Abstract
Background
Spray drying techniques are commonly utilized in the pharmaceutical, dairy and animal feed industries for processing liquids into powders but have not been applied to human blood products. Spray dried protein products are known to maintain stability during storage at room temperature.
Study design and methods
Plasma units collected at the donor facility were shipped overnight at room temperature to a processing facility where single-use spray drying occurred. After 48 hours storage at room temperature, the spray dried plasma product was split in two and rehydrated with 1.5% glycine or deionized water and assayed for chemistry analytes and coagulation factors. Matched fresh frozen plasma (FFP) was analyzed in parallel as controls.
Results
Reconstitution was achieved for both rehydration groups within five minutes (n=6). There was no statistically significant intergroup difference in recovery for total protein, albumin, IgG, IgA, and IgM (96% or higher). With the exception of factor VIII (58%), the recovery of clotting factors in the glycine reconstituted products ranged from 72% to 93%. Glycine reconstitution was superior to deionized water.
Conclusion
We documented proteins and coagulation activities were recovered in physiologic quantities in reconstituted spray dried plasma products. Further optimization of the spray drying method and reconstitution fluid may result in even better recoveries. Spray drying is a promising technique for preparing human plasma that can be easily stored at room temperature, shipped, and reconstituted. Rapid reconstitution of the microparticles results in a novel plasma product from single donors.
Introduction
Human plasma contains thousands of different proteins.1 A fraction of these proteins are critically important to the balance between procoagulation and anticoagulation hemostasis and serve both as the rationale for transfusion of plasma and for analysis of the quality of plasma components. Currently, plasma for transfusion in the United States is either collected by plasmapheresis or derived from collections of whole blood and generally stored in the frozen state2. Freeze-dried (lyophilized) plasma products were used in Europe for decades. Even today such products are available on a limited basis (Table 1)3–6, but the lyophilization process is lengthy and no product has been licensed in the US.6,7,8
Table 1.
Examples of alternatives to fresh frozen plasma (FFP) in Europe* suitable for room temperature storage
| Product details | License status | ||||||
|---|---|---|---|---|---|---|---|
| Product name |
Production process |
Infection safety | Pool size | Country | Year | Vendor | Reference |
| PCSD† | Lyophilization leukocyte reduced | Quarantine-stored beyond diagnostic window | At least 10 donations | France | 1994 | French Military Service | 3,4 |
| Human S/D-Plasma lyophilisiert | Lyophilization leukocyte reduced | Solvent/detergent process | Up to 1000 donations | Germany | 2003 | German Red Cross Blood Service West | 5 |
| LyoPlas N-w | Lyophilization leukocyte reduced | Quaratine-stored for at least 4 months | Single donation | Germany | 2007 | German Red Cross Blood Service West | 6 |
No alternative approved in the U.S.
Plasma cryodesséché sécurisé déleucocyté (PCSD) - freeze dried and secured plasma (FDSP)5
Commonly used in the pharmaceutical, dairy and animal feed industries to preserve protein constituents, spray drying is a well-established method for processing liquids into powders.9–12,13,14 Unlike lyophilization that relies upon ice sublimation principles to produce dried flakes, spray-drying procedures harness heat from a heated gas stream to evaporate microdispersed droplets created by atomization of a continuous liquid feed to produce a fine powder. Spray drying has not been used to store plasma for eventual reconstitution for transfusion.
We conducted a feasibility study in which plasma separated from units of whole blood collected at one institution was shipped to a processing laboratory for spray drying. The resulting powder was returned, reconstituted with 1.5% glycine or deionized water and compared to the matched FFP controls for quantitative and qualitative coagulation and chemistry analytes.
