Abstract
Studies over the past decade have highlighted important roles played by sensory receptors outside of traditionally sensory tissues; for example, taste receptors participate in pH sensing in the cerebrospinal fluid, bitter taste receptors mediate bronchodilation and ciliary beating in the lung (Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Nat Med 16: 1299–1304, 2010; Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Science 325: 1131–1134, 2009), and olfactory receptors play roles in both sperm chemotaxis and muscle cell migration (Griffin CA, Kafadar KA, Pavlath GK. Cell 17: 649–661, 2009). More recently, several studies have shown that sensory receptors also play important roles in the regulation of blood pressure. This review will focus on several recent studies examining the roles that sensory receptors play in blood pressure regulation, with an emphasis on three pathways: the adenylate cyclase 3 (AC3) pathway, the Gpr91-succinate signaling pathway, and the Olfr78/Gpr41 short-chain fatty acid signaling pathway. Together, these pathways demonstrate that sensory receptors play important roles in mediating blood pressure control and that blood pressure regulation is coupled to the metabolism of the host as well as the metabolism of the gut microbiota.
Keywords: succinate, short-chain fatty acids, olfactory receptors, Gpr91, Olfr78
Sensory Receptors and Blood Pressure Regulation
blood pressure homeostasis is regulated by a variety of inputs (23) including baroreceptors, chemoreceptors, sympathetic nerve activity, and hormones. Although these inputs are important regulators of blood pressure on a timescale of seconds (neural) or minutes (hormonal), long-term blood pressure control is dominated by the kidney (10, 22, 24, 76) via regulation of extracellular fluid volume. When blood pressure is high, the excess pressure promotes renal salt and water excretion and thus returns blood volume, and blood pressure, toward normal. Conversely, if blood pressure is low, extracellular fluid volume will be increased, thereby increasing both blood volume and blood pressure.
Recently, studies have highlighted roles that sensory receptors, particularly in the kidney, play in the regulation of blood pressure. Indeed, the idea that sensory receptors modulate blood pressure regulation is not new; chemoreceptors in the carotid and aortic bodies have long been known to play important roles in blood pressure regulation. However, as recent studies have demonstrated, sensory receptors, olfactory receptors (ORs), taste receptors, and other G protein-coupled receptors (often of the GPR family) play functional roles in traditionally “nonsensory” processes in many different tissues (13, 14, 25, 28, 30, 33, 34, 38, 39, 60, 64, 65, 73). Often, the ligands for these “sensory” receptors are metabolites produced by metabolic pathways or other physiological processes (25, 28, 33, 72). This review will focus on recent studies highlighting the roles that renal and cardiovascular sensory receptors play in blood pressure regulation, focusing on the role of adenylate cyclase 3 (AC3), as well as receptors that have been shown to be sensors for two important metabolites: succinate and short-chain fatty acids.
Succinate
Succinate is a metabolic intermediate of the citric acid cycle that is present in the plasma in the low micrometer range (25). In the 1980s, it was demonstrated that succinate alters various aspects of metabolism and transport in the proximal tubule (3, 7, 19–21). However, it was not until 2004 that G protein-coupled receptor 91 (Gpr91) was identified as a succinate receptor by He et al. (25); subsequently, Gpr91 is now also referred to as succinate receptor 1 (or Sucnr1). In agreement with earlier work on the effect of succinate in the kidney, Gpr91 was localized to the proximal tubule as well as the juxtaglomerular apparatus (JGA) and distal tubule. A subsequent study localized Gpr91 to the glomerulus (likely glomerular endothelial cells) and to the apical membranes of multiple distal segments, including the thick ascending limb, the macula densa (MD), and the principal cells of the cortical and medullary collecting ducts (56).
In the study of He et al. (25), activation of Gpr91 by succinate was identified as a novel stimulus for renin secretion. This study demonstrated that succinate delivery could induce hypertension in wild-type but not Gpr91−/− animals (although angiotensin II induced hypertension similarly in both genotypes). This group also reported that succinate delivery increased plasma renin levels and that the succinate-induced blood pressure increase in wild-type animals could be blocked by angiotension-coverting enzyme inhibitors, implicating the activation of the renin-angiotensin system. On a cellular level, it is thought that succinate activates Gpr91 on the apical membrane of MD cells to activate mitogen-activated protein kinases as well as cyclooxygenase-2 (72). This leads to increased synthesis and release of prostaglandin E2, which is both a vasodilator and an important paracrine mediator of renin release from the juxtaglomerular apparatus (63). In further support of the role of succinate to mediate renin release, it was shown that succinate induces renin release from ex vivo glomeruli/JGA preparations in Gpr91+/+ but not Gpr91−/− mice (72). Interestingly, however, Gpr91−/− mice have normal baseline blood pressures (25), likely due to long-term blood pressure counterregulatory mechanisms (23). Because renin is a key part of the ability of the kidney to increase blood pressure in the face of low blood volume, it is tempting to speculate that succinate may be acting via Gpr91 to support increases in blood volume (and therefore blood pressure).
