Abstract
Alterations in the ubiquitin-proteasome system (UPS) have been described in left ventricular hypertrophy and failure, although results have been inconsistent. The role of the UPS in right ventricular (RV) hypertrophy (RVH) and RV failure (RVF) is unknown. Given the greater percent increase in RV mass associated with RV afterload stress, as present in many congenital heart lesions, we hypothesized that alterations in the UPS could play an important role in RVH/RVF. UPS expression and activity were measured in the RV from mice with RVH/RVF secondary to pulmonary artery constriction (PAC). Epoxomicin and MG132 were used to inhibit the proteasome, and overexpression of the 11S PA28α subunit was used to activate the proteasome. PAC mice developed RVH (109.3% increase in RV weight to body weight), RV dilation with septal shift, RV dysfunction, and clinical RVF. Proteasomal function (26S β5 chymotrypsin-like activity) was decreased 26% (P < 0.05). Protein expression of 19S subunit Rpt5 (P < 0.05), UCHL1 deubiquitinase (P < 0.0001), and Smurf1 E3 ubiquitin ligase (P < 0.01) were increased, as were polyubiquitinated proteins (P < 0.05) and free-ubiquitins (P = 0.05). Pro-apoptotic Bax was increased (P < 0.0001), whereas anti-apoptotic Bcl-2 decreased (P < 0.05), resulting in a sixfold increase in the Bax/Bcl-2 ratio. Proteasomal inhibition did not accelerate RVF. However, proteasome enhancement by cardiac-specific proteasome overexpression partially improved survival. Proteasome activity is decreased in RVH/RVF, associated with upregulation of key UPS regulators and pro-apoptotic signaling. Enhancement of proteasome function partially attenuates RVF, suggesting that UPS dysfunction contributes to RVF.
Keywords: ubiquitin, proteasome, right ventricle, cardiac hypertrophy, heart failure
pathologic hypertrophy is an important adaptation to stressors varying from ischemia to hypertension as well as many forms of congenital heart disease. Chronic hypertrophy-inducing stress can lead to fibrosis, decreased contractile function, and ultimately heart failure. Pressure overload hypertrophy is associated with a marked increase in protein synthesis at a rate exceeding degradation (57), the most dramatic the heart experiences since early development. Under normal circumstances, the proteins of the heart turn over at a rate that would replace all in 30 days (15, 33, 50). More than 70% of protein turnover is regulated by the ubiquitin-proteasome system (UPS) (18, 56, 74), involving signaling the target by covalent attachment of ubiquitin and degradation of tagged proteins by the 26S proteasome complex with release of ubiquitins by deubiquitinating enzymes. Three peptidase activities, chymotrypsin-like, trypsin-like, and caspase-like activities, have been assigned to the β5, β2, and β1 subunits, respectively, of the 20S core proteasomal complex, capped by 19S regulatory and/or 11S activator complexes. The UPS also interacts with lysosomal (autophagy dependent or independent) and other protease-specific pathways within the cardiomyocyte (17, 71).
Abnormalities in proteasomal function have been described well in models of left ventricular (LV) hypertrophy (LVH), ischemia-reperfusion (2, 10, 16), and dilated and desmin-related cardiomyopathies (13, 49), as well as in human heart failure (47, 49, 72). However, studies have been contradictory, showing either increased or decreased proteasomal enzyme activity in the afterload stressed LV (31, 43). Other models have demonstrated both cardioprotective (9, 21, 25, 59) and cardiotoxic (12, 20, 27, 32, 45, 46) roles of the UPS via regulation of apoptosis, NF-κB signaling, and oxidative stress.
Although much is known about the molecular mechanisms of LVH and LV failure (LVF), the mechanisms underlying right ventricular (RV) hypertrophy (RVH) and RV failure (RVF) are less understood. RVH and RVF are a major cause of morbidity and mortality in patients with pulmonary hypertension and represent long-term risks for patients with surgically corrected congenital heart diseases such as tetralogy of Fallot, L-transposition of the great arteries, and hypoplastic left heart (4, 34). Standard treatments for LVF (ACE inhibitors, β-blockers) have shown limited success in RVF (22, 54), suggesting fundamental cellular-level differences between the RV and LV. The two ventricles derive embryologically from different heart fields (19) and manifest differences in calcium handling, inotropy, and patterns of gene expression in response to stress (7, 37, 67, 69). Little is known about the role of the UPS in the RV. A dominant mutation in the gene encoding plakoglobin in a patient with arrhythmogenic RV dysplasia has been shown to be associated with alterations in ubiquitination, possibly via changes in proteasomal degradation (1). Although ubiquitins and proteasomes have been shown to play a role in the RV (3, 66), the clinical significance of the UPS in RV health and disease remains unclear.
Given that the RV is a thinner-walled chamber than the LV, such that afterload stress would produce a greater stimulus for RVH, we hypothesized that the UPS could be even more critical for maintaining protein integrity in the rapidly hypertrophying RV. We used our previously reported model of pulmonary artery constriction (PAC) resulting in marked RVH [doubling of RV weight to body weight (RVW:BW)] and recapitulating the clinical features of RVH/RVF in patients (RV enlargement, septal shift, tricuspid regurgitation, right bundle branch block, increased RV end-diastolic pressure, decreased RV function, ventricular dyssynchrony, and peripheral edema) and transitioning to RVF and death (67). The purpose of the current study was to evaluate the role of the UPS in mediating RVH/RVF.
MATERIALS AND METHODS
Model of PAC.
RVH/RVF was created using our previously characterized murine model of PAC (67). Briefly, male FVB/N mice aged 8 to 10 wk were intubated, a left lateral thoracotomy was performed, and the main pulmonary trunk was identified and banded with a 7-0 suture, tied tight against a 26-gauge needle, which was then removed, all under isoflurane inhalation anesthesia. Age- and strain-matched sham-operated controls underwent an identical procedure, with the sole exception being placement of the band. Sham-operated animals were a separate group and not failed bandings. We have previously shown that this PAC model of pressure overload is characterized by a stable increase in RVW/BW without signs of failure by 4 days post-operative (post-op), and by clinical features of heart failure (maladaptive hypertrophy) progressing to death by 8 to 10 days post-op. (51, 67). Accordingly, animals were euthanized at two time points: at 4 days post-op and at 8 to 10 days post-op.
Echocardiography.
Serial two-dimensional and Doppler echocardiograms were performed at baseline and 1 wk after sham or PAC surgeries. Echocardiographic images were acquired with a GE Vivid 7 ultrasound system (GE Healthcare, Milwaukee, WI) with 13-MHz transducer. Doppler signals analyzed included maximum pulse wave Doppler and velocity-time integral in the RV outflow tract (RVOT) to estimate the peak pressure gradient between the RV and pulmonary artery. RVOT dimensions were measured at the level of the pulmonary valve in parasternal short-axis views, and RVOT fractional shortening (FS), an echo surrogate of RV function, was calculated (44, 67).
Peptidase activity assays.
Hearts of mice euthanized at 1 wk post-op were separated into RV free wall, LV free wall, and septum, weights were quickly recorded, and hearts were then frozen in liquid nitrogen and stored at −80°C for further processing. Homogenization of RV free wall was performed on ice beginning with mincing of the tissues for 10 s with a razor blade. The tissues were then immediately homogenized using a dounce homogenizer with appropriate volumes of 26S buffer of (in mmol/l) 50 (mM) Tris, 1 EDTA, 150 NaCl, and 5 MgCl2 (pH 7.5) and freshly added DTT (0.5 mmol/l) and subsequently centrifuged at 12,000 g for 30 min at 4°C.
The 26S proteasomal assay was carried out in a total volume of 100 μl in 96-well plates with 100 μmol/l (μM) ATP in 26S buffer of (mmol/l) 50 Tris, 1 EDTA, 150 NaCl, 5 MgCl2 (pH 7.5), and 0.5 DTT using 24 μg of protein supernatants. Assays were initiated by addition of succinyl-Leu-Leu-Val-Tyr-7-amido-4-methylcoumarin (Suc-LLVY-AMC; 100 μmol/l final concentration). This substrate is cleaved by the chymotrypsin-like (β5) activity of the proteasome releasing free AMC, which was then measured spectrofluorometrically using a Fluoroskan Ascent fluorometer (Thermo Fisher Scientific, Waltham, MA) or SpectraMax Microplate Reader (Molecular Devices, Sunnyvale, CA) at an excitation wavelength of 390 nm and an emission wavelength of 460 nm. Fluorescence was measured at 15-min intervals for 2 h. All assays were linear in this range. Each assay was conducted in the absence and presence of the specific proteasomal inhibitor 20 μmol/l epoxomicin. Proteasome activity results are expressed as means ± SE. Similar assays were performed to detect trypsin-like (β2) and caspase-like (β1) activities using Boc-LSTR-AMC and Z-LLE-AMC, respectively. For β2 trypsin-like activity 60 μmol/l of epoxomicin and for β1 caspase-like activity 40 μmol/l Z-Pro-Nle-Asp-al were used as inhibitors as previously described (29). The 20S β1 caspase-like proteasomal activity assays were performed in a similar fashion with 10 μl of 10× Chemicon buffer containing 250 mmol/l HEPES, 5 mmol/l EDTA, 0.5% Nonidet P-40, and 0.01% SDS. No ATP was used for the 20S activity assays.
