Abstract
The functional consequences of the familial hypertrophic cardiomyopathy A57G (alanine-to-glycine) mutation in the myosin ventricular essential light chain (ELC) were assessed in vitro and in vivo using previously generated transgenic (Tg) mice expressing A57G-ELC mutant vs. wild-type (WT) of human cardiac ELC and in recombinant A57G- or WT-protein-exchanged porcine cardiac muscle strips. Compared with the Tg-WT, there was a significant increase in the Ca2+ sensitivity of force (ΔpCa50 ≅ 0.1) and an ∼1.3-fold decrease in maximal force per cross section of muscle observed in the mutant preparations. In addition, a significant increase in passive tension in response to stretch was monitored in Tg-A57G vs. Tg-WT strips indicating a mutation-induced myocardial stiffness. Consistently, the hearts of Tg-A57G mice demonstrated a high level of fibrosis and hypertrophy manifested by increased heart weight-to-body weight ratios and a decreased number of nuclei indicating an increase in the two-dimensional size of Tg-A57G vs. Tg-WT myocytes. Echocardiography examination showed a phenotype of eccentric hypertrophy in Tg-A57G mice, enhanced left ventricular (LV) cavity dimension without changes in LV posterior/anterior wall thickness. Invasive hemodynamics data revealed significantly increased end-systolic elastance, defined by the slope of the pressure-volume relationship, indicating a mutation-induced increase in cardiac contractility. Our results suggest that the A57G allele causes disease by means of a discrete modulation of myofilament function, increased Ca2+ sensitivity, and decreased maximal tension followed by compensatory hypertrophy and enhanced contractility. These and other contributing factors such as increased myocardial stiffness and fibrosis most likely activate cardiomyopathic signaling pathways leading to pathologic cardiac remodeling.
Keywords: myosin essential light chain, hypertrophic cardiomyopathy, Ca2+ sensitivity of contraction, echocardiography, pressure-volume loops
the beating of the heart depends on ATP-controlled synchronous interactions between two major contractile proteins; myosin and actin (15). The myosin cross bridges are the molecular motors of the heart, which bind and hydrolyze Mg-ATP and cyclically attach and dissociate from actin-tropomyosin (Tm)-troponin (Tn) thin filaments in a Ca2+-dependent manner. The cycle of Ca2+ fluxes regulate the coupling between excitation and contraction and permit the highly synchronized action of cardiac sarcomeres causing the heart to contract (systole) or relax (diastole) (20). The two key components of the myosin cross bridge include the motor domain, consisting of the ATP and actin binding sites, and the neck domain also called the lever arm, which is structurally supported by two types of myosin light chains, the essential (ELC) and the regulatory (RLC) light chains (44). Attached to their respective IQ motifs on the myosin heavy chain (MHC), both light chains inherently participate in all biochemical steps of the acto-myosin cycle and execution of the power stroke (52). As components of the neck region of myosin, both ELC and RLC contribute to the movement of the lever arm in response to ATP hydrolysis and actin binding that ultimately result in myosin-elicited contractile force generation and sarcomere shortening (14).
The ELC constitutes an essential part of the myosin lever arm structure, but its contractile function is not entirely clear. A wealth of evidence suggests that it may play a role in force development and muscle contraction (17, 24, 36, 40, 54). The functional importance of myosin ELC in cardiac muscle has emerged through the identification of several mutations in the ELC gene (MYL3) shown to cause familial hypertrophic cardiomyopathy (FHC). FHC is an autosomal dominant disorder, manifested by asymmetric or symmetric cardiac hypertrophy, myofibrillar disarray, and fibrosis (2, 25). The clinical phenotype is highly variable and ranges from absence of symptoms to rapidly progressive heart failure and/or sudden cardiac death (SCD; Ref. 32). Notably, FHC has been considered the leading cause of SCD in the young, especially in athletes (33, 47). Compared with the β-MHC or cardiac myosin binding protein-C (cMyBP-C), FHC-linked mutations in the ventricular ELC are rare, but they are also associated with SCD (13, 22, 29, 37, 41, 43, 45). In this report, we focus on the A57G (alanine to glycine) mutation implicated in malignant FHC outcomes (29).
To examine the effect of the A57G mutation on heart function, we used our previously generated transgenic (Tg) mice expressing the A57G-ELC mutation (Tg-A57G; Ref. 40) and its effects were compared with those seen in Tg-wild-type (WT) mice expressing the full-length nonmutated human ventricular ELC (24). Previously performed histological examination of mouse hearts showed that the human phenotype including extensive disorganization of myocytes and interstitial fibrosis could be recapitulated in Tg-A57G mice (40). At the molecular level, we showed a mutation-mediated increase in fiber stiffness and a concomitant decrease in the interfilament lattice spacing by ∼1.5 nm compared with Tg-WT mouse papillary muscle fibers (40). In this report, we followed up on these important structural observations and characterized the function of Tg-A57G mouse myocardium in vitro using myosin and skinned papillary muscle preparations from the hearts of Tg-A57G mice and in vivo by echocardiography and invasive hemodynamics. We also determined the force-pCa relationship in ELC (A57G and WT recombinant proteins)-exchanged β-MHC-containing porcine cardiac fibers. Our results from both experimental systems used in this study, transgenic mice and reconstituted porcine muscle preparations, suggest that the key effects of this FHC-causing A57G mutation do not regard to changes in the enzymatic properties of the contractile apparatus of the heart. Rather, the A57G allele may cause FHC by means of a discrete modulation of myofilament Ca2+ sensitivity (increase) and maximal contractile force (decrease) followed by compensatory cardiac hypertrophy and age-dependent morphological abnormalities.
MATERIALS AND METHODS
Transgenic Mice
All animal studies were conducted in accordance with institutional guidelines, and the protocol was reviewed and approved by the Animal Care and Use Committee at the University of Miami Miller School of Medicine (UMMSM). UMMSM has an Animal Welfare Assurance (A-3224-01, effective July 11, 2007) on file with the Office of Laboratory Animal Welfare (OLAW), National Institutes of Health. The generation and characterization of Tg mice used in this study and Tg protein expression profiles have been described earlier by Kazmierczak et al. (24) and by Muthu et al. (40). Previously produced Tg-WT lines, L1, L3, and L4 expressing 88, 30, and 77% of WT-ELC (UniProtKB: P08590) and Tg-A57G lines, L1, L2, and L5 expressing 80, 55, and 75% of A57G-mutant, respectively, were used in this study. The percentage of protein expression indicates the amount of replacement of the endogenous mouse cardiac ELC by the human ventricular WT (UniProtKB: P08590) or its A57G-mutant. In all experiments, Tg-A57G mice were gender and age matched with Tg-WT.
Histopathology
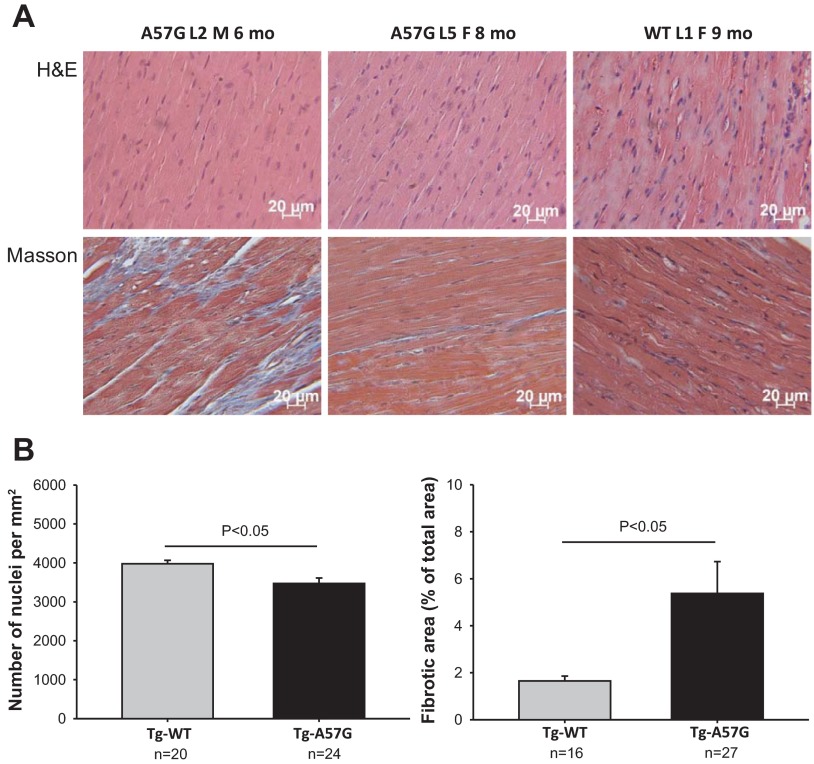
After euthanasia, the hearts from 6- to 9-mo-old Tg-A57G and Tg-WT mice were excised, weighed, and immersed in 10% buffered formalin. Slides of whole mouse hearts were prepared at the Histology Laboratory (University of Miami Miller School of Medicine, Miami FL). The paraffin-embedded longitudinal sections of whole mouse hearts stained with hematoxylin and eosin (H&E) and Masson's trichrome were examined for overall morphology and fibrosis using a Dialux 20 microscope, ×40/0.65 NA (numerical aperture) Leitz Wetzlar objective and an AxioCam HRc (Zeiss) as described previously (24, 40). Slides with H&E-stained sections from Tg-WT and Tg-A57G mouse hearts were analyzed for the number of nuclei. The nuclei count was taken from 9–10 segments (segment area of ∼0.033 mm2) of each slide, averaged, and expressed as number of nuclei per millimeters squared. Twenty segments from Tg-WT and twenty-four from Tg-A57G mouse left ventricles (LV) were analyzed. Slides with Masson's trichrome-stained sections were examined for the degree of fibrosis. Twenty-seven segments from Tg-A57G slides were analyzed using ImageJ software and compared with sixteen segments taken from Tg-WT slides. Fibrotic areas were expressed as percentage of total area analyzed.
Glycerinated Papillary Muscle Preparations
The papillary muscles from the LV of 4- to 6 mo-old Tg-A57G and from 4- to 5 mo-old Tg-WT were isolated, dissected into small muscle bundles, and chemically skinned in 50% glycerol and 50% pCa 8 solution {10−8 M [Ca2+], 1 mM free [Mg2+] [total MgPr (propionate) =3.88 mM], 7 mM EGTA, 2.5 mM [Mg-ATP2−], 20 mM MOPS pH 7.0, 15 mM creatine phosphate, and 15 U/ml of phosphocreatine kinase, ionic strength = 150 mM adjusted with KPr} containing 1% Triton X-100 for 24 h at 4°C. Mouse papillary muscle bundles were then transferred to the same solution without Triton X-100 and stored at −20°C for 5–10 days (23). Porcine papillary muscle preparations used in ELC-exchanged experiments were prepared according to the same protocol as Tg-mouse muscle bundles and stored at −20°C up to 6 wk.
Steady-State Force Measurements in Transgenic Mouse Papillary Muscle Strips
Small muscle strips composed of approximately three to six muscle fibers were isolated from a batch of glycerinated skinned mouse papillary muscle bundles and attached by tweezer clips to the force transducer of the Guth Muscle Research System (Heidelberg, Germany). They were placed in a 1-ml cuvette and skinned in 1% Triton X-100 dissolved in pCa 8 buffer for 30 min, as described in Kazmierczak et al. (23). Muscle strips were then washed three times for 5 min in pCa 8 buffer and tested for steady-state force development in pCa 4 solution (composition is the same as pCa 8 buffer except the [Ca2+] = 10−4 M). All experiments were carried out at 21°C. Maximal steady-state tension measured in pCa 4 solution was expressed in newtons per cross section of the muscle strip (N/m2; Ref. 38). The measurement of diameter was taken at approximately three points along the muscle strip length with an SZ6045 Olympus microscope (zoom ratio of 6.3:1, up to 189 × maximum magnification) and averaged (38). For the measurement of passive tension (in pCa 8 solution) in response to muscle stretch, the muscle strip was first released and stretched until it began generating tension (53). This point was set as zero for both the passive force and starting length of the muscle. Then, the muscle strip was stretched by 10% of its length × four consecutive times, and the passive tension in kilonewtons per meters squared was determined.