Recent studies have also tied the succinate-GPR91-renin pathway to various aspects of pathophysiology. Tissue succinate levels are known to be increased during ischemic hypoxia (whereas other TCA intermediates decrease; Refs. 17, 49); thus, an increase in succinate levels may indicate ischemia, oxidative stress, or renal energy deprivation. In addition, it has been suggested that accumulation of succinate in renal ischemia could contribute to stenosis-associated hypertension (25).
It has also been demonstrated (57) that circulating succinate levels are elevated in several rodent models of hypertension and metabolic disease, including the spontaneously hypertensive rats rat, ob/ob mice, and db/db mice. However, in humans, neither hypertensive nor diabetic patients presented with elevated succinate in the blood. As the authors note, however, this does not preclude a local signaling role of succinate, which is not reflected in the circulating levels. In the same vein, it has been suggested that localized succinate signaling may be relevant in the case of diabetic nephropathy, as hyperglycemia can lead to the local accumulation of succinate, which then can induce renin release from the JGA (49–51, 67). In addition, there is interest in urinary succinate as a potential early biomarker for diabetic nephropathy, as urinary succinate is increased one- to twofold in diabetic mice (67). Although much work remains to be done, succinate is clearly an important signaling molecule that plays important roles in both the physiology and pathophysiology of blood pressure control.
AC3 and the MD
AC3 is the “olfactory” isoform of adenylate cyclase and is a necessary component of OR signaling in the nose. In the nose, when an OR binds to its ligand, it activates downstream signaling by activating a specific subtype of G protein (Golfactory, or Golf) and subsequently, AC3. Golf and AC3 are obligatory for OR downstream signaling in the olfactory epithelium, as demonstrated by the fact that both Golf−/− and AC3−/− mice cannot smell (5, 77). In the kidney, Golf and AC3 colocalize with one another in both the distal convoluted tubule and in the MD (53). AC3−/− mice have a renal phenotype that is consistent with a dysregulation of MD function: decreased plasma renin and decreased glomerular filtration rate (GFR). Despite the decrease in plasma renin, however, blood pressure is normal in AC3−/− mice. Although a decrease in both plasma renin and GFR is consistent with an impairment in tubuloglomerular feedback (TGF), TGF (measured by stop-flow using an artificial perfusate) was normal in these animals (53). However, it is important to note that the ligand, and the OR, which mediate this signaling pathway in the MD, have not yet been identified. Therefore, it seems likely that TGF appeared normal in AC3+/+ and AC3−/− animals only because the artificial tubular fluid used for these studies was “missing” the ligand that activates this pathway (artificial tubular fluid is similar in electrolyte composition to tubular fluid but is missing other small molecule components). In the absence of the ligand, AC3+/+ and AC3−/− would indeed be expected to have similar TGF. Therefore, future studies must yet be done to understand if the GFR phenotype in AC3−/− is indeed due to altered TGF.
If AC3 does act on the TGF pathway, where might it impinge on this pathway to result in a chronic lowering of GFR? TGF is thought to be initiated by elevations in luminal NaCl, which leads to increased transport function (signaling) of the Na+-K+-2Cl− transporter (NKCC2) on the apical membrane of the MD (70). The MD isoform of NKCC2 is known to be inhibited by cAMP (35). AC3 is a potential source of this cAMP, thereby mediating a tonic “brake” on TGF by preventing NKCC2 from maximal signaling and keeping TGF in check. In an AC3−/− mouse, the brake on the pathway may be gone; if so, NKCC2 would be free to signal maximally. This would result in inappropriately high TGF, excess afferent arteriolar constriction, and therefore lowered GFR. Additionally, unrestrained NKCC2 signaling and excess TGF would decrease renin secretion, another phenotype seen in AC3−/− mice. In addition, the fact that AC3 is inhibited by increased intracellular Ca2+ concentrations (74) is consistent with reports in the literature that increased intracellular Ca2+ is required for the TGF response (55) and that the cAMP inhibition of NKCC2 is reversed by calcium ionophores (4). In this scenario, if GFR becomes elevated, then a higher flow rate near the MD will increase TGF both directly and indirectly via AC3: first, an increased GFR will result in increased NaCl delivery to the MD, which will increase NKCC2 signaling and will directly increase TGF. In addition, a high flow rate will increase intracellular Ca2+ concentration in the MD cells because 1) an increase in luminal NaCl transport would inhibit activity of the basolateral Na+/Ca2+ exchanger and therefore inhibit Ca2+ efflux, and 2) because distal segments are generally believed to respond to increased flow or shear stress with an increase in intracellular calcium (37, 66). This increase in intracellular calcium would serve to inhibit AC3 and release the brake on TGF, allowing TGF to increase and therefore for GFR to decrease (an appropriate response to the initially high GFR). Therefore, although much work remains to be done, it appears that previously published studies of TGF regulation fit well with a model in which AC3 acts as a modulator of TGF.
