Abstract
Hypochlorous acid (HOCl), the active ingredient of household bleach, is the most common disinfectant in medical, industrial, and domestic use and plays an important role in microbial killing in the innate immune system. Given the critical importance of the antimicrobial properties of chlorine to public health, it is surprising how little is known about the ways in which bacteria sense and respond to reactive chlorine species (RCS). Although the literature on bacterial responses to reactive oxygen species (ROS) is enormous, work addressing bacterial responses to RCS has begun only recently. Transcriptomic and proteomic studies now provide new insights into how bacteria mount defenses against this important class of antimicrobial compounds. In this review, we summarize the current knowledge, emphasizing the overlaps between RCS stress responses and other more well-characterized bacterial defense systems, and identify outstanding questions that represent productive avenues for future research.
Keywords: hypochlorous acid, chloramines, oxidation, protein folding, redox regulation
INTRODUCTION
Reactions of Reactive Chlorine Species with Biomolecules
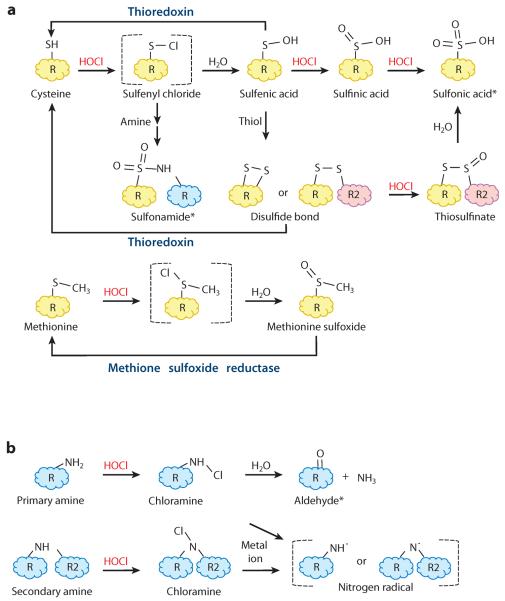
Hypochlorous acid (HOCl) is a powerful oxidant that interacts with most cellular macromolecules. Because the chemistry of reactive chlorine species (RCS) reactions, including detailed rate constants, has been summarized in several excellent reviews (30, 82, 107), we provide only a short overview here. Sulfur-containing compounds such as cysteine, methionine, or glutathione (γ-l-glutamyl-l-cysteinyl-glycine, GSH) react 100-fold more rapidly with HOCl than do any other cellular component. HOCl-mediated oxidation of cysteine thiols gives rise to unstable sulfenyl chloride (R-SCl) intermediates, which react with water to form oxidized cysteine sulfenic acids (R-SOH) (Figure 1a). These highly reactive intermediates can either be reduced by members of the thioredoxin family or be further oxidized to sulfinic (R-SO2H) and sulfonic (R-SO3H) acids, irreversible thiol modifications that typically lead to protein degradation. Cysteine sulfenic acids also readily react with other cysteine thiols (R-SH) in close proximity to form reversible disulfide bonds (R-S-S-R′). Although considered fairly stable, disulfide bonds are vulnerable to further HOCl-mediated oxidation, yielding thiosulfinates [R-S(O)-S-R′] or thiosulfonates [R-S(O2)-S-R′]. In addition, sulfenyl chlorides can react with primary or secondary amines to form irreversible sulfonamide linkages [R-S(O2)-NH-R′]. Reaction of HOCl with methionine is just as fast and primarily leads to methionine sulfoxide or dehydromethionine formation (Figure 1a). Whereas methionine sulfoxide formation can be repaired in vivo, dehydromethionine formation is considered to be irreversible (84).
Figure 1.
Reactions of hypochlorous acid (HOCl) with biomolecules. Reaction of HOCl with (a) sulfur-containing compounds or (b) amines. Brackets indicate unstable reactive intermediates. Enzymes known to repair oxidized cysteine or methionine residues in bacteria are indicated. Asterisks indicate irreversibly oxidized dead-end products.
The second most reactive targets of HOCl in proteins are primary and secondary amines (Figure 1b). Upon reaction with HOCl, amines are chlorinated to the respective chloramines (—NHCl). Chloramines, many of which have potent antimicrobial activities, are also considered RCS because they are capable of chlorinating and oxidizing other molecules. However, chloramines are four to five orders of magnitude less reactive than HOCl and appear to be more specific for cysteine and methionine oxidation (30, 82, 107). Whereas some chloramines are quite stable [notably N-chlorotaurine, an abundant chloramine associated with the innate immune system (79)], most are not. They instead rapidly decompose to their respective aldehydes. Chloramines can also react with iron or copper ions to generate nitrogen radicals, which are extremely reactive in their own right (45). A few amino acid modifications, including the formation of 2-oxo-histidine and 3-chlorotyrosine, have been characterized as specific biomarkers for HOCl stress in cells (82, 106). These reactions, however, are quite slow compared with other HOCl-mediated oxidations. Both tyrosine chlorination and aldehyde formation (i.e., carbonylation) are often associated with protein unfolding and irreversible aggregation (20).
HOCl and chloramines also react with nucleotides and lipids, albeit three to seven orders of magnitude more slowly than with amino acid side chains (30, 80, 85, 97). Primary and secondary amines of nucleotide bases appear to be the major targets in DNA and RNA, with the formation of chloramines preceding the formation of nitrogen radicals and stable chlorinated nucleotides. High doses of HOCl can eventually lead to DNA strand breakage. Chlorination of lipids can lead to the formation of several products. Double bonds in unsaturated fatty acids, for instance, can be chlorinated to form chlorohydrins, which are thought to contribute to HOCl-mediated cellular damage in eukaryotic cells (18). Amines in the head group of lipids, such as those in phosphatidylethanolamine, can form chloramines, nitrogen radicals, and aldehydes. Radicals derived from HOCl reactions with amines or peroxides can lead to lipid peroxidation, a complex oxidative breakdown pathway that degrades polyunsaturated fatty acids to aldehydes via organic hydroperoxide intermediates (80).
Reactive Chlorine Species in Microbial Environments
Bacteria encounter RCS not only in the form of household bleach (36, 94), but also in many natural environments. Cells of the animal innate immune system produce high quantities of oxidants, including HOCl, to eliminate invading pathogens (extensively reviewed in 50, 58, 107). The production of oxidants at toxic levels is initiated by the pathogen-mediated activation of NADPH oxidases located at the phagosome membrane. These oxidases reduce molecular oxygen to superoxide (O2˙−) at the expense of intracellular NADPH. Superoxide dismutases (SODs), in turn, dismutate O2˙− into hydrogen peroxide (H2O2). High concentrations of the heme-containing myeloperoxidase (MPO) are then released into the phagosome, where it generates HOCl by catalyzing the reaction of H2O2 with chloride (58). Neutrophils are the main producers of HOCl in the innate immune system, and MPO makes up 5% of their total protein content (50, 58, 107). Other oxidants, such as O2˙− and H2O2, appear to play only minor roles in bacterial killing by phagocytes, consistent with the observation that these reactive oxygen species (ROS) require much higher concentrations and prolonged incubation times in order to have lethal effects.
MPO is a haloperoxidase, oxidizing halides to the respective hypohalous acids (i.e., Cl− to HOCl, Br− to HOBr) (reviewed in 50, 58, 107). MPO also catalyzes the formation of a number of other reactive species in vivo, including nitrogen dioxide radicals and tyrosine hydroperoxide. Because Cl− is normally present at much higher concentrations than other halides under in vivo physiological conditions, HOCl is most likely the specific product of MPO. The toxicity of HOCl, which effectively eliminates invading pathogens, can also cause tremendous damage to human tissues. Incorrect cellular trafficking and processing of MPO lead to the release of HOCl into extracellular compartments, where it is involved in the progress of various human diseases, including atherosclerosis, chronic inflammation, and certain cancers (58).
RCS-generating haloperoxidases are also emerging as important modulators of both pathogenic and symbiotic bacteria-host interactions at mucosal surfaces. With Drosophila melanogaster as a model system, the dual oxidase (DUOX) was shown to play a central role in intestinal host defense. Fruit flies depleted of DUOX show increased levels of bacterial colonization in the intestine compared with wild-type D. melanogaster (6). Using HOCl-specific fluorescent probes, Chen et al. (21) showed that DUOX-mediated bacterial elimination is dependent on HOCl production. HOCl generated by haloperoxidase is also important for controlling symbiotic interactions between bioluminescent bacteria and the light organs of certain squid (92), indicating that HOCl production may be a general mechanism for controlling bacterial populations at epithelia in animals.
Haloperoxidases capable of generating HOCl as well as reactive bromine and iodine species are broadly distributed among fungi, plants, animals, and bacteria in both terrestrial and marine habitats, in which they are associated with the formation of a tremendous variety of halogenated metabolites, many of which have potent antimicrobial activities (reviewed in 11, 15, 41). It is likely, therefore, that nearly all bacteria may encounter RCS in their environments and that microbial communities of all types are shaped by the presence of RCS.