Steady-State Force Measurements in ELC-Exchanged Porcine Cardiac Muscle Preparations
Muscle strips consisting of approximately two to five porcine papillary muscle fibers were isolated from a batch of glycerinated skinned porcine papillary muscle bundles, attached to a force transducer and skinned for 30 min in a 1-ml cuvette in a solution containing pCa 8 buffer and 1% Triton X-100. Muscle strips were then washed three times for 5 min in pCa 8 before undergoing the ELC-protein exchange protocol. The reaction was performed in 200-μl solution containing 30 μM of recombinant human cardiac ELC-WT or ELC-A57G dissolved in 150 mM KCl, 10 mM KH2PO4, 10 mM imidazole (pH 6.5), 5 mM Mg-ATP, and 5 mM DTT in the presence of 1 mM TFP for 1 h at 21°C. The ELC exchanged fibers were washed three times for 5 min in pCa 8 buffer and reconstituted with human cardiac myosin RLC and troponin C (TnC) dissolved in 200 μl of pCa 8 solution containing 2 mM DTT (final concentration, 15 μM RLC and 15 μM TnC) for 30 min at 21°C. The latter step was necessary to ensure that muscle strips were fully reconstituted with proteins whose lack could alter the force-pCa relationship. ELC exchanged and RLC/TnC reconstituted preparations were then washed in pCa 8 buffer (3 times for 5 min) and subjected to force measurements.
Ca2+ Dependence of Force Development
After the initial steady-state force was determined, muscle strips (transgenic mouse or porcine reconstituted) were relaxed in pCa 8 buffer and then exposed to solutions of increasing Ca2+ concentrations from pCa 8 to pCa 4 (8). Steady-state force was measured in each “pCa” solution followed by relaxation in pCa 8 solution. Data were analyzed using the Hill equation (18), where “[Ca2+]50 or pCa50” is the free Ca2+ concentration that produces 50% force and “nH” is the Hill coefficient.
Rates of Muscle Relaxation in Tg-A57G and Tg-WT Muscle Strips
To monitor the relaxation rate, a photolabile derivative of BAPTA, Diazo-2 (6-nitro-1-diazo-2-naphthol-4-sulfonic acid), was used. Diazo-2 is able to rapidly chelate Ca2+ upon photolysis converting from a low affinity (Kd = 2.2 μmol/l) to a high affinity (Kd = 0.073 μmol/l) for Ca2+ (21). After testing for steady-state force, muscle strips were immersed in the solution of 2 mM Diazo-2, 0.5 mM CaCl2, 60 mM TES pH 7.0, 5 mM Mg-ATP, 1 mM [Mg2+], and 10 mM creatine phosphate along with 15 U/ml creatine phosphokinase, ionic strength = 200 mM. At this ratio of total added Ca2+ to Diazo-2, the resulting average initial force was ∼80% of the maximal force measured in the pCa 4 solution and the greatest extent of relaxation after photolysis of the Diazo-2 could be achieved. When force reached equilibrium, muscle strips were then exposed to a ultraviolet flash from a Xenon lamp. The photolysis-induced relaxation isotherms were fitted to a single exponential decay equation (Sigma Plot 11.0) yielding the relaxation rates in (s−1) for Tg-A57G vs. Tg-WT mice.
Preparation of Mouse Cardiac Myosin
Myosin was isolated from the left and right ventricles of 4- to 6 mo-old and 10- to 12 mo-old Tg-A57G and Tg-WT mice as described previously (24, 49). Briefly, after euthanasia, whole hearts were isolated and the atria were removed. Left and right ventricles were flash frozen and stored at −80°C until processed. The ventricular tissue was later thawed in an ice-cold Guba Straub-type buffer (pH 6.5) consisting of 300 mM NaCl, 100 mM NaH2PO4, 50 mM Na2HPO4, 1 mM MgCl2, 10 mM EDTA, 0.1% NaN3, 10 mM Na4P2O7, 1 mM DTT, and 1 μl/ml protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) in a volume of 0.75 ml buffer per 0.2 g tissue. Ventricles kept on ice were first minced by hand and then homogenized for 2 min at a frequency of 30 Hz in a Mixer-Mill MM301 (Retsch). The homogenate was then incubated on ice for 40 min before centrifugation for 1 h at 200,000 g. The supernatant was then diluted 60-fold (by volume) with 2 mM DTT and incubated on ice for 1 h. The samples were centrifuged again for 10 min at 8,000 g, and the pellets were then resuspended in a minimal volume of buffer containing 0.4 M KCl, 10 mM MOPS (pH 7.0), 5 mM DTT and 1 μl/ml protease inhibitor cocktail. Samples were then diluted 1:1 with glycerol, mixed gently, and stored at −20°C for up to ∼10 days. On average, one myosin preparation was obtained from a pool of approximately five hearts.
Actin-Activated Myosin ATPase Activity
Myosin used in the ATPase activity assays was purified from the hearts of Tg-A57G and Tg-WT mice as described above. Rabbit skeletal F-actin was prepared according to Kazmierczak et al. (24). Actin-activated myosin ATPase activity was measured as a function of actin concentration and the data analyzed as described in Kazmierczak et al. (23, 24). Briefly, 0.5 μM myosin dissolved in 0.4 M KCl was added to a 96-well microplate containing increasing concentrations of F-actin (in μM): 0.1, 0.5, 1.5, 3.0, 5, 7.5, 10, and 15. The assay was performed in a final volume of 120 μl in a buffer consisting of 25 mM imidazole pH 7.0, 4 mM MgCl2, 1 mM EGTA, and 1 mM DTT. The final KCl concentration was 77.7 mM. Protein mixtures were first incubated on ice for 10 min and then for another 10 min at 30°C. The reactions (run in triplicate) were initiated with the addition of 2.5 mM ATP with mixing in a Jitterbug incubator shaker (Boekel), allowed to proceed for 15 min at 30°C, and then terminated by the addition of 5% trichloroacetic acid. Precipitated proteins were cleared by centrifugation, and the inorganic phosphate was determined as described in Fiske and Subbarow (9). Data were analyzed using the Michaelis-Menten equation yielding Vmax and Km (16, 51).
Binding of Mouse-Purified Cardiac Myosin to Pyrene-Labeled F-Actin
Rabbit skeletal actin was labeled with pyrene iodoacetamide (Invitrogen/Molecular Probes) as described previously (23). Pyrene-labeled F-actin (at 0.5 μM), stabilized by 0.5 μM phalloidin, was titrated with increasing concentrations of mouse-purified Tg-A57G and Tg-WT myosins. Experiments were done in a 2-ml cuvette in a buffer containing 0.4 M KCl and 10 mM MOPS pH 7.0. Quenching of pyrene fluorescence of actin on myosin binding was monitored using a JASCO 6500 Fluorometer (Jasco), with excitation at 340 nm and emission at 407–409 nm. Titration profiles were analyzed with the nonlinear binding model as described in Kazmierczak et al. (23). In another set of experiments, dissociation of Tg-A57G vs. Tg-WT myosin from actin upon addition of AMP-PNP (adenylyl-imidodiphosphate; Roche) was monitored. Pyrene-labeled F-actin (0.5 μM) was complexed with 0.5 μM Tg-A57G or Tg-WT myosin in a 2-ml cuvette in a buffer containing 0.4 M KCl, 2 mM MgCl2, and 10 mM MOPS pH 7.0. Protein mixtures were incubated for 15 min (in the dark) at 21°C and then exposed to increasing concentrations of AMP-PNP from 0 to 100 μM. The recovery of pyrene fluorescence, due to AMP-PNP-induced dissociation of myosin from actin, was monitored at 407–409 nm with excitation wavelength of 340 nm. The dissociation isotherms were analyzed as described in Muthu et al. (40) and Kazmierczak et al. (23).
Fast Kinetic Experiments
Tg-A57G or Tg-WT myosins at 0.25 μM were mixed with 0.25 μM pyrene-labeled F-actin (stabilized by 0.25 μM phalloidin) in rigor buffer containing 0.4 M KCl, 1 mM DTT, and 10 mM MOPS pH 7.0. The complexes were mixed in a 1:1 ratio (vol/vol) with varying concentrations of Mg-ATP (10–80 μM) dissolved in the same buffer, and the dissociation of Tg-A57G vs. Tg-WT myosin from F-actin was observed by monitoring the time course of the change in pyrene fluorescence. Measurements were performed at 21°C using a BioLogic (Claix, France) model SFM-20 stopped-flow instrument outfitted with a Berger ball mixer and an FC-20 observation cuvette. The data were collected and digitized using a JASCO 6500 Fluorometer. The estimated dead time was 8.2 ms. The pyrene-F-actin was excited at 347 nm, and emission was monitored at 404 nm using monochromators set to 20-nm bandwidths. Typically, 5–13 stopped-flow records were averaged and fit to a single exponential equation to obtain the rate of a given Mg-ATP concentration. A plot of the observed myosin dissociation rates as a function of [Mg-ATP] was linear and the slope corresponded to the rate constant expressed in M−1·s−1.
SDS-PAGE Analysis of MHC-Isoform Expression in Myosin Preparations from Tg Mice
Preparations of cardiac myosin were tested for their MHC-isoform expression using SDS-PAGE (56). Briefly, myosin was dissolved in 0.4 M KCl, 10 mM MOPS pH 7, and 1 mM DTT and protein concentration was determined using Coomassie Plus reagent (Pierce). Myosin samples were mixed at 1:1 (vol/vol) ratio with Laemmli buffer (62.5 mM Tris·HCl pH 6.8, 25% glycerol, 2% SDS, and 0.01% bromophenol blue and 5% β-ME), heated, and loaded at 0.3 or 1.3 μg/lane and run on SDS-5% PAGE for ∼6 h at constant voltage of 70 V at 4°C. Bands were visualized by Coomassie blue G-250 or silver staining. Myosin ELC and RLC were used as loading controls and visualized on SDS-15% PAGE by silver staining or by Western blotting with protein specific antibodies (24).
Gene Expression Analysis Using Real-Time PCR in Mouse Cardiac Extracts from Tg Mice
Total RNA was isolated from ventricles of 4- to 6 mo-old (depicted “young”) and 11- to 12-mo-old (depicted “old”) Tg-A57G, Tg-WT, and nontransgenic (NTg) mice as described previously (24) and converted to a double stranded cDNA using Random Primers and a High-Capacity cDNA Reverse Transcription Kit (Applied Biosystems). Reverse transcription was performed in the MJ Research PTC-200 instrument according to the manufacturer's protocols. Quantitative PCR was conducted using SYBR Green I chemistry with gene-specific Quantitect Primer Sets (Qiagen) for murine Myl4 (myosin ELC atrial, NCBI accession no. NM_010858), Myh6 (cardiac α-myosin heavy chain, NCBI accession no. NM_010856) and Myh7 (cardiac β-myosin heavy chain, NCBI accession no. NM_080728) (24). Quantum RNA 18S internal standards (Ambion) and Power Sybr Green PCR Master-Mix (Applied Biosystems) were used according to manufacturer's protocols. All reactions were performed in triplicate and run using Bio-Rad iQ5 Multicolor Real-Time PCR Detection System with the following cycle parameters: cycle of 50°C (2 min) followed by 95°C (10 min), 40 cycles of 95°C (15 s) followed by 60°C (1 min). Raw data were analyzed using the Sequence Detection Software (ABI), and fold change in expression of each gene was calculated using the relative quantification ΔΔCt method with the levels of 18S ribosomal RNA as the normalizer gene (24).