Short-Chain Fatty Acids
The aforementioned study examining the role of AC3 in the kidney also identified several ORs that are expressed in murine kidney, including olfactory receptor 78 (Olfr78; Ref. 53). More recently, the localization, ligand, and subsequently the physiological role of this OR have been reported (52). Olfr78 localizes specifically to cell types known to play important roles in autoregulation of tissue blood flow and in blood pressure control, including vascular resistance beds in a variety of tissues (including skin, diaphragm, and skeletal muscle), as well as in the renal afferent (but not efferent) arteriole. The afferent arteriole is the site where renin (the initial, rate-limiting step in the renin-angiotensin-aldosterone pathway) is stored for eventual release into the bloodstream. Consistent with the reported localization of Olfr78, a recent study (16) reported that the human orthologue of Olfr78 (hOR51E2) is expressed in human kidney, as well as in a variety of other tissues (heart, skeletal muscle, etc.). The localization of Olfr78 to both vascular resistance beds and to the afferent arteriole seemed to suggest a role in the regulation of extracellular fluid volume and/or blood pressure.
The ligand for both murine Olfr78 and hOR51E2 has been shown to be short-chain fatty acids (SCFAs), specifically, acetate and propionate (52, 58). Intriguingly, the primary source of SCFAs in the bloodstream is metabolic production by the gut microbiota (9). Bacteria residing in the human gut are an important component of human physiology: the total wet weight of gut microbes in the human has been estimated to be between 175 g and 1.5 kg (9, 26), and these bacteria produce SCFAs (primarily acetate, propionate, and butyrate) such that the concentration in the colon itself is ∼100 mM (9). In the plasma, SCFAs have been reported to be in the 0.1- to 10-mM range (36, 39, 59). SCFAs produced via the gut microbiota have emerged as signaling molecules in the physiology of the host, where they play roles in physiological processes such as metabolism, immune responses, and susceptibility to HIV infection (26, 29, 36, 39, 60, 69).
The identification of Olfr78 as novel SCFA receptor (52), and the localization of Olfr78 to both the afferent arteriole and to vascular resistance beds (52), led to the novel hypothesis that Olfr78 may be playing a role in blood pressure regulation in response to SCFAs. Given the localization of Olfr78 to the afferent arteriole, it was hypothesized that SCFAs play a role in blood pressure regulation via Olfr78-mediated renin secretion. Consistent with this, propionate stimulated exocytosis from renin-containing juxtaglomerular cells in an Olfr78-dependent manner (52), and Olfr78−/− mice were found to have significantly lower plasma renin levels and baseline blood pressure compared with wild-type littermates (52).
Second, given the localization of Olfr78 to vascular resistance beds, it was hypothesized that SCFAs may also play a role in resistance vessels to acutely regulate blood pressure. Previous studies have shown that SCFAs, including propionate, cause vasodilatation of ex vivo vascular ring preparations from both rodents and humans (41–43) and that the inclusion of acetate in hemodialysis buffers causes hypotension in patients (31, 47). To test whether there is a systemic consequence of the reported ex vivo vasodilatory effect (41–43), blood pressure was assayed in mice during delivery of an intravenous dose of propionate. Upon propionate delivery, there was a rapid and dose-dependent drop in blood pressure (52) over a ∼1- to 2-min period, which recovered over ∼5 min (for a calculated plasma dose of 10 mM, the hypotensive response was ∼14 mmHg). This effect is consistent with the previously reported acute vasodilation of vascular ring preparations (41–43), and we therefore hypothesize that acute vasodilation of resistance vessels underlies the acute drop in blood pressure upon propionate administration. SCFAs have been reported to be in the 0.1- to 10-mM range in plasma (36, 39), a range that matches the hypotensive dose response seen in vivo, as well as the in vitro dose response of Olfr78 and hOR51E2 to propionate.
As noted above, the effect of propionate on renin release is absent in Olfr78−/− mice. In contrast to this, however, the acute hypotensive effect of propionate (presumably vasodilation) is accentuated in Olfr78−/− mice, indicating that Olfr78 activation antagonizes (rather than mediates) the acute hypotensive effects of propionate. In both the case of renin release and that of the smooth muscle cell responses, Olfr78 appears to be acting to support blood pressure in response to propionate. Therefore, Olfr78 acts to oppose the powerful hypotensive effects of propionate mediated by other receptors or pathways (the bulk of the acute hypotensive response must be mediated by a receptor other than Olfr78, as the hypotensive response is still present in Olfr78−/−). A likely candidate for these other receptors is Gpr41 and/or Gpr43, previously characterized SCFA receptors that also respond to gut flora-derived propionate to mediate physiological responses, such as adiposity (Gpr41; Ref. 60) and inflammatory responses (Gpr43; Refs. 36, 39).
