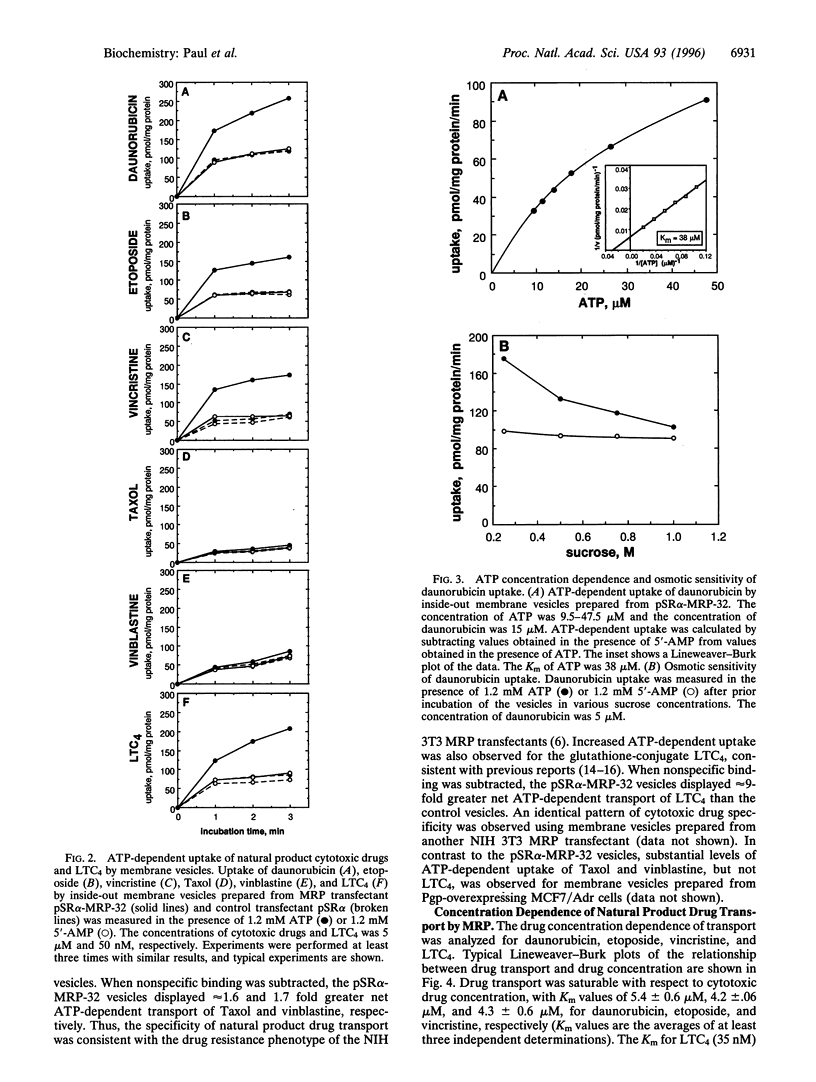
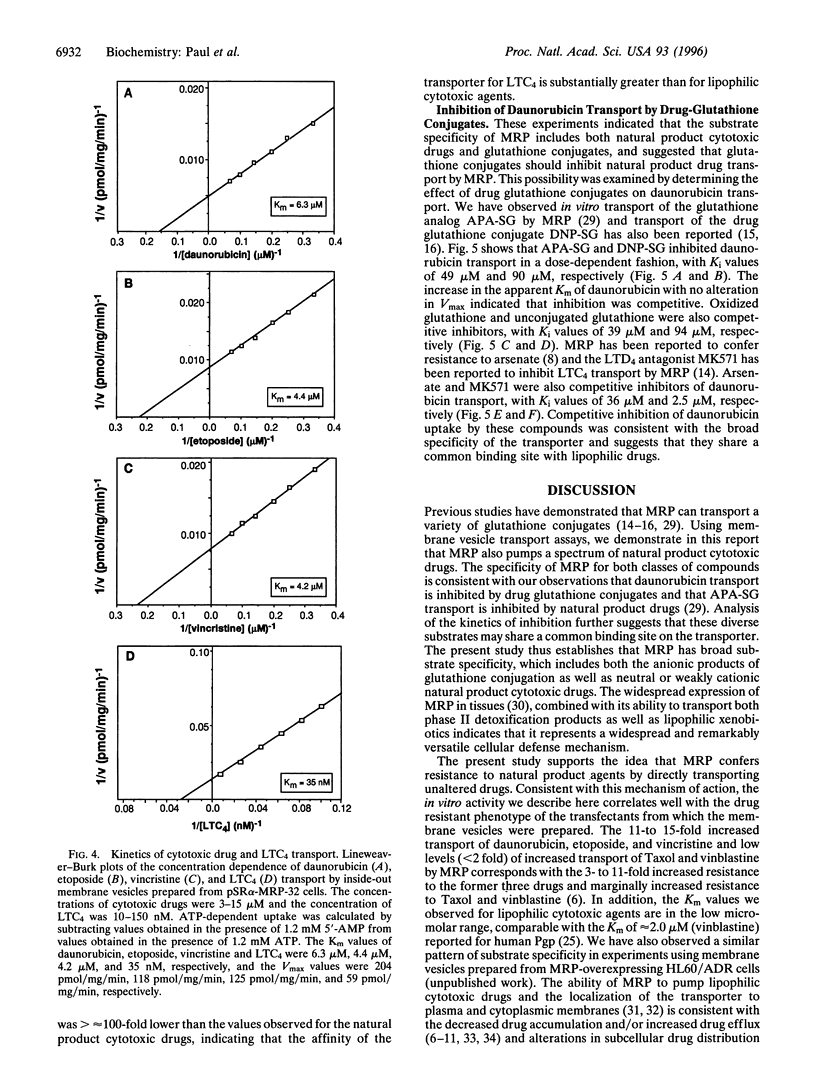
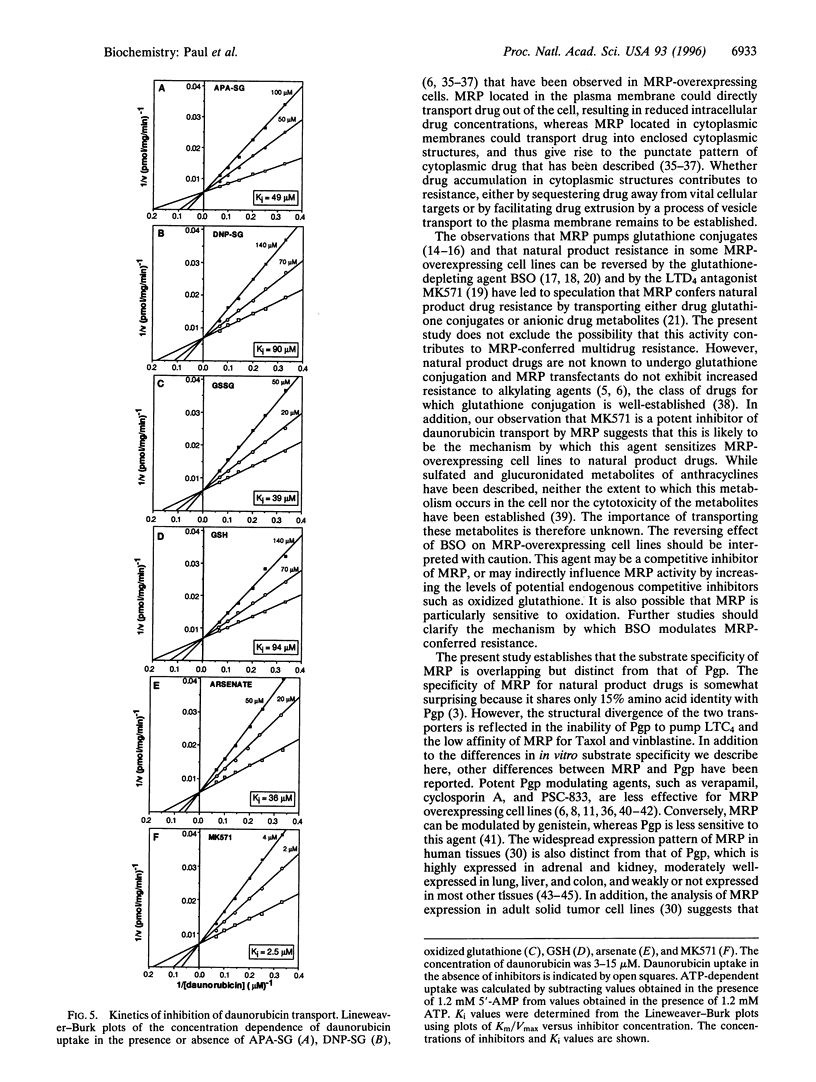
Abstract
MRP is a recently isolated ATP-binding cassette family transporter. We previously reported transfection studies that established that MRP confers multidrug resistance [Kruh, G. D., Chan, A., Myers, K., Gaughan, K., Miki, T. & Aaronson, S. A. (1994) Cancer Res. 54, 1649-1652] and that expression of MRP is associated with enhanced cellular efflux of lipophilic cytotoxic agents [Breuninger, L. M., Paul, S., Gaughan, K., Miki, T., Chan, A., Aaronson, S. A. & Kruh, G. D. (1995) Cancer Res. 55, 5342-5347]. To examine the biochemical mechanism by which MRP confers multidrug resistance, drug uptake experiments were performed using inside-out membrane vesicles prepared from NIH 3T3 cells transfected with an MRP expression vector. ATP-dependent transport was observed for several lipophilic cytotoxic agents including daunorubicin, etoposide, and vincristine, as well as for the glutathione conjugate leukotriene C4 (LTC4). However, only marginally increased uptake was observed for vinblastine and Taxol. Drug uptake was osmotically sensitive and saturable with regard to substrate concentration, with Km values of 6.3 microM, 4.4 microM, 4.2 microM, 35 nM, and 38 microM, for daunorubicin, etoposide, vincristine, LTC4, and ATP, respectively. The broad substrate specificity of MRP was confirmed by the observation that daunorubicin transport was competitively inhibited by reduced and oxidized glutathione, the glutathione conjugates S-(p-azidophenacyl)-glutathione (APA-SG) and S-(2,4-dinitrophenyl)glutathione (DNP-SG), arsenate, and the LTD4 antagonist MK571. This study establishes that MRP pumps unaltered lipophilic cytotoxic drugs, and suggests that this activity is an important mechanism by which the transporter confers multidrug resistance. The present study also indicates that the substrate specificity of MRP is overlapping but distinct from that of P-glycoprotein, and includes both the neutral or mildly cationic natural product cytotoxic drugs and the anionic products of glutathione conjugation. The widespread expression of MRP in tissues, combined with its ability to transport both lipophilic xenobiotics and the products of phase II detoxification, indicates that the transporter represents a widespread and remarkably versatile cellular defense mechanism.