Assessment of Protein Phosphorylation in Mouse Cardiac Myofibrils
After euthanasia, the hearts from ∼6-mo-old NTg, Tg-WT, and Tg-A57G mice were immediately isolated and frozen in liquid nitrogen. Before the experiment, the tissue was thawed in the CMF (cardiac myofibril) buffer consisting of 5 mM NaH2PO4, 5 mM Na2HPO4 (pH 7.0), 0.1 mM NaCl, 5 mM MgCl2, 0.5 mM EGTA, 5 mM ATP, 5 nM microcystin, 0.1% Triton X-100, 20 mM NaF (phosphatase inhibitor), 5 mM DTT, and 1 μl/ml protease inhibitor cocktail. The tissue was then homogenized in a Mixer-Mill MM301 until homogenous. The homogenate was then centrifuged for 4 min at 8,000 g, and the supernatant was discarded. After centrifugation, the pellets were left on ice for 4 min. This step was repeated three times until the pellet turned white. The pellets were then resuspended in the CMF buffer and the myofibrils were subsequently dissolved in SDS-PAGE sample buffer, and loaded on 12% SDS-PAGE. Phosphorylation of troponin (TnT, TnI) and myosin RLC was determined using Pro-Q Diamond phosphoprotein gel stain reagent (Invitrogen) as described in the manufacturer's manual. The total protein was further detected in the same gel using the SYPRO Ruby protein gel stain (Invitrogen). A transilluminator was used to view, image and photograph the gel and the band intensities were quantified using the ImageJ software.
In Vivo Studies
Echocardiography and invasive hemodynamics studies were performed on 6- to 7-mo-old male and female Tg-A57G mice, and the results were compared with those obtained for 5- to 7-mo-old Tg-WT mice. If no sex-dependent differences were noted, the results were pooled.
Echocardiography
In vivo cardiac morphology and function in Tg-A57G and Tg-WT mice were assessed with a Vevo-770 imaging system (Visual Sonics, Toronto, ON, Canada) equipped with 17.5 MHz transducer. Heart images were recorded from mice under isoflurane inhalation anesthesia (1–2%) when heart rates (>500 beats/min) and body temperature (37 ± 1°C) were controlled. Parameters of the heart structure and function were saved as M-mode and B-mode images, and all images were analyzed using Vevo 770 3.0.0 software (Visual Sonics). Determined parameters included the following: LV end-diastolic (d) and end-systolic (s) dimensions: LV posterior wall (LVPWd, LVPWs) and anterior wall (LVAWd, LVAWs). LV end-diastolic and end-systolic endocardial volumes (LVVd, LVVs) and ejection fraction (EF) were calculated from bidimensional long-axis parasternal views.
Hemodynamic Measurements
Mice were anesthetized in a chamber saturated with isoflurane and transferred to a surgical bench where anesthesia was maintained with 1–2% isoflurane, and the body temperature controlled at 37 ± 1°C. During all procedures, a 6% albumin solution was infused into the jugular vein at the rate of 5 μl/min. The micro-tip catheter transducer (SPR-839; Millar Instruments, Houston, TX) was introduced into the LV through the right carotid. The LV pressure-volume loops were recorded at steady state and during inferior vena cava occlusion. The volume calibration was done using echocardiographic measurements: end-diastolic volume (EDV) and stroke volume (SV). Cardiac preload was indexed as the EDV and end-diastolic pressure (EDP). Cardiac afterload was evaluated as effective arterial elastance (Ea): ESP/SV. Myocardial contractility was indexed by the peak rate of rise in LV pressure (dP/dtmax) and the load-independent end-systolic elastance (Ees), which is the slope of the end-systolic pressure-volume relationship (ESPVR). Diastolic performance was measured by the peak rate of LV relaxation, −dP/dtmin, and the time constant of ventricular relaxation (tau).
Statistical Analysis
All values are shown as means ± SE. Statistically significant differences between two groups of mice (WT and A57G) were determined using an unpaired Student's t-test (Sigma Plot 11; Systat Software, San Jose, CA), with significance defined as P < 0.05. Comparisons between multiple groups were performed using one-way ANOVA or ANOVA for repeated measures. In vivo tests were analyzed using GraphPad Prism software (San Diego, CA) version 5.0 for Windows.
RESULTS
Previously generated transgenic mice (24, 40), expressing the A57G-mutated or WT human ventricular ELC, were used to characterize the functional consequences of the FHC-linked Ala → Gly (A57G) ELC mutation in vitro and in vivo. In all experiments, the results obtained on mouse cardiac preparations from Tg-A57G mice were compared with those obtained on age- and sex-matched Tg-WT mice. As we showed previously there were no functional differences between different lines of Tg-WT and between Tg-WT and NTg littermates (24); therefore, NTg mice were not included in functional studies but were included in transcript analysis and SDS-PAGE experiments.
Assessment of Cardiac Hypertrophy and Morphology in Tg Mice
Representative H&E- and Masson's trichrome-stained LV sections from Tg-A57G and Tg-WT mouse hearts are presented in Fig. 1A. The heart tissue morphology pictured in the H&E stained sections showed no mutation-induced abnormalities, but the lower nuclei count revealed cardiac hypertrophy in the hearts of Tg-A57G mice compared with Tg-WT (Fig. 1B, left). A decreased number of nuclei indicated an increase in the two-dimensional size of Tg-A57G myocytes compared with the two-dimensional images of myocytes from Tg-WT. Notably, as demonstrated in Fig. 1A, bottom, and Fig. 1B, right, the hearts of Tg-A57G mice manifested severe fibrosis, an inherent feature of FHC, in animals as young as 6 mo of age. Histopathology data collected in this study are in accord with those observed previously and obtained on older (∼12 mo of age) animals (40). The heart weight-to-body weight ratio (HW/BW × 1,000) was determined for male and female Tg-A57G vs. Tg-WT mice of various ages. Significantly higher values were noted for the mutant mice (5.4 ± 0.2, n = 33) compared with Tg-WT controls (4.8 ± 0.2; n = 42; P < 0.05), supporting the hypertrophic phenotype in Tg-A57G mice. Compared with previously reported data on HW/BW for Tg-A57G vs. Tg-WT mice showing no statistical significance (40), the current study used a 2.2-fold larger sample size for Tg-WT and 3.3-fold for Tg-A57G. Noteworthy, while the HW/BW ratio for Tg-WT remained the same in this report as in (40), it increased in Tg-A57G mice.
Fig. 1.
Assessment of cardiac morphology and hypertrophy in transgenic (Tg)-A57G and Tg-wild-type (WT) mice. A: histopathology images of heart samples from representative Tg-A57G and Tg-WT mice stained with hematoxylin and eosin (H&E) and Masson's trichrome (Masson). Left ventricular sections from the hearts of 2 lines of Tg-A57G (L2 and L5) were compared with Tg-WT L1 mice. Note extensive collagen-stained fibrotic lesions in 6-mo-old Tg-A57G L2 mice. L, mouse line; M, male; F, female. B: assessment of cardiac hypertrophy by nuclei count (left) and fibrosis by Masson's trichrome-stained (right) heart sections from Tg-A57G vs. Tg-WT mice listed in A. The nuclei count (per mm2) was taken from 24 segments of an approximate area of 0.033 mm2/segment of H&E-stained slides from Tg-A57G hearts and compared with the number of nuclei analyzed in 20 segments of Tg-WT slides. Myocardial fibrosis was assessed in the same area of 0.033 mm2/section (27 total) of Masson's trichrome stained Tg-A57G slides and compared with fibrotic areas assessed in 16 Tg-WT sections using the ImageJ program. Note significant differences in both the nuclei count and the assessment of fibrotic areas between the groups.
Functional Consequences of the A57G Mutation Determined in Transgenic Cardiac Muscle Preparations
Force and kinetic measurements in skinned mouse papillary muscle strips.
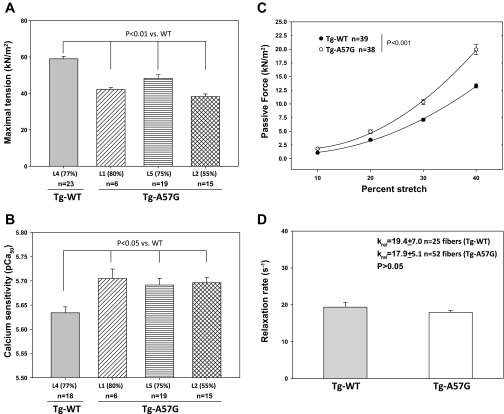
Measurements of steady-state force generation and muscle relaxation rates were performed on skinned papillary muscle strips from age- and gender-matched Tg-A57G vs. Tg-WT mice. Five to eight mice per group were used with each ventricle yielding six to nine papillary muscle strips that were subjected to mechanic measurements. A large (20–35%) decrease in maximal tension per cross section of muscle strip was observed in all tested lines of Tg-A57G mice compared with age and gender matched Tg-WT mice (Fig. 2A). The values of maximal force in kilonewtons per meters squared, and the number of all tested fibers are listed in the Fig. 2A legend. Particularly, the A57G L2 expressing 55% of transgene displayed the lowest level of force (38.21 ± 1.49 kN/m2; n = 15) among all mutant lines, which was decreased by ∼35% compared with Tg-WT mice (59.00 ± 1.31 kN/m2; n = 23). The A57G mutation also affected the Ca2+ sensitivity of force, and a small (ΔpCa50 ∼0.07) but significant (P < 0.05) increase in the Ca2+ sensitivity of force was observed between Tg-A57G animals compared with Tg-WT (Fig. 2B). No change in the Hill coefficient of the force-pCa dependence was noted between all groups of mice (nH = 3.9 ± 0.3).
Fig. 2.
Measurements of steady-state force in skinned papillary muscle fibers from Tg-A57G vs. Tg-WT mice. A: maximal tension assessment at saturating (pCa 4) calcium concentrations in skinned papillary muscle strips from Tg-WT L4 (line 4) vs. Tg-A57G L1, L5, and L2 (lines 1, 5, and 2) mice. A significant (P < 0.01) decrease in maximal force (pCa 4) per cross-sectional area of muscle strip was observed in all mutant lines compared with WT. The following values of tension (in kN/m2) were monitored in Tg-A57G mice: 42.17 ± 1.04 (L1), 48.30 ± 1.96 (L5), and 38.21 ± 1.49 (L2) compared with 59.00 ± 1.31 for Tg-WT (L4). B: calcium sensitivity of force in skinned papillary muscle strips from Tg-WT L4 vs. Tg-A57G L1, L5, and L2 mice. A small but significant (P < 0.05) increase in the Ca2+ sensitivity of force was observed between the strips from Tg-A57G animals compared with Tg-WT. The pCa50 values were as follows: Tg-A57G, pCa50 = 5.705 ± 0.048 (L1), 5.691 ± 0.006 (L5), and 5.696 ± 0.043 (L2); and Tg-WT L4, pCa50 = 5.634 ± 0.052. No mutation-dependent change in the Hill coefficient between the groups. C: assessment of passive tension (stiffness) under relaxation conditions (pCa 8). Tg-A57G L1 fibers demonstrated significantly increased levels of passive tension at 20, 30, and 40% of fiber stretch. Specific values of tension at pCa 8 were as follows (in kN/m2): 10%, 1.813 ± 0.173; 20%, 4.922 ± 0.337; 30%, 10.333 ± 0.486; and 40% and 19.926 ± 0.899 for n = 38 compared with Tg-WT L1 strips: 10%, 1.073 ± 0.085; 20%, 3.420 ± 0.156; 30%, 7.096 ± 0.218; and 40%, 13.285 ± 0.367 for n = 39 muscle strips. D: relaxation rates in Tg-A57G vs. Tg-WT papillary muscle strips assessed with Diazo-2. A mutation-induced 8% decrease in relaxation rates was observed, but the difference between relaxation kinetics in Tg-A57G and Tg-WT mice was not statistically significant (P > 0.05). The animals used were as follows: A and B: 3 lines of ∼5-mo-old female Tg-A57G (L1, L5, and L2) and Tg-WT L4; C: ∼6.5-mo-old female Tg-A57G L1 and Tg-WTL1; and D: ∼4-mo-old male Tg-A57G L1 and Tg-WT L1 mice.