Antimicrobial Effects of Reactive Chlorine Species
Like other disinfectants, RCS kill microbes most likely by damaging multiple cellular components simultaneously. The identity of the targets directly responsible for cell death upon RCS exposure has been an active area of research for many years (reviewed in 50, 74, 107). However, how RCS kill bacteria remains incompletely defined and probably varies depending on bacterial species, RCS type, and exposure conditions. Early studies noted that HOCl treatment causes a rapid loss of glucose respiration and converts most of the cellular ATP pool to AMP (7, 43). Most evidence now points toward the inner membrane as the site of lethal damage to vegetative cells. Inactivation of RCS-sensitive cytoplasmic enzymes requires doses far in excess of those required for killing (1, 16), and oxidation of outer membrane and periplasmic proteins correlates only poorly with killing (90). However, the doses of HOCl necessary for loss of ATP, inhibition of F1 ATPase, loss of DNA replication (via loss of association of the origin of replication with the inner membrane), and failure of metabolite and protein transport across the inner membrane closely parallel the doses necessary for killing (1, 7, 8, 75, 90, 91). Killing by HOCl is remarkably fast, with lethal damage occurring within 100 ms of exposure (2). An alternative mechanism of cell killing by RCS might be through the oxidative unfolding and aggregation of essential bacterial proteins (104). This conclusion is based on the observation that bacteria depleted of the HOCl-activated molecular chaperone Hsp33 (see below) accumulate high amounts of aggregated proteins and are significantly more sensitive to bleach treatment than wild-type bacteria are.
BACTERIAL RESPONSES TO REACTIVE CHLORINE SPECIES
By definition, bacteria fail to mount successful defenses against lethal doses of RCS. The response to sublethal doses, however, defines the types of damage that cells are capable of repairing and recovering from. This is of interest not only for understanding interactions between bacteria and various RCS-generating eukaryotes, but also potentially for developing strategies to protect human cells from the MPO-generated RCS damage that contributes to inflammatory disease. The study of the molecular mechanisms that bacteria use to sense and respond to RCS stress is still in its infancy, especially when compared with the well-characterized responses to ROS, such as H2O2 and O2˙− (reviewed extensively in 28, 53, 96, 100), but strong common themes have emerged (Figure 2). We first discuss the best-characterized RCS responses and then present what is known about other, less well-understood cellular processes that seem to be involved in RCS resistance. Note that the types of damage repaired during sublethal stresses may not necessarily be the same as those responsible for killing by lethal doses of RCS (see above), although this is a common assumption.
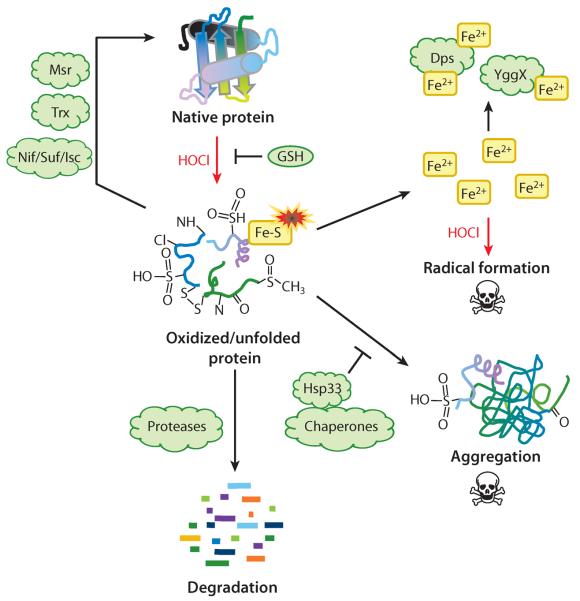
Figure 2.
Bacterial responses to protein damage by reactive chlorine species (RCS). Hypochlorous acid (HOCl) reacts rapidly with proteins, leading to side chain oxidation and unfolding. Low-molecular-weight thiols (e.g., GSH) react with RCS and reduce their effective concentration. Repair enzymes, including methionine sulfoxide reductase (Msr), thioredoxin (Trx), and Fe-S cluster repair systems (Nif/Suf/Isc), can reverse oxidative protein damage. Metal ions released from damaged proteins promote radical formation. Dps and YggX sequester metals and minimize metal-catalyzed oxidative damage. Chaperones, particularly Hsp33, prevent aggregation of unfolded proteins, and proteases degrade irreversibly damaged proteins.
Summary of Experimental Conditions
Most of the literature on the molecular responses of bacteria to RCS focuses on laboratory strains of Escherichia coli, but more recent work has now also extended to other strains and species. The most notable differences are in the experimental setups that researchers use to treat bacteria with RCS; whereas in some studies actively growing cells are treated with RCS in rich media, other studies use rinsed cells resuspended in nonreactive buffers, such as phosphate. As described above, RCS (especially HOCl) react rapidly with the amino acids and nucleotides in rich media, giving rise to secondary reactive species, which might elicit responses very different from those triggered by the initially added RCS. We therefore note the experimental conditions where appropriate.
Defenses Against Reactive Oxygen Species
As outlined above, RCS are powerful oxidants. Many of the well-characterized bacterial responses to ROS-derived oxidative stress (reviewed in 53) appear to also be involved in resisting RCS stress.
Catalases and peroxidases
Catalases and peroxidases are H2O2-detoxifying enzymes which also play roles in resisting RCS. Catalase-deficient mutants of E. coli, Helicobacter pylori, and Staphylococcus aureus, for example, are more sensitive to HOCl than the respective wild-type strains are (34, 69, 71). These results agree with gene expression studies in E. coli, Mycobacterium bovis, Bacillus subtilis, Pseudomonas aeruginosa, and Bacillus cereus that showed RCS-induced upregulation of catalases (19, 23, 56, 95, 101). Whereas catalases are the primary H2O2-scavenging enzymes at high H2O2 concentrations, peroxidases, the second major class of H2O2-scavenging enzymes, work best at low H2O2 concentrations. Like catalases, peroxidases also are upregulated by RCS in many species (13, 19, 23, 35, 56, 81, 83, 95, 101, 102), but few experiments have directly tested whether peroxidases protect bacteria against RCS stress. A mutant of Burkholderia pseudomallei lacking the bifunctional catalase-peroxidase fusion protein KatG is quite sensitive to HOCl (68). What remains unclear at this point is precisely how H2O2-detoxifying enzymes protect bacteria against RCS; does RCS stress lead to H2O2 accumulation in vivo? If so, by what mechanism? Both peroxidases and organic hydroperoxide reductases (e.g., OhrA, OsmC) detoxify organic peroxide compounds, which can potentially be formed by RCS or MPO-stimulated lipid or amino acid peroxidation (see above) (29, 107). Mutants of B. subtilis that lack OhrA are substantially more sensitive to HOCl (23), and organic hydroperoxide reductases are upregulated by RCS in many bacteria, including B. subtilis, E. coli, P. aeruginosa, B. cereus, and Burkholderia cenocepacia (19, 23, 83, 95, 101).
Methionine sulfoxide reductase
Methionine sulfoxide reductase (Msr) is an enzyme conserved in all three domains of life (14). It too is upregulated by RCS stress in B. subtilis, B. cereus, E. coli, and P. aeruginosa (19, 23, 95, 101). E. coli mutants lacking Msr are more sensitive to HOCl stress, whereas overexpression of Msr increases HOCl resistance above wild-type levels (90). H. pylori mutants lacking Msr are more sensitive to killing by neutrophils, HOCl, and chloramine-T (71). This resistance appears to be due to the ability of Msr to repair oxidized methionines in catalase and is dependent on the presence of the molecular chaperone GroEL (see below) (71), which is sensitive to HOCl inactivation by methionine oxidation in E. coli (57).
Repair of iron-sulfur clusters
Another major class of upregulated genes in RCS-stressed cells are those involved in the repair and biosynthesis of iron-sulfur (Fe-S) clusters, including the Nif, Isc, and Suf machinery (reviewed in 108) (Figure 2). Fe-S clusters are ubiquitous redox cofactors, consisting of iron atoms liganded by amino acids in a variety of different configurations. Because cysteine is the most common amino acid involved in the formation of Fe-S clusters, it is not surprising that RCS treatment of bacteria can lead to their oxidative destruction (51, 88). Curiously, RCS-mediated induction of genes involved in repairing damaged Fe-S clusters has been observed in the gram-negative species E. coli, P. aeruginosa, Salmonella enterica, B. cenocepacia, and Legionella pneumophila (12, 13, 38, 83, 95, 101, 102), but not in the gram-positive species S. aureus, B. subtilis, and B. cereus (19, 23, 81).
Glutathione and other low-molecular-weight thiols
Low-molecular-weight thiols play an important role in maintaining the reducing potential of the cytoplasm and in defending cells against a variety of different oxidants (reviewed in 47, 73). The major low-molecular-weight thiol in eukaryotes and proteobacteria such as E. coli is glutathione (GSH). Other bacteria employ different molecules for the same purpose, for example, bacillithiol (BSH) in Bacillus spp., mycothiol in Mycobacterium spp., and coenzyme A in S. aureus. Genes related to GSH metabolism are upregulated by RCS in E. coli, P. aeruginosa, and L. pneumophila (13, 95, 101). Both GSH and BSH play roles in the RCS stress response. E. coli mutants lacking GSH are much more sensitive to HOCl, N-chlorotaurine, and monochloramine, and to neutrophil-mediated killing (22). Protection of E. coli by GSH seems to depend on its very high reactivity with RCS (30, 44). As many as four molecules of RCS react with one molecule of GSH in vivo, effectively reducing the amount of RCS that can interact with other essential cellular components (22, 32).