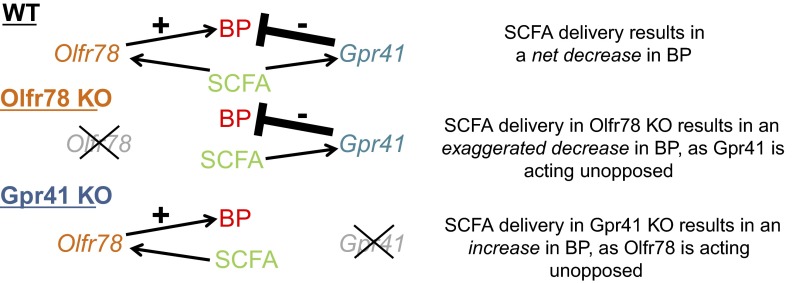
RT-PCR experiments demonstrated that both Gpr41 and Gpr43 colocalize to blood vessels, along with Olfr78 (52). It was therefore hypothesized that Gpr41 and/or Gpr43 act to mediate the bulk of the hypotensive response, while Olfr78 opposes this response. In this case, the contrasting responses of Olfr78 and Gpr41/43 would produce a “buffering” effect to guard against wide swings in blood pressure (Fig. 1). In support of this hypothesis, it was found that although 10 mM propionate (a concentration at the high end of the physiologically relevant range; Ref. 39) produce a decrease in blood pressure of 13.9 mmHg in wild-type mice, this effect is attenuated in Gpr41+/− animals and is absent in Gpr41−/− (in fact, Gpr41−/− animals have a modest hypertensive response; Ref. 52). These data indicate that Gpr41 likely mediates the hypotensive effects of propionate, whereas Olfr78 functions to raise blood pressure and to antagonize the hypotensive effects of propionate.
Fig. 1.
Working model for the acute hypotensive effect of short-chain fatty acid (SCFA) delivery on blood pressure (BP, blood pressure; WT, wild type; KO, knockout). Gpr41 acts to decrease blood pressure in response to SCFA, whereas Olfr78 acts in opposition to increase blood pressure. In WT animals, the hypotensive response of Gpr41 prevails. When Olfr78 is genetically deleted, the hypotensive response is further accentuated as the “brake” on the pathway has been removed. However, when Gpr41 is genetically deleted, the hypotensive response is absent, and instead a slight hypertensive response (presumably mediated by Olfr78) remains.
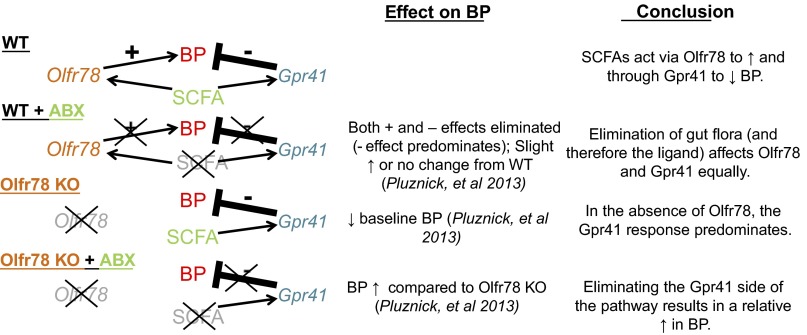
To implicate gut microbiota more directly in these processes, antibiotic treatment was used to dramatically reduce gut microbiota. This antibiotic treatment resulted in an increase in the systolic, diastolic, and mean blood pressure in Olfr78−/− mice but did not significantly affect blood pressure in wild-type littermates (52). This is consistent with the idea (Fig. 1) that Olfr78 and Gpr41 respond to SCFAs to effect opposing changes in blood pressure. This model predicts that when the activation of both Olfr78 and Gpr41 are decreased simultaneously (via antibiotic treatment suppression of SCFA production), there is little effect in a wild-type animal (see Fig. 2). However, in an Olfr78−/− animal, propionate is acting solely on Gpr41 to lower blood pressure; therefore, removing the source of this ligand would allow for a relative rise in blood pressure. Although future studies must be done to better understand these pathways, it is clear that metabolites of gut microbiota can play a role in blood pressure regulation and that these effects are mediated in a complex manner via at least two SCFA receptors.
Fig. 2.
When WT mice are treated with antibiotics (ABX) to reduce gut flora and therefore the circulating SCFA, the Olfr78 and Gpr41 sides of the pathway are eliminated simultaneously, and therefore there is little change in blood pressure. However, in mice null for Olfr78, SCFA are signaling solely through the hypotensive side of the pathway. Therefore, if the ligand is reduced by antibiotic treatment in these mice, this hypotensive pathway is disrupted and there is a relative increase in blood pressure.
It is intriguing to consider “why” the metabolites of gut flora would affect vascular resistance. One possibility is that this facilitates nutrient absorption after a meal. The majority of absorption occurs in the small intestine; however, a significant quantity of nutrients (including starches, lactose, and lactulose) is absorbed from the large intestine in animals (2, 9, 11, 12, 27, 32, 44–46, 68) and in humans (1, 6, 15, 40, 48, 54, 61, 62, 75). After a meal, SCFA concentrations in the vessels serving the large intestine would peak, and the resulting vasodilation would facilitate efficient absorption from the gut into the circulation, ensuring that nutrients are not lost in the stool. Although it unclear whether there is a beneficial effect of having a systemic change in BP after a meal, it is interesting to note that there is evidence for this drop in BP in humans: postprandial hypotension is common among the elderly, even among healthy elderly populations (71).