Since our previous mechanical study demonstrated a significantly increased rigor stiffness in Tg-A57G vs. Tg-WT mice (40), we then pursued measurements of passive tension (in pCa 8 solution) in response to fiber stretch (Fig. 2C). As demonstrated, the level of passive tension was significantly increased in Tg-A57G strips compared with Tg-WT (P < 0.001), suggesting higher stiffness (increased resistance to stretch) in Tg-A57G myocardium. It is important to note that the mutation did not cause any differences in the resting length of the muscle strip allowing for direct comparison of the effects elicited by Tg-A57G and Tg-WT in response to stretch (40). After steady-state characterization, we then examined the effect of the A57G mutation on the kinetics of muscle relaxation using Diazo-2, a chelator of Ca2+ upon photolysis (Fig. 2D). A small (but not significant) decrease (by ∼8%) in the rate of force relaxation was observed in Tg-A57G preparations compared with Tg-WT (Fig. 2D).
Actin-activated myosin ATPase activity.
Two age groups of mice were used to obtain myosin from Tg-A57G and Tg-WT mice (see materials and methods). No age-dependent differences within each group were noted in solution experiments using various batches of myosin (data not shown). The steady-state actin-activated myosin ATPase activity was determined as a function of increasing F-actin concentrations using myosin purified from the hearts of Tg-A57G and Tg-WT mice. The ATPase isotherms were obtained by plotting the ATPase activity vs. [F-actin], with data points expressed as averages ± SE of six to seven experiments performed on different myosin preparations. No change in Vmax (in s−1), representing the rate constant of the transition from the weakly (A·M·ATP ↔ A·M·ADP·Pi) to strongly (A·M·ADP ↔ A·M) bound myosin cross bridges was observed between Tg-A57G (0.340 ± 0.032) and Tg-WT (0.364 ± 0.031) myosins. Likewise, similar Km (Michaelis-Menten constant) values (in μM) were observed between Tg-A57G (6.26 ± 1.54) and Tg-WT (7.43 ± 1.52) myosins.
Steady-state binding/dissociation of mouse myosins to F-actin.
The effect of A57G on the binding of myosin to F-actin was examined using a fluorescence-based assay as described in our recent publications (23, 31). Two to three different preparations of myosin were used and the assay was performed in triplicate. The fluorescence of pyrene-labeled F-actin was quenched in response to the binding of myosin heads. The experimental points were fitted to a nonlinear binding model as described previously (23, 31), yielding apparent dissociation constants, Kd = 16.8 ± 0.01 nM (n = 3) for Tg-A57G myosin and Kd = 11.8 ± 0.01 nM (n = 2) for Tg-WT myosin. The binding of myosin to actin under rigor (no nucleotide) conditions was strong and the binding isotherms basically overlapped (not shown). We then studied the effect of A57G on the dissociation profile of Tg-A57G myosin from pyrene-labeled F-actin on the addition of nonhydrolysable ATP analog, AMP-PNP. Upon nucleotide addition and the dissociation of Tg myosin from F-actin, the intensity of pyrene fluorescence increased. Similar to the binding in rigor, the results showed no difference in the dissociation profiles of Tg-A57G (Kd = 6.4 ± 6.0 μM; n = 4) and/or Tg-WT (Kd = 6.8 ± 3.4 μM, n = 4) myosin from F-actin, plotted as a function of increasing concentrations of AMP-PNP (not shown).
Stopped flow kinetics of acto-myosin interaction.
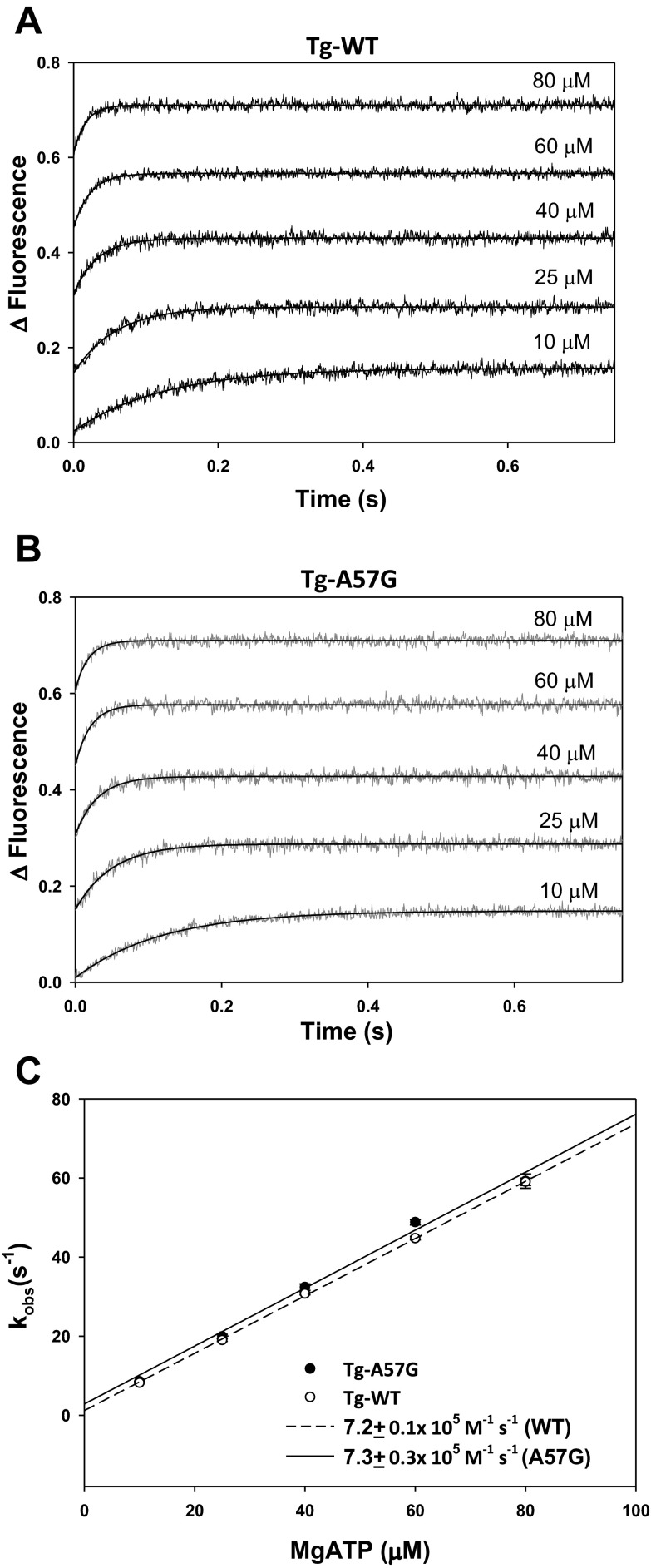
To further examine the effect of A57G on the interaction of myosin with actin, we measured the dissociation rates (kobs) of Tg-A57G vs. Tg-WT myosin bound to pyrene-labeled F-actin (Fig. 3). The time course of the change in pyrene fluorescence was monitored as a function of ATP concentrations. Myosins were stoichiometrically mixed with pyrene-F-actin (2 heads of myosin per actin monomer), and the complexes were mixed in a 1:1 volume ratio with increasing concentrations of Mg-ATP (10–80 μM) in a stopped flow apparatus. An increase in the fluorescence intensity was monitored as a function of time as the myosin heads dissociated from pyrene-F-actin on the addition of Mg-ATP. The representative time-dependent dissociation profiles for Tg-WT and Tg-A57G myosins are presented in Fig. 3, A and B, respectively. The observed dissociation rates (kobs) derived from the averaged fluorescence traces and fitted with a single exponential are presented in Table 1. The results revealed significant differences in kobs between Tg-A57G and Tg-WT myosin for 10, 40, and 60 μM Mg-ATP (Table 1), indicating faster dissociation rates in A57G compared with WT. Linear regression analysis of the plot of kobs vs. increasing [Mg-ATP] yielded the effective second-order ATP binding rate for the A57G equal to 7.3 ± 0.3 × 105 M−1·s−1 compared with 7.2 ± 0.1 × 105 M−1·s−1 for Tg-WT (Fig. 3C) indicating no overall effect of the A57G mutation on the rate of myosin cross-bridge dissociation from actin.
Fig. 3.
Fast kinetics of interaction between Tg-A57G and Tg-WT myosins with pyrene-labeled F-actin. A: representative averaged traces of pyrene-fluorescence on ATP-dependent dissociation of Tg-WT from F-actin. Increasing Mg-ATP concentrations (10–80 μM) were used. B: averaged traces of pyrene-fluorescence for Tg-A57G myosin. C: dissociation rates (kobs)-[Mg-ATP] dependence and the effective second-order ATP binding rates for Tg-A57G vs. Tg-WT myosins. The values of kobs ± SE for each Mg-ATP concentration are presented in Table 1.
Table 1.
Acto-myosin dissociation rates
| [Mg-ATP]/System | 10 μM | 25 μM | 40 μM | 60 μM | 80 μM |
|---|---|---|---|---|---|
| Tg-A57G | 8.596 ± 0.061† | 19.915 ± 0.565 | 32.415 ± 0.820* | 48.814 ± 0.682‡ | 59.232 ± 1.801 |
| n = 6 | n = 4 | n = 5 | n = 22 | n = 14 | |
| Tg-WT | 8.219 ± 0.074† | 19.044 ± 0.379 | 30.765 ± 0.313* | 44.775 ± 0.434‡ | 59.036 ± 0.906 |
| n = 8 | n = 8 | n = 8 | n = 27 | n = 26 |
Values are means ± SE. Dissociation rates (kobs) are in s−1.
P = 0.0495;
P = 0.0028;
P = 4.62E-06.
Effect of A57G on MHC Isoform Expression and Myofilament Protein Phosphorylation in Tg Cardiac Myofibrils
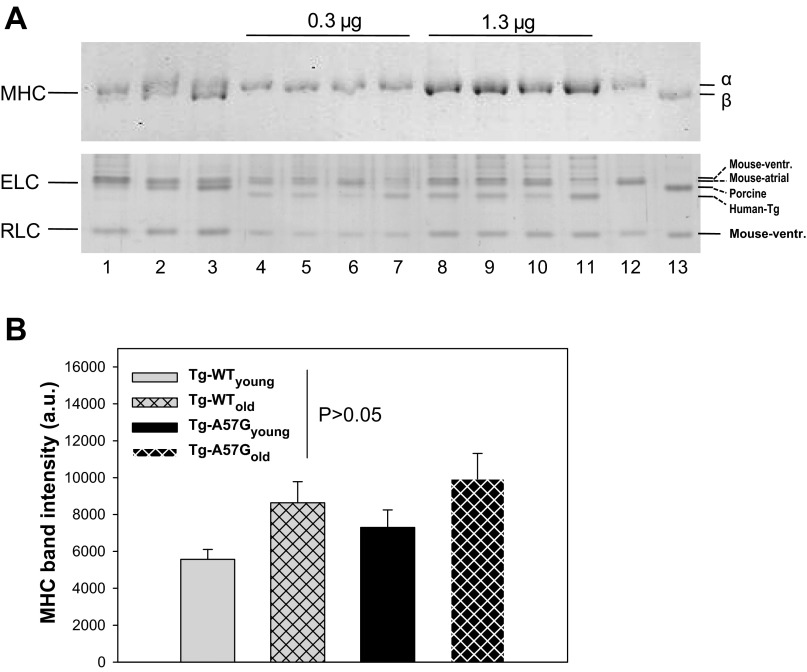
Considering that the MHC isoform switch (α to β) can significantly impact myosin cross-bridge kinetics (50), and especially muscle relaxation (7, 10, 19), we examined an age-dependent MHC expression profile in myosin purified from young and old Tg-A57G and Tg-WT mice. This experiment was also important considering that pathologic hypertrophy is often indicated by an upregulation of the β-MHC isoform (30). As shown in Fig. 4A, no significant changes in the α-MHC protein expression profile were detected in any of tested myosin preparations from Tg-A57G and Tg-WT mice. A visible separation of the α- vs. β -MHC isoforms was achieved in the mixtures of α-containing NTg myosin with β-porcine myosin (Fig. 4A, lanes 1–3). To increase detection of MHC isoforms, two different loading amounts (0.3 and 1.3 μg per lane) of myosin purified from the hearts of young and old Tg-A57G and Tg-WT mice were used (56). However, no double bands containing both the α- and β-MHC were observed in any tested transgenic myosins. Assessment of MHC band intensity corrected for loading showed a slight increase in the MHC band intensity in the older animals but the differences were not statistically significant (Fig. 4B). To further explore the possibility of an age- or disease-causing mutation-dependent upregulation of the β-MHC isoform, we pursued gene expression profiling in heart tissue from 4- to 6 mo-old (depicted as young) and 11- to 12 mo-old (depicted as old) Tg-A57G, Tg-WT, and NTg mice using a real-time quantitative PCR technique (24). Compared with NTg mice, ∼2.4- and ∼4.6-fold upregulation of the β-MHC in the hearts of Tg-A57Gyoung and Tg-WTyoung, respectively, was observed, but the difference was only statistically significant for Tg-WTyoung mice (Table 2). Older animals showed ∼0.5- and ∼0.1-fold downregulation of the β-MHC in Tg-A57Gold and Tg-WTold mice compared with age-matched NTgold mice (Table 2). Interestingly, there was a fivefold upregulation of the β-MHC in the hearts of old vs. young NTg mice showing an age-dependent effect. In addition, we also observed a significant upregulation of the α-MHC in the young group of the mutant and WT mice compared with NTg (Table 1). Noteworthy, similar to what was observed at the protein level (Fig. 4A), we found a significant upregulation of the atrial myosin ELC, another hypertrophic marker, in the young group of experimental mice (Table 2).
