doi: 10.1083/jcb.200905153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moldoveanu T, Grace CR, Llambi F, Nourse A, Fitzgerald P, Gehring K, et al. BID-induced structural changes in BAK promote apoptosis. Nat Struct Mol Biol. 2013;20:589–597. doi: 10.1038/nsmb.2563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, et al. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher JI, Meusburger S, Hawkins CJ, Riglar DT, Lee EF, Fairlie WD, et al. Apoptosis is triggered when prosurvival Bcl-2 proteins cannot restrain Bax. Proc Natl Acad Sci USA. 2008;105:18081–18087. doi: 10.1073/pnas.0808691105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Fletcher JI, Kaufmann T, van Delft MF, Chen L, Czabotar PE, et al. Apoptosis initiated when BH3 ligands engage multiple Bcl-2 homologs, not Bax or Bak.[see comment] Science. 2007;315:856–859. doi: 10.1126/science.1133289. [DOI] [PubMed] [Google Scholar]
- Billen LP, Kokoski CL, Lovell JF, Leber B, Andrews DW. Bcl-XL inhibits membrane permeabilization by competing with Bax. PLoS Biol. 2008;6:e147. doi: 10.1371/journal.pbio.0060147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovell JF, Billen LP, Bindner S, Shamas-Din A, Fradin C, Leber B, et al. Membrane binding by tBid initiates an ordered series of events culminating in membrane permeabilization by Bax. Cell. 2008;135:1074–1084. doi: 10.1016/j.cell.2008.11.010. [DOI] [PubMed] [Google Scholar]
- Shamas-Din A, Bindner S, Zhu W, Zaltsman Y, Campbell C, Gross A, et al. tBid undergoes multiple conformational changes at the membrane required for Bax activation. J Biol Chem. 2013;288:22111–22127. doi: 10.1074/jbc.M113.482109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber B, Lin J, Andrews DW. Still embedded together binding to membranes regulates Bcl-2 protein interactions. Oncogene. 2010;29:5221–5230. doi: 10.1038/onc.2010.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallenne T, Gautier F, Oliver L, Hervouet E, Noel B, Hickman JA, et al. Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members. J Cell Biol. 2009;185:279–290. doi: 10.1083/jcb.200809153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, London E. The membrane topography of the diphtheria toxin T domain linked to the a chain reveals a transient transmembrane hairpin and potential translocation mechanisms. Biochemistry. 2009;48:10446–10456. doi: 10.1021/bi9014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Coraiola M, Dalla Serra M, Mingarro I, Menestrina G, Salgado J. Peptides derived from apoptotic Bax and Bid reproduce the poration activity of the parent full-length proteins. Biophys J. 2005;88:3976–3990. doi: 10.1529/biophysj.104.058008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Saez AJ, Coraiola M, Serra MD, Mingarro I, Muller P, Salgado J. Peptides corresponding to helices 5 and 6 of Bax can independently form large lipid pores. FEBS J. 2006;273:971–981. doi: 10.1111/j.1742-4658.2006.05123.x. [DOI] [PubMed] [Google Scholar]
- Qian S, Wang W, Yang L, Huang HW. Structure of transmembrane pore induced by Bax-derived peptide: evidence for lipidic pores. Proc Natl Acad Sci USA. 2008;105:17379–17383. doi: 10.1073/pnas.0807764105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yethon JA, Epand RF, Leber B, Epand RM, Andrews DW. Interaction with a membrane surface triggers a reversible conformational change in Bax normally associated with induction of apoptosis. J Biol Chem. 2003;278:48935–48941. doi: 10.1074/jbc.M306289200. [DOI] [PubMed] [Google Scholar]
- Hsu Y-T, Youle RJ. Bax in murine thymus is a soluble monomeric protein that displays differential detergent-induced conformations. J Biol Chem. 1998;273:10777–10783. doi: 10.1074/jbc.273.17.10777. [DOI] [PubMed] [Google Scholar]
- Vogel S, Raulf N, Bregenhorn S, Biniossek ML, Maurer U, Czabotar P, et al. Cytosolic Bax: does it require binding proteins to keep its pro-apoptotic activity in check. J Biol Chem. 2012;287:9112–9127. doi: 10.1074/jbc.M111.248906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dussmann H, Rehm M, Concannon CG, Anguissola S, Wurstle M, Kacmar S, et al. Single-cell quantification of Bax activation and mathematical modelling suggest pore formation on minimal mitochondrial Bax accumulation. Cell Death Differ. 2010;17:278–290. doi: 10.1038/cdd.2009.123. [DOI] [PubMed] [Google Scholar]