For measurement of cathepsin L activity, we used 34 μg of protein sample in cathepsin L buffer (at pH 5.5) of (in mmol/l) 100 sodium acetate, 1 EDTA, and 1 DTT added fresh before use. Cathepsin L substrate Z-Phe-Arg-MCA (100 μmol/l final concentration) was used in the presence and absence of 10 μmol/l cathepsin L inhibitor I (Calbiochem). For measurement of calpain activity, 50 μg of protein sample was used in 100 μl of activation buffer of (in mmol/l) 50 Tris, 1 EDTA, 10 CaCl2, and 150 NaCl (at pH 7.5) with 0.5 DTT added fresh before use. The β5-substrate Suc-LLVY-AMC (200 μmol/l) was used in the presence and absence of 50 μmol/l of calpain inhibitor IV (EMD Biosciences).
Western blotting.
RV protein expression was determined by Western blot at 4 days (early RVH) and 8 to 10 days (late RVH and RVF). Western blots were performed based on established procedures (28, 76). Protein concentration was analyzed using Spectronic Genesys (Thermo Electron, Madison, WI) and/or NanoDrop (Thermo Fisher Scientific, Wilmington, DE). After one-dimensional separation, the proteins were transferred to nitrocellulose membranes with traditional sandwich methods or using Trans-Blot Turbo Transfer System (Bio-Rad Laboratories, Hercules, CA). Blots were sequentially probed with primary antibodies and appropriate horseradish peroxidase-conjugated IgG secondary antibodies and detected using Versadoc or Chemidoc XRS (Bio-Rad Laboratories) and quantified using Quantity One software program. Blots were normalized to GAPDH (Advanced Immunochemical; 1:1,000), Lamin B (Santa Cruz Biotechnologies), α-Actinin (Abcam; 1:125), or Ponceau-stained images. The primary antibodies include 20S proteasome (Epitomics; 1:10,000; Abcam), Ubiquitin (Sigma Aldrich; 1:100; Santa Cruz Biotechnologies 1:500 and 1:1,000; Life Sensors 1:1,000), UCHL1 (Enzo), 19S Rpt5 (Enzo; 1:2,500), 11S (Enzo; 1:1,000), NF-κB (1:2,000), and Smurf1 (1:200) from Santa Cruz Biotechnologies. UbcH5C (1:1,000), IκBα (1:1,000), Bax (1:1,000), and Bcl-2 (1:1,000) were from Cell Signaling Technologies. Oxyblot carbonylation was performed using a modified procedure based upon dinitrophenylhydrazine modification of oxidized carbonyl groups (21).
Gene microarray analysis.
Gene expression was evaluated using standard microarray methods (67) for altered regulation of transcripts pertaining to the UPS and related systems. These experiments were performed comparing 10-day post-op RVs of PAC (late RVH and RVF) and sham-operated mice (n = 4) using Agilent microarrays (Agilent Technologies, Santa Clara, CA) and analyzed using Genespring GX (Agilent Technologies) and Stanford's Significance Analysis of Microarrays (SAM) following similar procedures (64, 67).
Administration of proteasomal inhibitors.
Studies using proteasomal inhibitors were performed in comparison with vehicle (10% DMSO/saline) as the control beginning 1 day before surgery until the 8th post-op day. The β5-specific proteasome inhibitor epoxomicin was given by daily intraperitoneal injection at the rate of 0.5 mg·kg−1·day−1, a dose which we tested and found to be nonlethal in control mice (data not shown) and that also does not independently induce apoptosis (32). Because of concern that the short half-life of the drug would result in incomplete inhibition of the targeted enzymes, we conducted additional studies comparing three methods of administration: once daily intraperitoneally, three times daily intraperitoneally, or via continuous subcutaneous delivery via Alzet osmotic minipump (Durect, Cupertino, CA), delivering the same total daily dose. These studies demonstrated equal efficacy of enzyme inhibition, so all further studies with epoxomicin were conducted using the once daily intraperitoneal approach (data not shown). The inhibitor MG132 was given via Alzet pump with a nonlethal dose of 10 μg·kg−1·day−1 that is known to not affect nonproteasomal protein degradation pathways like calpain-mediated muscle proteolysis (6).
Cardiomyocyte-specific proteasome enhancement using PA28α overexpression.
The creation and baseline characterization of binary transgenic mice with cardiac-specific Tet-suppressible (Tet-off system) overexpression of the PA28α subunit of the 11S proteasome activator were previously described (41). The binary mice were generated by breeding single transgenic PA28α responder mice with single transgenic tTA mice, all on the FVB/N background. The resulting double transgenic mice with cardiomyocyte-restricted proteasome enhancement were identified by PCR genotyping for the presence of both transgenes. Both the double transgenic mice and tTA single transgenic littermates (for controls) were subjected to PAC as detailed above.
Histopathology.
Terminal deoxynucleotidyl transferase dUTP-mediated nick-end labeling (TUNEL) assay was performed using DeadEnd calorimetric TUNEL system (Promega) (52). About 1,000 nuclei per RV were counted and analyzed using Image-Pro Analyzer (Bethesda, MD).
Statistical analysis.
Results are expressed as means ± SE unless mentioned otherwise. Statistical significance of differences in parameters from sham- and PAC-operated mice was assessed by t-test or one-way ANOVA with post hoc testing (Bonferroni/Dunnett's multiple comparison) using GraphPad Prism (GraphPad Software, La Jolla, CA) and Microsoft Excel (Redmond, WA). A P value less than 0.05 was considered significant. For gene microarrays, SAM incorporates a false discovery rate correction for multiple testing errors and calculates a d statistic for each gene based on the ratio of change in gene expression to SD in the data for that gene (64, 67). Survival studies were analyzed using Gehan-Breslow-Wilcoxon and χ2-square tests.
Animal care.
All procedures were performed in accordance with National Institutes of Health standards and were approved by the Administrative Panel on Laboratory Animal Care at Stanford University.
RESULTS
RV hypertrophy and failure.
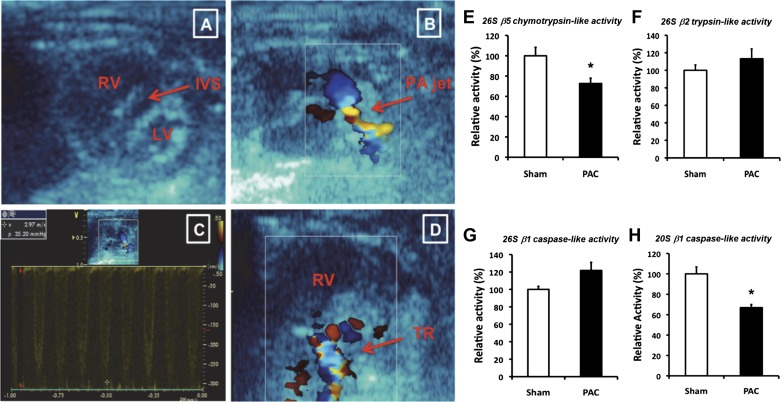
Echocardiography showed severe pulmonary stenosis at the site of the band and marked RV enlargement with flattening of the interventricular septum or septal encroachment into the LV and tricuspid regurgitation (Fig. 1). Doppler showed peak pressure gradients between 28 and 50 mmHg. PAC mice showed marked RVH by 4 days [the early RVH group, RVW/BW of 1.44 ± 0.1 mg/g in PAC vs. 0.83 ± 0.09 mg/g in sham (73% increase; P < 0.0001; n = 6/group)] and even greater RVH by 8 days [decompensated RVH with clinical RVF group (109.3% increase; P < 0.0001; n = 16 to 17/group; Table 1)]. LV weights and interventricular septal weights did not change. Consistent with our prior results in this model, the RVF mice began dying beginning the second week after surgery.
Fig. 1.
Decreased proteasomal enzyme activities 8 days (8d) after pulmonary artery (PA) constriction (PAC). A: 2-dimensional parasternal short-axis image in a mouse with severe pulmonary stenosis showing a markedly enlarged right ventricle (RV), small left ventricle (LV), and flattening of the interventricular septum (IVS). B: color Doppler showing flow acceleration across main PA. C: pulsed Doppler showing gradient of 35 mmHg. D: color Doppler showing tricuspid regurgitation (TR). E: 26S β5 chymotrypsin-like activity. F: 26S β2 trypsin-like activity. G: 26S β1 caspase-like activity. H: 20S β1 caspase-like activity. Results are shown as percent change from sham-operated controls. *P < 0.05; n = 6 to 7.
Table 1.