In Bacillus spp., BSH is also strongly protective against RCS stress (23), but the mechanism of protection seems to be different. A critical cysteine residue of methionine synthase (MetE), which catalyzes the final step in methionine synthesis, appears to be specifically modified by bacillithiolation in HOCl-treated B. subtilis, B. megaterium, B. pumilus, and B. amyloliquefaciens (23, 24). Bacillithiolation of MetE is thought to protect the enzyme from irreversible overoxidation while halting translation and preventing protein synthesis under conditions that inevitably lead to damaging protein modifications. The resulting growth arrest may be important to allow cells to recover effectively from cellular damage. A similar protective role has been proposed for glutathionylation of MetE in oxidatively stressed E. coli cells (73), although this has not been tested under RCS stress conditions. Both bacillithiolation and glutathionylation of MetE lead to methionine auxotrophy (23, 49), and HOCl-induced growth arrest of B. subtilis can be rescued by the addition of methionine (23). Two caveats to this result are that methionine is an efficient scavenger of both HOCl and chloramines (30), and that redox proteomic studies of HOCl-treated E. coli and Staphylococcus carnosus did not show significant modification of MetE, suggesting that this model may not be universally applicable (24, 64). Low-molecular-weight thiols are probably generally protective both as nonspecific sacrificial targets and as specific modifiers of oxidation sensitive proteins.
Other cellular antioxidants
In S. aureus, the carotenoid staphyloxanthin is protective against a variety of oxidative stress conditions, including HOCl and neutrophil-mediated killing (25). Thioredoxin and thioredoxin reductase, responsible for maintaining the reduced state of cytoplasmic cysteines (4), are upregulated by RCS treatment in a number of organisms (13, 19, 23, 38, 56, 83, 95), but to our knowledge no one has tested whether thioredoxin plays a protective role in RCS-stressed bacteria. Another major antioxidant enzyme, SOD (53), which is upregulated by RCS in many bacteria (see below) (19, 23, 33, 35, 95, 101), does not appear to be protective (32, 69). Indeed, SOD seems to be sensitive to inactivation by RCS (69, 70).
Defenses Against Protein Unfolding
In contrast to stress conditions induced by ROS or RNS (reactive nitrogen species), which generally have little global effect on cellular protein folding (53), RCS induce significant protein unfolding and aggregation (see above). It is therefore not surprising that the second major stress response pathway induced by RCS treatment in bacteria involves upregulation of molecular chaperones and proteases devoted to preventing the formation of cytotoxic protein aggregates and degrading irreversibly damaged or unfolded proteins (reviewed in 42, 77) (Figure 2).
Although there have been few direct measurements of protein aggregation in RCS-stressed bacteria in vivo (104), on the basis of transcriptional and proteomic data it is clear that the unfolded protein response is strongly activated by RCS in E. coli, B. cereus, B. cenocepacia, P. aeruginosa, L. pneumophila, B. subtilis, and M. bovis (13, 19, 23, 33, 35, 38, 56, 83, 95, 101). There is limited information available about whether this is also true for other RCS. N-chlorotaurine treatment increases GroEL levels in E. coli (5), although monochloramine treatment did not (12).
One bacterial chaperone that seems particularly well adapted to combating the unfolding effects of HOCl stress is the redox-regulated protein Hsp33 (55). Inactive under nonstress conditions, Hsp33 becomes readily activated under oxidative stress conditions that lead to protein unfolding. HOCl sensing in Hsp33 is mediated by four cysteines, which coordinate zinc under nonstress conditions (54). Upon exposure to HOCl, the cysteines form two intramolecular disulfide bonds, causing the release of zinc and the unfolding of the C terminus of Hsp33 (27, 52, 104). The fact that Hsp33 gains function upon unfolding makes Hsp33 a member of a new class of chaperones that are active when partially unfolded (86). This very rapid, stress-specific activation allows bacteria to defend themselves instantaneously against severe protein unfolding stress conditions without the need for time-consuming gene expression. Because the chaperone function of Hsp33 does not rely on nucleotide binding or hydrolysis, Hsp33 is ideally suited to function under HOCl-induced stress conditions, which cause severe ATP depletion (see above) and transiently inactivate common ATP-dependent chaperone systems, such as the DnaK system (105).
One central HOCl target protein appears to be the translation elongation factor Tu (EF-Tu), one of the most abundant proteins in the cell (59). Its sensitivity to in vivo oxidation by HOCl treatment has been shown in both gram-positive and gram-negative bacteria (24, 64). EF-Tu-deficient E. coli cells are significantly more sensitive to HOCl (64). Vibrio cholerae EF-Tu is particularly sensitive to oxidation and appears to be the critical substrate of Hsp33 in that organism (103). Bacillithiolation of conserved EF-Tu cysteines was observed after a variety of gram-positive species were treated with HOCl (24), potentially protecting EF-Tu against irreversible overoxidation. Taken together, these data suggest that EF-Tu is an important in vivo target of sublethal doses of RCS and that bacteria employ a number of mechanisms to protect their protein synthesis machinery against irreversible damage by RCS.
Changes in Cysteine and Methionine Metabolism
Given the high reactivity of RCS with sulfur-containing cellular components, it is not surprising that bacterial pathways responsible for resisting sulfur starvation and replenishing cysteine and methionine pools are upregulated by RCS. Expression of these genes in E. coli is largely controlled by intracellular cysteine and S-adenosylmethionine concentrations (60, 61), suggesting that RCS-mediated oxidation of cellular thiol and methionine pools mimics sulfur starvation. Cysteine and methionine synthesis and transport genes are upregulated by RCS in E. coli, B. subtilis, B. cenocepacia, P. aeruginosa, S. enterica, and M. bovis (23, 38, 83, 95, 101, 102). Also upregulated are genes encoding transporters for exogenous sulfur sources such as sulfate, thiosulfate, or sulfonates (including taurine) (38, 56, 95, 101, 102). Unfortunately, we do not know whether upregulation of these genes protects cells against RCS. The upregulation of taurine transporters is particularly interesting, given the high amounts of taurine and N-chlorotaurine produced by RCS-generating neutrophils (79).
Defenses Against DNA Damage
Although RCS damage DNA in vitro, it is unclear to what extent DNA damage contributes to RCS toxicity in vivo. Bacteria have a well-studied and complex response to DNA damage, including systems for homologous recombination, repair of damaged nucleotides, and mutagenic DNA polymerases that bypass lesions that block normal DNA synthesis (reviewed in 65). Slight upregulation of some of these systems has been seen in RCS-treated bacteria, but there is little evidence for a systematic DNA damage response associated with RCS stress. RCS upregulate DNA repair polymerases in B. subtilis, L. pneumophila, and P. aeruginosa, as well as a few genes involved in repair of damaged nucleotides inxs B. subtilis, B. cenocepacia, and L. pneumophila (13, 23, 83, 95).
There is slightly better evidence that homologous recombination might play a role in RCS response. Whereas RecA, an essential factor for homologous recombination, is not induced by HOCl in wild-type E. coli (33), recA mutants are more sensitive to HOCl in aerobic conditions, especially in combination with mutations in other RCS-resistant factors (e.g., dps, yjiE) (32, 34, 38). In contrast, RecA is upregulated by RCS in L. pneumophila (13), and RecN, which is involved in the recombinational repair of DNA double-strand breaks, is upregulated by HOCl treatment in both E. coli O157:H7 and P. aeruginosa (95, 101). These results, combined with the known ability of RCS to react with nucleotides (see above), suggest that RCS can cause DNA damage and strand breaks in bacteria but that this is probably not a major contributor to the high toxicity of RCS. The baseline level of DNA repair enzymes in bacteria is probably sufficient to prevent RCS-mediated DNA damage.
Defenses Against Reactive Metals
HOCl treatment of bacteria leads to the release of cellular iron, which is due at least partially to the HOCl-mediated oxidation of Fe-S clusters (3, 51, 88, 89). Because HOCl undergoes hydroxyl radical (OH˙)-producing Fenton chemistry with Fe2+ and Cu+ orders of magnitude faster than with H2O2 (17, 85), control of cellular metal pools likely plays an important role in the RCS stress response. Indeed, fur mutants of E. coli, which contain higher than normal levels of intracellular iron, are more sensitive to HOCl than are wild-type cells (34). E. coli mutants lacking Dps, an iron-sequestering DNA-binding protein with peroxidase activity (109), are sensitive to HOCl, and mutants that overproducing Dps are protected (9, 34). YggX, a protein thought to be involved in Fe2+ trafficking and reduction of OH˙ formation during ROS stress (39), also appears to play some role in resisting RCS stress (Figure 2). Although the precise function of YggX is still unclear, ΔyggX mutants of E. coli are more sensitive to HOCl killing, and the conserved and critical cysteine of YggX is sensitive to HOCl oxidation in vivo (64).
Microarray analyses in RCS-treated bacteria support the hypothesis that RCS induce substantial changes in metal homeostasis, although the details seem to vary among species. Dps, for example, is upregulated in B. subtilis, B. cereus, and S. aureus (19, 23, 81), but not in E. coli (12, 38, 101), despite its strong protective phenotype in that organism. In addition, whereas RCS downregulate iron-uptake genes in response to HOCl in E. coli (12, 38), iron transporters were upregulated in Bacillus spp. and S. aureus (19, 23, 81). It is unclear how many of these differences are due to differences in the respective growth or treatment conditions or to variations in the protective responses among species.