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barrand M. A., Rhodes T., Center M. S., Twentyman P. R. Chemosensitisation and drug accumulation effects of cyclosporin A, PSC-833 and verapamil in human MDR large cell lung cancer cells expressing a 190k membrane protein distinct from P-glycoprotein. Eur J Cancer. 1993;29A(3):408–415. doi: 10.1016/0959-8049(93)90397-x. [DOI] [PubMed] [Google Scholar]
- Bizub-Bender D., Kulkosky J., Skalka A. M. Monoclonal antibodies against HIV type 1 integrase: clues to molecular structure. AIDS Res Hum Retroviruses. 1994 Sep;10(9):1105–1115. doi: 10.1089/aid.1994.10.1105. [DOI] [PubMed] [Google Scholar]
- Breuninger L. M., Paul S., Gaughan K., Miki T., Chan A., Aaronson S. A., Kruh G. D. Expression of multidrug resistance-associated protein in NIH/3T3 cells confers multidrug resistance associated with increased drug efflux and altered intracellular drug distribution. Cancer Res. 1995 Nov 15;55(22):5342–5347. [PubMed] [Google Scholar]
- Cole S. P., Bhardwaj G., Gerlach J. H., Mackie J. E., Grant C. E., Almquist K. C., Stewart A. J., Kurz E. U., Duncan A. M., Deeley R. G. Overexpression of a transporter gene in a multidrug-resistant human lung cancer cell line. Science. 1992 Dec 4;258(5088):1650–1654. doi: 10.1126/science.1360704. [DOI] [PubMed] [Google Scholar]
- Cole S. P., Chanda E. R., Dicke F. P., Gerlach J. H., Mirski S. E. Non-P-glycoprotein-mediated multidrug resistance in a small cell lung cancer cell line: evidence for decreased susceptibility to drug-induced DNA damage and reduced levels of topoisomerase II. Cancer Res. 1991 Jul 1;51(13):3345–3352. [PubMed] [Google Scholar]
- Cole S. P., Downes H. F., Slovak M. L. Effect of calcium antagonists on the chemosensitivity of two multidrug-resistant human tumour cell lines which do not overexpress P-glycoprotein. Br J Cancer. 1989 Jan;59(1):42–46. doi: 10.1038/bjc.1989.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole S. P., Sparks K. E., Fraser K., Loe D. W., Grant C. E., Wilson G. M., Deeley R. G. Pharmacological characterization of multidrug resistant MRP-transfected human tumor cells. Cancer Res. 1994 Nov 15;54(22):5902–5910. [PubMed] [Google Scholar]