Morphometric characteristics at 1 wk post-operative
| Sham | PAC | Sham + MG132 | PAC + MG132 | PAC + Epoxomicin | PAC on CR/tTA | PAC on CR/PA28α/tTA | |
|---|---|---|---|---|---|---|---|
| N | 16 | 17 | 6 | 7 | 3 | 4 | 4 |
| Body weight, g | 29.0 ± 2.2 | 25.9 ± 4.3 | 28.1 ± 1.2 | 26.1 ± 1.6 | 23.5 ± 2.6 | 25.2 ± 1.3 | 25.9 ± 1.2 |
| RV weight, mg | 22.45 ± 3.2 | 42.06 ± 10.3* | 23.48 ± 2.7 | 41.26 ± 2.9*† | 37.13 ± 8.2‡ | 42.3 ± 2.9*† | 45.3 ± 4.5*† |
| Heart weight/body weight, mg/g | 4.01 ± 0.2 | 5.25 ± 0.5* | 4.38 ± 0.4 | 5.35 ± 0.1*† | 5.29 ± 0.7* | 5.65 ± 0.4*† | 5.66 ± 0.9*† |
| RV weight/body weight, mg/g | 0.77 ± 0.1 | 1.62 ± 0.3* | 0.84 ± 0.1 | 1.58 ± 0.1*† | 1.57 ± 0.2* | 1.65 ± 0.1*† | 1.79 ± 0.2*† |
| LV weight/body weight, mg/g | 2.05 ± 0.2 | 1.91 ± 0.4 | 2.11 ± 0.4 | 1.73 ± 0.2 | 2.00 ± 0.2 | 2.37 ± 0.15 | 2.10 ± 0.3 |
| Interventricular septal weight/body weight, mg/g | 0.78 ± 0.1 | 0.79 ± 0.1 | 0.82 ± 0.2 | 0.90 ± 0.2 | 0.65 ± 0.1 | 0.71 ± 0.1 | 0.77 ± 0.1 |
Values are means ± SD; N, number of mice per group. Left: sham vs. pulmonary artery constriction (PAC). Center: proteasome-inhibited mice (MG132 or epoxomicin). Right: PA28α overexpressors.
P < 0.0001 vs. sham (+ vehicle);
P < 0.0001 vs. sham + MG132;
P < 0.01 vs. sham (+ vehicle). CR-tTA, cardiac restricted Tet trans-activator transgenic control mice; CR-PA28α/tTA, cardiac restricted PA28α overexpression transgenic mice; LV, left ventricular; RV, right ventricular.
Altered proteasomal enzyme activities after PAC.
At 8 days, 26S chymotrypsin-like (β5) activity and 20S caspase-like (β1) activity were significantly decreased in PAC compared with sham-operated controls (Fig. 1, E and H). However, 26S trypsin-like (β2) enzyme activity and 26S caspase-like (β1) activity (Fig. 1, F and G) were not changed, indicating differential regulation of each component of UPS proteolytic function.
Alterations in regulators of UPS.
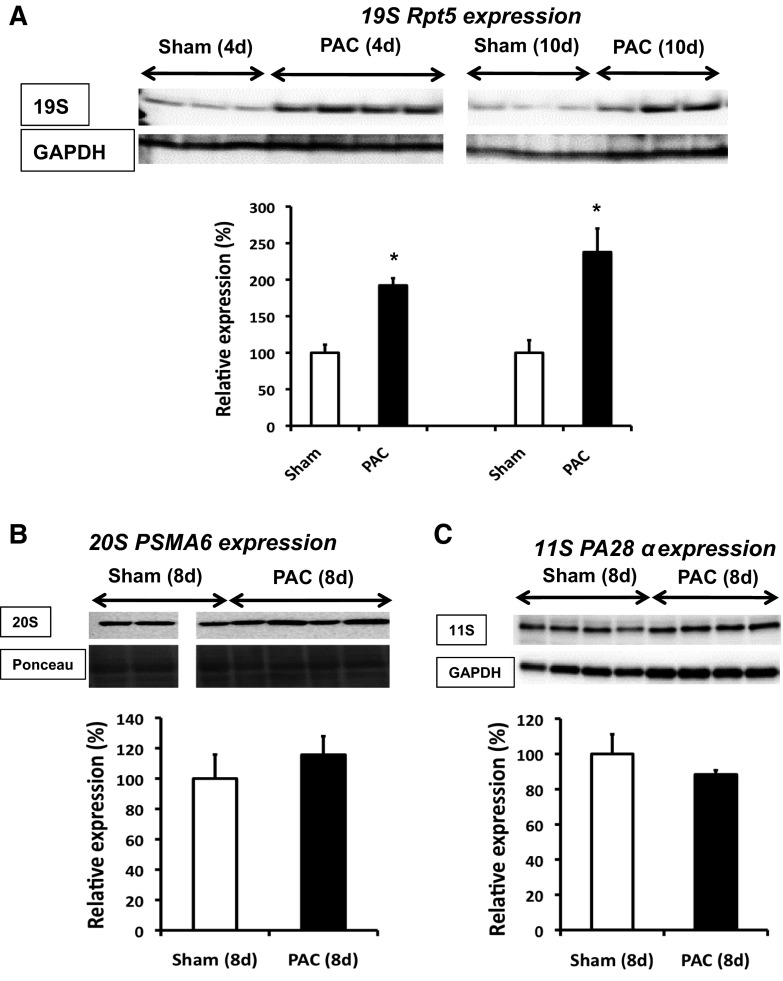
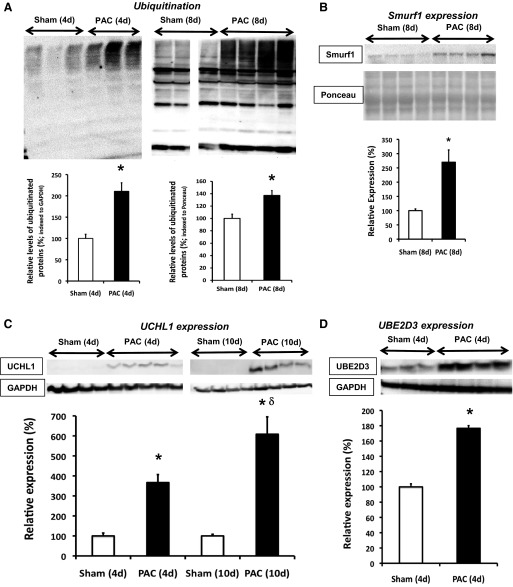
Several UPS and related genes involved in protein degradation were altered in the RV of PAC versus sham at 10 days (Table 2). Nineteen genes were significantly upregulated and 21 downregulated. We also characterized expression of representative protein targets at various levels of the UPS cascade. Expression of the 19S base regulatory subunit Rpt5 (S6a ATPase) was significantly increased at 4 and 10 days in PAC (Fig. 2A). 11S and 20S proteasome expression were not significantly altered [20S studied using 2 different antibodies, one targeting the α1-subunit and another, the anti-core proteasome (α5/α7, β1, β5, β5i, β7)] (Fig. 2, B and C). Consistent with the decrease in proteasome peptidase activities, relative levels of ubiquitinated proteins were significantly increased both at 4 and 8 days after PAC (Fig. 3A) associated with a 110% increase in free ubiquitin (P = 0.05; Fig. 5A). The physiologic significance of these results is supported by the fact that other regulators in the ubiquitin pathway were also increased. The ubiquitin ligase Smurf1 (SMAD specific E3 ubiquitin protein ligase 1) was increased at 8 days (Fig. 3B). Microarrays showed several deubiquitinating enzymes (Table 2) involved in the conversion of ubiquitin polymers to monomers were altered, including a 7.6-fold increase in UCHL1 (ubiquitin carboxy-terminal hydrolase 1). Correspondingly, protein expression of UCHL1 was increased 3.7- and 6.1-fold at 4 and 10 days, respectively (Fig. 3C). The E2 conjugation enzyme ubiquitin conjugation enzyme E2D 3 (UBE2D3/UbcH5C) was increased at 4 days (Fig. 3D).
Table 2.