Changes in Cell Envelope Composition
The cell envelope is one of the primary targets of RCS (see above). It is therefore not unexpected that bacteria respond to RCS stress by modifying the properties of their outermost layers. One gene strongly induced by HOCl treatment in both E. coli and S. enterica is ycfR (also known as bhsA or comC) (38, 101, 102), whose gene product reduces the permeability and increases the hydrophobicity of the outer membrane (31, 76). Strains lacking ycfR are more sensitive to several stress conditions, including exposure to HOCl (31). HOCl treatment leads to the repression of outer membrane porin (omp) genes in S. enterica and P. aeruginosa (78, 95, 102). OmpW increases the HOCl permeability of S. enterica, making a △ompW mutant more resistant to HOCl treatment (78). Consistent with the model that the presence of Omp family members correlates with increased HOCl permeability and sensitivity, acid-adapted S. enterica cells, which have an increased complement of those proteins (66), are more sensitive to HOCl than nonadapted cells (67). Repression of omp genes by HOCl treatment has not been observed in other bacteria, making generalization difficult. In fact, although RCS treatment changes the expression of some predicted transport proteins in every species tested so far, no obvious patterns have emerged that would suggest that up- or downregulation of any particular class of transport proteins is universally protective. It is interesting, however, to note that HOCl induces the expression of several members of the diverse proline-proline-glutamate (PPE) family of outer membrane antigenic variation and transport proteins in M. bovis (56). The dense outer layers of mycobacteria have been hypothesized to protect these cells against RCS (43), but this has not been tested directly.
Changes in Carbon Metabolism
Metabolic flux analysis of cells experiencing ROS stress has revealed that bacteria respond to ROS such as H2O2 with dramatic changes in central carbon metabolism (48, 93, 99). Briefly, flux of glucose through the NADPH-generating pentose phosphate pathway increases at the expense of glycolysis and the tricarboxylic acid cycle, increasing the pool of NADPH available for reductive detoxification reactions. These changes appear to be largely due to posttranslational redox regulation of enzyme activities. Although detailed metabolomic analyses have not been performed in RCS-stressed cells, available data are largely consistent with the existence of a similar response (32). A number of enzymes involved in glycolysis contain conserved critical cysteine residues that are sensitive to HOCl-mediated oxidation in vivo (24, 64). These include glyceraldehyde 3′-phosphate dehydrogenase, phosphoglycerate kinase, and dihydrolipoyl dehydrogenase. Inactivation of these enzymes negatively affects carbon flux through glycolysis. Several tricarboxylic acid cycle enzymes are also sensitive to RCS oxidation in vivo, including aconitase (AcnB), malate synthase (GlcB), and malate dehydrogenase (Mdh) (24, 64). Mutants of E. coli lacking AcnB or Mdh are more sensitive to HOCl (64). Expression of genes for enzymes related to electron transport, oxidative phosphorylation, and carbon-utilization pathways is regulated by RCS in E. coli, P. aeruginosa, B. subtilis, B. cereus, S. enterica, and L. pneumophila (13, 19, 23, 38, 95, 102). In addition, the catabolite repression system appears to play some role in HOCl resistance in E. coli (9). Although these studies are an excellent start, a great deal of work remains to be done to elucidate how central carbon and energy metabolism are regulated in response to RCS stress.
Other Stress Response Pathways
In addition to targeted responses against particular stress conditions, bacteria have general stress responses, which are broadly protective against multiple stresses. The details of activation differ among bacterial species; however, these stress responses are generally coordinated by alternative sigma factors (RpoS in gram-negative bacteria or σB in gram-positive bacteria) (10, 46). RCS treatment of bacteria with well-defined RpoS or σB regulons, such as E. coli or B. subtilis, leads to upregulation of many general stress response factors (23, 38, 101). E. coli cells lacking RpoS are very sensitive to HOCl, especially at stationary phase (34), whereas cells with higher than normal RpoS levels are substantially protected against HOCl treatment (9). These phenotypes were traced to RpoS-dependent expression of Dps and catalase (9, 34), suggesting that upregulation of other general stress response factors (e.g., multidrug efflux pumps, trehalose biosynthesis) may be relatively unimportant in resisting RCS.
TRANSCRIPTION FACTORS INVOLVED IN SENSING REACTIVE CHLORINE SPECIES
Genetic control of the RCS response is still poorly understood. This is in striking contrast to the well-characterized sensor proteins that detect ROS and RNS and the regulatory networks controlled by those sensors (28, 96, 100). Although several regulators are known to respond to RCS, in most cases, neither the specific nature of the signal to which they respond nor the protective role(s) of their downstream genes has been fully characterized. This may reflect the variety of physiological targets of RCS. Although a dedicated RCS-responsive regulator would be expected to respond only to RCS and to control genes specific to RCS tolerance, most known RCS-responsive regulators also respond to other stresses, including ROS, RNS, or electrophilic toxins, and control defenses against those stresses, some of which could be generated as secondary effects of RCS treatment (see above). The relative contributions of primary and secondary RCS effects to either lethal or sublethal RCS damage are not currently known.
YjiE
To date, the only transcription factor described as responding specifically to RCS is E. coli YjiE (38). Identified from a genomic expression library as a gene that confers increased HOCl resistance when overexpressed, yjiE encodes a LysR family dual regulator conserved in a variety of gram-negative bacteria. A ΔyjiE mutant is more sensitive than the wild type to HOCl, but not to H2O2 or to the cysteine-modifying reagent diamide. Microarray analysis of wild-type and ΔyjiE cells revealed that YjiE is a global regulator involved in the upregulation of genes engaged in cysteine and methionine metabolism and the downregulation of iron acquisition genes after HOCl treatment (38). The specific downstream targets of YjiE that confer increased HOCl resistance have yet to be identified. Unlike many other redox-sensitive transcription factors (100), YjiE does not contain any highly conserved cysteine residues, and the mechanism by which YjiE senses RCS has not yet been established.
OhrR
OhrR, a MarA family repressor, is conserved in gram-positive and gram-negative bacteria. It has been best characterized in the soil organism B. subtilis as a regulator of resistance to organic hydroperoxides (100). OhrR contains conserved cysteines that are oxidized by organic hydroperoxides, leading to derepression of the organic hydroperoxide reductase OhrA (29, 37). HOCl induces ohr gene expression in B. subtilis, B. cereus, and B. cenocepacia (19, 23, 83); the most highly upregulated gene in HOCl-treated B. subtilis is ohrA. It is not known whether OhrR responds directly to RCS or to organic hydroperoxides generated as oxidation products by RCS. In either case, both ohrR and ohrA mutants are more sensitive to HOCl stress than the isogenic wild-type strain is (23).
NemR
E. coli NemR, a broadly conserved TetR family repressor, was originally described as a sensor of cysteine-modifying electrophiles (98). We (40) recently showed that NemR uses redox-sensitive cysteine residues to directly sense HOCl and N-chlorotaurine (but not ROS or RNS) both in vivo and in vitro. The NemR-regulated electrophile detoxification enzymes N-ethylmaleimide reductase and glyoxalase 1 are required for survival of HOCl treatment. RCS reactions can result in the production of a variety of toxic electrophiles in vivo (see above). Although the importance of electrophile production in bacterial RCS toxicity has not been well studied, RCS stress does lead to upregulation of aldehyde reductases and other electrophile detoxifying enzymes in many bacteria, including E. coli, B. subtilis, B. cereus, B. cenocepacia, and P. aeruginosa (19, 23, 38, 83, 95, 101).
OxyR
OxyR, a LysR family repressor, is one of the best-characterized redox-sensitive transcription factors known to date. It controls the response to H2O2 in many gram-negative bacteria (53, 100). Oxidation of a conserved cysteine leads to changes in the secondary structure of OxyR and activates transcription of key antioxidant defenses, including catalases, peroxidases, disulfide reductases, Dps, and metal transporters (53). Genes regulated by OxyR are strongly upregulated after HOCl treatment of E. coli, S. enterica, and P. aeruginosa when grown in rich media (38, 95, 101, 102), but not in E. coli treated with monochloramine (12) or when E. coli was treated with HOCl in phosphate buffer (33). Pretreatment of E. coli in phosphate buffer with H2O2 did, however, increase resistance to subsequent HOCl treatment in an oxyR-dependent manner, indicating that genes under OxyR control can contribute to increased HOCl tolerance (34). Under the same conditions, pretreatment of oxyR+ E. coli with HOCl also increased H2O2 resistance, suggesting that HOCl did activate OxyR in these experiments. Curiously, E. coli mutants lacking the OxyR-regulated genes for catalase and peroxidase were still protected from HOCl by preexposure to H2O2, suggesting that other members of the OxyR regulon (e.g., Dps) are important for RCS tolerance.