- Coley H. M., Amos W. B., Twentyman P. R., Workman P. Examination by laser scanning confocal fluorescence imaging microscopy of the subcellular localisation of anthracyclines in parent and multidrug resistant cell lines. Br J Cancer. 1993 Jun;67(6):1316–1323. doi: 10.1038/bjc.1993.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coley H. M., Workman P., Twentyman P. R. Retention of activity by selected anthracyclines in a multidrug resistant human large cell lung carcinoma line without P-glycoprotein hyperexpression. Br J Cancer. 1991 Mar;63(3):351–357. doi: 10.1038/bjc.1991.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Flens M. J., Izquierdo M. A., Scheffer G. L., Fritz J. M., Meijer C. J., Scheper R. J., Zaman G. J. Immunochemical detection of the multidrug resistance-associated protein MRP in human multidrug-resistant tumor cells by monoclonal antibodies. Cancer Res. 1994 Sep 1;54(17):4557–4563. [PubMed] [Google Scholar]
- Fojo A. T., Ueda K., Slamon D. J., Poplack D. G., Gottesman M. M., Pastan I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc Natl Acad Sci U S A. 1987 Jan;84(1):265–269. doi: 10.1073/pnas.84.1.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gekeler V., Ise W., Sanders K. H., Ulrich W. R., Beck J. The leukotriene LTD4 receptor antagonist MK571 specifically modulates MRP associated multidrug resistance. Biochem Biophys Res Commun. 1995 Mar 8;208(1):345–352. doi: 10.1006/bbrc.1995.1344. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Gottesman M. M., Pastan I. Expression of the MDR1 gene in human cancers. Cancer Treat Res. 1991;57:101–119. doi: 10.1007/978-1-4615-3872-1_5. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Grant C. E., Valdimarsson G., Hipfner D. R., Almquist K. C., Cole S. P., Deeley R. G. Overexpression of multidrug resistance-associated protein (MRP) increases resistance to natural product drugs. Cancer Res. 1994 Jan 15;54(2):357–361. [PubMed] [Google Scholar]
- Horio M., Gottesman M. M., Pastan I. ATP-dependent transport of vinblastine in vesicles from human multidrug-resistant cells. Proc Natl Acad Sci U S A. 1988 May;85(10):3580–3584. doi: 10.1073/pnas.85.10.3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa T. ATP/Mg2+-dependent cardiac transport system for glutathione S-conjugates. A study using rat heart sarcolemma vesicles. J Biol Chem. 1989 Oct 15;264(29):17343–17348. [PubMed] [Google Scholar]