As alluded to above, the opposing actions of Gpr41 and Olfr78 in response to propionate may act to buffer blood pressure changes. Specifically, Gpr41 (EC50 11 μM for propionate; Refs. 8, 36) would be expected to be tonically active under basal conditions (plasma SCFAs are 0.1–10 mM; Refs. 36, 39, 59). Therefore, although a transient hypotensive response is observed after the delivery of a bolus of propionate, it may in fact be that Gpr41 is exerting a tonic hypotensive influence, involved in setting blood pressure. When plasma SCFA concentrations begin to rise (i.e., after a meal), Gpr41 would further lower blood pressure; however, as plasma SCFAs elevate further, Olfr78 (EC50 0.9 mM for propionate; Ref. 52) would activate as well, acting as a homeostatic brake to prevent an inappropriate level of hypotension.
Summary
In summary, although the complexity of extracellular fluid volume and blood pressure regulation has been long appreciated (23), recent studies have revealed new roles for sensory receptors in these processes. The examples reviewed here highlight the fact that fluid, electrolyte, and blood pressure regulation is complex and that it is affected by the metabolism of not only the host (succinate) but also of the gut microbiota (SCFAs). Clearly, future studies are needed to better understand not only each individual pathway, but how these pathways, and others, interact in the integrative control of extracellular fluid volume and blood pressure.
GRANTS
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases Grant ROO-DK-081610 (to J. L. Pluznick).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.L.P. prepared figures; J.L.P. drafted manuscript; J.L.P. edited and revised manuscript; J.L.P. approved final version of manuscript.
REFERENCES
- 1. Anderson IH, Levine AS, Levitt MD. Incomplete absorption of the carbohydrate in all-purpose wheat flour. N Engl J Med 304: 891–892, 1981 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DG, Beever DE. Post-abomasal digestion of carbohydrate in the adult ruminant. Proc Nutr Soc 28: 121–131, 1969 [DOI] [PubMed] [Google Scholar]
- 3. Balaban RS, Mandel LJ. Metabolic substrate utilization by rabbit proximal tubule. An NADH fluorescence study. Am J Physiol Renal Fluid Electrolyte Physiol 254: F407–F416, 1988 [DOI] [PubMed] [Google Scholar]
- 4. Bell PD. Cyclic AMP-calcium interaction in the transmission of tubuloglomerular feedback signals. Kidney Int 28: 728–732, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Belluscio L, Gold GH, Nemes A, Axel R. Mice deficient in G(olf) are anosmic. Neuron 20: 69–81, 1998 [DOI] [PubMed] [Google Scholar]
- 6. Bond JH, Levitt MD. Quantitative measurement of lactose absorption. Gastroenterology 70: 1058–1062, 1976 [PubMed] [Google Scholar]
- 7. Brazy PC, Mandel LJ, Gullans SR, Soltoff SP. Interactions between phosphate and oxidative metabolism in proximal renal tubules. Am J Physiol Renal Fluid Electrolyte Physiol 247: F575–F581, 1984 [DOI] [PubMed] [Google Scholar]
- 8. Brown AJ, Goldsworthy SM, Barnes AA, Eilert MM, Tcheang L, Daniels D, Muir AI, Wigglesworth MJ, Kinghorn I, Fraser NJ, Pike NB, Strum JC, Steplewski KM, Murdock PR, Holder JC, Marshall FH, Szekeres PG, Wilson S, Ignar DM, Foord SM, Wise A, Dowell SJ. The Orphan G protein-coupled receptors GPR41 and GPR43 are activated by propionate and other short chain carboxylic acids. J Biol Chem 278: 11312–11319, 2003 [DOI] [PubMed] [Google Scholar]
- 9. Bugaut M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp Biochem Physiol B 86: 439–472, 1987 [DOI] [PubMed] [Google Scholar]
- 10. Curtis JJ, Luke RG, Dustan HP, Kashgarian M, Whelchel JD, Jones P, Diethelm AG. Remission of essential hypertension after renal transplantation. N Engl J Med 309: 1009–1015, 1983 [DOI] [PubMed] [Google Scholar]
- 11. Demigne C, Remesy C. Influence of unrefined potato starch on cecal fermentations and volatile fatty acid absorption in rats. J Nutr 112: 2227–2234, 1982 [DOI] [PubMed] [Google Scholar]
- 12. Demigne C, Remesy C, Rayssiguier Y. Effect of fermentable carbohydrates on volatile fatty acids, ammonia and mineral absorption in the rat caecum. Reprod Nutr Dev 20: 1351–1359, 1980 [DOI] [PubMed] [Google Scholar]
- 13. Deshpande DA, Wang WC, McIlmoyle EL, Robinett KS, Schillinger RM, An SS, Sham JS, Liggett SB. Bitter taste receptors on airway smooth muscle bronchodilate by localized calcium signaling and reverse obstruction. Nat Med 16: 1299–1304, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Elliott RA, Kapoor S, Tincello DG. Expression and distribution of the sweet taste receptor isoforms T1R2 and T1R3 in human and rat bladders. J Urol 186: 2455–2462, 2011 [DOI] [PubMed] [Google Scholar]