SoxR
SoxR is a MerR family activator that contains an Fe-S cluster whose oxidation leads to the activation of genes encoding SOD, efflux proteins, and nitroreductases and the repression of porin expression (28, 53). Given the ability of RCS to oxidize cysteine residues and destroy Fe-S clusters, it is easy to fathom how RCS treatment could lead to SoxR activation. Yet the classic SoxR-controlled enzyme is SOD, which itself appears to be sensitive to HOCl-mediated inactivation (see above). Microarray analysis of HOCl-treated E. coli O157:H7 in rich medium did reveal induction of the SoxR regulon by HOCl treatment (101), but no induction was observed in HOCl- or monochloramine-treated nonpathogenic E. coli or in HOCl-treated S. enterica (12, 38, 102). Studies of E. coli treated with HOCl in phosphate buffer showed rapid induction of micF, a SoxR-regulated small RNA that represses production of outer membrane porins (see above) (33). A study of HOCl-treated S. enterica in rich medium showed both induction of the Sox regulon and decreased survival of mutants lacking SoxS, a transcription factor directly under the control of SoxR (26). These apparently contradictory results may be due to differences in growth conditions or in HOCl concentrations, which can dramatically affect the level of micF induced by HOCl in E. coli (33). The results clearly indicate that the role of SoxR in RCS defense remains an open question, as does the identity of any genes controlled by SoxR which protect cells against RCS.
PerR
PerR is a Fur family repressor conserved in gram-positive and gram-negative bacteria (96). Best characterized in B. subtilis, PerR responds to H2O2 and RNS. It contains two metal centers, a structural Zn2+ and a regulatory Fe2+. In the presence of H2O2, an OH˙ is formed at the Fe2+ site and reacts with nearby amino acids in PerR, resulting in the formation of 2-oxo-histidine and the irreversible inactivation of PerR. PerR regulates the expression of catalase, peroxidase, and other factors involved in defense against ROS and metal stresses.
HOCl treatment of both B. subtilis and B. cereus resulted in the induction of the PerR regulon (19, 23). However, a perR mutant of B. subtilis appeared no more sensitive to HOCl than wild type (23). Whereas the cysteine residues coordinating the Zn2+ ion in PerR are not oxidized during the H2O2 response (62), in vivo studies in B. subtilis demonstrated that they are oxidized by HOCl treatment (23). A more detailed analysis of the derepression of PerR in response to HOCl treatment in S. aureus revealed an unexpected link between PerR regulation and SOD activity (69). S. aureus cells exposed to HOCl in phosphate buffer and then transferred to rich medium showed clear HOCl-dependent induction of several genes of the PerR regulon. A mutant lacking SOD showed higher basal levels of PerR-regulated gene expression, revealed a peak in PerR-regulated gene expression at lower HOCl concentrations, and, most importantly, was more resistant to HOCl treatment than the wild-type was. Because SOD is rapidly inactivated by HOCl (32), these results led to the conclusion that HOCl-dependent inactivation of PerR is due at least in part to an increase in cellular O2˙− concentrations. Nevertheless, whether PerR has different mechanisms for sensing ROS and RCS, and whether induction of the PerR regulon is physiologically relevant for surviving RCS stress, remains to be determined.
ArcA
A study has identified the ArcAB two-component system, which regulates the switch between aerobic and anaerobic metabolism (72), as a potential HOCl-responsive regulator in S. enterica (78). ArcA negatively regulates expression of OmpW in response to both H2O2 and HOCl, thereby reducing influx of these oxidants into the cell and improving survival (see above). The ArcAB system senses the oxidation state of the membrane-localized quinone pool (72), indicating that HOCl treatment might have an effect on the ratio of reduced quinone to oxidized quinone. Although this would be consistent with the fact that HOCl disrupts respiration (see above), to our knowledge, the direct effect of RCS on the quinone oxidation state has not been measured in any bacterium. In vitro studies have shown that HOCl is unreactive toward at least one model quinone (ubiquinone-10) (3).
Spx
Spx is an unusual RNA polymerase-binding regulator that controls expression of a variety of different stress response genes in gram-positive bacteria (87). Spx contains two conserved cysteines. Oxidation of these cysteines to a disulfide bond in response to either reactive electrophiles or ROS changes the activity of Spx, causing the induction or repression of nearly 300 genes. The downstream targets include genes encoding Msr, aldehyde reductases, and thioredoxin. HOCl-treated B. subtilis also accumulates disulfide-bonded Spx (23), and both B. subtilis and B. cereus induce the Spx regulon in response to HOCl (19, 23). Expression of spx is regulated by PerR (63), which may also contribute to the observed effect of RCS on the Spx regulon (see above). A B. subtilis spx mutant shows some increased sensitivity to HOCl, but due to the pleiotropic effects of that mutation, it is unclear whether this effect is specific to RCS stress or due to a general defect in viability (23).
ComR
As mentioned above, the outer membrane protein-encoding gene ycfR is strongly upregulated by HOCl in E. coli. Its expression is controlled by the TetR family repressor ComR (YcfQ) (31, 76), which appears to directly sense metal ions (primarily copper, but also silver and gold) (76). It is therefore likely that ComR detects RCS stress indirectly via the accumulation of metals released from oxidized metal centers (see above). Release and control of reactive metal ions might serve both as a signal and as a determinant of survival during RCS stress.
CONCLUSIONS
RCS are powerful antimicrobials capable of damaging multiple cellular components and are encountered by bacteria in many different habitats. Bacteria appear to employ overlapping stress response systems to counteract the toxic effects of RCS. These systems include defenses against oxidative stress, protein unfolding, and sulfur starvation, suggesting that these effects are the most characteristic consequences of sublethal RCS treatment in bacteria. The roles of other RCS-induced defense systems, including defenses against reactive metals, changes in cell envelope composition, and changes in central metabolism, remain poorly defined. Future research is required to explore the ways in which all these defenses contribute to RCS survival in different species and under different conditions. The RCS response is coordinated by multiple regulators, some of which are well-known sensors of particular stress conditions. However, the discovery of YjiE in E. coli demonstrates the existence of regulators specific for RCS, a potentially fruitful new avenue of research likely to lead to new insights into how bacteria respond to this important class of toxins.
SUMMARY POINTS
RCS are powerful antimicrobials encountered by bacteria in many different environments.
The most universal responses of bacteria to RCS stress involve defenses against peroxides, methionine oxidation, protein unfolding, and sulfur starvation.
Bacteria have specific defenses against RCS, including the chaperone Hsp33 and the HOCl-sensing transcription factor YjiE.
FUTURE ISSUES
Why and how do peroxide-detoxifying enzymes protect against HOCl?
Do other stress response pathways contribute to RCS defense? If so, how?
Are there RCS-specific transcription factors other than YjiE?
ACKNOWLEDGMENTS
We thank the members of the Jakob lab and Dr. Robert Bender for critical manuscript review. Support comes from the National Institute of Allergy and Infectious Disease grant R21-AI097893 (U.J.) and National Research Service Award fellowship F32GM096613 (M.J.G.).
Glossary
- Reactive chlorine species (RCS)
chlorine-containing compounds, including HOCl and chloramines, capable of chlorinating and oxidizing other molecules
- Glutathione (GSH)
the tripeptide γ-l-glutamyl-l-cysteinyl-glycine, involved in maintaining the reducing redox potential of the cytoplasm in eukaryotes and many bacteria
- HOCl
hypochlorous acid
- Superoxide dismutase (SOD)
an enzyme that dismutates O2˙− to O2 and H2O2
- Myeloperoxidase (MPO)
the enzyme responsible for synthesis of HOCl during the oxidative burst of neutrophils
- Reactive oxygen species (ROS)
strong oxidants containing oxygen
- Dual oxidase (DUOX)
an HOCl-generating enzyme with fused NADPH oxidase and peroxidase domains
- Catalase
an H2O2-detoxifying enzyme that catalyzes the decomposition of H2O2 to H2O and O2
- Peroxidase
an H2O2-detoxifying enzyme that reduces H2O2 to H2O with concomitant oxidation of an electron donor
- Methionine sulfoxide reductase (Msr)