- Lee EF, Czabotar PE, van Delft MF, Michalak E, Boyle M, Willis SN, et al. A novel BH3 ligand that selectively targets Mcl-1 reveals that apoptosis can proceed without Mcl-1 degradation. J Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranovich A, Liu Q, Collins T, Geng F, Dixit S, Leber B, et al. Differences in the mechanisms of proapoptotic BH3 proteins binding to Bcl-XL and Bcl-2 quantified in live MCF-7 cells. Mol Cell. 2012;45:754–763. doi: 10.1016/j.molcel.2012.01.030. [DOI] [PubMed] [Google Scholar]
- Wilson-Annan J, O'Reilly LA, Crawford SA, Hausmann G, Beaumont JG, Parma LP, et al. Proapoptotic BH3-only proteins trigger membrane integration of prosurvival Bcl-w and neutralize its activity. J Cell Biol. 2003;162:877–888. doi: 10.1083/jcb.200302144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim PK, Annis MG, Dlugosz PJ, Leber B, Andrews DW. During apoptosis bcl-2 changes membrane topology at both the endoplasmic reticulum and mitochondria. Mol Cell. 2004;14:523–529. doi: 10.1016/s1097-2765(04)00263-1. [DOI] [PubMed] [Google Scholar]
- Dlugosz PJ, Billen LP, Annis MG, Zhu W, Zhang Z, Lin J, et al. Bcl-2 changes conformation to inhibit Bax oligomerization. EMBO J. 2006;25:2287–2296. doi: 10.1038/sj.emboj.7601126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogner C, Leber B, Andrews DW. Apoptosis: embedded in membranes. Curr Opin Cell Biol. 2010;22:845–851. doi: 10.1016/j.ceb.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Bouchier-Hayes L, Chipuk JE, Bonzon C, Sullivan BA, Green DR, et al. BH3 domains of BH3-only proteins differentially regulate Bax-mediated mitochondrial membrane permeabilization both directly and indirectly. Mol Cell. 2005;17:525–535. doi: 10.1016/j.molcel.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Kuwana T, Mackey MR, Perkins G, Ellisman MH, Latterich M, Schneiter R, et al. Bid, Bax, and lipids cooperate to form supramolecular openings in the outer mitochondrial membrane. Cell. 2002;111:331–342. doi: 10.1016/s0092-8674(02)01036-x. [DOI] [PubMed] [Google Scholar]
- Uren RT, Dewson G, Chen L, Coyne SC, Huang DCS, Adams JM, et al. Mitochondrial permeabilization relies on BH3 ligands engaging multiple pro-survival Bcl-2 relatives, not Bak. J Cell Biol. 2007;177:277–287. doi: 10.1083/jcb.200606065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, et al. Proapoptotic Bak is sequestered by Mcl-1 and Bcl-xL, but not Bcl-2, until displaced by BH3-only proteins. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed JC. Proapoptotic multidomain Bcl-2/Bax-family proteins: mechanisms, physiological roles, and therapeutic opportunities. Cell Death Differ. 2006;13:1378–1386. doi: 10.1038/sj.cdd.4401975. [DOI] [PubMed] [Google Scholar]
- Pang YP, Dai H, Smith A, Meng XW, Schneider PA, Kaufmann SH. Bak conformational changes induced by ligand binding: insight into BH3 domain binding and Bak homo-oligomerization. Sci Rep. 2012;2:257. doi: 10.1038/srep00257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Zhu W, Lapolla SM, Miao Y, Shao Y, Falcone M, et al. Bax forms an oligomer via separate, yet interdependent, surfaces. J Biol Chem. 2010;285:17614–17627. doi: 10.1074/jbc.M110.113456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewson G, Ma S, Frederick P, Hockings C, Tan I, Kratina T, et al. Bax dimerizes via a symmetric BH3:groove interface during apoptosis. Cell Death Differ. 2012;194:P001074530. doi: 10.1038/cdd.2011.