- 15. Englyst HN, Cummings JH. Digestion of the polysaccharides of some cereal foods in the human small intestine. Am J Clin Nutr 42: 778–787, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Flegel C, Manteniotis S, Osthold S, Hatt H, Gisselmann G. Expression profile of ectopic olfactory receptors determined by deep sequencing. PLoS One 8: e55368, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goldberg ND, Passonneau JV, Lowry OH. Effects of changes in brain metabolism on the levels of citric acid cycle intermediates. J Biol Chem 241: 3997–4003, 1966 [PubMed] [Google Scholar]
- 18. Griffin CA, Kafadar KA, Pavlath GK. MOR23 promotes muscle regeneration and regulates cell adhesion and migration. Dev Cell 17: 649–661, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gullans SR, Brazy PC, Dennis VW, Mandel LJ. Interactions between gluconeogenesis and sodium transport in rabbit proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 246: F859–F869, 1984 [DOI] [PubMed] [Google Scholar]
- 20. Gullans SR, Brazy PC, Mandel LJ, Dennis VW. Stimulation of phosphate transport in the proximal tubule by metabolic substrates. Am J Physiol Renal Fluid Electrolyte Physiol 247: F582–F587, 1984 [DOI] [PubMed] [Google Scholar]
- 21. Gullans SR, Kone BC, Avison MJ, Giebisch G. Succinate alters respiration, membrane potential, and intracellular K+ in proximal tubule. Am J Physiol Renal Fluid Electrolyte Physiol 255: F1170–F1177, 1988 [DOI] [PubMed] [Google Scholar]
- 22. Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 23. Guyton AC, Coleman TG, Granger HJ. Circulation: overall regulation. Annu Rev Physiol 34: 13–46, 1972 [DOI] [PubMed] [Google Scholar]
- 24. Hamm LL, Hering-Smith KS. Pivotal role of the kidney in hypertension. Am J Med Sci 340: 30–32, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. He W, Miao FJ, Lin DC, Schwandner RT, Wang Z, Gao J, Chen JL, Tian H, Ling L. Citric acid cycle intermediates as ligands for orphan G-protein-coupled receptors. Nature 429: 188–193, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Hill MJ, Drasar BS. The normal colonic bacterial flora. Gut 16: 318–323, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hintz HF, Hogue DE, Walker EF, Jr, Lowe JE, Schryver HF. Apparent digestion in various segments of the digestive tract of ponies fed diets with varying roughage-grain ratios. J Anim Sci 32: 245–248, 1971 [DOI] [PubMed] [Google Scholar]
- 28. Huang AL, Chen X, Hoon MA, Chandrashekar J, Guo W, Trankner D, Ryba NJ, Zuker CS. The cells and logic for mammalian sour taste detection. Nature 442: 934–938, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94: 58–65, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kaske S, Krasteva G, Konig P, Kummer W, Hofmann T, Gudermann T, Chubanov V. TRPM5, a taste-signaling transient receptor potential ion-channel, is a ubiquitous signaling component in chemosensory cells. BMC Neurosci 8: 49, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Keshaviah PR. The role of acetate in the etiology of symptomatic hypotension. Artif Organs 6: 378–387, 1982 [DOI] [PubMed] [Google Scholar]
- 32. Keys JE, Jr, DeBarthe JV. Site and extent of carbohydrate, dry matter, energy and protein digestion and the rate of passage of grain diets in swine. J Anim Sci 39: 57–62, 1974 [DOI] [PubMed] [Google Scholar]
- 33. Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G. Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci USA 108: 8030–8035, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Krasteva G, Hartmann P, Papadakis T, Bodenbenner M, Wessels L, Weihe E, Schutz B, Langheinrich AC, Chubanov V, Gudermann T, Ibanez-Tallon I, Kummer W. Cholinergic chemosensory cells in the auditory tube. Histochem Cell Biol 137: 483–497, 2012 [DOI] [PubMed] [Google Scholar]
- 35. Laamarti MA, Bell PD, Lapointe JY. Transport and regulatory properties of the apical Na-K-2Cl cotransporter of macula densa cells. Am J Physiol Renal Physiol 275: F703–F709, 1998 [DOI] [PubMed] [Google Scholar]
- 36. Le PE, Loison C, Struyf S, Springael JY, Lannoy V, Decobecq ME, Brezillon S, Dupriez V, Vassart G, Van DJ, Parmentier M, Detheux M. Functional characterization of human receptors for short chain fatty acids and their role in polymorphonuclear cell activation. J Biol Chem 278: 25481–25489, 2003 [DOI] [PubMed] [Google Scholar]