Materials and Methods
Spray dry instrument
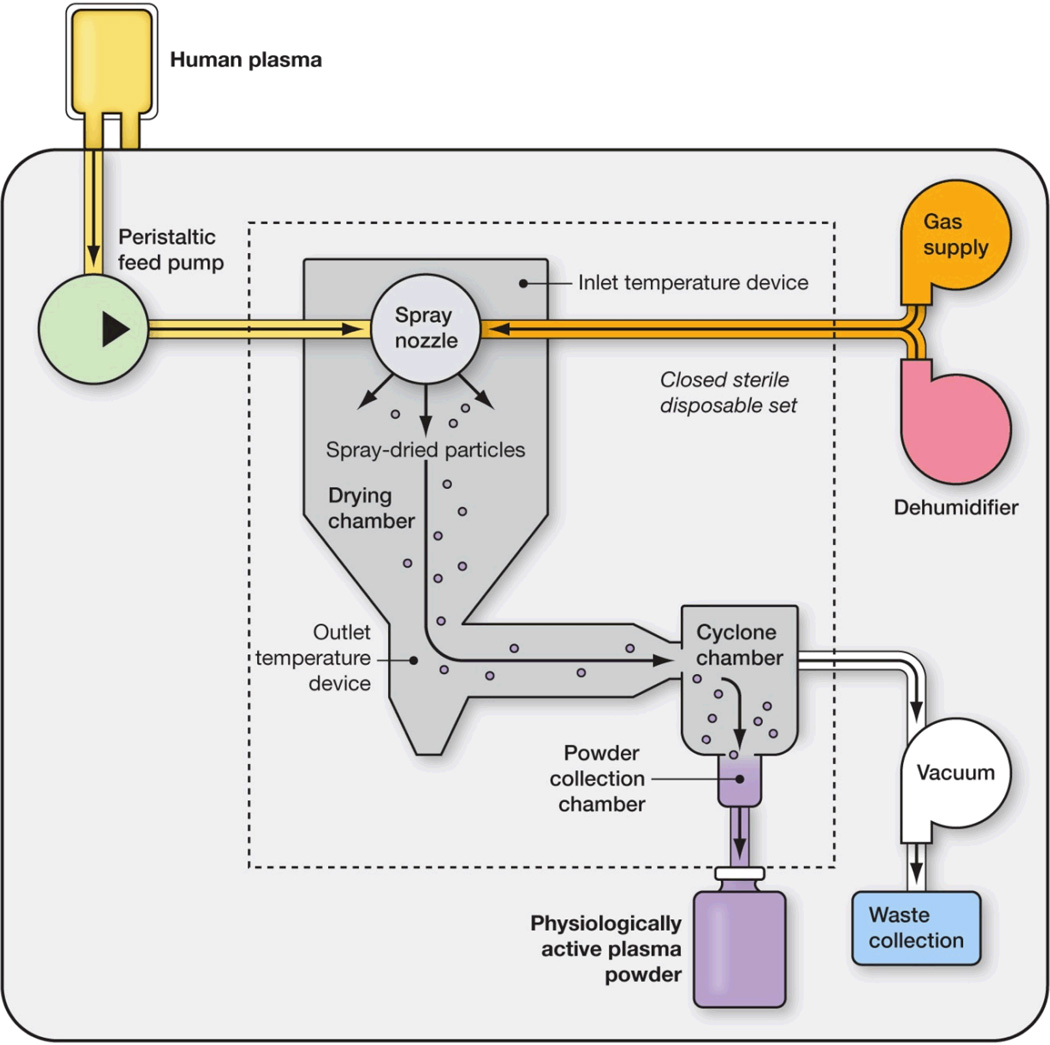
The spray dryer is comprised of an atomizer, gas disperser, drying chamber, and systems for gas and moisture exhaust and powder recovery (Fig. 1). The spray drying process consists of feeding the liquid plasma into a stream of pressurized drying gas that passes through an aerosolizing nozzle to produce atomized plasma. This plasma is then injected into a drying chamber as a cloud of aerosolized plasma of a controlled particle size. Next, the particles pass through a field of heated nonreactive gas in the drying chamber. Finally, the aerosolized droplets of liquid plasma are dried rapidly by the heated drying gas through an evaporative process, resulting in fine dried plasma particles suspended within the drying gas stream. The dried plasma is then collected into a plasma storage bag and the drying gas and evaporated water are exhausted.
Figure 1.
Schematic depiction of a spray drying technique for single donor plasma. Illustrated is a research apparatus with an optional single use sterile cartridge that renders human plasma into small microparticles. This desktop research unit occupies approximately four square feet of space.
Contact between spray droplets and the heated drying gas is managed by precise flow rates of both plasma and the drying gas, which, in turn, control evaporation rates and product temperatures in the dryer. Consequently, high heat transfer coefficients are generated, and stabile, rapid drying of the atomized plasma droplets at moderate temperatures takes place.