Alterations in UPS gene expression in RV hypertrophy/RV failure
| Gene Symbol | Gene Name | Entrez Gene ID | Score(d) | Fold Change |
|---|---|---|---|---|
| Genes significantly upregulated in PAC RV vs. sham RV | ||||
| Ctsk | Cathepsin K | 13038 | 5.778 | 7.97 |
| Uch1l | Ubiquitin carboxy-terminal hydrolase L1 | 22223 | 7.751 | 7.63 |
| Ctss | Cathepsin S | 13040 | 5.309 | 3.84 |
| Rnf149 | Ring finger protein 149 | 67702 | 7.362 | 2.95 |
| Ctsl | Cathepsin L | 13039 | 4.015 | 2.32 |
| Atp6ap2 | ATPase, H+ transporting, lysosomal accessory protein 2 | 70495 | 6.005 | 2.17 |
| Lipa | Lysosomal acid lipase A | 16889 | 4.821 | 2.14 |
| Ctsz | Cathcpsin Z | 64138 | 4.519 | 2.07 |
| Ube2e2 | Ubiquitin-conjugating enzyme E2E 2 (UBC4/5 homolog, yeast) | 218793 | 7.351 | 1.93 |
| Rnf2 | Ring finger protein 2 | 19821 | 6.280 | 1.79 |
| Wwp2 | WW domain containing E3 ubiquitin protein ligase 2 | 66894 | 3.923 | 1.75 |
| Lamp1 | Lysosomal-associated membrane protein 1 | 16783 | 12.960 | 1.47 |
| Ctsd | Cathepsin D | 13033 | 4.612 | 1.44 |
| Fbx114 | F-box and leucine-rich repeat protein 14 | 101358 | 3.877 | 1.44 |
| Cand1 | Cullin associated and neddylation disassociated 1 | 71902 | 4.151 | 1.44 |
| Laptm4a | Lysosomal-associated protein transmembrane 4A | 17775 | 4.173 | 1.41 |
| Ndfip2 | Nedd4 family interacting protein 2 | 76273 | 4.080 | 1.40 |
| Psma2 | Proteasome (prosome, macropain) subunit, α type 2 | 19166 | 3.739 | 1.40 |
| Rnf19a | Ring finger protein 19A | 30945 | 5.336 | 1.36 |
| Genes significantly downregulated in PAC RV vs. sham RV | ||||
| Atg10 | Autophagy-related 10 (yeast) | 66795 | −4.389 | 2.73 |
| Ubq1n4 | Ubiquilin 4 | 94232 | −4.751 | 2.69 |
| Ctsf | Cathepsin F | 56464 | −3.816 | 2.52 |
| Rilp11 | Rab interacting lysosomal protein-like 1 | 75695 | −4.892 | 2.43 |
| Dcun1d2 | DCN1, defective in cullin neddylation 1, domain containing 2 (S. cerevisiae) | 102323 | −5.176 | 2.34 |
| Mul1 | Mitochondrial ubiquitin ligase activator of NFKB 1 | 68350 | −4.290 | 2.31 |
| Cul9 | Cullin 9 | 78309 | −3.927 | 2.23 |
| Psme1 | Proteasome (prosome, macropain) 28 subunit, α | 19186 | −5.325 | 1.95 |
| Ubac1 | Ubiquitin associated domain containing 1 | 98766 | −5.386 | 1.93 |
| Uchl4 | Ubiquitin carboxyl-terminal esterase L4 | 93841 | −4.016 | 1.79 |
| Rnf31 | Ring finger protein 31 | 268749 | −7.617 | 1.76 |
| Ubc2b | Ubiquitin-conjugating enzyme E2B, RAD6 homology (S. cerevisiac) | 22210 | −5.482 | 1.70 |
| Usp10 | Ubiquitin specific peptidase 10 | 22224 | −4.559 | 1.63 |
| Dcun1d5 | DCN1, defective in cullin neddylation 1, domain containing 5 (S. cerevisiae) | 76863 | −4.875 | 1.49 |
| Uch13 | Ubiquitin carboxy1-terminal esterase L3 (ubiquitin thiolesterase) | 50933 | −5.435 | 1.41 |
| Rnf114 | Ring finger protein 114 | 81018 | −6.888 | 1.37 |
| Pcgf1 | Polycomb group ring finger 1 | 69837 | −4.247 | 1.37 |
| Dram2 | VDNA-damage regulated autophagy modulator 2 | 67171 | −5.308 | 1.37 |
| Cops7a | COP9 (constitutive photomorphogenic) homolog, subunit 7a (Arabidopsis thaliana) | 26894 | −5.191 | 1.36 |
| Rnf187 | Ring finger protein 187 | 108660 | −5.423 | 1.32 |
| Ubl5 | Ubiquitin-like 5 | 66177 | −3.881 | 1.22 |
Stanford's Significance Analysis of Microarrays identified genes with statistically different expression between PAC and sham RV at 10 days post-operative using Agilent gene chips with a false discovery rate of <5%.
Fig. 2.
Altered proteasome expression after PAC. Immunoblot analyses of 19S Rpt5 (S6a ATPase) subunit at 4 days (4d) and 10 days (10d) (A), 20S PSMA6 (α1) subunit proteasome at 8d (B), and 11S (PA28α) subunit at 8d (C) are shown. PSMA6 was normalized with Ponceau staining. Results are shown as percent change from respective sham-operated controls. *P < 0.05 vs. sham; n = 3 to 4. All samples in B were derived from the same immunoblot and analyzed with a common reference sample; 1 of the sham lanes was removed because the sample was defective. Thus the sham samples in B are shown with a break between the lanes.
Fig. 3.
Increased ubiquitin signaling following PAC. Immunoblot analyses of signaling targets along the ubiquitin cascade are shown. Polyubiquitins at 4d and 8d (A), Smurf1 (SMAD specific E3 ubiquitin protein ligase 1) at 8d (B), de-ubiquitinating enzyme Uchl1 (Ubiquitin carboxy-terminal hydrolase 1) at 4d and 10d (C), and UBE2D3 (Ubiquitin conjugation enzyme E2D 3, UbcH5C) at 4d (D) are shown. Smurf1 and ubiquitins (8d) were normalized with Ponceau staining. Ubiquitin (4d) was normalized with GAPDH. Results are shown as percent change from sham-operated controls. *P < 0.05 vs. sham; δP < 0.05 vs. PAC (4d); n = 3–6. All samples in A were derived from the same immunoblot and analyzed with a common reference sample; 1 of the sham lanes was removed because the sample was defective. Thus the sham samples in A are shown with a break between the lanes.
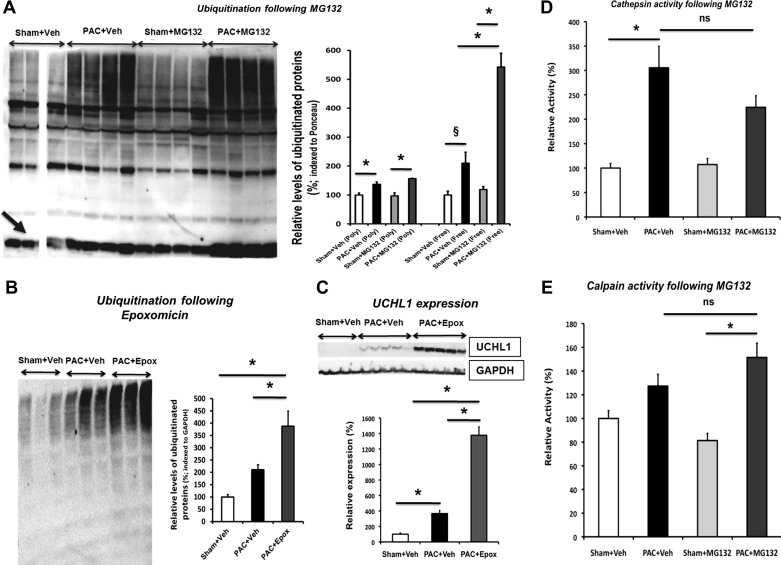
Fig. 5.
Altered ubiquitin-proteasomal signaling following proteasomal inhibition. Immunoblot analyses of RV of sham, PAC, and proteasome-inhibited mice are shown. A: polyubiquitins (Poly) and free-ubiquitins (Free; black arrow) at 8d. B: polyubiquitins at 4d. C: de-ubiquitinating enzyme Uchl1 at 4d. Ubiquitins were normalized with Ponceau staining (A) or GAPDH (B). Cathepsin (D) and calpain peptidase activities (E) were not altered following MG132 treatment. Proteolytic activities were studied at 8d post-operative in both sham-operated and PAC groups treated with vehicle or MG132. Results are shown as percent change from sham + vehicle (Veh). *P < 0.05; §P = 0.059; n = 3–7. Epox, epoxomicin; ns, not significant. All samples in A were derived from the same immunoblot and analyzed with a common reference sample; 1 of the sham lanes was removed because the sample was defective. Thus the sham samples in A are shown with a break between the lanes.
Alterations in UPS targets in RV failure after PAC.
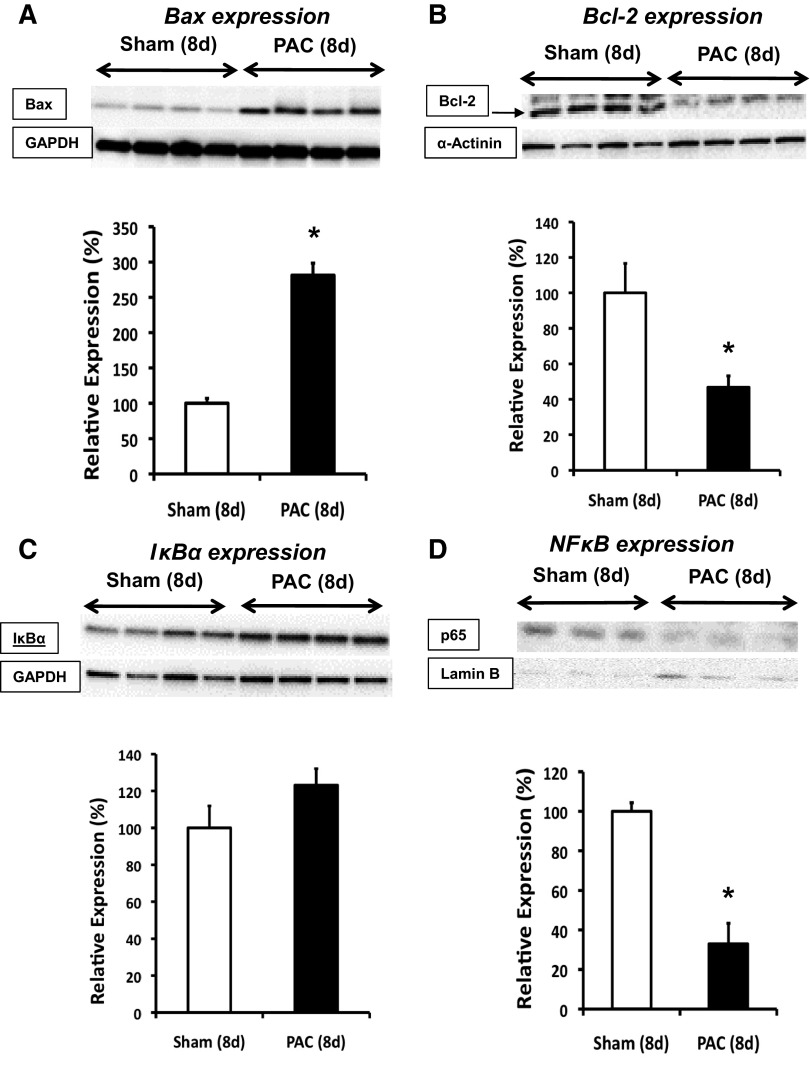
At 8 days, expression levels of ubiquitination targets were altered. The pro-apoptotic Bax levels were increased, and anti-apoptotic Bcl-2 levels were decreased. The Bax-to-Bcl-2 ratio was increased sixfold, suggesting that altered regulation of apoptosis may be an important mechanism in the development of RVF in this model (Fig. 4, A and B).