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleicken S, Classen M, Padmavathi PV, Ishikawa T, Zeth K, Steinhoff HJ, et al. Molecular details of Bax activation, oligomerization, and membrane insertion. J Biol Chem. 2010;285:6636–6647. doi: 10.1074/jbc.M109.081539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh KJ, Singh P, Lee K, Foss K, Lee S, Park M, et al. Conformational changes in BAK, a pore-forming proapoptotic Bcl-2 family member, upon membrane insertion and direct evidence for the existence of BH3-BH3 contact interface in BAK homo-oligomers. J Biol Chem. 2010;285:28924–28937. doi: 10.1074/jbc.M110.135293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Hockings C, Anwari K, Kratina T, Fennell S, Lazarou M, et al. Assembly of the Bak apoptotic pore: A critical role for the Bak alpha6 helix in the multimerization of homodimers during apoptosis. The Journal of biological chemistry. 2013;288:26027–26038. doi: 10.1074/jbc.M113.490094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George NM, Evans JJ, Luo X. A three-helix homo-oligomerization domain containing BH3 and BH1 is responsible for the apoptotic activity of Bax. Genes Dev. 2007;21:1937–1948. doi: 10.1101/gad.1553607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy A, Thennarasu S, Lee DK, Tan A, Maloy L. Solid-state NMR investigation of the membrane-disrupting mechanism of antimicrobial peptides MSI-78 and MSI-594 derived from magainin 2 and melittin. Biophys J. 2006;91:206–216. doi: 10.1529/biophysj.105.073890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogden KA. Antimicrobial peptides: pore formers or metabolic inhibitors in bacteria? Nature reviews. Microbiology. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- Dewson G, Kratina T, Czabotar P, Day CL, Adams JM, Kluck RM. Bak activation for apoptosis involves oligomerization of dimers via their alpha6 helices. Mol Cell. 2009;36:696–703. doi: 10.1016/j.molcel.2009.11.008. [DOI] [PubMed] [Google Scholar]
- Garcia-Saez AJ. The secrets of the Bcl-2 family. Cell Death Differ. 2012;19:1733–1740. doi: 10.1038/cdd.2012.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrones O, Antonsson B, Yamaguchi H, Wang HG, Liu J, Lee RM, et al. Lipidic pore formation by the concerted action of proapoptotic BAX and tBID. J Biol Chem. 2004;279:30081–30091. doi: 10.1074/jbc.M313420200. [DOI] [PubMed] [Google Scholar]
- Ke F, Bouillet P, Kaufmann T, Strasser A, Kerr J, Voss AK. Consequences of the combined loss of BOK and BAK or BOK and BAX. Cell Death Dis. 2013;4:e650. doi: 10.1038/cddis.2013.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards AL, Gavathiotis E, Labelle JL, Braun CR, Opoku-Nsiah KA, Bird GH, et al. Multimodal interaction with BCL-2 family proteins underlies the proapoptotic activity of PUMA BH3. Chem Biol. 2013;20:888–902. doi: 10.1016/j.chembiol.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walensky LD, Pitter K, Morash J, Oh KJ, Barbuto S, Fisher J, et al. A stapled BID BH3 helix directly binds and activates BAX. Mol Cell. 2006;24:199–210. doi: 10.1016/j.molcel.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Cartron PF, Oliver L, Mayat E, Meflah K, Vallette FM. Impact of pH on Bax alpha conformation, oligomerisation and mitochondrial integration. FEBS Lett. 2004;578:41–46. doi: 10.1016/j.febslet.2004.10.080. [DOI] [PubMed] [Google Scholar]
- Khaled AR, Kim K, Hofmeister R, Muegge K, Durum SK. Withdrawal of IL-7 induces bax translocation from cytosol to mitochondria through a rise in intracellular pH. Proc Natl Acad Sci USA. 1999;96:14476–14481. doi: 10.1073/pnas.96.25.14476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nie C, Tian C, Zhao L, Petit PX, Mehrpour M, Chen Q. Cysteine 62 of Bax is critical for its conformational activation and its proapoptotic activity in response to H2O2-induced apoptosis. J Biol Chem. 2008;283:15359–15369. doi: 10.1074/jbc.M800847200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliari LJ, Kuwana T, Bonzon C, Newmeyer DD, Tu S, Beere HM, et al. The multidomain proapoptotic molecules Bax and Bak are directly activated by heat. Proc Natl Acad Sci USA. 2005;102:17975–17980. doi: 10.1073/pnas.0506712102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, et al. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- Etxebarria A, Terrones O, Yamaguchi H, Landajuela A, Landeta O, Antonsson B, et al. Endophilin B1/Bif-1 stimulates BAX activation independently from its capacity to produce large scale membrane morphological rearrangements. J Biol Chem. 2009;284:4200–4212. doi: 10.1074/jbc.M808050200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi Y, Karbowski M, Yamaguchi H, Kazi A, Wu J, Sebti SM, et al. Loss of Bif-1 suppresses Bax/Bak conformational change and mitochondrial apoptosis. Mol Cell Biol. 2005;25:9369–9382. doi: 10.1128/MCB.25.21.9369-9382.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azad A, Fox J, Leverrier S, Storey A. Blockade of the BAK hydrophobic groove by inhibitory phosphorylation regulates commitment to apoptosis. PLoS One. 2012;7:e49601. doi: 10.1371/journal.pone.0049601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox JL, Ismail F, Azad A, Ternette N, Leverrier S, Edelmann MJ, et al. Tyrosine dephosphorylation is required for Bak activation in apoptosis. EMBO J. 2010;29:3853–3868. doi: 10.1038/emboj.2010.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin M, Deng X. Nicotine inactivation of the proapoptotic function of Bax through phosphorylation. J Biol Chem. 2005;280:10781–10789. doi: 10.1074/jbc.M500084200. [DOI] [PubMed] [Google Scholar]
- Antonsson B, Conti F, Ciavatta A, Montessuit S, Lewis S, Martinou I, et al. Inhibition of Bax channel-forming activity by Bcl-2. Science. 1997;277:370–372. doi: 10.1126/science.277.5324.370. [DOI] [PubMed] [Google Scholar]
- Landeta O, Landajuela A, Gil D, Taneva S, Di Primo C, Sot B, et al. Reconstitution of proapoptotic BAK function in liposomes reveals a dual role for mitochondrial lipids in the BAK-driven membrane permeabilization process. J Biol Chem. 2011;286:8213–8230. doi: 10.1074/jbc.M110.165852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran VH, Bartolo R, Westphal D, Alsop A, Dewson G, Kluck RM. Bak apoptotic function is not directly regulated by phosphorylation. Cell Death Dis. 2013;4:e452. doi: 10.1038/cddis.2012.191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinou JC, Youle RJ. Mitochondria in apoptosis: Bcl-2 family members and mitochondrial dynamics. Dev Cell. 2011;21:92–101. doi: 10.1016/j.devcel.2011.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipuk JE, McStay GP, Bharti A, Kuwana T, Clarke CJ, Siskind LJ, et al. Sphingolipid metabolism cooperates with BAK and BAX to promote the mitochondrial pathway of apoptosis. Cell. 2012;148:988–1000. doi: 10.1016/j.cell.2012.01.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Lazarou M, Stojanovski D, Frazier AE, Kotevski A, Dewson G, Craigen WJ, et al. Inhibition of Bak activation by VDAC2 is dependent on the Bak transmembrane anchor. J Biol Chem. 2010;285:36876–36883. doi: 10.1074/jbc.M110.159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Kim H, Tu HC, Westergard TD, Fisher JK, Rubens JA, et al. The VDAC2-BAK rheostat controls thymocyte survival. Sci Signal. 2009;2:ra48. doi: 10.1126/scisignal.2000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benard G, Neutzner A, Peng G, Wang C, Livak F, Youle RJ, et al. IBRDC2, an IBR-type E3 ubiquitin ligase, is a regulatory factor for Bax and apoptosis activation. EMBO J. 2010;29:1458–1471. doi: 10.1038/emboj.2010.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen ZJ, Esnault S, Schinzel A, Borner C, Malter JS. The peptidyl-prolyl isomerase Pin1 facilitates cytokine-induced survival of eosinophils by suppressing Bax activation. Nat Immunol. 2009;10:257–265. doi: 10.1038/ni.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karbowski M, Lee YJ, Gaume B, Jeong SY, Frank S, Nechushtan A, et al. Spatial and temporal association of Bax with mitochondrial fission sites, Drp1, and Mfn2 during apoptosis. J Cell Biol. 2002;159:931–938. doi: 10.1083/jcb.200209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montessuit S, Somasekharan SP, Terrones O, Lucken-Ardjomande S, Herzig S, Schwarzenbacher R, et al. Membrane remodeling induced by the dynamin-related protein Drp1 stimulates Bax oligomerization. Cell. 2010;142:889–901. doi: 10.1016/j.cell.2010.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]