- Ishikawa T., Akimaru K., Kuo M. T., Priebe W., Suzuki M. How does the MRP/GS-X pump export doxorubicin? J Natl Cancer Inst. 1995 Nov 1;87(21):1639–1640. doi: 10.1093/jnci/87.21.1639. [DOI] [PubMed] [Google Scholar]
- Ishikawa T., Müller M., Klünemann C., Schaub T., Keppler D. ATP-dependent primary active transport of cysteinyl leukotrienes across liver canalicular membrane. Role of the ATP-dependent transport system for glutathione S-conjugates. J Biol Chem. 1990 Nov 5;265(31):19279–19286. [PubMed] [Google Scholar]
- Jedlitschky G., Leier I., Buchholz U., Center M., Keppler D. ATP-dependent transport of glutathione S-conjugates by the multidrug resistance-associated protein. Cancer Res. 1994 Sep 15;54(18):4833–4836. [PubMed] [Google Scholar]
- Kamimoto Y., Gatmaitan Z., Hsu J., Arias I. M. The function of Gp170, the multidrug resistance gene product, in rat liver canalicular membrane vesicles. J Biol Chem. 1989 Jul 15;264(20):11693–11698. [PubMed] [Google Scholar]
- Krishnamachary N., Center M. S. The MRP gene associated with a non-P-glycoprotein multidrug resistance encodes a 190-kDa membrane bound glycoprotein. Cancer Res. 1993 Aug 15;53(16):3658–3661. [PubMed] [Google Scholar]
- Kruh G. D., Chan A., Myers K., Gaughan K., Miki T., Aaronson S. A. Expression complementary DNA library transfer establishes mrp as a multidrug resistance gene. Cancer Res. 1994 Apr 1;54(7):1649–1652. [PubMed] [Google Scholar]
- Kruh G. D., Gaughan K. T., Godwin A., Chan A. Expression pattern of MRP in human tissues and adult solid tumor cell lines. J Natl Cancer Inst. 1995 Aug 16;87(16):1256–1258. doi: 10.1093/jnci/87.16.1256. [DOI] [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Cole S. P., Deeley R. G., Keppler D. The MRP gene encodes an ATP-dependent export pump for leukotriene C4 and structurally related conjugates. J Biol Chem. 1994 Nov 11;269(45):27807–27810. [PubMed] [Google Scholar]
- Leier I., Jedlitschky G., Buchholz U., Keppler D. Characterization of the ATP-dependent leukotriene C4 export carrier in mastocytoma cells. Eur J Biochem. 1994 Mar 1;220(2):599–606. doi: 10.1111/j.1432-1033.1994.tb18661.x. [DOI] [PubMed] [Google Scholar]
- Lelong I. H., Padmanabhan R., Lovelace E., Pastan I., Gottesman M. M. ATP and GTP as alternative energy sources for vinblastine transport by P-170 in KB-V1 plasma membrane vesicles. FEBS Lett. 1992 Jun 15;304(2-3):256–260. doi: 10.1016/0014-5793(92)80632-q. [DOI] [PubMed] [Google Scholar]