In its commercial configuration, the spray dryer will be approximately 2(w) × 3(d) × 5(h) feet with a closed, single-use disposable set of approximately 14 × 9 × 4 inches.
Producing spray dried plasma
Units of whole blood (500 mL in AS-1 anticoagulant) were collected by standard venipuncture techniques from randomly selected research donors of blood group O D positive. Leukocyte filtration was not performed. Within 1 hour of collection, plasma was separated by centrifugation (20–24°C, 4654 × g, 6:30 minutes) and a manual plasma extractor separated the plasma from the red cell mass, as per standard FFP procedure. A 60 mL aliquot of plasma was frozen immediately at −20°C in a 300mL blood storage bag (Transfer pack, 4R2014, Fenwal, Inc., Lake Zurich, IL) and the remaining plasma unit was shipped at room temperature overnight to the processing laboratory for single-use spray drying preservation. Room temperature shipment was conducted to obviate losses from repetitive freeze/thaw cycles. The frozen aliquots were stored at −20°C as matched controls until the time of testing. The shipped plasma underwent continuous flow spray drying in an open-system plastic disposable that was integrally attached to the finished product bag. All products were collected, shipped, processed, and subsequently returned within 48 hours.
Reconstitution process
After 48 hours storage at room temperature, the spray dried product was aliquoted to two samples of 3 grams for rehydration with 30 mL of 1.5% glycine or deionized water, while matched FFP controls were thawed in a 37°C warm water bath (Helmar QuickThaw, Helmar Inc., Noblesville, IN); samples were then analyzed in parallel. Per protocol, all samples were allotted five minutes for reconstitution, with a visual check for no residual precipitate matter.
Clinical laboratory tests
Coagulation factor activity as assayed by mixing 1:10 and 1:20 dilutions of test plasma with plasma deficient in the factor to be assayed. The activated partial thromboplastin time (for factor VIII and factor IX) or prothrombin time (for factor V and factor VII) in the test plasma were compared to standard curves and are shown as percentages of the standards.
Fibrinogen concentration, PT, aPTT, and thrombin times were assayed by an automated coagulation analyzer (STAR Evolution; Diagnostica Stago, Parsipany, NJ). vWF antigen was assayed by immunoturbidometry (Liatest; Diagnostica Stago). vWF activity was determined by a ristocetin-dependent platelet agglutination method on a platelet aggregometer (Chronolog Model 700; Chrono-Log, Havertwon, PA). ADAMTS13 enzyme activity was measured by fluorescence resonance energy transfer using commercial reagents (ELISA; GTI, Milwaukee, WI).
Quantitative and qualitative coagulation parameters (prothrombin time/INR, partial prothromplastin time, thrombin time, fibrinogen, factor V, factor VII, factor VIII, factor IX, protein C, protein S, vWF antigen, vWF activity, and ADAMTS13) and quantitative clinical chemistry analytes (total protein, albumin, IgG, IgA, and IgM) were determined in parallel for the matched FFP control, 1.5% glycine, and deionized water samples (Dimension Vista 1500; Siemens, Newark, DE).
Pilot study
Initially, samples from three donors were prepared, shipped, and tested with a limited coagulation panel (PT, aPPT, TT; data not shown) to determine the logistics and feasibility of the two site cooperative study. Following successful completion of the trial samples, all subsequent products were analyzed for coagulation factors specifically to determine a more accurate coagulation profile of the spray dried product.
Statistical analysis
Statistical analysis was done with MedCalc (Mariakerke, Belgium). The Kruskal-Wallis test and the sign test were used. P < 0.05 was considered statistically significant.
Results
Six O D positive whole blood units from healthy research donors were utilized. Single donor plasma was spray dried using a spray dry instrument (Fig. 1). We obtained a powder of small microparticles (Fig. 2). The spray dried plasma was reconstituted to an aqueous solution within five minutes in 1.5% glycine and deionized water to identify both feasibility and functionally active plasma proteins. Once reconstituted, both samples were compared to matched FFP controls. Coagulation activities and quantitative clinical chemistry analytes were batch tested within one hour of reconstitution.
Figure 2.