an enzyme that regenerates methionine from methionine sulfoxide
- Bacillithiol (BSH)
the α-anomeric glycoside of l-cysteinyl-d-glucosamine with l-malic acid, found in many gram-positive bacteria instead of glutathione
Footnotes
DISCLOSURE STATEMENT The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- 1.Albrich JM, Gilbaugh JH, 3rd, Callahan KB, Hurst JK. Effects of the putative neutrophil-generated toxin, hypochlorous acid, on membrane permeability and transport systems of Escherichia coli. J. Clin. Invest. 1986;78:177–84. doi: 10.1172/JCI112548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albrich JM, Hurst JK. Oxidative inactivation of Escherichia coli by hypochlorous acid. Rates and differentiation of respiratory from other reaction sites. FEBS Lett. 1982;144:157–61. doi: 10.1016/0014-5793(82)80591-7. [DOI] [PubMed] [Google Scholar]
- 3.Albrich JM, McCarthy CA, Hurst JK. Biological reactivity of hypochlorous acid: implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA. 1981;78:210–14. doi: 10.1073/pnas.78.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arner ES, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur. J. Biochem. 2000;267:6102–9. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- 5.Arnitz R, Sarg B, Ott HW, Neher A, Lindner H, Nagl M. Protein sites of attack of N-chlorotaurine in Escherichia coli. Proteomics. 2006;6:865–69. doi: 10.1002/pmic.200500054. [DOI] [PubMed] [Google Scholar]
- 6.Bae YS, Choi MK, Lee WJ. Dual oxidase in mucosal immunity and host-microbe homeostasis. Trends Immunol. 2010;31:278–87. doi: 10.1016/j.it.2010.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Barrette WC, Albrich JM, Hurst JK. Hypochlorous acid-promoted loss of metabolic energy in Escherichia coli. Infect. Immun. 1987;55:2518–25. doi: 10.1128/iai.55.10.2518-2525.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barrette WC, Jr, Hannum DM, Wheeler WD, Hurst JK. General mechanism for the bacterial toxicity of hypochlorous acid: abolition of ATP production. Biochemistry. 1989;28:9172–78. doi: 10.1021/bi00449a032. [DOI] [PubMed] [Google Scholar]
- 9.Barth E, Gora KV, Gebendorfer KM, Settele F, Jakob U, Winter J. Interplay of cellular cAMP levels, σS activity and oxidative stress resistance in Escherichia coli. Microbiology. 2009;155:1680–89. doi: 10.1099/mic.0.026021-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Battesti A, Majdalani N, Gottesman S. The RpoS-mediated general stress response in Escherichia coli. Annu. Rev. Microbiol. 2011;65:189–213. doi: 10.1146/annurev-micro-090110-102946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bengtson P, Bastviken D, de Boer W, Oberg G. Possible role of reactive chlorine in microbial antagonism and organic matter chlorination in terrestrial environments. Environ. Microbiol. 2009;11:1330–39. doi: 10.1111/j.1462-2920.2009.01915.x. [DOI] [PubMed] [Google Scholar]
- 12.Berry D, Holder D, Xi C, Raskin L. Comparative transcriptomics of the response of Escherichia coli to the disinfectant monochloramine and to growth conditions inducing monochloramine resistance. Water Res. 2010;44:4924–31. doi: 10.1016/j.watres.2010.07.026. [DOI] [PubMed] [Google Scholar]
- 13.Bodet C, Sahr T, Dupuy M, Buchrieser C, Hechard Y. Legionella pneumophila transcriptional response to chlorine treatment. Water Res. 2012;46:808–16. doi: 10.1016/j.watres.2011.11.059. [DOI] [PubMed] [Google Scholar]
- 14.Boschi-Muller S, Gand A, Branlant G. The methionine sulfoxide reductases: catalysis and substrate specificities. Arch. Biochem. Biophys. 2008;474:266–73. doi: 10.1016/j.abb.2008.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Butler A, Walker JV. Marine haloperoxidases. Chem. Rev. 1993;93:1937–44. [Google Scholar]
- 16.Camper AK, McFeters GA. Chlorine injury and the enumeration of waterborne coliform bacteria. Appl. Environ. Microbiol. 1979;37:633–41. doi: 10.1128/aem.37.3.633-641.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Candeias LP, Stratford MR, Wardman P. Formation of hydroxyl radicals on reaction of hypochlorous acid with ferrocyanide, a model iron(II) complex. Free Radic. Res. 1994;20:241–49. doi: 10.3109/10715769409147520. [DOI] [PubMed] [Google Scholar]
- 18.Carr AC, Vissers MC, Domigan NM, Winterbourn CC. Modification of red cell membrane lipids by hypochlorous acid and haemolysis by preformed lipid chlorohydrins. Redox Rep. 1997;3:263–71. doi: 10.1080/13510002.1997.11747122. [DOI] [PubMed] [Google Scholar]
- 19.Ceragioli M, Mols M, Moezelaar R, Ghelardi E, Senesi S, Abee T. Comparative transcriptomic and phenotypic analysis of the responses of Bacillus cereus to various disinfectant treatments. Appl. Environ. Microbiol. 2010;76:3352–60. doi: 10.1128/AEM.03003-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chapman AL, Winterbourn CC, Brennan SO, Jordan TW, Kettle AJ. Characterization of non-covalent oligomers of proteins treated with hypochlorous acid. Biochem. J. 2003;375:33–40. doi: 10.1042/BJ20030685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chen X, Lee KA, Ha EM, Lee KM, Seo YY, et al. A specific and sensitive method for detection of hypochlorous acid for the imaging of microbe-induced HOCl production. Chem. Commun. (Camb.) 2011;47:4373–75. doi: 10.1039/c1cc10589b. [DOI] [PubMed] [Google Scholar]
- 22.Chesney JA, Eaton JW, Mahoney JR., Jr Bacterial glutathione: a sacrificial defense against chlorine compounds. J. Bacteriol. 1996;178:2131–35. doi: 10.1128/jb.178.7.2131-2135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chi BK, Gronau K, Mader U, Hessling B, Becher D, Antelmann H. S-bacillithiolation protects against hypochlorite stress in Bacillus subtilis as revealed by transcriptomics and redox proteomics. Mol. Cell Proteomics. 2011;10:M111.009506. doi: 10.1074/mcp.M111.009506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chi BK, Roberts AA, Huyen TT, Bäsell K, Becher D, et al. S-bacillithiolation protects conserved and essential proteins against hypochlorite stress in Firmicutes bacteria. Antioxid. Redox Signal. 2013;18:1273–95. doi: 10.1089/ars.2012.4686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clauditz A, Resch A, Wieland KP, Peschel A, Gotz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 2006;74:4950–53. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Collao B, Morales EH, Gil F, Polanco R, Calderón IL, Saavedra CP. Differential expression of the transcription factors MarA, Rob, and SoxS of Salmonella Typhimurium in response to sodium hypochlorite: down-regulation of rob by MarA and SoxS. Arch. Microbiol. 2012;194:933–42. doi: 10.1007/s00203-012-0828-8. [DOI] [PubMed] [Google Scholar]
- 27.Cook NL, Pattison DI, Davies MJ. Myeloperoxidase-derived oxidants rapidly oxidize and disrupt zinc-cysteine/histidine clusters in proteins. Free Radic. Biol. Med. 2012;53:2072–80. doi: 10.1016/j.freeradbiomed.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 28.Crack JC, Green J, Hutchings MI, Thomson AJ, Le Brun NE. Bacterial iron-sulfur regulatory proteins as biological sensor-switches. Antioxid. Redox Signal. 2012;17:1215–31. doi: 10.1089/ars.2012.4511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cussiol JR, Alves SV, de Oliveira MA, Netto LE. Organic hydroperoxide resistance gene encodes a thiol-dependent peroxidase. J. Biol. Chem. 2003;278:11570–78. doi: 10.1074/jbc.M300252200. [DOI] [PubMed] [Google Scholar]
- 30.Deborde M, von Gunten U. Reactions of chlorine with inorganic and organic compounds during water treatment—kinetics and mechanisms: a critical review. Water Res. 2008;42:13–51. doi: 10.1016/j.watres.2007.07.025. [DOI] [PubMed] [Google Scholar]
- 31.Deng K, Wang S, Rui X, Zhang W, Tortorello ML. Functional analysis of ycfR and ycfQ in Escherichia coli O157:H7 linked to outbreaks of illness associated with fresh produce. Appl. Environ. Microbiol. 2011;77:3952–59. doi: 10.1128/AEM.02420-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dukan S, Belkin S, Touati D. Reactive oxygen species are partially involved in the bacteriocidal action of hypochlorous acid. Arch. Biochem. Biophys. 1999;367:311–16. doi: 10.1006/abbi.1999.1265. [DOI] [PubMed] [Google Scholar]
- 33.Dukan S, Dadon S, Smulski DR, Belkin S. Hypochlorous acid activates the heat shock and soxRS systems of Escherichia coli. Appl. Environ. Microbiol. 1996;62:4003–8. doi: 10.1128/aem.62.11.4003-4008.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dukan S, Touati D. Hypochlorous acid stress in Escherichia coli: resistance, DNA damage, and comparison with hydrogen peroxide stress. J. Bacteriol. 1996;178:6145–50. doi: 10.1128/jb.178.21.6145-6150.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dukan S, Turlin E, Biville F, Bolbach G, Touati D, et al. Coupling 2D SDS-PAGE with CNBr cleavage and MALDI-TOFMS: a strategy applied to the identification of proteins induced by a hypochlorous acid stress in Escherichia coli. Anal. Chem. 1998;70:4433–40. doi: 10.1021/ac980132z. [DOI] [PubMed] [Google Scholar]