- Marquardt D., Center M. S. Drug transport mechanisms in HL60 cells isolated for resistance to adriamycin: evidence for nuclear drug accumulation and redistribution in resistant cells. Cancer Res. 1992 Jun 1;52(11):3157–3163. [PubMed] [Google Scholar]
- McGrath T., Center M. S. Mechanisms of multidrug resistance in HL60 cells: evidence that a surface membrane protein distinct from P-glycoprotein contributes to reduced cellular accumulation of drug. Cancer Res. 1988 Jul 15;48(14):3959–3963. [PubMed] [Google Scholar]
- McGrath T., Latoud C., Arnold S. T., Safa A. R., Felsted R. L., Center M. S. Mechanisms of multidrug resistance in HL60 cells. Analysis of resistance associated membrane proteins and levels of mdr gene expression. Biochem Pharmacol. 1989 Oct 15;38(20):3611–3619. doi: 10.1016/0006-2952(89)90134-2. [DOI] [PubMed] [Google Scholar]
- Meijer C., Mulder N. H., Timmer-Bosscha H., Peters W. H., de Vries E. G. Combined in vitro modulation of adriamycin resistance. Int J Cancer. 1991 Oct 21;49(4):582–586. doi: 10.1002/ijc.2910490419. [DOI] [PubMed] [Google Scholar]
- Müller M., Meijer C., Zaman G. J., Borst P., Scheper R. J., Mulder N. H., de Vries E. G., Jansen P. L. Overexpression of the gene encoding the multidrug resistance-associated protein results in increased ATP-dependent glutathione S-conjugate transport. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):13033–13037. doi: 10.1073/pnas.91.26.13033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider E., Yamazaki H., Sinha B. K., Cowan K. H. Buthionine sulphoximine-mediated sensitisation of etoposide-resistant human breast cancer MCF7 cells overexpressing the multidrug resistance-associated protein involves increased drug accumulation. Br J Cancer. 1995 Apr;71(4):738–743. doi: 10.1038/bjc.1995.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen H., Paul S., Breuninger L. M., Ciaccio P. J., Laing N. M., Helt M., Tew K. D., Kruh G. D. Cellular and in vitro transport of glutathione conjugates by MRP. Biochemistry. 1996 May 7;35(18):5719–5725. doi: 10.1021/bi960098n. [DOI] [PubMed] [Google Scholar]
- Slovak M. L., Hoeltge G. A., Dalton W. S., Trent J. M. Pharmacological and biological evidence for differing mechanisms of doxorubicin resistance in two human tumor cell lines. Cancer Res. 1988 May 15;48(10):2793–2797. [PubMed] [Google Scholar]