Finalized spray dried plasma product. The microparticles of spray dried plasma are smaller than lyophilized preparations (not shown), which facilitates a very rapid reconstitution within a minute.
Physiologically active analytes
The spray dried plasma reconstituted in 1.5% glycine contained the coagulation factors in physiologic quantities (Table 2). While the recovery was reduced for all factors in the spray dried preparation compared with control FFP aliquots, statistically significant differences occurred only for Factor VIII and vWF, which was anticipated given the heat lability and room temperature transportation of Factor VIII.15 Deionized water yielded significantly lower recovery for most coagulation factors compared to 1.5% glycine (Table 2). We did not detect a statistically significant difference between the three plasma products for total protein, albumin, IgG, IgA, and IgM (Table 3).
Table 2.
Coagulation factor analysis of spray dried plasma*
| Spray dried plasma | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standard FFP | ||||||||
| (control) | 1.5% glycine† | Deionized water‡ | ||||||
| Parameter | mean | ± SD | mean | ± SD | P compared to control |
mean | ± SD | P compared to 1.5% glycine |
| Fibrinogen (Factor I) (mg/dL) | 372.8 | 154.7 | 320.8 | 99.0 | NS†† | 267.3 | 84.2 | 0.03 |
| Factor V (%) | 70.2 | 20.0 | 55.7 | 19.7 | NS | 17.5 | 8.1 | 0.03 |
| Factor VII (%) | 72.8 | 23.7 | 62.8 | 27.1 | NS | 58.3 | 18.4 | NS |
| Factor VIII (%) | 108.0 | 33.0 | 62.7 | 19.6 | 0.03 | 36.8 | 14.0 | 0.03 |
| Factor IX (%) | 101.0 | 23.0 | 91.2 | 28.5 | NS | 70.3 | 19.4 | 0.03 |
| Protein C (%) | 81.2 | 11.9 | 75.5 | 14.8 | NS | 74.5 | 9.3 | 0.03 |
| Protein S (%) | 102.7 | 17.1 | 73.5 | 25.2 | NS | 77.7 | 17.6 | 0.03 |
| vWF antigen (IU/dL) | 112.3 | 52.2 | 84.8 | 30.1 | 0.03 | 97.8 | 39.2 | NS |
| vWF activity (IU/dL) | 110.7 | 34.4 | 82.7 | 35.6 | 0.03 | 58.0 | 25.9 | 0.03 |
| ADAMTS-13 (%) | 108.3 | 23.5 | 98.3 | 20.2 | NS | 89.3 | 8.1 | NS |
Single donor products were analyzed (n = 6; sign test).
The lowest recovery rates were 58% for factor VIII, 72% for protein S and 75% for both vWF assays compared to control. The recovery rates for the remaining parameters ranged from 79% to 93%.
The lowest recovery rates were 25% for factor V and factor VIII compared to FFP controls. The recovery rates for the remaining parameters ranged from 52% to 92%.
NS – not statistically significant (P ≥ 0.05)
Table 3.
Clinical chemistry analytes in spray dried plasma
| Spray Dried Plasma* | ||||||
|---|---|---|---|---|---|---|
| Standard FFP | ||||||
| control† | 1.5% glycine† | Deionized water† | ||||
| Parameter | mean | ± SD | mean | ± SD | mean | ± SD |
| Total protein (g/dL) | 6.0 | 0.5 | 6.2 | 0.5 | 6.1 | 0.7 |
| Albumin (g/dL) | 3.1 | 0.2 | 3.1 | 0.2 | 3.1 | 0.2 |
| IgG (mg/dL) | 975.7 | 305.5 | 942.8 | 241.4 | 975.6 | 302.6 |
| IgA (mg/dL) | 222.3 | 94.2 | 220.5 | 83.2 | 222.3 | 94.3 |
| IgM (mg/dL) | 68.0 | 30.9 | 71.5 | 38.3 | 67.3 | 32.4 |
The recovery for all parameters in either spray dried plasma product was 96% or higher.
Single donor products were analyzed. There was no statistically significant difference for the five parameters between the three groups (n = 6; P ≥ 0.05; Kruskal-Wallis test).