- 37. Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM. Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285: F998–F1012, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Magistroni R, Furci L, Albertazzi A. [Autosomal dominant polycystic kidney disease: from genes to cilium]. G Ital Nefrol 25: 183–191, 2008 [PubMed] [Google Scholar]
- 39. Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, Schilter HC, Rolph MS, Mackay F, Artis D, Xavier RJ, Teixeira MM, Mackay CR. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature 461: 1282–1286, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. McNeil NI, Bingham S, Cole TJ, Grant AM, Cummings JH. Diet and health of people with an ileostomy. 2. Ileostomy function and nutritional state. Br J Nutr 47: 407–415, 1982 [DOI] [PubMed] [Google Scholar]
- 41. Mortensen FV, Nielsen H, Mulvany MJ, Hessov I. Short chain fatty acids dilate isolated human colonic resistance arteries. Gut 31: 1391–1394, 1990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nutting CW, Islam S, Daugirdas JT. Vasorelaxant effects of short chain fatty acid salts in rat caudal artery. Am J Physiol Heart Circ Physiol 261: H561–H567, 1991 [DOI] [PubMed] [Google Scholar]
- 43. Nutting CW, Islam S, Ye MH, Batlle DC, Daugirdas JT. The vasorelaxant effects of acetate: role of adenosine, glycolysis, lyotropism, and pHi and Cai2+. Kidney Int 41: 166–174, 1992 [DOI] [PubMed] [Google Scholar]
- 44. Orskov ER, Fraser C, Kay RN. Dietary factors influencing the digestion of starch in the rumen and small and large intestine of early weaned lambs. Br J Nutr 23: 217–226, 1969 [DOI] [PubMed] [Google Scholar]
- 45. Orskov ER, Fraser C, Mason VC, Mann SO. Influence of starch digestion in the large intestine of sheep on caecal fermentation, caecal microflora and faecal nitrogen excretion. Br J Nutr 24: 671–682, 1970 [DOI] [PubMed] [Google Scholar]
- 46. Orskov ER, Fraser C, McDonald I. Digestion of concentrates in sheep. 3. Effects of rumen fermentation of barley and maize diets on protein digestion. Br J Nutr 26: 477–486, 1971 [DOI] [PubMed] [Google Scholar]
- 47. Pagel MD, Ahmad S, Vizzo JE, Scribner BH. Acetate and bicarbonate fluctuations and acetate intolerance during dialysis. Kidney Int 21: 513–518, 1982 [DOI] [PubMed] [Google Scholar]
- 48. Perman JA, Modler S. Role of the intestinal microflora in disposition of nutrients in the gastrointestinal tract. J Pediatr Gastroenterol Nutr 2, Suppl 1: S193–S196, 1983 [DOI] [PubMed] [Google Scholar]
- 49. Peti-Peterdi J. High glucose and renin release: the role of succinate and GPR91. Kidney Int 78: 1214–1217, 2010 [DOI] [PubMed] [Google Scholar]
- 50. Peti-Peterdi J, Gevorgyan H, Lam L, Riquier-Brison A. Metabolic control of renin secretion. Pflügers Arch 465: 53–58, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Peti-Peterdi J, Kang JJ, Toma I. Activation of the renal renin-angiotensin system in diabetes–new concepts. Nephrol Dial Transplant 23: 3047–3049, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Pluznick JL, Protzko RJ, Gevorgyan H, Peterlin Z, Sipos A, Han J, Brunet I, Wan LX, Rey F, Wang T, Firestein SJ, Yanagisawa M, Gordon JI, Eichmann A, Peti-Peterdi J, Caplan MJ. Olfactory receptor responding to gut microbiota-derived signals plays a role in renin secretion and blood pressure regulation. Proc Natl Acad Sci USA 110: 4410–4415, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Pluznick JL, Zou DJ, Zhang X, Yan Q, Rodriguez-Gil DJ, Eisner C, Wells E, Greer CA, Wang T, Firestein S, Schnermann J, Caplan MJ. Functional expression of the olfactory signaling system in the kidney. Proc Natl Acad Sci USA 106: 2059–2064, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Pomare EW, Branch WJ, Cummings JH. Carbohydrate fermentation in the human colon and its relation to acetate concentrations in venous blood. J Clin Invest 75: 1448–1454, 1985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Ren Y, Liu R, Carretero OA, Garvin JL. Increased intracellular Ca2+ in the macula densa regulates tubuloglomerular feedback. Kidney Int 64: 1348–1355, 2003 [DOI] [PubMed] [Google Scholar]
- 56. Robben JH, Fenton RA, Vargas SL, Schweer H, Peti-Peterdi J, Deen PM, Milligan G. Localization of the succinate receptor in the distal nephron and its signaling in polarized MDCK cells. Kidney Int 76: 1258–1267, 2009 [DOI] [PubMed] [Google Scholar]