Fig. 4.
Mechanisms of remodeling in hypertrophied and failing RV. Immunoblot analyses show expression profile of targets affected by ubiquitin-proteasome system. Pro-apoptotic Bax at 8d (A), anti-apoptotic Bcl-2 at 8d (B), IκBα at 8d (C), and p65 subunit of nuclear NF-κB at 8d (D) are shown. Results are shown as percent change from sham-operated controls. *P < 0.05; n = 3 to 4.
NF-κB is a transcription factor mediating LVH, and IκBα, an inhibitor of NF-κB, is degraded by the UPS. Prior studies in LVH have suggested that UPS degradation of IκBα alters LV remodeling by disinhibition of NF-κB (32). In contrast with these findings in LVH/LVF, we did not observe NF-κB-mediated roles in RVH/RVF. Cytosolic IκBα expression was slightly increased and nuclear NF-κB expression was markedly decreased after 8 days PAC (Fig. 4, C and D).
Effects of proteasomal inhibition on key UPS regulators and function.
Studies using proteasomal inhibitors in LVH/LVF have been controversial, showing both cardiotoxic and cardioprotective effects (20, 59). Given our findings of decreased UPS activity in RVH/RVF, we tested whether inhibition of proteasome activity could further accelerate RV dysfunction or modify RV hypertrophy. To begin with, we found that there was an even greater increase (2.6-fold) in free ubiquitins following MG132 (nonspecific proteasome inhibitor) versus PAC + vehicle (Fig. 5A). With the administration of epoxomicin (a β5-specific proteasomal inhibitor), ubiquitin conjugates further increased, indicating increased accumulation of polyubiqutinated proteins that are not degraded by the inhibited proteasome (Fig. 5B). Correspondingly, the levels of UCHL1 deubiquitinase increased 3.7-fold following proteasomal inhibition versus PAC + vehicle (Fig. 5C). Because of its wider spectrum of activity, we investigated the effect of MG132 on other proteases influencing nonproteasomal degradative pathways including autophagy, cathepsins (lysosomal protease, increased in microarray data; Table 2, genes significantly upregulated in PAC RV vs. sham RV), and calpains (nonlysosomal protease) (35). Neither system was significantly affected (Fig. 5, D and E). Importantly, administration of inhibitors in the PAC mice did not hasten mortality. Both the increase in RV weight (Table 1) and the decrease in RVOT FS following PAC were similar in both vehicle- and inhibitor-treated PAC groups (sham: 46.73 ± 2%; PAC: 23.3 ± 1.3%; sham + MG132: 49.19 ± 1.8%; PAC + MG132: 23.8 ± 1%; PAC + epoxomicin: 28.14 ± 4.2%). These data suggest that further proteasomal inhibition does not affect the development of RVH and does not hasten RVF.
Proteasome enhancement via PA28α overexpression partially attenuates RVF.
Given that proteasomal activity was decreased in the failing RV, we hypothesized that proteasomal activation would attenuate pressure overload-induced RVF. We have recently demonstrated that cardiac-restricted overexpression of PA28α of the 11S proteasome complex (CR-PA28αOE) inhibited aberrant protein aggregation, attenuated cardiac hypertrophy, and delayed death in desmin-related cardiomyopathy as well as protected against ischemia/reperfusion injury (41). We performed PAC on CR-PA28αOE mice. To control for the effect of tTA transgene, we compared survival of CR-PA28αOE versus tTA transgenic mice subjected to PAC.
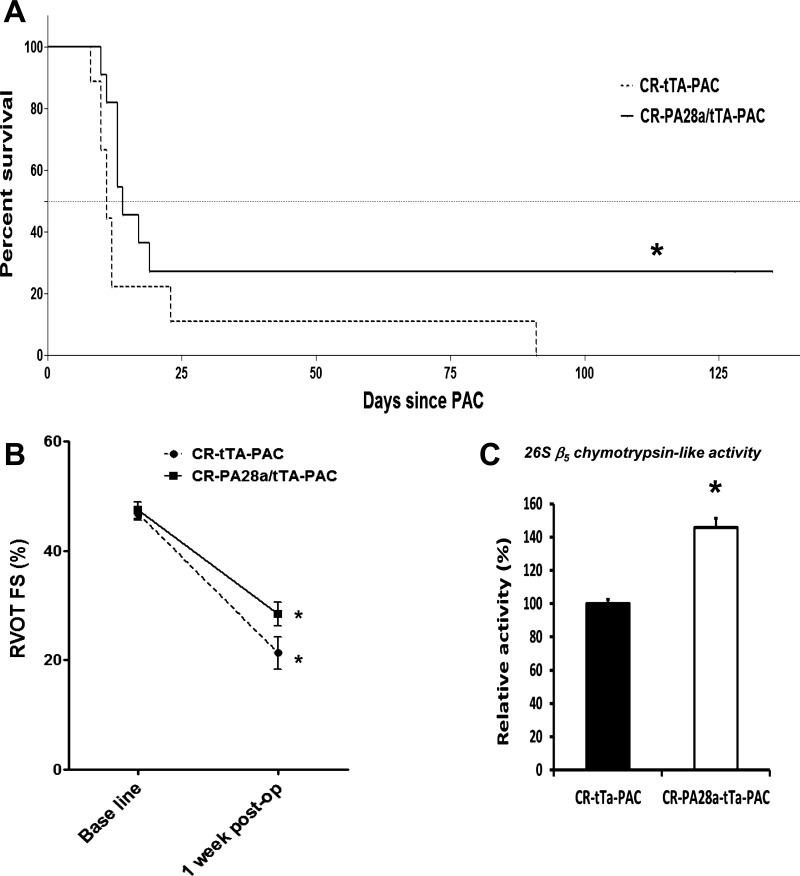
As expected, PAC on cardiac restricted Tet trans-activator transgenic control mice resulted in early mortality beginning at 8 days in the controls. In the CR-PA28α/tTA-PAC group, RVH was unaltered (Table 1). Nevertheless, PA28α overexpression significantly delayed mortality and extended survival after PAC compared with tTA controls (P < 0.05). Median survival was significantly extended by 21.4%. Although the majority of transgenic mice also died, late survival, beyond 3 wk, was most improved by proteasomal enhancement (P < 0.0001) (Fig. 6A); RVOT FS was improved by 25% in the PA28α overexpression group, although the latter did not reach statistical significance (Fig. 6B). Given the increase in Bax-to-Bcl2 ratio in PAC, we assessed apoptosis in our PAC model histologically. The amount of TUNEL-positive nuclei were not significantly altered in both myocytes and nonmyocytes following proteasome overexpression (data not shown), indicating that UPS may not attenuate RVF by altering apoptotic cell death mechanisms. The survival and functional changes were associated with a significant increase in the 26S chymotrypsin-like (β5) activity following conditional proteasome overexpression (Fig. 6C). Correspondingly, the relative levels of ubiquitin conjugates were decreased by 16%, although they did not reach statistical significance (data not shown). Overall, we conclude that enhancement of proteasomal function has a partial protective effect in RVH/RVF.
Fig. 6.
Enhancement of proteasomal activity by overexpression of PA28α extends survival in RV hypertrophy (RVH)/RV failure (RVF). A: shift to the right of Kaplan-Meier survival curve in PA28α overexpressors vs. control tTA transgenic mice after PAC. *P < 0.05 by Gehan-Breslow-Wilcoxon survival analysis; n = 9–11. B: decrease in RV outflow tract fractional shortening (RVOT FS) is less in PA28α overexpressors vs. control tTA transgenic mice subjected to PAC. *P < 0.001 vs. respective baselines; n = 4–6. C: increased 26S β5 chymotrypsin-like activity in PA28α overexpressors vs. control tTA transgenic mice subjected to PAC; n = 3–5. CR-tTA, cardiac restricted Tet trans-activator transgenic control mice; CR-PA28α/tTA, cardiac restricted PA28α overexpression transgenic mice.
DISCUSSION
UPS activity has been shown to be decreased (19, 49) in human LVF; however, in rodent models of LVH and LVF, studies have shown either increased or decreased UPS activity (31, 43, 71). Thus the role of the UPS in LVH/LVF is still unclear. We hypothesized that the UPS could play an even greater role in RVH/RVF due to the much greater increase in hypertrophy in the normally thin-walled RV after pulmonary artery banding compared with the LV after aortic banding, a 109% increase in RVW/BW in PAC versus a 54% increase in LVW/BW in transverse aortic constriction (76). The current study demonstrates downregulation of proteasome function during RV remodeling: decreased chymotrypsin-like 26S proteasomal activity, decreased caspase-like 20S proteasome activity, and alterations throughout the ubiquitination and proteasomal regulatory cascade. This includes increased ubiquitin conjugates and increase in expression of representative targets, namely UCHL1 deubiquitinase, 19S regulatory subunit, and Smurf1 E3 ubiquitin ligase. These alterations were associated with a sixfold increase in pro-apoptotic Bax to anti-apoptotic Bcl-2 ratio. To confirm whether these alterations in UPS activity played a role in the transition from RVH to RVF, mice with cardiac-restricted PA28α proteasome overexpression were subjected to PAC. Proteasome enhancement via PA28α overexpression resulted in partially improved survival.