Discussion
The development of a plasma product that can easily be stored, transported, rapidly reconstituted, and transfused is key to advancing treatment in coagulopathic patients.16 Spray dried plasma would have a number of crucial advantages: rapid formation of very small particles, ability to utilize single donor plasma, option for autologous plasma, option for drying in a closed, sterile single-use disposable kit, and relatively inexpensive cost (Table 4). Utilizing a single donor source provides an additional safety measure by reducing the number of potential donor exposures. It thus creates an advantage over lyophilized plasma products that can require pooling from multiple donors. Furthermore, given volume constraints, spray drying plasma would be sufficient for creating an autologous stable plasma product that negates the use of a cold chain.
Table 4.
Comparison between spray dried plasma and lyophilized plasma products
| Human plasma |
||
|---|---|---|
| Parameter | Spray Dried | Lyophilized |
| Particle size | Powder from droplets | Bulk material (no droplets) |
| Rate of formation | Few hours or less | One to several days |
| Reconstitution time | One to few minutes | Few minutes |
| Plasma source | Intended for single donor | Single or pooled donors |
| Autologous plasma | Easy to apply | Possible |
| Throughput | Low to medium | Medium to high |
| Equipment | Desk top | Mainframe |
| Production sites | Possibly many, distributed | Often few, centralized |
A preparation of a small volume dried plasma product that is stable for long periods at room temperature and readily reconstituted at the point of use would have many advantages for civilian and military transfusion needs. The current two site study, which approximates the collection of blood at a mobile site and shipment to a processing laboratory, illustrates a novel methodology for creating an alternative to frozen human plasma, thereby obviating the need for maintaining an expensive cold chain and negating losses of plasma products from cracking or breaking of plasma bags during handling, shipping and thaw. As many as a third of FFP bags fracture during transit to remote areas, as well as, the thawing process.17 Elimination of the shipping, storage, and thawing processes could enable first responders to utilize life saving plasma in physiologic quantities at the initial point of care.
Despite controversy on the appropriate amount, ratio, and setting for transfusion of plasma in both civilian and military patients, the literature demonstrates early intervention is critical in the coagulopathic patient.18–20 Clearly aggressive management with FFP administration has been shown to be life saving, but component therapy may not always be available and logistic limitations and technical requirements are often too cumbersome for adequate plasma replacement. In such situations, fresh whole blood (FWB) has sometimes been utilized.21 US military personal reported transfusing 13% FWB to soldiers in Operation Iraqi Freedom.22 As such, alternative plasma sources that are easily maintained and rapidly available hold great promise.
Spray dried plasma allows for a rapid dehydration process that results in very small particles that dissolve quickly. We allotted 5 minutes for reconstitution and do not anticipate any practically relevant difference in reconstitution time between spray dried and freeze-dried plasma products. 5 The brief heat exposure must not adversely affect heat labile proteins, as exemplified by our excellent factor V recovery. Although alternative products may have higher recovery rates for factor VIII, hemostasis can be achieved with factor VIII levels of 30%.23 The spray dry process is conducted in a single use system, and is an ideal template for establishing a novel technique for creating a clinically effective transfusable blood product that is stable at room temperature until time of reconstitution. Moreover, beyond the traditional therapeutic transfusion practice for reversing coagulopathies, spray dried plasma could serve as an important source for autologous plasma use in in vitro cultures to generate cellular therapy products.
Although a small sample size, this preliminary proof of principle study highlights an important step to developing an FFP alternative. Utilization of 1.5% glycine was shown to be an effective reconstitution fluid, and previous reports have highlighted its relative ease and safety.13,24,25 Future studies are necessary to establish the optimum reconstitution solution and fluid ratio, potential immunogenicity changes to spray dried human plasma, isohemagglutinin influence, as well as determine long term stability at refrigerated and room temperatures for the spray dried plasma microparticles.
Acknowledgement
We would like to thank Jürgen Bux and Klaus Koerner for sharing information on German lyophilized products and Sherry Sheldon for technical and logistical support of this project.
Footnotes
Authorship contribution. G.S.B. participated in study design, sample collection, data analysis, and wrote the manuscript. K.N. performed and J.L. supervised the clinical chemistry and coagulation testing. D.C. supervised the spray drying of the plasma units. H.G.K. initiated the study and contributed to study design, discussion, and manuscript preparation. W.A.F. contributed to study design, data discussion, and writing of the manuscript.