Fig. 4.
Assessment of age-dependent expression of α-myosin heavy chain (α-MHC) vs. β-MHC isoforms in Tg-A57G and Tg-WT mice. A, top: representative Coomassie-stained SDS-5% PAGE of α-MHC and β-MHC preparations. Two different loadings (0.3 and 1.3 μg per lane) of myosin purified from the hearts of young and old Tg-A57G and Tg-WT mice were used (lanes 4–11). A, bottom: loading controls of myosin essential (ELC) and regulatory (RLC) light chains separated on silver-stained SDS-15% PAGE. Lanes 1–3: clear separation of α- vs. β-MHC isoforms in mixtures of α-NTg (top band) to β-porcine myosin (bottom band). Lane 1: 1:0.2 (wt/wt); lane 2, 1:1 (wt/wt); and lane 3: 1:2 (wt/wt). Lanes 4 and 8, Tg-A57G L2, 4-mo-old; lanes 5 and 9: Tg-A57G L2, 12-mo-old; lanes 6 and 10: Tg-WT L3, 5-mo-old; lanes 7 and 11: Tg-WT L4, 11-mo-old. Protein loading in lanes 4–7 was 0.3 μg/well and in lanes 8–11 was 1.3 μg/well. Lane 12: α-NTg myosin standard; and lane 13: β-porcine myosin standard. As presented at top, no doublets of α- and β-MHCs were observed in any of transgenic myosins, loaded at 0.3 or 1.3 μg/well. Bottom: note that all transgenic animals expressed the atrial ELC isoform in their ventricles. Gel migration of ELC isoforms was according to their molecular mass: mouse ventricular > mouse atrial > porcine > human transgenic. B: assessment of MHC band intensities in SDS-5% PAGE. Data are derived from 16 SDS-5% PAGE gels ran in parallel with Coomassie- or silver-stained SDS-15% PAGE gels to correct for loading with respect to ELC, RLC, and actin. In addition ELC and RLC, loading controls were Western blotted and visualized with protein specific antibodies (24). On average 90–100 determinations of each MHC band in Tg-A57G and Tg-WT myosins were performed (a.u., arbitrary units).
Table 2.
Gene expression profile (fold change) in the hearts from young and old Tg-A57G and Tg-WT compared with NTg mice
| Gene (Protein) | Myl4 (ELC Atrial) | Myh6 (α-MHC) | Myh7 (β-MHC) |
|---|---|---|---|
| Tg-A57Gyoung | 4.787 P = 0.022 | 3.307 P = 0.043 | 2.424 P = 0.061 |
| Tg-WTyoung | 13.413 P = 0.009 | 4.596 P = 0.026 | 4.569 P = 0.011 |
| NTgyoung | 1 | 1 | 1 |
| Tg-A57Gold | 1.757 P = 0.125 | 0.730 P = 0.381 | 0.518 P = 0.172 |
| Tg-WTold | 4.917 P = 0.005 | 1.091 P = 0.839 | 0.119 P = 0.001 |
| NTgold | 1 | 1 | 1 |
| Tg-A57Gold vs. Tg-A57Gyoung | 2.623 P = 0.026 | 0.575 P = 0.211 | 1.079 P = 0.860 |
| Tg-WTold vs. Tg-WTyoung | 2.619 P = 0.132 | 0.618 P = 0.351 | 0.132 P = 0.001 |
| NTgold vs. NTgyoung | 7.146 P = 0.007 | 2.605 P = 0.061 | 5.052 P = 0.007 |
Values are means ± SE.
ELC, essential light chain; MHC, myosin heavy chain; Tg, transgenic; NTg, nontransgenic; WT, wild type; young, 4- to 5-mo-old mice; old, 11- to 12-mo-old mice.
To test whether the A57G mutation in myosin ELC had any effect on phosphorylation of other sarcomeric proteins, we examined endogenous phosphorylation of troponin (TnT, TnI) and myosin RLC in Tg-WT and Tg-A57G mouse cardiac myofibrils using the Pro-Q Diamond-SYPRO Ruby system, designed to quantitatively assess sarcomeric protein phosphorylation. NTg myofibrils were used as controls. As demonstrated in Fig. 5, A–D, the A57G mutation produced no changes in phosphorylation of TnT, TnI, or RLC compared with NTg preparations. Band intensities of the ProQ Diamond-stained gels demonstrating the extent of protein phosphorylation (Fig. 5A) were normalized for protein loading detected with SYPRO Ruby (Fig. 5B) or Coomassie (Fig. 5C) stained TnT, TnI, or RLC protein bands. Three sets of preparations were run on multiple Pro-Q and SYPRO gels, and the band intensities were averaged. We also tested the potential effect of the A57G mutation on MyBP-C phosphorylation using a 4–15% gel gradient allowing visualization of MyBP-C, and no any changes between Tg-A57G vs. Tg-WT preparations were noted (data not shown).
Fig. 5.
Assessment of protein phosphorylation in Tg-A57G and Tg-WT mouse myofibrils. Representative images of Pro-Q Diamond (A)-, SYPRO Ruby (B)-, and Coomassie brilliant blue-stained (C) SDS-15% PAGE containing Tg-A57G and Tg-WT mouse myofibrils. Phosphorylation status of TnT, TnI, and RLC in NTg myofibrils served as a control. Degree of protein phosphorylation recorded in the ProQ Diamond stained gel was normalized for protein loading detected in the same gel by subsequent SYPRO Ruby stain. Alternatively, protein loading was monitored on SDS-14% PAGE (resolving gel acrylamide:bisacrylamide ratio of 180:1) stained with Coomassie brilliant blue. D: degree of RLC phosphorylation monitored in Tg-A57G, Tg-WT, and NTg preparations corrected for loading using SYPRO (black bars) or Coomassie (dashed bars) staining. Compared with NTg myofibrils (100%) the following phosphorylation values were monitored in Tg-WT vs. Tg-A57G, respectively (in %): 104 ± 6, n = 8 vs. 117 ± 8, n = 9 (TnT); 113 ± 8, n = 17 vs. 113 ± 9, n = 22 (TnI); and 111 ± 8, n = 17 vs. 101 ± 2, n = 22 (RLC); n represents number of analyzed protein bands from 2–15 gels. MW Marker, 75- and 25-kDa standard protein bands; +P TnI, phosphorylated TnI standard; Tm, tropomyosin, TnT, troponin T; ELCend, endogenous porcine cardiac ELC, ELCTg, transgenic human cardiac ELC (WT or A57G) expressed in mice.
Functional Effects of A57G Assessed in Recombinant Protein A57G- or WT-Exchanged Porcine Cardiac Muscle Preparations
ELC-exchanged porcine papillary muscle strips.
To assess the effect of A57G mutation on force generation in β-MHC-containing porcine papillary muscle strips, we replaced the endogenous ELC protein with A57G or WT using an ELC-exchange protocol, shown schematically in Fig. 6A. This experiment was critical to assure that the effect of FHC-linked mutation can be examined in the background of β-MHC, the isoform of cardiac MHC (MYH7) that is observed in humans. Following ELC-protein exchange, porcine papillary muscle strips were subsequently reconstituted with exogenous TnC and RLC to secure the functionality of the reconstituted system (Fig. 6A). As shown in Fig. 6B, the amino acid sequence of porcine ventricular ELC (UniProtKB: F1SNW4) is 95% identical to the human ELC, especially in the vicinity of the A57G mutation. Unexpectedly, there was a difference in the level of A57G vs. WT reconstitution assessed in 7 individual exchange experiments showing a 1.6-fold higher yield for the mutant (Fig. 6C). The extent of A57G-exchange in porcine strips was in accord with the degree of A57G-exchange observed previously in porcine myosin (40), but WT-exchange efficiency was lower in porcine muscle strips (Fig. 6D, inset) compared with myosin (40).
Fig. 6.

Steady-state force measurements in skinned porcine papillary muscle fibers reconstituted with recombinant human cardiac ELC-WT or ELC-A57G mutant proteins. A: schematic representation of the force-pCa experimental protocol: 1) exchange process of recombinant ELC-WT or ELC-A57G mutant proteins for endogenous porcine cardiac ELC; 2) restoration of maximal pCa 4 developed force; and 3) assessment of the force-pCa relationship before and after ELC-exchange. B: ventricular ELC protein sequences: human (UniProtKB: P08590) and porcine (UniProtKB: F1SNW4). The Ala residue that is mutated to Gly in FHC is bolded and underlined. Amino acid differences between porcine and human ELC are highlighted in gray. C: extent of ELC protein replacement (exogenous for endogenous) in porcine papillary muscle strips. Note that A57G was more efficient in exchanging the endogenous porcine ELC protein than WT. D: force-pCa relationship assessed in ELC-exchanged porcine cardiac muscle strips. Note the leftward shift by ΔpCa50 = 0.11 in A57G-exchanged preparations compared with WT-exchanged fibers. Inset: representative SDS-PAGE of control (lane 1), WT-reconstituted (lane 2), and A57G-reconstituted (lane 3) porcine papillary muscle strips. ELCmut, human cardiac ELC (WT or A57G)-exchanged muscle strips.
Ca2+ sensitivity of force in ELC-exchanged porcine papillary muscle strips.
Similar to the results observed for Tg mouse papillary muscle strips, the Ca2+ sensitivity of force in A57G-exchanged porcine strips was increased compared with WT-exchanged muscles (Fig. 6D). The pCa50 values were pCa50 = 5.75 ± 0.02 for A57G-exchanged strips (derived from n = 12 individual experiments) compared with WT-reconstituted with pCa50 = 5.64 ± 0.01 (n = 9). A slight but not significant decrease in maximal (pCa 4) force was also noted in A57G-exchanged (30.6 ± 1.2 kN/m2) vs. WT-exchanged (32.4 ± 2.1 kN/m2) porcine cardiac muscle strips. These changes are not likely to be due to a higher level of reconstitution with A57G vs. WT proteins, as the same increase in the Ca2+ sensitivity of force was observed in transgenic preparations expressing similar amounts of human ventricular ELC (WT or A57G) protein in mice.
In Vivo Measurements in Tg-A57G and Tg-WT Mice
Echocardiographic characterization of Tg mice.