- 57. Sadagopan N, Li W, Roberds SL, Major T, Preston GM, Yu Y, Tones MA. Circulating succinate is elevated in rodent models of hypertension and metabolic disease. Am J Hypertens 20: 1209–1215, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Saito H, Chi Q, Zhuang H, Matsunami H, Mainland JD. Odor coding by a Mammalian receptor repertoire. Sci Signal 2: ra9, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Samuel BS, Gordon JI. A humanized gnotobiotic mouse model of host-archaeal-bacterial mutualism. Proc Natl Acad Sci USA 103: 10011–10016, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Samuel BS, Shaito A, Motoike T, Rey FE, Backhed F, Manchester JK, Hammer RE, Williams SC, Crowley J, Yanagisawa M, Gordon JI. Effects of the gut microbiota on host adiposity are modulated by the short-chain fatty-acid binding G protein-coupled receptor, Gpr41. Proc Natl Acad Sci USA 105: 16767–16772, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Sandberg AS, Andersson H, Kivisto B, Sandstrom B. Extrusion cooking of a high-fibre cereal product. 1. Effects on digestibility and absorption of protein, fat, starch, dietary fibre and phytate in the small intestine. Br J Nutr 55: 245–254, 1986 [DOI] [PubMed] [Google Scholar]
- 62. Saunders DR, Wiggins HS. Conservation of mannitol, lactulose, and raffinose by the human colon. Am J Physiol Gastrointest Liver Physiol 241: G397–G402, 1981 [DOI] [PubMed] [Google Scholar]
- 63. Schweda F, Klar J, Narumiya S, Nusing RM, Kurtz A. Stimulation of renin release by prostaglandin E2 is mediated by EP2 and EP4 receptors in mouse kidneys. Am J Physiol Renal Physiol 287: F427–F433, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Shah AS, Ben-Shahar Y, Moninger TO, Kline JN, Welsh MJ. Motile cilia of human airway epithelia are chemosensory. Science 325: 1131–1134, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Spehr M, Gisselmann G, Poplawski A, Riffell JA, Wetzel CH, Zimmer RK, Hatt H. Identification of a testicular odorant receptor mediating human sperm chemotaxis. Science 299: 2054–2058, 2003 [DOI] [PubMed] [Google Scholar]
- 66. Taniguchi J, Guggino WB. Membrane stretch: a physiological stimulator of Ca2+-activated K+ channels in thick ascending limb. Am J Physiol Renal Fluid Electrolyte Physiol 257: F347–F352, 1989 [DOI] [PubMed] [Google Scholar]
- 67. Toma I, Kang JJ, Sipos A, Vargas S, Bansal E, Hanner F, Meer E, Peti-Peterdi J. Succinate receptor GPR91 provides a direct link between high glucose levels and renin release in murine and rabbit kidney. J Clin Invest 118: 2526–2534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Topping DL, Illman RJ, Taylor MN, McIntosh GH. Effects of wheat bran and porridge oats on hepatic portal venous volatile fatty acids in the pig. Ann Nutr Metab 29: 325–331, 1985 [DOI] [PubMed] [Google Scholar]
- 69. Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, Sogin ML, Jones WJ, Roe BA, Affourtit JP, Egholm M, Henrissat B, Heath AC, Knight R, Gordon JI. A core gut microbiome in obese and lean twins. Nature 457: 480–484, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Vallon V, Muhlbauer B, Osswald H. Adenosine and kidney function. Physiol Rev 86: 901–940, 2006 [DOI] [PubMed] [Google Scholar]
- 71. Van Orshoven NP, Jansen PA, Oudejans I, Schoon Y, Oey PL. Postprandial hypotension in clinical geriatric patients and healthy elderly: prevalence related to patient selection and diagnostic criteria. J Aging Res 2010: 243752, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Vargas SL, Toma I, Kang JJ, Meer EJ, Peti-Peterdi J. Activation of the succinate receptor GPR91 in macula densa cells causes renin release. J Am Soc Nephrol 20: 1002–1011, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wang J, Li X, Ke Y, Lu Y, Wang F, Fan N, Sun H, Zhang H, Liu R, Yang J, Ye L, Liu M, Ning G. GPR48 increases mineralocorticoid receptor gene expression. J Am Soc Nephrol 23: 281–293, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Wei J, Zhao AZ, Chan GC, Baker LP, Impey S, Beavo JA, Storm DR. Phosphorylation and inhibition of olfactory adenylyl cyclase by CaM kinase II in Neurons: a mechanism for attenuation of olfactory signals. Neuron 21: 495–504, 1998 [DOI] [PubMed] [Google Scholar]
- 75. Wiggins HS. Nutritional value of sugars and related compounds undigested in the small gut. Proc Nutr Soc 43: 69–75, 1984 [DOI] [PubMed] [Google Scholar]
- 76. Wilson FH, Disse-Nicodeme S, Choate KA, Ishikawa K, Nelson-Williams C, Desitter I, Gunel M, Milford DV, Lipkin GW, Achard JM, Feely MP, Dussol B, Berland Y, Unwin RJ, Mayan H, Simon DB, Farfel Z, Jeunemaitre X, Lifton RP. Human hypertension caused by mutations in WNK kinases. Science 293: 1107–1112, 2001 [DOI] [PubMed] [Google Scholar]
- 77. Wong ST, Trinh K, Hacker B, Chan GC, Lowe G, Gaggar A, Xia Z, Gold GH, Storm DR. Disruption of the type III adenylyl cyclase gene leads to peripheral and behavioral anosmia in transgenic mice. Neuron 27: 487–497, 2000 [DOI] [PubMed] [Google Scholar]