- Sugawara I., Kataoka I., Morishita Y., Hamada H., Tsuruo T., Itoyama S., Mori S. Tissue distribution of P-glycoprotein encoded by a multidrug-resistant gene as revealed by a monoclonal antibody, MRK 16. Cancer Res. 1988 Apr 1;48(7):1926–1929. [PubMed] [Google Scholar]
- Tew K. D. Glutathione-associated enzymes in anticancer drug resistance. Cancer Res. 1994 Aug 15;54(16):4313–4320. [PubMed] [Google Scholar]
- Twentyman P. R., Fox N. E., Bleehen N. M. Drug resistance in human lung cancer cell lines: cross-resistance studies and effects of the calcium transport blocker, verapamil. Int J Radiat Oncol Biol Phys. 1986 Aug;12(8):1355–1358. doi: 10.1016/0360-3016(86)90170-7. [DOI] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Bagrij T., Scheper R. J., Twentyman P. R. Regulation by glutathione of drug transport in multidrug-resistant human lung tumour cell lines overexpressing multidrug resistance-associated protein. Br J Cancer. 1995 Jul;72(1):82–89. doi: 10.1038/bjc.1995.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Versantvoort C. H., Broxterman H. J., Pinedo H. M., de Vries E. G., Feller N., Kuiper C. M., Lankelma J. Energy-dependent processes involved in reduced drug accumulation in multidrug-resistant human lung cancer cell lines without P-glycoprotein expression. Cancer Res. 1992 Jan 1;52(1):17–23. [PubMed] [Google Scholar]
- Versantvoort C. H., Schuurhuis G. J., Pinedo H. M., Eekman C. A., Kuiper C. M., Lankelma J., Broxterman H. J. Genistein modulates the decreased drug accumulation in non-P-glycoprotein mediated multidrug resistant tumour cells. Br J Cancer. 1993 Nov;68(5):939–946. doi: 10.1038/bjc.1993.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaman G. J., Flens M. J., van Leusden M. R., de Haas M., Mülder H. S., Lankelma J., Pinedo H. M., Scheper R. J., Baas F., Broxterman H. J. The human multidrug resistance-associated protein MRP is a plasma membrane drug-efflux pump. Proc Natl Acad Sci U S A. 1994 Sep 13;91(19):8822–8826. doi: 10.1073/pnas.91.19.8822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlstra J. G., de Vries E. G., Mulder N. H. Multifactorial drug resistance in an adriamycin-resistant human small cell lung carcinoma cell line. Cancer Res. 1987 Apr 1;47(7):1780–1784. [PubMed] [Google Scholar]