Baseline echocardiography documented similar parameters of LVPW and LVAW wall dimensions in diastole and systole in Tg-A57G and Tg-WT mice (Table 3). However, LV volumes in diastole and systole were significantly larger in Tg-A57G mice, indicating a mutation-induced chamber enlargement and the development of eccentric hypertrophy in Tg-A57G mice (Table 3).
Table 3.
Echo analysis of Tg-A57G vs. Tg-WT mice
| Parameter | Tg-A57G | Tg-WT | P |
|---|---|---|---|
| n (number of mice) | 8 | 9 | |
| HR, beats/min | 514 ± 17 | 537 ± 15 | 0.318 |
| LVPWd, mm | 0.84 ± 0.04 | 0.83 ± 0.04 | 0.753 |
| LVPWs, mm | 1.24 ± 0.07 | 1.20 ± 0.04 | 0.629 |
| LVAWd, mm | 0.95 ± 0.05 | 1.02 ± 0.05 | 0.396 |
| LVAWs, mm | 1.52 ± 0.09 | 1.58 ± 0.04 | 0.526 |
| LVVd, μl | 74.6 ± 3.3* | 57.1 ± 3.6* | *0.003 |
| LVVs, μl | 31.4 ± 3.0* | 20.9 ± 2.9* | *0.023 |
Values are means ± SE.
HR, heart rate; LV, left ventricle; PW, posterior wall; AW, anterior wall; LVV, LV end-diastolic (d) and end-systolic (s) endocardial volumes.
P < 0.05, statistical significance.
Impact of A57G on cardiac performance in vivo.
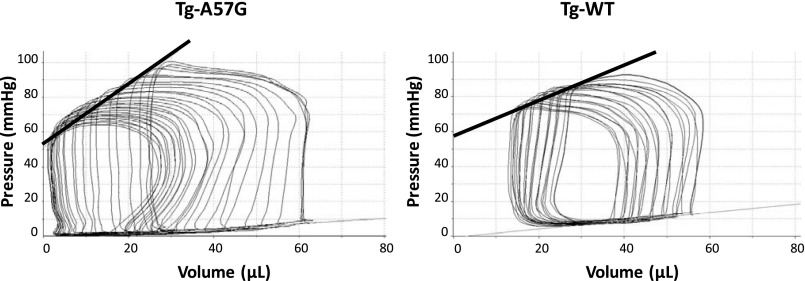
Table 4 summarizes the analyses of hemodynamic parameters derived from pressure volume loops at a steady state and following the inferior vena cava occlusion. The concentration of the anesthetic (1–2% isoflurane) was controlled to maintain the same heart rates in both groups of mice (Tg-A57G and Tg-WT). Load-dependent parameters of systolic function such as stroke work (SW), stroke volume (SV), and cardiac output were significantly increased in Tg-A57G mice compared with Tg-WT (Table 4). However, EF and cardiac efficiency were decreased in Tg-A57G vs. Tg-WT mice, but the differences between the groups were not statistically significant (Table 4). Consistently with the relationship between the LV filling volume, which produces the stretch of the heart fibers and a subsequent contraction of the next cycle, we observed increased LVEDV with higher LVESP in Tg-A57G mice compared with Tg-WT (Table 4). These data suggest higher heart contractility in Tg-A57G mice, which is in agreement with higher Ees, a parameter analyzed during inferior vena cava occlusion. Representative LV pressure-volume loops recorded during the inferior vena cava occlusion are presented in Fig. 7. The load-independent end-systolic elastance, Ees, defined as the slope of the ESPVR was higher in Tg-A57G vs. Tg-WT mice (Fig. 7). The time constant of ventricular relaxation tau (in ms) was twofold faster in Tg-A57G mice, but the difference compared with Tg-WT did not reach statistical significance (Table 4). Because end-systolic pressure was higher in Tg-A57G (104.7 ± 2.9 mmHg) vs. Tg-WT (91.4 ± 4.0 mmHg) mice (Table 4), tau was expected to be faster in the mutant mice. These changes were most likely associated with the type of cardiac remodeling observed in Tg-A57G animals manifested by eccentric hypertrophy and sphericalization of the LV leading toward systolic dysfunction.
Table 4.
Hemodynamic parameters derived from PV relations in Tg-A57G and Tg-WT mice
| Parameter | Tg-A57G | Tg-WT | P |
|---|---|---|---|
| n (number of mice) | 6 | 9 | |
| HR, beats/min | 463 ± 20 | 478 ± 22 | 0.648 |
| Integrated performance | |||
| EF, % | 57.9 ± 3.2 | 64.6 ± 3.0 | 0.147 |
| SW, mmHg × μl | 3,435 ± 294* | 2,233 ± 214* | *0.005 |
| SV, μl | 43.3 ± 2.4* | 33.4 ± 1.4* | *0.002 |
| CO, ml/min | 20.2 ± 1.7* | 15.9 ± 0.9* | *0.033 |
| CE | 0.286 ± 0.062 | 0.428 ± 0.068 | 0.170 |
| Afterload | |||
| LVESV, μl | 34.0 ± 3.6 | 27.3 ± 3.1 | 0.183 |
| LVESP, mmHg | 104.7 ± 2.9* | 91.4 ± 4.0* | *0.032 |
| Ea, mmHg/μl | 2.45 ± 0.12 | 2.78 ± 0.18 | 0.186 |
| Preload | |||
| LVEDV, μl | 69.4 ± 3.2* | 52.6 ± 2.9* | *0.002 |
| LVEDP, mmHg | 11.5 ± 2.1 | 12.6 ± 1.9 | 0.720 |
| Contractility | |||
| dP/dtmax, mmHg/s | 8073 ± 793 | 6951 ± 589 | 0.267 |
| Ees (slope), mmHg/μl | 3.21 ± 0.58* | 1.52 ± 0.24* | *0.016 |
| Relaxation | |||
| −dP/dtmin, mmHg/s | 8576 ± 934 | 6873 ± 582 | 0.125 |
| Tau, ms | 8.5 ± 0.9 | 17.1 ± 4.8 | 0.175 |
Values are means ± SE.
PV, pressure-volume; EF, ejection fraction; SW, stroke work; SV, stroke volume; CO, cardiac output; CE, cardiac efficiency calculated as SW/PV area; LVESV and LVESP, LV-end systolic volume and pressure, respectively; Ea, arterial elastance: ratio of LV-end systolic pressure to stroke volume (ESP/SV); LVEDV and LVEDP, LV-end diastolic volume and pressure; dP/dtmax, the peak rate of rise in LV pressure; Ees, measure of myocardial contractility defined by the slope of the end-systolic pressure–volume relationship; tau, isovolumic relaxation constant.
Fig. 7.
Invasive hemodynamic measurements in Tg-A57G vs. Tg-WT mice. Representative left ventricular pressure-volume loops recorded during the inferior vena cava occlusion. The load-independent end-systolic elastance, Ees (marked with black thick line), was defined as the slope of the end-systolic pressure-volume relationship. Note higher Ees monitored in Tg-A57G compared with Tg-WT mice.
DISCUSSION
Research interests in the ELC of myosin as a potential regulator of cardiac muscle contraction have been high since the discovery of several FHC-associated mutations in the MYL3 gene that encodes for the ventricular myosin ELC (13, 22, 29, 37, 41, 43, 45). Compared with the β-MHC, genetic mutations in the ELC are rare but they are also associated with malignant outcomes (3, 13, 17, 29, 42). In this report we followed up on our structural observations associated with the alanine to glycine mutation occurring at residue 57 of the human ventricular ELC shown to cause hypertrophic cardiomyopathy and SCD (29). Our previous study demonstrated a mutation-induced decrease in the interfilament lattice spacing and increased rigor stiffness in skinned papillary muscles from Tg-A57G mice compared with Tg-WT controls (40). In the current report, we studied the effect of the A57G mutation on contractile force, muscle relaxation, and myofilament Ca2+ sensitivity in skinned cardiac papillary muscle strips and in vivo by echocardiography and invasive hemodynamics. We present evidence for the mutant-elicited alterations in contractile function in Tg-A57G animals compared with Tg-WT controls.
Molecular Mechanisms of the A57G Mutation Studied in Mouse and Porcine Papillary Muscle Strips and in Solution by Stopped Flow Kinetics and Binding Profiles
This study is the first to provide insight into the structure-function relationship of a disease-causing mutation in the myosin ELC assessed in vitro and in vivo. Consistent with other malignant FHC-mutations, but in the myosin RLC that we have studied to date (26, 48, 55), an increase in the Ca2+ sensitivity of force and a decrease in maximal tension were also determined in skinned papillary muscle strips from Tg-A57G mice. Other common abnormalities observed in these RLC and ELC animal models of FHC included cardiac hypertrophy, abnormal myocardial appearance, fiber disarray, and increased loose connective tissue and fibrosis, all thought to affect contractile force and cardiac performance (26, 40, 55).
Despite significant progress in unraveling the genetic basis of hypertrophic cardiomyopathy, there is still limited understanding of the molecular events and signaling pathways associated with sarcomeric mutations and their clinical FHC phenotypes. Initially, impaired myofilament contractile function was suggested to be the most important mechanism, accounting for compensatory hypertrophy and diastolic dysfunction forming two of the hallmarks of the clinical FHC phenotype (12). Diastolic dysfunction was evidenced in our murine RLC models of FHC, and the observed changes in mice correlated with the malignant outcomes in humans (1, 55). As monitored in Tg-RLC mouse preparations, the impaired muscle relaxation that is likely responsible for the diastolic dysfunction was paralleled by decreased rates of cross-bridge attachment and dissociation (1, 5, 26, 35, 39).
Unlike in FHC-RLC mice, in this report we did not observe any significant changes in the rates of muscle relaxation in Tg-A57G compared with Tg-WT animals (Fig. 2D). In fact, the Mg-ATP binding rates (kobs), measured in the stopped-flow assay, were significantly faster in A57G compared with WT at 10, 40, and 60 μM ATP, suggesting that under these conditions the myosin cross bridges detach quicker in the A57G mutant than in WT (Table 1). However, no difference was noted in the effective second-order ATP binding rate (Fig. 3C), indicating no overall effect of the A57G mutation on the rate of myosin cross-bridge dissociation. No diastolic dysfunction was also monitored in Tg-A57G mice in vivo (Table 4 and Fig. 7). Future studies using Doppler ultrasound examination, as demonstrated for our Tg-RLC models (1), are necessary to conclude on the effect of A57G on diastolic function in mice.
Several additional molecular mechanisms have been proposed that might explain some of the clinical and pathological manifestations of FHC, including perturbations in calcium cycling and myofibrillar Ca2+ sensitization potentially leading to cardiac arrhythmias (4). Our current results on A57G papillary muscle fibers, showing a mutation-induced increase in myofilament Ca2+ sensitivity, support the hypothesis that the A57G -mediated sensitization to calcium (Figs. 2B and 6D) may be the primary sarcomeric mechanism leading to the pathologic cardiac remodeling. As we predicted earlier, the A57G mutation is anticipated to influence the local conformation of the ELC and interfere with the function of myosin lever arm to produce force (40). Considering its Ca2+-sensitizing effect on muscle contraction, it is likely that the A57G mutated N-terminal-ELC extension can directly communicate with the actin-Tm-Tn regulatory system (24, 36). Noteworthy, in agreement with the model of Ca2+-dependent regulation of contraction proposed by McKillop and Geeves (34), we showed that myosin cross bridges can directly affect the Ca2+-dependent regulation of force development in the heart (15).
Clinical data indicate that fibrosis, a secondary phenomenon indicative of myocardial damage, may be inherent to FHC disease (12). As we observed in this study, the hearts of Tg-A57G mice manifested substantial fibrosis most likely due to accumulation of collagen aggregates (Fig. 1). In support of this, passive tension/stiffness was increased in Tg-A57G fibers compared with Tg-WT (Fig. 2C). This result conforms to our earlier observations of increased stiffness in Tg-A57G myocardium (40) leading us to the conclusion that increased myocardial stiffness and fibrosis are intrinsic mechanisms of A57G-mediated cardiac disease.