- 36.Freese SD, Nozaic DJ. Chlorine: Is it really so bad and what are the alternatives? Water South Afr. 2004;30:18–24. [Google Scholar]
- 37.Fuangthong M, Atichartpongkul S, Mongkolsuk S, Helmann JD. OhrR is a repressor of ohrA, a key organic hydroperoxide resistance determinant in Bacillus subtilis. J. Bacteriol. 2001;183:4134–41. doi: 10.1128/JB.183.14.4134-4141.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gebendorfer KM, Drazic A, Le Y, Gundlach J, Bepperling A, et al. Identification of a hypochlorite-specific transcription factor from Escherichia coli. J. Biol. Chem. 2012;287:6892–903. doi: 10.1074/jbc.M111.287219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gralnick JA, Downs DM. The YggX protein of Salmonella enterica is involved in Fe(II) trafficking and minimizes the DNA damage caused by hydroxyl radicals: Residue CYS-7 is essential for YggX function. J. Biol. Chem. 2003;278:20708–15. doi: 10.1074/jbc.M301577200. [DOI] [PubMed] [Google Scholar]
- 40.Gray MJ, Wholey WY, Parker BM, Kim M, Jakob U. NemR is a bleach-sensing transcription factor. J. Biol. Chem. 2013;288:13789–98. doi: 10.1074/jbc.M113.454421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gribble GW. Science Dossiers Prepared for Euro Chlor. Euro Chlor; Brussels: 2004. Natural Organohalogens. http://www.eurochlor.org/media/41291/sd6-organohalogens-final.pdf. [Google Scholar]
- 42.Guisbert E, Yura T, Rhodius VA, Gross CA. Convergence of molecular, modeling, and systems approaches for an understanding of the Escherichia coli heat shock response. Microbiol. Mol. Biol. Rev. 2008;72:545–54. doi: 10.1128/MMBR.00007-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Haas CN, Engelbrecht RS. Physiological alterations of vegetative microorganisms resulting from chlorination. J. Water Pollut. Control. Fed. 1980;52:1976–89. [PubMed] [Google Scholar]
- 44.Harwood DT, Kettle AJ, Winterbourn CC. Production of glutathione sulfonamide and dehydroglutathione from GSH by myeloperoxidase-derived oxidants and detection using a novel LC-MS/MS method. Biochem. J. 2006;399:161–68. doi: 10.1042/BJ20060978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hawkins CL, Davies MJ. Hypochlorite-induced damage to DNA, RNA, and polynucleotides: formation of chloramines and nitrogen-centered radicals. Chem. Res. Toxicol. 2002;15:83–92. doi: 10.1021/tx015548d. [DOI] [PubMed] [Google Scholar]
- 46.Hecker M, Pane-Farre J, Volker U. SigB-dependent general stress response in Bacillus subtilis and related gram-positive bacteria. Annu. Rev. Microbiol. 2007;61:215–36. doi: 10.1146/annurev.micro.61.080706.093445. [DOI] [PubMed] [Google Scholar]
- 47.Helmann JD. Bacillithiol, a new player in bacterial redox homeostasis. Antioxid. Redox Signal. 2011;15:123–33. doi: 10.1089/ars.2010.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Henard CA, Bourret TJ, Song M, Vazquez-Torres A. Control of redox balance by the stringent response regulatory protein promotes antioxidant defenses of Salmonella. J. Biol. Chem. 2010;285:36785–93. doi: 10.1074/jbc.M110.160960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hondorp ER, Matthews RG. Oxidation of cysteine 645 of cobalamin-independent methionine synthase causes a methionine limitation in Escherichia coli. J. Bacteriol. 2009;191:3407–10. doi: 10.1128/JB.01722-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hurst JK. What really happens in the neutrophil phagosome? Free Radic. Biol. Med. 2012;53:508–20. doi: 10.1016/j.freeradbiomed.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hurst JK, Barrette WC, Jr, Michel BR, Rosen H. Hypochlorous acid and myeloperoxidasecatalyzed oxidation of iron-sulfur clusters in bacterial respiratory dehydrogenases. Eur. J. Biochem. 1991;202:1275–82. doi: 10.1111/j.1432-1033.1991.tb16500.x. [DOI] [PubMed] [Google Scholar]
- 52.Ilbert M, Horst J, Ahrens S, Winter J, Graf PC, et al. The redox-switch domain of Hsp33 functions as dual stress sensor. Nat. Struct. Mol. Biol. 2007;14:556–63. doi: 10.1038/nsmb1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Imlay JA. Cellular defenses against superoxide and hydrogen peroxide. Annu. Rev. Biochem. 2008;77:755–76. doi: 10.1146/annurev.biochem.77.061606.161055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jakob U, Eser M, Bardwell JC. Redox switch of hsp33 has a novel zinc-binding motif. J. Biol. Chem. 2000;275:38302–10. doi: 10.1074/jbc.M005957200. [DOI] [PubMed] [Google Scholar]
- 55.Jakob U, Muse W, Eser M, Bardwell JC. Chaperone activity with a redox switch. Cell. 1999;96:341–52. doi: 10.1016/s0092-8674(00)80547-4. [DOI] [PubMed] [Google Scholar]
- 56.Jang HJ, Nde C, Toghrol F, Bentley WE. Global transcriptome analysis of the Mycobacterium bovis BCG response to sodium hypochlorite. Appl. Microbiol. Biotechnol. 2009;85:127–40. doi: 10.1007/s00253-009-2208-0. [DOI] [PubMed] [Google Scholar]
- 57.Khor HK, Fisher MT, Schoneich C. Potential role of methionine sulfoxide in the inactivation of the chaperone GroEL by hypochlorous acid (HOCl) and peroxynitrite (ONOO–) J. Biol. Chem. 2004;279:19486–93. doi: 10.1074/jbc.M310045200. [DOI] [PubMed] [Google Scholar]
- 58.Klebanoff SJ. Myeloperoxidase: friend and foe. J. Leukoc. Biol. 2005;77:598–625. doi: 10.1189/jlb.1204697. [DOI] [PubMed] [Google Scholar]
- 59.Krab IM, Parmeggiani A. EF-Tu, a GTPase odyssey. Biochim. Biophys. Acta. 1998;1443:1–22. doi: 10.1016/s0167-4781(98)00169-9. [DOI] [PubMed] [Google Scholar]
- 60.Kredich NM. The molecular basis for positive regulation of cys promoters in Salmonella typhimurium and Escherichia coli. Mol. Microbiol. 1992;6:2747–53. doi: 10.1111/j.1365-2958.1992.tb01453.x. [DOI] [PubMed] [Google Scholar]
- 61.LaMonte BL, Hughes JA. In vivo hydrolysis of S-adenosylmethionine induces the met regulon of Escherichia coli. Microbiology. 2006;152:1451–59. doi: 10.1099/mic.0.28489-0. [DOI] [PubMed] [Google Scholar]
- 62.Lee JW, Helmann JD. Functional specialization within the Fur family of metalloregulators. Biometals. 2007;20:485–99. doi: 10.1007/s10534-006-9070-7. [DOI] [PubMed] [Google Scholar]
- 63.Leelakriangsak M, Kobayashi K, Zuber P. Dual negative control of spx transcription initiation from the P3 promoter by repressors PerR and YodB in Bacillus subtilis. J. Bacteriol. 2007;189:1736–44. doi: 10.1128/JB.01520-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Leichert LI, Gehrke F, Gudiseva HV, Blackwell T, Ilbert M, et al. Quantifying changes in the thiol redox proteome upon oxidative stress in vivo. Proc. Natl. Acad. Sci. USA. 2008;105:8197–202. doi: 10.1073/pnas.0707723105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lenhart JS, Schroeder JW, Walsh BW, Simmons LA. DNA repair and genome maintenance in Bacillus subtilis. Microbiol. Mol. Biol. Rev. 2012;76:530–64. doi: 10.1128/MMBR.05020-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Leyer GJ, Johnson EA. Acid adaptation induces cross-protection against environmental stresses in Salmonella typhimurium. Appl. Environ. Microbiol. 1993;59:1842–47. doi: 10.1128/aem.59.6.1842-1847.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leyer GJ, Johnson EA. Acid adaptation sensitizes Salmonella typhimurium to hypochlorous acid. Appl. Environ. Microbiol. 1997;63:461–67. doi: 10.1128/aem.63.2.461-467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loprasert S, Whangsuk W, Sallabhan R, Mongkolsuk S. Regulation of the katG-dpsA operon and the importance of KatG in survival of Burkholderia pseudomallei exposed to oxidative stress. FEBS Lett. 2003;542:17–21. doi: 10.1016/s0014-5793(03)00328-4. [DOI] [PubMed] [Google Scholar]
- 69.Maalej S, Dammak I, Dukan S. The impairment of superoxide dismutase coordinates the derepression of the PerR regulon in the response of Staphylococcus aureus to HOCl stress. Microbiology. 2006;152:855–61. doi: 10.1099/mic.0.28385-0. [DOI] [PubMed] [Google Scholar]
- 70.MacMillan-Crow LA, Crow JP, Thompson JA. Peroxynitrite-mediated inactivation of manganese superoxide dismutase involves nitration and oxidation of critical tyrosine residues. Biochemistry. 1998;37:1613–22. doi: 10.1021/bi971894b. [DOI] [PubMed] [Google Scholar]
- 71.Mahawar M, Tran V, Sharp JS, Maier RJ. Synergistic roles of Helicobacter pylori methionine sulfoxide reductase and GroEL in repairing oxidant-damaged catalase. J. Biol. Chem. 2011;286:19159–69. doi: 10.1074/jbc.M111.223677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Malpica R, Sandoval GR, Rodriguez C, Franco B, Georgellis D. Signaling by the arc two-component system provides a link between the redox state of the quinone pool and gene expression. Antioxid. Redox Signal. 2006;8:781–95. doi: 10.1089/ars.2006.8.781. [DOI] [PubMed] [Google Scholar]
- 73.Masip L, Veeravalli K, Georgiou G. The many faces of glutathione in bacteria. Antioxid. Redox Signal. 2006;8:753–62. doi: 10.1089/ars.2006.8.753. [DOI] [PubMed] [Google Scholar]