Our principal finding that the chymotrypsin-like (β5) and caspase-like (β1) proteasomal activity is decreased in RVH/RVF indicates that the proteasomes are hypofunctioning in this model of RV failure. The results are in concordance with a number of studies on LVH remodeling in both mice and humans (33, 49, 63), in aging hearts (8), and ischemia-reperfusion injury (21, 60) but is in discordance with other studies (5, 12, 20, 32, 45, 46). The explanations for the varying responses in the LV have been debated (31, 43) and may be related to differences in the animal models or in techniques used in assessing proteasome activity. In accordance with other models of cardiac stress, the changes among the proteasome components following PAC indicate differential regulation of specific proteasomal enzyme activities in cardiac remodeling offering opportunities for targeted therapeutic interventions (23, 30, 33).
The decrease in UPS enzyme function in our model was accompanied by an increase in total ubiquitinated proteins, indicating inadequate protein degradation. This parallels the results seen in failing human hearts (49). Although ubiquitin has been implicated in nonproteasomal protein recycling and other functions (38, 65), the increase in ubiquitinated proteins and free ubiquitins following proteasomal inhibition points to their role in proteasome-mediated protein recycling. The increase in ubiquitin conjugates in PAC was accompanied by a 7.6-fold increase in gene expression of the deubiquitinating enzyme, UCHL1, responsible for recycling of ubiquitin polymers to monomers. This is usually accompanied by an increase in protein expression, as reported in human dilated cardiomyopathy (47, 49, 72), viral myocarditis (27), and balloon injured carotid artery (58). We confirmed a 6.1-fold increase in UCHL1 protein 10 days after PAC, indicating a possible compensatory role of UCHL1 in RVH/RVF. The specificity of substrates for proteasomal degradation is primarily conferred by the E3 ubiquitin ligases. MuRF1 has been reported to be upregulated in hypertrophic and failing hearts (71). We found that the Smurf1 ubiquitin ligase expression is upregulated in PAC, the first report identifying a role of Smurf1 in cardiac hypertrophy. Smurf1 is responsible for proteasomal degradation of substrates including RhoA, an important F-actin filament modulator and participant in serum response factor-mediated cardiac gene transcription (39, 73); RhoA is also important in modulating TGF-regulated Smad signaling and cardiac fibrosis (24, 53, 62, 70). Finally, we have recently demonstrated that microRNAs associated with the UPS are altered in our model of RVH and RVF (51). These include downregulation of miR-1 (known to target E2, Ube2h) and upregulation of miR-199a-5p (known to target E2, Ube2g1, Ube2i). In addition, miR-28 (predicted to target E3, Fbxl18) and miR-93 (predicted to target deubiquitinase, Usp24) were upregulated in RVH but downregulated in LVH. We have also shown excellent correlation using RT-PCR for a number of corresponding target genes in the UPS, namely F-Box and leucine-rich repeat E3 ubiquitin ligases, ubiquitin-conjugating enzymes, etc. Other genes altered in hypertrophy and heart failure include myosin heavy chain, atrial natriuretic peptide, and sarco-endoplasmic reticulum ATPase.
The post-translational modification of apoptotic proteins by ubiquitination regulates key components of programmed cell death signaling cascades (48, 55, 63, 68). Similar to the findings of Tsukamoto et al. (63) in LVH/LVF, decreases in proteasome activity were associated with decreases in anti-apoptotic proteins and increases in pro-apoptotic proteins and TUNEL-positive nuclei. In contrast with the LVH model of Hedhli et al. (32), where proteasomal activity was found to be increased, in RVH IκBα levels were not decreased, consistent with the decrease in proteasome activity observed. However, unlike UPS studies in human cardiomyopathies (49) and rodent models of LV ischemia-reperfusion (21), we did not find any difference in oxidative carbonylation in RVs of the PAC group compared with sham (data not shown).
The role of proteasomal inhibition of LVH/LVF has also been controversial. In studies in LVH/LVF models where UPS activity was found to be increased, proteasomal inhibition attenuates the development of hypertrophy and partially, but not totally, rescues LV function (20). In contrast, Tang et al. (59) have shown that proteasome inhibition with bortezomib decreased LV function in pressure overload-induced LVH/LVF (59). To avoid the differential inhibitory effects on the various cardiac proteasome subtypes (11, 36), we tested proteasome inhibitors from two diverse classes in vivo using standard tested nontoxic doses. Neither the irreversible/β5-specific (epoxomicin) nor the reversible/nonspecific (MG132) proteasomal inhibitors altered the degree of RVH or hastened the development of RVF, suggesting that the failing RV is not responsive to further proteasomal inhibition in this model of RV failure. Nevertheless, future studies are needed to study the effect of inhibitors being developed against the induced isoforms of proteasomes (10).
There are several possible reasons for the differences in our results from those in some (but not all) studies in the LV. First, our results in the RV are consistent with the results of the groups that have also found decreases in proteasome activity in the afterloaded LV (63), suggesting a possibility that downregulation of proteasome activity may be a generalized process in both ventricles. Alternatively, the UPS response to RV pressure overload may be fundamentally different from that in the LV, reflecting other key differences in the stress responses of the two ventricles (7, 22, 37, 67), as may be the case with Smurf1 upregulation. Some of the discrepancies among the different studies of LVH may reflect the use of mice of different background strains (20, 32, 63), and a similar effect in our study cannot be ruled out. Notably, both our study and the study by Tang et al. (59) used mice from the FVB/N background, and have both shown no change in cardiac hypertrophy following proteasomal inhibition. Finally, even in studies showing a benefit of proteasomal inhibitors in LVF, the improvement in LV function is not huge (32).
Although a pharmacologic activator of proteasomal function has not been tested in vivo (40), we were able to test whether proteasome enhancement could attenuate the progression from RVH to RVF using mice overexpressing the proteasome activator subunit PA28α of the 11S proteasome complex, CR-PA28αOE. 11S is considered to play a greater role in intracellular protein degradation than in antigen processing (14, 26, 75). We have recently demonstrated that CR-PA28αOE did not alter the homeostasis of normal proteins and cardiac function but did facilitate the degradation of a surrogate misfolded protein in the heart. Importantly, we had shown that PA28α overexpression inhibits aberrant protein aggregation, attenuates LVH, and delays premature death in desmin cardiomyopathy as well as protects against LV ischemia-reperfusion injury (41, 42). Our current results show that overexpression of PA28α partially prolongs survival in RVH/RVF, suggesting that proteasome functional insufficiency contributes in part to the pathogenesis of RVF (60). However, contrary to our initial hypothesis, this effect was not dramatic, indicating that the decrease in proteasomal activity in our PAC model is only a partial contributor to the transition from RVH to RVF. These results could still be consistent with prior observations in the LV, suggesting that therapeutic approaches to modulating UPS activity that might work in conditions of LV stress may not be effective for RV stress. Alternatively, our model of RVH/RVF may be too rapid or severe to be fully rescued by the restoration of proteasome function. However, our model is similar to most transverse aortic constriction models of LVH/LVF, where the rapid progression from hypertrophy to heart failure provides a powerful tool to study basic mechanisms of disease. Given that humans with RVH often develop RVF only over decades, it is possible that proteasomal enhancement would have better efficacy in a more chronic setting. Future studies will be required to dissect how differential changes in subunit-specific proteasomal activities affect substrate selectivity and specificity, and, ultimately, how these affect cardiac phenotypes. Given the incomplete improvement in survival following proteasome overexpression, it would also be important to understand the contribution of alternative components that may be rate-limiting in RVF including autophagy and interplay between ubiquitin-proteasome-mediated proteolysis and selective autophagy (71).
GRANTS
This work was supported by National Institutes of Health Grants HL-061535 (to D. Bernstein), HL-072166 (to X. Wang), and HL-096819 (to A. V. Gomes) and an American Heart Association Western States Affiliate Postdoctoral Fellowship, Stanford Dean's Postdoctoral Fellowship, and Alex Vibber Endowed Fellowship in Pediatric Cardiology Research by Lucile Packard Children's Hospital at Stanford (to V. Rajagopalan).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: V.R., X.W., A.V.G., and D.B. provided the conception and design of research; V.R., M.Z., S.R., and S.D. performed experiments; V.R., M.Z., S.R., G.A.F., X.W., S.D., A.V.G., and D.B. analyzed data; V.R., S.R., G.A.F., X.W., S.D., A.V.G., and D.B. interpreted results of experiments; V.R. and D.B. prepared figures; V.R. and D.B. drafted manuscript; V.R., M.Z., S.R., G.A.F., X.W., A.V.G., and D.B. edited and revised manuscript; V.R., M.Z., S.R., G.A.F., X.W., S.D., A.V.G., and D.B. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Prof. Ron Kopito for expert advice on the UPS, Dr. Joshua Spin for helpful suggestions on microarray analyses, and Dong-Qing Hu on histology staining. All are from Stanford University.