Mutant-Induced Ventricular Remodeling in Tg-A57G Mice
Characteristic pathologic features of FHC include asymmetric or symmetric cardiac hypertrophy, fibrosis, and cardiomyocyte disarray (12). Measurements of the HW/BW ratios in transgenic mice showed significantly larger values in Tg-A57G compared with Tg-WT mice confirming a mutation-induced hypertrophic phenotype. Consistent with this observation, mutant animals demonstrated larger LV cavity size during diastole and systole without any differences in the anterior and posterior wall thickness suggesting a phenotype of eccentric hypertrophy in Tg-A57G-mutated mice (Table 3). Higher LVEDV values with no changes in LVESV resulted in elevated blood ejection during ventricular contraction. These data were confirmed by hemodynamic analyses showing higher stroke volume in Tg-A57G mice compared with controls, which ultimately resulted in increased cardiac output (Table 4). End systolic elastance or the slope of the ESPVR was higher in Tg-A57G compared with Tg-WT mice indicating enhanced contractility. As shown by the Borlaug and Kass (6), Ees is a measure of contractility, but it is also influenced by chamber geometry and passive myocardial stiffening. The authors noted that ventricular and arterial stiffening are common in heart failure patients whose systolic function is preserved (6). Passive tension was enhanced in Tg-A57G mice compared with Tg-WT controls (Fig. 2C) suggesting its possible contribution to the observed changes in Ees. Increased Ees was paralleled by a decrease in cardiac efficiency in the mutant mice, but the differences between the groups were not statistically significant (Table 4). Hence, the pathogenic mechanisms inherent to the A57G myocardium include its compromised contractile function at the level of sarcomeres, compensatory hypertrophy, and the altered contractility of the heart.
As demonstrated by Lee et al. (29), occurrences of SCD were reported in family members carrying the A57G mutation. However, it is not uncommon that hypertrophic cardiomyopathy patients have normal systolic function and yet may die suddenly. In fact, significantly higher end-systolic pressure monitored in Tg-A57G vs. Tg-WT mice (Table 4) and a faster relaxation parameter tau suggest that A57G-induced cardiac remodeling may result in eccentric hypertrophy and sphericalization of the LV, both indicative of potential systolic dysfunction. Therefore, it is possible that the A57G mutation in myosin ELC may not follow a common trait of FHC and result in systolic rather than diastolic dysfunction contributing to malignancy observed in mutation-positive family members (29). There are several factors that may trigger SCD in FHC patients. Abnormal heart rhythms, namely atrial and ventricular fibrillation, are the leading causes of sudden death (27). The mechanism for an abnormal arrhythmic activity is not fully understood, but the evidence supports a prominent role for abnormal cycling of intracellular calcium ions (27). Baudenbacher et al. (4) showed that in mice expressing FHC-linked mutations in troponin T, the risk of developing ventricular tachycardia was directly proportional to the degree of Ca2+ sensitization caused by the troponin T mutation. In another study and using cardiomyocytes isolated from mice expressing troponin T mutants, the authors demonstrated that myofilament Ca2+ sensitization increased cytosolic Ca2+ buffering resulting in arrhythmogenic changes in Ca2+ homeostasis (46). Strong evidence for abnormal calcium handling underlying FHC pathology comes from the recent study on hypertrophic cardiomyopathy patient-specific-induced pluripotent stem cells (28). The authors generated pluripotent stem cell cardiomyocytes from a 10-member family cohort carrying a hereditary FHC mutation in the MYH7 gene and using Ca2+ imaging techniques showed that dysregulation of Ca2+ cycling and elevation in intracellular Ca2+ were central mechanisms for disease pathogenesis (28). Based on these reports we speculate that by altering Ca2+ sensitivity of contraction the A57G mutation may increase the propensity of A57G-positive patients to cardiac arrhythmia. The structural perturbations of the A57G myocardium and increased myocardial fibrosis are likely to be the contributing factors.
Gene Expression Profile and Myofilament Protein Phosphorylation in Tg-A57G Mice
Besides disruption of the biophysical properties of the sarcomere and causing calcium-induced mechanical alterations, the A57G mutation is also expected to activate gene transcription remodeling of the heart. Transcript expression examination of the Myl4, Myh6, and Myh7 genes encoding the atrial isoform of myosin ELC, α-MHC, and β-MHC, respectively (Table 2), revealed a significant upregulation of the atrial ELC (Myl4) gene, a hypertrophy marker, in the hearts of Tg-A57G and Tg-WT mice compared with age-matched NTg animals. Interestingly, Myl4 was also increased in old vs. young NTg mice indicating an age-dependent phenotype. As expected, Myh6 was increased in young Tg mice driven by the α-MHC promoter, but no further increase in Myh6 in older animals was monitored (Table 2). No significant changes in the β-MHC transcript expression profile (Table 2) or in β-MHC protein expression (Fig. 4) were observed in Tg-A57G animals. An age-dependent upregulation or downregulation of Myh7 was observed in Tg-WT mice, but these differences were not present at the protein level, where the α-MHC was the major detected MHC isoform. These data may explain the lack of A57G-induced alterations in the myosin cross-bridge kinetics (Figs. 2D and 3). Another factor that we have taken into consideration was the possibility of mutation exerted secondary effects on the phosphorylation of other proteins of the sarcomere and/or phosphorylation-mediated regulation of cardiac muscle contraction. However, again, Pro-Q Diamond/SYPRO Ruby studies showed no changes in phosphorylation of the key regulatory proteins, TnI, TnT, and myosin RLC that could possibly account for observed functional changes in Tg-A57G vs. Tg-WT hearts. This implies that the mechanism of disease for this A57G mutation in ELC does not involve changes in myofilament protein phosphorylation. Further investigation of posttranslational modifications will possibly shed more light on the origin of A57G-induced cardiomyopathy.
Limitations
Using the structure-function approach to study FHC-linked mutations entails several limitations. Pathologic cardiac hypertrophy is associated with ventricular remodeling through alterations beyond the sarcomeric structures and involving the extracellular matrix and many intracellular signaling pathways that ultimately result in changes in cardiomyocyte function. Sarcomere proteins are directly engaged in signal reception and transmission among the plasma membrane, cytosolic organelles, and the nuclear envelope. Their Z-discs provide docking sites for kinases, phosphatases, and transcription factors, which shuttle back and forth to their sites of regulation in complex pathways (11). Therefore, investigations of FHC mutations should include these signaling networks and characterization of their roles in excitation, contraction, and relaxation and in adaptive and maladaptive responses of heart muscle.
Conclusions
Gene-manipulated mouse models that closely recapitulate the clinical phenotypes of human inherited cardiomyopathies are invaluable in demonstrating the true cause of the disease and exploring pathogenic mechanisms. Despite limitations intrinsic to the discrepancies between humans and mice (e.g., heart rate, heart size, etc.), mice are still the best tools offering an integrated understanding of the processes involved in FHC. Results from this study suggest that the A57G allele may cause FHC by means of a discrete modulation of myofilament function, reduced maximal force, and increased myofilament Ca2+ sensitivity. As a consequence, the A57G hearts might be prone to compensatory hypertrophy, enhanced cardiac contractility, and ultimately pathological ventricular remodeling. Anatomical changes observed in Tg-A57G hearts that mostly develop later in the cardiomyopathic syndrome are anticipated to contribute to disease progression. Future studies are needed to establish whether myofilament Ca2+ sensitization observed in Tg-A57G papillary muscle fibers is linked to proarrhythmic effects. Efforts should also be directed toward elucidating the complex signaling mechanisms responsible for the observed myofilament phenotypes. At present we can only speculate that the origin of heart dysfunction and SCD observed in A57G-positive patients most likely resides in the Ca2+-dependent myofilament dysfunction, and therapeutic efforts should be put forward to restore Ca2+ homeostasis to prevent development of hypertrophy and potential electrophysiological defects.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grants R01-HL-108343 and HL-071778 (to D. Szczesna-Cordary) and R01-HL-107110 (to J. M. Hare) and American Heart Association Grant 12PRE12030412 (to W. Huang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.K., E.P., W.H., P.M., J.L., C.-C.Y., and A.I.R. performed experiments; K.K., E.P., W.H., P.M., J.L., J.M.H., and D.S.-C. analyzed data; K.K., E.P., W.H., P.M., J.M.H., and D.S.-C. interpreted results of experiments; K.K., E.P., W.H., P.M., C.-C.Y., and D.S.-C. prepared figures; K.K., E.P., W.H., P.M., and D.S.-C. drafted manuscript; K.K., W.H., P.M., J.L., and D.S.-C. conception and design of research; K.K., E.P., W.H., P.M., J.L.,C.-C.Y., J.M.H., and D.S.-C. edited and revised manuscript; D.S.-C. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Dr. David Dweck for expert assistance with stopped-flow experiments and Michelle Jones for editorial help with the manuscript.