- 74.McDonnell G, Russell AD. Antiseptics and disinfectants: activity, action, and resistance. Clin. Microbiol. Rev. 1999;12:147–79. doi: 10.1128/cmr.12.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McKenna SM, Davies KJ. The inhibition of bacterial growth by hypochlorous acid. Possible role in the bactericidal activity of phagocytes. Biochem. J. 1988;254:685–92. doi: 10.1042/bj2540685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mermod M, Magnani D, Solioz M, Stoyanov JV. The copper-inducible ComR (YcfQ) repressor regulates expression of ComC (YcfR), which affects copper permeability of the outer membrane of Escherichia coli. Biometals. 2012;25:33–43. doi: 10.1007/s10534-011-9510-x. [DOI] [PubMed] [Google Scholar]
- 77.Meyer AS, Baker TA. Proteolysis in the Escherichia coli heat shock response: a player at many levels. Curr. Opin. Microbiol. 2011;14:194–99. doi: 10.1016/j.mib.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Morales EH, Calderon IL, Collao B, Gil F, Porwollik S, et al. Hypochlorous acid and hydrogen peroxide-induced negative regulation of Salmonella enterica serovar Typhimurium ompW by the response regulator ArcA. BMC Microbiol. 2012;12:63. doi: 10.1186/1471-2180-12-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nagl M, Hess MW, Pfaller K, Hengster P, Gottardi W. Bactericidal activity of micromolar N-chlorotaurine: evidence for its antimicrobial function in the human defense system. Antimicrob. Agents Chemother. 2000;44:2507–13. doi: 10.1128/aac.44.9.2507-2513.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niki E. Lipid peroxidation: physiological levels and dual biological effects. Free Radic. Biol. Med. 2009;47:469–84. doi: 10.1016/j.freeradbiomed.2009.05.032. [DOI] [PubMed] [Google Scholar]
- 81.Palazzolo-Ballance AM, Reniere ML, Braughton KR, Sturdevant DE, Otto M, et al. Neutrophil microbicides induce a pathogen survival response in community-associated methicillin-resistant Staphylococcus aureus. J. Immunol. 2008;180:500–9. doi: 10.4049/jimmunol.180.1.500. [DOI] [PubMed] [Google Scholar]
- 82.Pattison DI, Davies MJ, Hawkins CL. Reactions and reactivity of myeloperoxidase-derived oxidants: differential biological effects of hypochlorous and hypothiocyanous acids. Free Radic. Res. 2012;46:975–95. doi: 10.3109/10715762.2012.667566. [DOI] [PubMed] [Google Scholar]
- 83.Peeters E, Sass A, Mahenthiralingam E, Nelis H, Coenye T. Transcriptional response of Burkholderia cenocepacia J2315 sessile cells to treatments with high doses of hydrogen peroxide and sodium hypochlorite. BMC Genomics. 2010;11:90. doi: 10.1186/1471-2164-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Peskin AV, Turner R, Maghzal GJ, Winterbourn CC, Kettle AJ. Oxidation of methionine to dehydromethionine by reactive halogen species generated by neutrophils. Biochemistry. 2009;48:10175–82. doi: 10.1021/bi901266w. [DOI] [PubMed] [Google Scholar]
- 85.Prutz WA. Hypochlorous acid interactions with thiols, nucleotides, DNA, and other biological substrates. Arch. Biochem. Biophys. 1996;332:110–20. doi: 10.1006/abbi.1996.0322. [DOI] [PubMed] [Google Scholar]
- 86.Reichmann D, Xu Y, Cremers CM, Ilbert M, Mittelman R, et al. Order out of disorder: working cycle of an intrinsically unfolded chaperone. Cell. 2012;148:947–57. doi: 10.1016/j.cell.2012.01.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rochat T, Nicolas P, Delumeau O, Rabatinova A, Korelusova J, et al. Genome-wide identification of genes directly regulated by the pleiotropic transcription factor Spx in Bacillus subtilis. Nucleic Acids Res. 2012;40:9571–83. doi: 10.1093/nar/gks755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rosen H, Klebanoff SJ. Oxidation of Escherichia coli iron centers by the myeloperoxidase-mediated microbicidal system. J. Biol. Chem. 1982;257:13731–35. [PubMed] [Google Scholar]
- 89.Rosen H, Klebanoff SJ. Oxidation of microbial iron-sulfur centers by the myeloperoxidase-H2O2-halide antimicrobial system. Infect. Immun. 1985;47:613–18. doi: 10.1128/iai.47.3.613-618.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rosen H, Klebanoff SJ, Wang Y, Brot N, Heinecke JW, Fu X. Methionine oxidation contributes to bacterial killing by the myeloperoxidase system of neutrophils. Proc. Natl. Acad. Sci. USA. 2009;106:18686–91. doi: 10.1073/pnas.0909464106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rosen H, Orman J, Rakita RM, Michel BR, VanDevanter DR. Loss of DNA-membrane interactions and cessation of DNA synthesis in myeloperoxidase-treated Escherichia coli. Proc. Natl. Acad. Sci. USA. 1990;87:10048–52. doi: 10.1073/pnas.87.24.10048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ruby EG, McFall-Ngai MJ. Oxygen-utilizing reactions and symbiotic colonization of the squid light organ by Vibrio fischeri. Trends Microbiol. 1999;7:414–20. doi: 10.1016/s0966-842x(99)01588-7. [DOI] [PubMed] [Google Scholar]
- 93.Rui B, Shen T, Zhou H, Liu J, Chen J, et al. A systematic investigation of Escherichia coli central carbon metabolism in response to superoxide stress. BMC Syst. Biol. 2010;4:122. doi: 10.1186/1752-0509-4-122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rutala WA, Weber DJ. Uses of inorganic hypochlorite (bleach) in health-care facilities. Clin. Microbiol. Rev. 1997;10:597–610. doi: 10.1128/cmr.10.4.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Small DA, Chang W, Toghrol F, Bentley WE. Toxicogenomic analysis of sodium hypochlorite antimicrobial mechanisms in Pseudomonas aeruginosa. Appl. Microbiol. Biotechnol. 2007;74:176–85. doi: 10.1007/s00253-006-0644-7. [DOI] [PubMed] [Google Scholar]
- 96.Spiro S, D'Autreaux B. Non-heme iron sensors of reactive oxygen and nitrogen species. Antioxid. Redox Signal. 2012;17:1264–76. doi: 10.1089/ars.2012.4533. [DOI] [PubMed] [Google Scholar]
- 97.Stanley NR, Pattison DI, Hawkins CL. Ability of hypochlorous acid and N-chloramines to chlorinate DNA and its constituents. Chem. Res. Toxicol. 2010;23:1293–302. doi: 10.1021/tx100188b. [DOI] [PubMed] [Google Scholar]
- 98.Umezawa Y, Shimada T, Kori A, Yamada K, Ishihama A. The uncharacterized transcription factor YdhM is the regulator of the nemA gene, encoding N-ethylmaleimide reductase. J. Bacteriol. 2008;190:5890–97. doi: 10.1128/JB.00459-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Valdivia-González M, Pérez-Donoso JM, Vásquez CC. Effect of tellurite-mediated oxidative stress on the Escherichia coli glycolytic pathway. Biometals. 2012;25:451–58. doi: 10.1007/s10534-012-9518-x. [DOI] [PubMed] [Google Scholar]
- 100.Vázquez-Torres A. Redox active thiol sensors of oxidative and nitrosative stress. Antioxid. Redox Signal. 2012;17:1201–14. doi: 10.1089/ars.2012.4522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang S, Deng K, Zaremba S, Deng X, Lin C, et al. Transcriptomic response of Escherichia coli O157:H7 to oxidative stress. Appl. Environ. Microbiol. 2009;75:6110–23. doi: 10.1128/AEM.00914-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Wang S, Phillippy AM, Deng K, Rui X, Li Z, et al. Transcriptomic responses of Salmonella enterica serovars Enteritidis and Typhimurium to chlorine-based oxidative stress. Appl. Environ. Microbiol. 2010;76:5013–24. doi: 10.1128/AEM.00823-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wholey WY, Jakob U. Hsp33 confers bleach resistance by protecting elongation factor Tu against oxidative degradation in Vibrio cholerae. Mol. Microbiol. 2012;83:981–91. doi: 10.1111/j.1365-2958.2012.07982.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Winter J, Ilbert M, Graf PC, Ozcelik D, Jakob U. Bleach activates a redox-regulated chaperone by oxidative protein unfolding. Cell. 2008;135:691–701. doi: 10.1016/j.cell.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Winter J, Linke K, Jatzek A, Jakob U. Severe oxidative stress causes inactivation of DnaK and activation of the redox-regulated chaperone Hsp33. Mol. Cell. 2005;17:381–92. doi: 10.1016/j.molcel.2004.12.027. [DOI] [PubMed] [Google Scholar]
- 106.Winterbourn CC, Kettle AJ. Biomarkers of myeloperoxidase-derived hypochlorous acid. Free Radic. Biol. Med. 2000;29:403–9. doi: 10.1016/s0891-5849(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 107.Winterbourn CC, Kettle AJ. Redox reactions and microbial killing in the neutrophil phagosome. Antioxid. Redox Signal. 2013;18:642–60. doi: 10.1089/ars.2012.4827. [DOI] [PubMed] [Google Scholar]
- 108.Xu XM, Moller SG. Iron-sulfur clusters: biogenesis, molecular mechanisms, and their functional significance. Antioxid. Redox Signal. 2011;15:271–307. doi: 10.1089/ars.2010.3259. [DOI] [PubMed] [Google Scholar]
- 109.Zeth K. Dps biomineralizing proteins: multifunctional architects of nature. Biochem. J. 2012;445:297–311. doi: 10.1042/BJ20120514. [DOI] [PubMed] [Google Scholar]