Present address for V. Rajagopalan: Dept. of Biomedical Sciences, College of Osteopathic Medicine, New York Institute of Technology, Old Westbury, NY.
REFERENCES
- 1.Asimaki A, Syrris P, Wichter T, Matthias P, Saffitz JE, McKenna WJ. A novel dominant mutation in plakoglobin causes arrhythmogenic right ventricular cardiomyopathy. Am J Hum Genet 81: 964–973, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker TA, Geng Q, Romero J, Picken MM, Gamelli RL, Majetschak M. Prolongation of myocardial viability by proteasome inhibition during hypothermic organ preservation. Biochem Biophys Res Commun 401: 548–553, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balasubramanian S, Mani S, Shiraishi H, Johnston RK, Yamane K, Willey CD, Cooper Iv G, Tuxworth WJ, Kuppuswamy D. Enhanced ubiquitination of cytoskeletal proteins in pressure overloaded myocardium is accompanied by changes in specific E3 ligases. J Mol Cell Cardiol 41: 669–679, 2006 [DOI] [PubMed] [Google Scholar]
- 4.Bernstein D, Webber S. New directions in basic research in hypertrophy and heart failure: relevance for pediatric cardiology. Prog Pediatr Cardiol 32: 5–9, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, Yacoub MH, Evans PC. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res 79: 472–480, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Bonuccelli G, Sotgia F, Schubert W, Park DS, Frank PG, Woodman SE, Insabato L, Cammer M, Minetti C, Lisanti MP. Proteasome inhibitor (MG-132) treatment of mdx mice rescues the expression and membrane localization of dystrophin and dystrophin-associated proteins. Am J Pathol 163: 1663–1675, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brunet S, Aimond F, Li H, Guo W, Eldstrom J, Fedida D, Yamada KA, Nerbonne JM. Heterogeneous expression of repolarizing, voltage-gated K+ currents in adult mouse ventricles. J Physiol 559: 103–120, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bulteau AL, Szweda LI, Friguet B. Age-dependent declines in proteasome activity in the heart. Arch Biochem Biophys 397: 298–304, 2002 [DOI] [PubMed] [Google Scholar]
- 9.Cai ZP, Shen Z, Van Kaer L, Becker LC. Ischemic preconditioning-induced cardioprotection is lost in mice with immunoproteasome subunit low molecular mass polypeptide-2 deficiency. FASEB J 22: 4248–4257, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Calise J, Powell SR. The ubiquitin proteasome system and myocardial ischemia. Am J Physiol Heart Circ Physiol 304: H337–H349, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Carrier L. Too much of a good thing is bad: proteasome inhibition induces stressed hearts to fail. Cardiovasc Res 88: 389–390, 2010 [DOI] [PubMed] [Google Scholar]
- 12.Chen B, Ma Y, Meng R, Xiong Z, Zhang C, Chen G, Zhang A, Dong Y. MG132, a proteasome inhibitor, attenuates pressure-overload-induced cardiac hypertrophy in rats by modulation of mitogen-activated protein kinase signals. Acta Biochim Biophys Sin (Shanghai) 42: 253–258, 2010 [DOI] [PubMed] [Google Scholar]
- 13.Chen Q, Liu JB, Horak KM, Zheng H, Kumarapeli AR, Li J, Li F, Gerdes AM, Wawrousek EF, Wang X. Intrasarcoplasmic amyloidosis impairs proteolytic function of proteasomes in cardiomyocytes by compromising substrate uptake. Circ Res 97: 1018–1026, 2005 [DOI] [PubMed] [Google Scholar]
- 14.Chen X, Barton LF, Chi Y, Clurman BE, Roberts JM. Ubiquitin-independent degradation of cell-cycle inhibitors by the REGgamma proteasome. Mol Cell 26: 843–852, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cho H, Thorvaldsen JL, Chu Q, Feng F, Birnbaum MJ. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J Biol Chem 276: 38349–38352, 2001 [DOI] [PubMed] [Google Scholar]
- 16.Churchill EN, Ferreira JC, Brum PC, Szweda LI, Mochly-Rosen D. Ischaemic preconditioning improves proteasomal activity and increases the degradation of Œ¥PKC during reperfusion. Cardiovasc Res 85: 385–394, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ciechanover A. The ubiquitin proteolytic system: from a vague idea, through basic mechanisms, and onto human diseases and drug targeting. Neurology 66: S7–S19, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Dantuma NP, Lindsten K. Stressing the ubiquitin-proteasome system. Cardiovasc Res 85: 263–271, 2010 [DOI] [PubMed] [Google Scholar]
- 19.Day SM, Divald A, Wang P, Davis F, Bartolone S, Jones R, Powell SR. Impaired assembly and post-translational regulation of 26S proteasome in human end stage heart failure. Circ Heart Fail 6: 544–549, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Depre C, Wang Q, Yan L, Hedhli N, Peter P, Chen L, Hong C, Hittinger L, Ghaleh B, Sadoshima J, Vatner DE, Vatner SF, Madura K. Activation of the cardiac proteasome during pressure overload promotes ventricular hypertrophy. Circulation 114: 1821–1828, 2006 [DOI] [PubMed] [Google Scholar]
- 21.Divald A, Kivity S, Wang P, Hochhauser E, Roberts B, Teichberg S, Gomes AV, Powell SR. Myocardial ischemic preconditioning preserves postischemic function of the 26S proteasome through diminished oxidative damage to 19S regulatory particle subunits. Circ Res 106: 1829–1838, 2010 [DOI] [PubMed] [Google Scholar]
- 22.Dore A, Houde C, Chan KL, Ducharme A, Khairy P, Juneau M, Marcotte F, Mercier LA. Angiotensin receptor blockade and exercise capacity in adults with systemic right ventricles: a multicenter, randomized, placebo-controlled clinical trial. Circulation 112: 2411–2416, 2005 [DOI] [PubMed] [Google Scholar]
- 23.Drews O, Tsukamoto O, Liem D, Streicher J, Wang Y, Ping P. Differential regulation of proteasome function in isoproterenol-induced cardiac hypertrophy. Circ Res 107: 1094–1101, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dupont S, Inui M, Newfeld SJ. Regulation of TGF-beta signal transduction by mono- and deubiquitylation of Smads. FEBS Lett 586: 1913–1920, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Enrico O, Gabriele B, Nadia C, Sara G, Daniele V, Giulia C, Antonio S, Mario P. Unexpected cardiotoxicity in haematological bortezomib treated patients. Br J Haematol 138: 396–397, 2007 [DOI] [PubMed] [Google Scholar]
- 26.Ferrington DA, Husom AD, Thompson LV. Altered proteasome structure, function, and oxidation in aged muscle. FASEB J 19: 644–646, 2005 [DOI] [PubMed] [Google Scholar]
- 27.Gao G, Zhang J, Si X, Wong J, Cheung C, McManus B, Luo H. Proteasome inhibition attenuates coxsackievirus-induced myocardial damage in mice. Am J Physiol Heart Circ Physiol 295: H401–H408, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gomes AV, Waddell DS, Siu R, Stein M, Dewey S, Furlow JD, Bodine SC. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J 26: 2986–2999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gomes AV, Waddell DS, Siu R, Stein M, Dewey S, Furlow JD, Bodine SC. Upregulation of proteasome activity in muscle RING finger 1-null mice following denervation. FASEB J 26: 2986–2999, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gomes AV, Young GW, Wang Y, Zong C, Eghbali M, Drews O, Lu H, Stefani E, Ping P. Contrasting proteome biology and functional heterogeneity of the 20S proteasome complexes in mammalian tissues. Mol Cell Proteomics 8: 302–315, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hedhli N, Depre C. Proteasome inhibitors and cardiac cell growth. Cardiovasc Res 85: 321–329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedhli N, Lizano P, Hong C, Fritzky LF, Dhar SK, Liu H, Tian Y, Gao S, Madura K, Vatner SF, Depre C. Proteasome inhibition decreases cardiac remodeling after initiation of pressure overload. Am J Physiol Heart Circ Physiol 295: H1385–H1393, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hwee DT, Gomes AV, Bodine SC. Cardiac proteasome activity in muscle ring finger-1 null mice at rest and following synthetic glucocorticoid treatment. Am J Physiol Endocrinol Metab 301: E967–E977, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaufman BD, Desai M, Reddy S, Osorio JC, Chen JM, Mosca RS, Ferrante AW, Mital S. Genomic profiling of left and right ventricular hypertrophy in congenital heart disease. J Card Fail 14: 760–767, 2008 [DOI] [PubMed] [Google Scholar]
- 35.Kisselev AF, Goldberg AL. Proteasome inhibitors: from research tools to drug candidates. Chem Biol 8: 739–758, 2001 [DOI] [PubMed] [Google Scholar]
- 36.Kloss A, Meiners S, Ludwig A, Dahlmann B. Multiple cardiac proteasome subtypes differ in their susceptibility to proteasome inhibitors. Cardiovasc Res 85: 367–375, 2010 [DOI] [PubMed] [Google Scholar]