Statement of disclaimer. The views expressed do not necessarily represent the view of the National Institutes of Health, the Department of Health and Human Services, or the U.S. Federal Government.
Conflict-of-interest disclosure. D.C. discloses he is an employee and stakeholder in Velico Medical. The other authors have nothing to disclose.
References
- 1.Hu S, Loo JA, Wong DT. Human body fluid proteome analysis. Proteomics. 2006;6:6326–6353. doi: 10.1002/pmic.200600284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Association of Blood Banks. Technical Manual: 50th Anniversary AABB Edition 1953-2003. 14th ed. Bethesda, Md: American Association of Blood Banks; 2002. [Google Scholar]
- 3.Daban JL, Clapson P, Ausset S, Deshayes AV, Sailliol A. Freeze dried plasma: a French army specialty. Crit Care. 2010;14:412. doi: 10.1186/cc8937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dagan JL. Le plasma cryodesséché : un produit stable et rapidement disponible pour les opérations militares. Ann Fr Anesth Reanim 2009, 28:S143.DRK-Blutspendedienst West. Humanes S/D-Plasma, lyophilisiert. Hämotherapie. 2004;2:R1–R8. [Google Scholar]
- 5.DRK-Blutspendedienst West. Humanes S/D-Plasma, lyophilisiert. Hämotherapie. 2004;2:R1–R8. [Google Scholar]
- 6.Bux J. Gefriergetrocknetes Plasma - Renaissance eines Klassikers. Hämotherapie. 2009;13:33–37. [Google Scholar]
- 7.Steil L, Thiele T, Hammer E, Bux J, Kalus M, Völker U, Greinacher A. Proteomic characterization of freeze-dried human plasma: providing treatment of bleeding disorders without the need for a cold chain. Transfusion. 2008;48:2356–2363. doi: 10.1111/j.1537-2995.2008.01856.x. [DOI] [PubMed] [Google Scholar]
- 8.Heim MU, Meyer B, Hellstern P. Recommendations for the use of therapeutic plasma. Curr Vasc Pharmacol. 2009;7:110–119. doi: 10.2174/157016109787455671. [DOI] [PubMed] [Google Scholar]
- 9.Torrallardona D, Conde MR, Badiola I, Polo J, Brufau J. Effect of fishmeal replacement with spray-dried animal plasma and colistin on intestinal structure, intestinal microbiology, and performance of weanling pigs challenged with Escherichia coli K99. J Anim Sci. 2003;81:1220–1226. doi: 10.2527/2003.8151220x. [DOI] [PubMed] [Google Scholar]
- 10.Quigley JD, Wolfe TM. Effects of spray-dried animal plasma in calf milk replacer on health and growth of dairy calves. J Dairy Sci. 2003;86:586–592. doi: 10.3168/jds.S0022-0302(03)73637-6. [DOI] [PubMed] [Google Scholar]
- 11.Van Dijk AJ, Enthoven PM, Van den Hoven SG, Van Laarhoven MM, Niewold TA, Nabuurs MJ, Beynen AC. The effect of dietary spray-dried porcine plasma on clinical response in weaned piglets challenged with a pathogenic Escherichia coli. Vet Microbiol. 2002;84:207–218. doi: 10.1016/s0378-1135(01)00463-1. [DOI] [PubMed] [Google Scholar]
- 12.Frank JW, Carroll JA, Allee GL, Zannelli ME. The effects of thermal environment and spray-dried plasma on the acute-phase response of pigs challenged with lipopolysaccharide. J Anim Sci. 2003;81:1166–1176. doi: 10.2527/2003.8151166x. [DOI] [PubMed] [Google Scholar]
- 13.Bothiraja C, Shinde MB, Rajalakshmi S, Pawar AP. Evaluation of molecular pharmaceutical and in-vivo properties of spray-dried isolated andrographolide-PVP. J Pharm Pharmacol. 2009;61:1465–1472. doi: 10.1211/jpp/61.11.0005. [DOI] [PubMed] [Google Scholar]