REFERENCES
- 1.Abraham TP, Jones M, Kazmierczak K, Liang HY, Pinheiro AC, Wagg CS, Lopaschuk GD, Szczesna-Cordary D. Diastolic dysfunction in familial hypertrophic cardiomyopathy transgenic model mice. Cardiovasc Res 82: 84–92, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alcalai R, Seidman JG, Seidman CE. Genetic basis of hypertrophic cardiomyopathy: from bench to the clinics. J Cardiovasc Electrophysiol 19: 104–110, 2008 [DOI] [PubMed] [Google Scholar]
- 3.Andersen PS, Hedley PL, Page SP, Syrris P, Moolman-Smook JC, McKenna WJ, Elliott PM, Christiansen M. A novel Myosin essential light chain mutation causes hypertrophic cardiomyopathy with late onset and low expressivity. Biochem Res Int 2012: 685108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudenbacher F, Schober T, Pinto JR, Sidorov VY, Hilliard F, Solaro RJ, Potter JD, Knollmann BC. Myofilament Ca2+ sensitization causes susceptibility to cardiac arrhythmia in mice. J Clin Invest 118: 3893–3903, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borejdo J, Szczesna-Cordary D, Muthu P, Metticolla P, Luchowski R, Gryczynski Z, Gryczynski I. Single molecule detection approach to muscle study: kinetics of a single cross-bridge during contraction of muscle. Methods Mol Biol 875: 311–334, 2012 [DOI] [PubMed] [Google Scholar]
- 6.Borlaug BA, Kass DA. Ventricular-vascular interaction in heart failure. Heart Fail Clin 4: 23–36, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deacon JC, Bloemink MJ, Rezavandi H, Geeves MA, Leinwand LA. Erratum to: Identification of functional differences between recombinant human alpha and beta cardiac myosin motors. Cell Mol Life Sci 69: 4239–4255, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dweck D, Reyes-Alfonso A, Jr, Potter JD. Expanding the range of free calcium regulation in biological solutions. Anal Biochem 347: 303–315, 2005 [DOI] [PubMed] [Google Scholar]
- 9.Fiske CH, Subbarow Y. The colorimetric determination of phosphorus. J Biol Chem 66: 375–400, 1925 [Google Scholar]
- 10.Fitzsimons DP, Patel JR, Moss RL. Role of myosin heavy chain composition in kinetics of force development and relaxation in rat myocardium. J Physiol 513: 171–183, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Force T, Bonow RO, Houser SR, Solaro RJ, Hershberger RE, Adhikari B, Anderson ME, Boineau R, Byrne BJ, Cappola TP, Kalluri R, LeWinter MM, Maron MS, Molkentin JD, Ommen SR, Regnier M, Tang WH, Tian R, Konstam MA, Maron BJ, Seidman CE. Research priorities in hypertrophic cardiomyopathy: report of a Working Group of the National Heart, Lung, and Blood Institute. Circulation 122: 1130–1133, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Frey N, Luedde M, Katus HA. Mechanisms of disease: hypertrophic cardiomyopathy. Nat Rev Cardiol 9: 91–100, 2012 [DOI] [PubMed] [Google Scholar]
- 13.Garcia-Pavia P, Vazquez ME, Segovia J, Salas C, Avellana P, Gomez-Bueno M, Vilches C, Gallardo ME, Garesse R, Molano J, Bornstein B, Alonso-Pulpon L. Genetic basis of end-stage hypertrophic cardiomyopathy. Eur J Heart Fail 13: 1193–1201, 2011 [DOI] [PubMed] [Google Scholar]
- 14.Geeves MA. Molecular motors: stretching the lever-arm theory. Nature 415: 129–131, 2002 [DOI] [PubMed] [Google Scholar]
- 15.Geeves MA, Holmes KC. The molecular mechanism of muscle contraction. Adv Protein Chem 71: 161–193, 2005 [DOI] [PubMed] [Google Scholar]
- 16.Hanson KR, Ling R, Havir E. A computer program for fitting data to the Michaelis-Menten equation. Biochem Biophys Res Commun 29: 194–197, 1967 [DOI] [PubMed] [Google Scholar]
- 17.Hernandez OM, Jones M, Guzman G, Szczesna-Cordary D. Myosin essential light chain in health and disease. Am J Physiol Heart Circ Physiol 292: H1643–H1654, 2007 [DOI] [PubMed] [Google Scholar]
- 18.Hill TL, Einsenberg E, Greene LE. Theoretical model for the cooperative equilibrium binding of myosin subfragment-1 to the actin-troponin-tropomyosin complex. Proc Natl Acad Sci USA 77: 3186–3190, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Janssen PM. Kinetics of cardiac muscle contraction and relaxation are linked and determined by properties of the cardiac sarcomere. Am J Physiol Heart Circ Physiol 299: H1092–H1099, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Janssen PM. Myocardial contraction-relaxation coupling. Am J Physiol Heart Circ Physiol 299: H1741–H1749, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johns EC, Ryder KO, Hodson EA, Hart G, Mulligan IP, Lipscomb S, Ashley CC. Investigating the relaxation rate, following diazo-2 photolysis, of a skinned trabecular preparation from guinea-pig hypertrophied left ventricle. Pflügers Arch 438: 771–777, 1999 [DOI] [PubMed] [Google Scholar]
- 22.Kaski JP, Syrris P, Esteban MT, Jenkins S, Pantazis A, Deanfield JE, McKenna WJ, Elliott PM. Prevalence of sarcomere protein gene mutations in preadolescent children with hypertrophic cardiomyopathy. Circ Cardiovasc Genet 2: 436–441, 2009 [DOI] [PubMed] [Google Scholar]
- 23.Kazmierczak K, Muthu P, Huang W, Jones M, Wang Y, Szczesna-Cordary D. Myosin regulatory light chain mutation found in hypertrophic cardiomyopathy patients increases isometric force production in transgenic mice. Biochem J 442: 95–103, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kazmierczak K, Xu Y, Jones M, Guzman G, Hernandez OM, Kerrick WG, Szczesna-Cordary D. The role of the N-terminus of the myosin essential light chain in cardiac muscle contraction. J Mol Biol 387: 706–725, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keren A, Syrris P, McKenna WJ. Hypertrophic cardiomyopathy: the genetic determinants of clinical disease expression. Nat Clin Pract Cardiovasc Med 5: 158–168, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Kerrick WG, Kazmierczak K, Xu Y, Wang Y, Szczesna-Cordary D. Malignant familial hypertrophic cardiomyopathy D166V mutation in the ventricular myosin regulatory light chain causes profound effects in skinned and intact papillary muscle fibers from transgenic mice. FASEB J 23: 855–865, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knollmann BC, Roden DM. A genetic framework for improving arrhythmia therapy. Nature 451: 929–936, 2008 [DOI] [PubMed] [Google Scholar]
- 28.Lan F, Lee AS, Liang P, Sanchez-Freire V, Nguyen PK, Wang L, Han L, Yen M, Wang Y, Sun N, Abilez OJ, Hu S, Ebert AD, Navarrete EG, Simmons CS, Wheeler M, Pruitt B, Lewis R, Yamaguchi Y, Ashley EA, Bers DM, Robbins RC, Longaker MT, Wu JC. Abnormal calcium handling properties underlie familial hypertrophic cardiomyopathy pathology in patient-specific induced pluripotent stem cells. Cell Stem Cell 12: 101–113, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee W, Hwang TH, Kimura A, Park SW, Satoh M, Nishi H, Harada H, Toyama J, Park JE. Different expressivity of a ventricular essential myosin light chain gene Ala57Gly mutation in familial hypertrophic cardiomyopathy. Am Heart J 141: 184–189, 2001 [DOI] [PubMed] [Google Scholar]
- 30.Lopez JE, Myagmar BE, Swigart PM, Montgomery MD, Haynam S, Bigos M, Rodrigo MC, Simpson PC. Beta-myosin heavy chain is induced by pressure overload in a minor subpopulation of smaller mouse cardiac myocytes. Circ Res 109: 629–638, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lu X, Kazmierczak K, Jiang X, Jones M, Watt J, Helfman DM, Moore JR, Szczesna-Cordary D, Lossos IS. Germinal center-specific protein human germinal center associated lymphoma directly interacts with both myosin and actin and increases the binding of myosin to actin. FEBS J 278: 1922–1931, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maron BJ. Hypertrophic cardiomyopathy: a systematic review. JAMA 287: 1308–1320, 2002 [DOI] [PubMed] [Google Scholar]
- 33.Maron BJ. The young competitive athlete with cardiovascular abnormalities: causes of sudden death, detection by preparticipation screening, and standards for disqualification. Card Electrophysiol Rev 6: 100–103, 2002 [DOI] [PubMed] [Google Scholar]
- 34.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: evidence for three states of the thin filament. Biophys J 65: 693–701, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mettikolla P, Calander N, Luchowski R, Gryczynski I, Gryczynski Z, Zhao J, Szczesna-Cordary D, Borejdo J. Cross-bridge kinetics in myofibrils containing familial hypertrophic cardiomyopathy R58Q mutation in the regulatory light chain of myosin. J Theor Biol 284: 71–81, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Michael JJ, Gollapudi SK, Ford SJ, Kazmierczak K, Szczesna-Cordary D, Chandra M. Deletion of 1–43 amino acids in cardiac myosin essential light chain blunts length dependency of Ca2+ sensitivity and cross-bridge detachment kinetics. Am J Physiol Heart Circ Physiol 304: H253–H259, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morita H, Rehm HL, Menesses A, McDonough B, Roberts AE, Kucherlapati R, Towbin JA, Seidman JG, Seidman CE. Shared genetic causes of cardiac hypertrophy in children and adults. N Engl J Med 358: 1899–1908, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Muthu P, Kazmierczak K, Jones M, Szczesna-Cordary D. The effect of myosin RLC phosphorylation in normal and cardiomyopathic mouse hearts. J Cell Mol Med 16: 911–919, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muthu P, Mettikolla P, Calander N, Luchowski R, Gryczynski I, Gryczynski Z, Szczesna-Cordary D, Borejdo J. Single molecule kinetics in the familial hypertrophic cardiomyopathy D166V mutant mouse heart. J Mol Cell Cardiol 48: 989–998, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Muthu P, Wang L, Yuan CC, Kazmierczak K, Huang W, Hernandez OM, Kawai M, Irving TC, Szczesna-Cordary D. Structural and functional aspects of the myosin essential light chain in cardiac muscle contraction. FASEB J 25: 4394–4405, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Olson TM, Karst ML, Whitby FG, Driscoll DJ. Myosin light chain mutation causes autosomal recessive cardiomyopathy with mid-cavitary hypertrophy and restrictive physiology. Circulation 105: 2337–2340, 2002 [DOI] [PubMed] [Google Scholar]
- 42.Page SP, Kounas S, Syrris P, Christiansen M, Frank-Hansen R, Andersen PS, Elliott PM, McKenna WJ. Cardiac myosin binding protein-C mutations in families with hypertrophic cardiomyopathy: disease expression in relation to age, gender, and long term outcome. Circ Cardiovasc Genet 5: 156–166, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Poetter K, Jiang H, Hassanzadeh S, Master SR, Chang A, Dalakas MC, Rayment I, Sellers JR, Fananapazir L, Epstein ND. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet 13: 63–69, 1996 [DOI] [PubMed] [Google Scholar]
- 44.Rayment I, Rypniewski WR, Schmidt-Base K, Smith R, Tomchick DR, Benning MM, Winkelmann DA, Wesenberg G, Holden HM. Three-dimensional structure of myosin subfragment-1: a molecular motor. Science 261: 50–58, 1993 [DOI] [PubMed] [Google Scholar]
- 45.Richard P, Charron P, Carrier L, Ledeuil C, Cheav T, Pichereau C, Benaiche A, Isnard R, Dubourg O, Burban M, Gueffet JP, Millaire A, Desnos M, Schwartz K, Hainque B, Komajda M; EUROGENE Heart Failure Project Hypertrophic cardiomyopathy: distribution of disease genes, spectrum of mutations, and implications for a molecular diagnosis strategy. Circulation 107: 2227–2232, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Schober T, Huke S, Venkataraman R, Gryshchenko O, Kryshtal D, Hwang HS, Baudenbacher FJ, Knollmann BC. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res 111: 170–179, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spirito P, Bellone P, Harris KM, Bernabo P, Bruzzi P, Maron BJ. Magnitude of left ventricular hypertrophy and risk of sudden death in hypertrophic cardiomyopathy. N Engl J Med 342: 1778–1785, 2000 [DOI] [PubMed] [Google Scholar]
- 48.Szczesna-Cordary D, Guzman G, Zhao J, Hernandez O, Wei J, Diaz-Perez Z. The E22K mutation of myosin RLC that causes familial hypertrophic cardiomyopathy increases calcium sensitivity of force and ATPase in transgenic mice. J Cell Sci 118: 3675–3683, 2005 [DOI] [PubMed] [Google Scholar]
- 49.Szczesna-Cordary D, Jones M, Moore JR, Watt J, Kerrick WGL, Xu Y, Wang Y, Wagg C, Lopaschuk GD. Myosin regulatory light chain E22K mutation results in decreased cardiac intracellular calcium and force transients. FASEB J 21: 3974–3985, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Tardiff JC, Hewett TE, Factor SM, Vikstrom KL, Robbins J, Leinwand LA. Expression of the beta (slow)-isoform of MHC in the adult mouse heart causes dominant-negative functional effects. Am J Physiol Heart Circ Physiol 278: H412–H419, 2000 [DOI] [PubMed] [Google Scholar]
- 51.Trybus KM. Biochemical studies of myosin. Methods 22: 327–335, 2000 [DOI] [PubMed] [Google Scholar]
- 52.Trybus KM. Role of myosin light chains. J Muscle Res Cell Motil 15: 587–594, 1994 [DOI] [PubMed] [Google Scholar]
- 53.Ueta CB, Oskouei BN, Olivares EL, Pinto JR, Correa MM, Simovic G, Simonides WS, Hare JM, Bianco AC. Absence of myocardial thyroid hormone inactivating deiodinase results in restrictive cardiomyopathy in mice. Mol Endocrinol 26: 809–818, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wang L, Muthu P, Szczesna-Cordary D, Kawai M. Characterizations of myosin essential light chain's N-terminal truncation mutant Delta43 in transgenic mouse papillary muscles by using tension transients in response to sinusoidal length alterations. J Muscle Res Cell Motil 34: 93–105, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang Y, Xu Y, Kerrick WGL, Wang Y, Guzman G, Diaz-Perez Z, Szczesna-Cordary D. Prolonged Ca2+ and force transients in myosin RLC transgenic mouse fibers expressing malignant and benign FHC mutations. J Mol Biol 361: 286–299, 2006 [DOI] [PubMed] [Google Scholar]
- 56.Warren CM, Greaser ML. Method for cardiac myosin heavy chain separation by sodium dodecyl sulfate gel electrophoresis. Anal Biochem 320: 149–151, 2003 [DOI] [PubMed] [Google Scholar]