- 37.Kondo RP, Dederko DA, Teutsch C, Chrast J, Catalucci D, Chien KR, Giles WR. Comparison of contraction and calcium handling between right and left ventricular myocytes from adult mouse heart: a role for repolarization waveform. J Physiol 571: 131–146, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kraft C, Peter M, Hofmann K. Selective autophagy: ubiquitin-mediated recognition and beyond. Nat Cell Biol 12: 836–841, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Kuwahara K, Barrientos T, Pipes GC, Li S, Olson EN. Muscle-specific signaling mechanism that links actin dynamics to serum response factor. Mol Cell Biol 25: 3173–3181, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee BH, Lee MJ, Park S, Oh DC, Elsasser S, Chen PC, Gartner C, Dimova N, Hanna J, Gygi SP, Wilson SM, King RW, Finley D. Enhancement of proteasome activity by a small-molecule inhibitor of USP14. Nature 467: 179–184, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J, Horak KM, Su H, Sanbe A, Robbins J, Wang X. Enhancement of proteasomal function protects against cardiac proteinopathy and ischemia/reperfusion injury in mice. J Clin Invest 121: 3689–3700, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Li J, Powell SR, Wang X. Enhancement of proteasome function by PA28α overexpression protects against oxidative stress. FASEB J 25: 883–893, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Li YF, Wang X. The role of the proteasome in heart disease. Biochim Biophys Acta 1809: 141–149, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lindqvist P, Henein M, Kazzam E. Right ventricular outflow-tract fractional shortening: an applicable measure of right ventricular systolic function. Eur J Echocardiogr 4: 29–35, 2003 [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, Chen B, Liu D, Yang Y, Xiong Z, Zeng J, Dong Y. MG132 treatment attenuates cardiac remodeling and dysfunction following aortic banding in rats via the NF-κB/TGF-β1 pathway. Biochem Pharmacol 81: 1228–1236, 2011 [DOI] [PubMed] [Google Scholar]
- 46.Meiners S, Dreger H, Fechner M, Bieler S, Rother W, Gunther C, Baumann G, Stangl V, Stangl K. Suppression of cardiomyocyte hypertrophy by inhibition of the ubiquitin-proteasome system. Hypertension 51: 302–308, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Otsuka K, Terasaki F, Shimomura H, Tsukada B, Horii T, Isomura T, Suma H, Shibayama Y, Kitaura Y. Enhanced expression of the ubiquitin-proteasome system in the myocardium from patients with dilated cardiomyopathy referred for left ventriculoplasty: an immunohistochemical study with special reference to oxidative stress. Heart Vessels 25: 474–484, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Portbury AL, Ronnebaum SM, Zungu M, Patterson C, Willis MS. Back to your heart: ubiquitin proteasome system-regulated signal transduction. J Mol Cell Cardiol 52: 526–537, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Predmore JM, Wang P, Davis F, Bartolone S, Westfall MV, Dyke DB, Pagani F, Powell SR, Day SM. Ubiquitin proteasome dysfunction in human hypertrophic and dilated cardiomyopathies. Circulation 121: 997–1004, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Razeghi P, Taegtmeyer H. Cardiac remodeling: UPS lost in transit. Circ Res 97: 964–966, 2005 [DOI] [PubMed] [Google Scholar]
- 51.Reddy S, Zhao M, Hu DQ, Fajardo G, Hu S, Ghosh Z, Rajagopalan V, Wu JC, Bernstein D. Dynamic microRNA expression during the transition from right ventricular hypertrophy to failure. Physiol Genomics 44: 562–575, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Reddy SZM, Hu DQ, Fajardo G, Katznelson E, Punn R, Spin JM, Chan FP, Bernstein D. Physiologic and molecular characterization of a murine model of right ventricular volume overload. Am J Physiol Heart Circ Physiol 304: H1314–H1327, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanchez NS, Barnett JV. TGFbeta and BMP-2 regulate epicardial cell invasion via TGFbetaR3 activation of the Par6/Smurf1/RhoA pathway. Cell Signal 24: 539–548, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, Ross RD, Pahl E, Blume ED, Dodd DA, Rosenthal DN, Burr J, LaSalle B, Holubkov R, Lukas MA, Tani LY, Pediatric Carvedilol Study Group Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA 298: 1171–1179, 2007 [DOI] [PubMed] [Google Scholar]
- 55.Sohns W, van Veen TA, van der Heyden MA. Regulatory roles of the ubiquitin-proteasome system in cardiomyocyte apoptosis. Curr Mol Med 10: 1–13, 2010 [DOI] [PubMed] [Google Scholar]
- 56.Su H, Wang X. The ubiquitin-proteasome system in cardiac proteinopathy: a quality control perspective. Cardiovasc Res 85: 253–262, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sugden PH, Clerk A. Cellular mechanisms of cardiac hypertrophy. J Mol Med 76: 725–746, 1998 [DOI] [PubMed] [Google Scholar]
- 58.Takami Y, Nakagami H, Morishita R, Katsuya T, Cui TX, Ichikawa T, Saito Y, Hayashi H, Kikuchi Y, Nishikawa T, Baba Y, Yasuda O, Rakugi H, Ogihara T, Kaneda Y. Ubiquitin carboxyl-terminal hydrolase L1, a novel deubiquitinating enzyme in the vasculature, attenuates NF-kappaB activation. Arterioscler Thromb Vasc Biol 27: 2184–2190, 2007 [DOI] [PubMed] [Google Scholar]
- 59.Tang M, Li J, Huang W, Su H, Liang Q, Tian Z, Horak KM, Molkentin JD, Wang X. Proteasome functional insufficiency activates the calcineurin-NFAT pathway in cardiomyocytes and promotes maladaptive remodelling of stressed mouse hearts. Cardiovasc Res 88: 424–433, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian Z, Zheng H, Li J, Li Y, Su H, Wang X. Genetically induced moderate inhibition of the proteasome in cardiomyocytes exacerbates myocardial ischemia-reperfusion injury in mice. Circ Res 111: 532–542, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Townsend TA, Wrana JL, Davis GE, Barnett JV. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem 283: 13834–13841, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tsukamoto O, Minamino T, Okada K, Shintani Y, Takashima S, Kato H, Liao Y, Okazaki H, Asai M, Hirata A, Fujita M, Asano Y, Yamazaki S, Asanuma H, Hori M, Kitakaze M. Depression of proteasome activities during the progression of cardiac dysfunction in pressure-overloaded heart of mice. Biochem Biophys Res Commun 340: 1125–1133, 2006 [DOI] [PubMed] [Google Scholar]
- 64.Tusher VG, Tibshirani R, Chu G. Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci USA 98: 5116–5121, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ulrich HD, Walden H. Ubiquitin signalling in DNA replication and repair. Nat Rev Mol Cell Biol 11: 479–489, 2010 [DOI] [PubMed] [Google Scholar]
- 66.Umar SDS, Lorga A, Gomes A, Eghbali M. Severe pulmonary hypertension and right ventricular dysfunction in rats are associated with changes in ubiquitin proteasome system (Abstract). Circulation 124: A14660, 2011 [Google Scholar]
- 67.Urashima T, Zhao M, Wagner R, Fajardo G, Farahani S, Quertermous T, Bernstein D. Molecular and physiological characterization of RV remodeling in a murine model of pulmonary stenosis. Am J Physiol Heart Circ Physiol 295: H1351–H1368, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vucic D, Dixit VM, Wertz IE. Ubiquitylation in apoptosis: a post-translational modification at the edge of life and death. Nat Rev Mol Cell Biol 12: 439–452, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Wang GY, Yeh CC, Jensen BC, Mann MJ, Simpson PC, Baker AJ. Heart failure switches the RV α1-adrenergic inotropic response from negative to positive. Am J Physiol Heart Circ Physiol 298: H913–H920, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wang S, Sun A, Li L, Zhao G, Jia J, Wang K, Ge J, Zou Y. Upregualtion of BMP-2 antagonizes TGF-beta1/ROCK-enhanced cardiac fibrotic signaling through activating of Smurf1/Smad6 complex. J Cell Mol Med 16: 2301–2310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wang X, Li J, Zheng H, Su H, Powell SR. Proteasome functional insufficiency in cardiac pathogenesis. Am J Physiol Heart Circ Physiol 301: H2207–H2219, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weekes J, Morrison K, Mullen A, Wait R, Barton P, Dunn MJ. Hyperubiquitination of proteins in dilated cardiomyopathy. Proteomics 3: 208–216, 2003 [DOI] [PubMed] [Google Scholar]
- 73.Wei L, Wang L, Carson JA, Agan JE, Imanaka-Yoshida K, Schwartz RJ. β1 Integrin and organized actin filaments facilitate cardiomyocyte-specific RhoA-dependent activation of the skeletal alpha-actin promoter. FASEB J 15: 785–796, 2001 [DOI] [PubMed] [Google Scholar]
- 74.Young GW, Wang Y, Ping P. Understanding proteasome assembly and regulation: importance to cardiovascular medicine. Trends Cardiovasc Med 18: 93–98, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang Z, Krutchinsky A, Endicott S, Realini C, Rechsteiner M, Standing KG. Proteasome activator 11S REG or PA28: recombinant REG alpha/REG beta hetero-oligomers are heptamers. Biochemistry 38: 5651–5658, 1999 [DOI] [PubMed] [Google Scholar]
- 76.Zhao M, Fajardo G, Urashima T, Spin JM, Poorfarahani S, Rajagopalan V, Huynh D, Connolly A, Quertermous T, Bernstein D. Cardiac pressure overload hypertrophy is differentially regulated by β-adrenergic receptor subtypes. Am J Physiol Heart Circ Physiol 301: H1461–H1470, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]