- 14.Ohtake S, Martin RA, Yee L, Chen D, Kristensen DD, Lechuga-Ballesteros D, Truong-Le V. Heat-stable measles vaccine produced by spray drying. Vaccine. 2010;28:1275–1284. doi: 10.1016/j.vaccine.2009.11.024. [DOI] [PubMed] [Google Scholar]
- 15.Smak Gregoor PJ, Harvey MS, Briët E, Brand A. Coagulation parameters of CPD fresh-frozen plasma and CPD cryoprecipitate-poor plasma after storage at 4 degrees C for 28 days. Transfusion. 1993;33:735–738. doi: 10.1046/j.1537-2995.1993.33994025023.x. [DOI] [PubMed] [Google Scholar]
- 16.Shuja FS, Finkelstein RA, Fukedome E, Duggan M, Kheirbek T, Hamwi K, Fischer TH, Fikry K, Demoya M, Velmahos GC, Alam HB. Development and Testing of Low- Volume Hyperoncotic, Hyperosmotic Spray-Dried Plasma for the Treatment of Trauma- Associated Coagulopathy. J Trauma. 2011;70:664–671. doi: 10.1097/TA.0b013e31820e83be. [DOI] [PubMed] [Google Scholar]
- 17.Mabry RL, Holcomb JB, Baker AM, Cloonan CC, Uhorchak JM, Perkins DE, Canfield AJ, Hagmann JH. United States Army Rangers in Somalia: an analysis of combat casualties on an urban battlefield. J Trauma. 2000;49:515–528. doi: 10.1097/00005373-200009000-00021. discussion 528-529. [DOI] [PubMed] [Google Scholar]
- 18.Bormanis J. Development of a massive transfusion protocol. Transfus Apher Sci. 2008;38:57–63. doi: 10.1016/j.transci.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 19.Nunez TC, Dutton WD, May AK, Holcomb JB, Young PP, Cotton BA. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. Transfusion. 2010;50:1914–1920. doi: 10.1111/j.1537-2995.2010.02682.x. [DOI] [PubMed] [Google Scholar]
- 20.Spinella PC, Holcomb JB. Resuscitation and transfusion principles for traumatic hemorrhagic shock. Blood Rev. 2009;23:231–240. doi: 10.1016/j.blre.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gonzalez EA, Moore FA, Holcomb JB, Miller CC, Kozar RA, Todd SR, Cocanour CS, Balldin BC, McKinley BA. Fresh frozen plasma should be given earlier to patients requiring massive transfusion. J Trauma. 2007;62:112–119. doi: 10.1097/01.ta.0000250497.08101.8b. [DOI] [PubMed] [Google Scholar]
- 22.Kauvar DS, Holcomb JB, Norris GC, Hess JR. Fresh whole blood transfusion: a controversial military practice. J Trauma. 2006;61:181–184. doi: 10.1097/01.ta.0000222671.84335.64. [DOI] [PubMed] [Google Scholar]
- 23.Gilbert MS. Musculoskeletal complications of haemophilia: the joint. Haemophilia. 2000;6(Suppl 1):34–37. doi: 10.1046/j.1365-2516.2000.00044.x. [DOI] [PubMed] [Google Scholar]
- 24.Spoerke N, Zink K, Cho SD, Differding J, Muller P, Karahan A, Sondeen J, Holcomb JB, Schreiber M. Lyophilized plasma for resuscitation in a swine model of severe injury. Arch Surg. 2009;144:829–834. doi: 10.1001/archsurg.2009.154. [DOI] [PubMed] [Google Scholar]
- 25.Shuja F, Shults C, Duggan M, Tabbara M, Butt MU, Fischer TH, Schreiber MA, Tieu B, Holcomb JB, Sondeen JL, Demoya M, Velmahos GC, Alam HB. Development and Testing of Freeze-Dried Plasma for the Treatment of Trauma-Associated Coagulopathy. J Trauma. 2008;65:975–985. doi: 10.1097/TA.0b013e3181801cd9. [DOI] [PubMed] [Google Scholar]; Schoenfeld H, Pruss A, Keller M, Schuster M, Meinck K, Neuner B, von Heymann C. Lyophilised plasma: evaluation of clotting factor activity over 6 days after reconstitution for transfusion. J Clin Pathol. 2010;63:726–730. doi: 10.1136/jcp.2010.079293. [DOI] [PubMed] [Google Scholar]